Two Players in the Field: Hierarchical Model of Interaction between the Dopamine and Acetylcholine Signaling Systems in the Striatum
Abstract
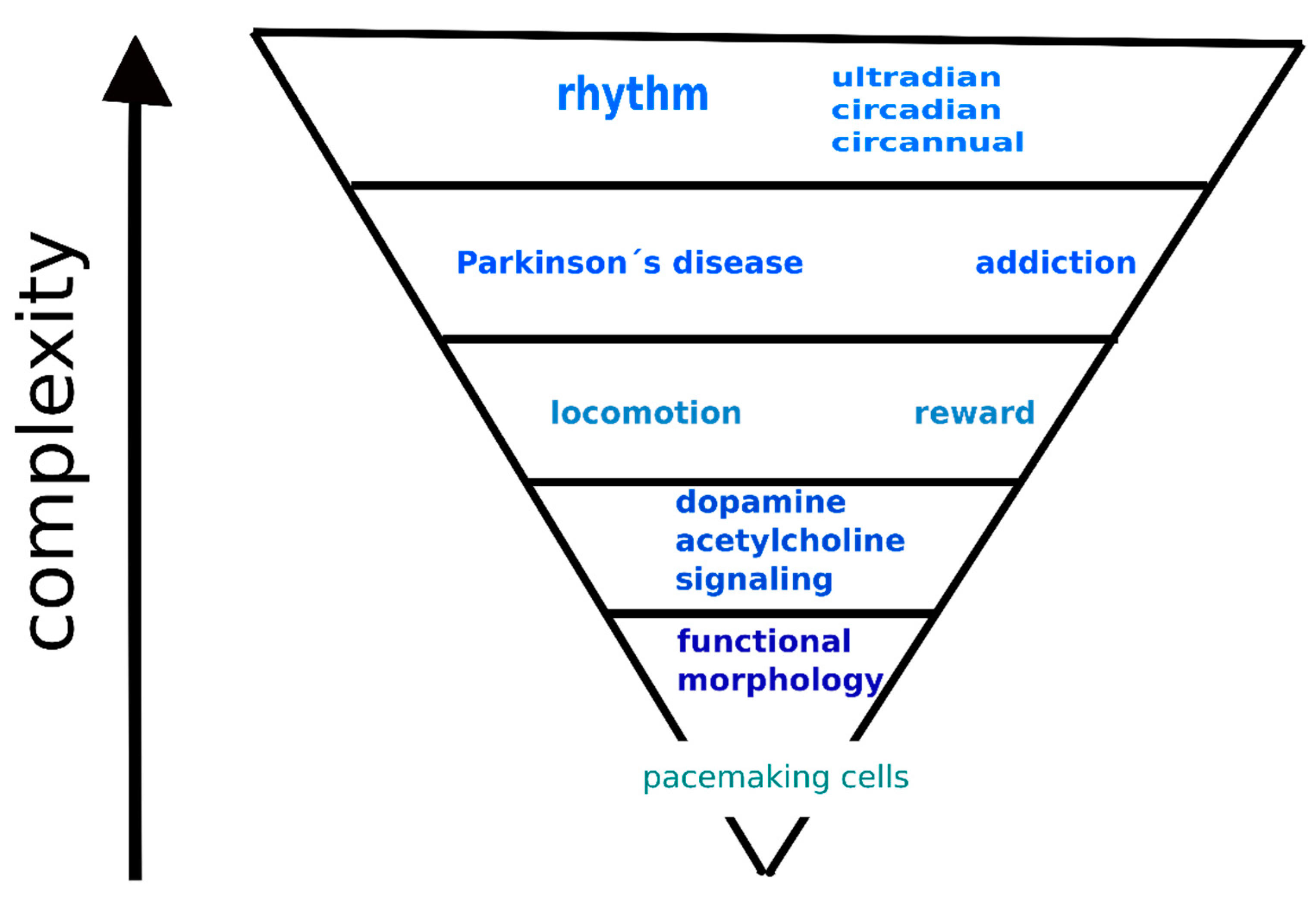
:1. Introduction
2. Functional Morphology of Striatal Connections
2.1. Classic Scheme of the Interaction between the Dopamine Pathway and Cholinergic Interneurons in the Striatum
2.2. Expression of Specific Receptors and Signaling through Receptors
2.3. Some Peculiarities in Striatal Neurotransmitter Function
3. Specific Functional Interconnection between Dopamine Receptors and Muscarinic Receptors
3.1. Locomotor Activity
3.2. Some Aspects of Addiction/Reward
3.3. Biological Rhythm
3.4. Contribution of Signaling Interactions to the Regulation of Behavioral Outcomes
4. Hierarchical Model of the Interaction between Dopamine and Acetylcholine Signaling Systems
5. Conclusions
Funding
Conflicts of Interest
References
- McCutcheon, R.A.; Abi-Dargham, A.; Howes, O.D. Schizophrenia, Dopamine and the Striatum: From Biology to Symptoms. Trends Neurosci. 2019, 42, 205–220. [Google Scholar] [CrossRef] [Green Version]
- Myslivecek, J.; Farar, V.; Valuskova, P. M(4) muscarinic receptors and locomotor activity regulation. Physiol. Res. 2017, 66, S443–S455. [Google Scholar] [CrossRef]
- Yager, L.M.; Garcia, A.F.; Wunsch, A.M.; Ferguson, S.M. The ins and outs of the striatum: Role in drug addiction. Neuroscience 2015, 301, 529–541. [Google Scholar] [CrossRef] [Green Version]
- Clos, M.; Bunzeck, N.; Sommer, T. Dopamine is a double-edged sword: Dopaminergic modulation enhances memory retrieval performance but impairs metacognition. Neuropsychopharmacology 2019, 44, 555–563. [Google Scholar] [CrossRef] [Green Version]
- Lloyd, K.; Dayan, P. Safety out of control: Dopamine and defence. Behav. Brain Funct. 2016, 12, 1–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valjent, E.; Gangarossa, G. The Tail of the Striatum: From Anatomy to Connectivity and Function. Trends Neurosci. 2021, 44. [Google Scholar] [CrossRef]
- Jaušovec, N. The neural code of intelligence: From correlation to causation. Phys. Life Rev. 2019, 31, 171–187. [Google Scholar] [CrossRef] [PubMed]
- Jabourian, M.; Venance, L.; Bourgoin, S.; Ozon, S.; Pérez, S.; Godeheu, G.; Glowinski, J.; Kemel, M.-L. Functional mu opioid receptors are expressed in cholinergic interneurons of the rat dorsal striatum: Territorial specificity and diurnal variation. Eur. J. Neurosci. 2005, 21, 3301–3309. [Google Scholar] [CrossRef]
- Saga, Y.; Hoshi, E.; Tremblay, L. Roles of Multiple Globus Pallidus Territories of Monkeys and Humans in Motivation, Cognition and Action: An Anatomical, Physiological and Pathophysiological Review. Front. Neuroanat. 2017, 11. [Google Scholar] [CrossRef] [Green Version]
- Aosaki, T.; Miura, M.; Suzuki, T.; Nishimura, K.; Masuda, M. Acetylcholine–dopamine balance hypothesis in the striatum: An update. Geriatr. Gerontol. Int. 2010, 10, S148–S157. [Google Scholar] [CrossRef]
- Tanimura, A.; Pancani, T.; Lim, S.A.O.; Tubert, C.; Melendez, A.E.; Shen, W.; Surmeier, D.J. Striatal cholinergic interneurons and Parkinson’s disease. Eur. J. Neurosci. 2018, 47, 1148–1158. [Google Scholar] [CrossRef]
- Pancani, T.; Foster, D.J.; Moehle, M.S.; Bichell, T.J.; Bradley, E.; Bridges, T.M.; Klar, R.; Poslusney, M.; Rook, J.M.; Daniels, J.S.; et al. Allosteric activation of M4 muscarinic receptors improve behavioral and physiological alterations in early symptomatic YAC128 mice. Proc. Natl. Acad. Sci. USA 2015, 112, 14078–14083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farar, V.; Mohr, F.; Legrand, M.; d’Incamps, B.L.; Cendelin, J.; Leroy, J.; Abitbol, M.; Bernard, V.; Baud, F.; Fournet, V.; et al. Near-complete adaptation of the PRiMA knockout to the lack of central acetylcholinesterase. J. Neurochem. 2012, 122, 1065–1080. [Google Scholar] [CrossRef] [PubMed]
- Esterlis, I.; Hannestad, J.O.; Bois, F.; Sewell, R.A.; Tyndale, R.F.; Seibyl, J.P.; Picciotto, M.R.; Laruelle, M.; Carson, R.E.; Cosgrove, K.P. Imaging changes in synaptic acetylcholine availability in living human subjects. J. Nucl. Med. 2013, 54, 78–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lane, T.A.; Boerner, T.; Bannerman, D.M.; Kew, J.N.; Tunbridge, E.M.; Sharp, T.; Harrison, P.J. Decreased striatal dopamine in group II metabotropic glutamate receptor (mGlu2/mGlu3) double knockout mice. BMC Neurosci. 2013, 14, 102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gatica, R.I.; Aguilar-Rivera, M.Í.; Azocar, V.H.; Fuentealba, J.A. Individual Differences in Amphetamine Locomotor Sensitization are Accompanied with Changes in Dopamine Release and Firing Pattern in the Dorsolateral Striatum of Rats. Neuroscience 2020, 427, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Nutt, D.J.; Lingford-Hughes, A.; Erritzoe, D.; Stokes, P.R.A. The dopamine theory of addiction: 40 years of highs and lows. Nat. Rev. Neurosci. 2015, 16, 305–312. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Pozzo-Miller, L. Dysfunction of the corticostriatal pathway in autism spectrum disorders. J. Neurosci. Res. 2020, 98, 2130–2147. [Google Scholar] [CrossRef]
- Foster, D.J.; Gentry, P.R.; Lizardi-Ortiz, J.E.; Bridges, T.M.; Wood, M.R.; Niswender, C.M.; Sulzer, D.; Lindsley, C.W.; Xiang, Z.; Conn, P.J. M5 receptor activation produces opposing physiological outcomes in dopamine neurons depending on the receptor’s location. J. Neurosci. 2014, 34, 3253–3262. [Google Scholar] [CrossRef] [Green Version]
- Gomeza, J.; Zhang, L.; Kostenis, E.; Felder, C.C.; Bymaster, F.P.; Brodkin, J.; Shannon, H.; Xia, B.; Duttaroy, A.; Deng, C.X.; et al. Generation and pharmacological analysis of M2 and M4 muscarinic receptor knockout mice. Life Sci. 2001, 68, 2457–2466. [Google Scholar] [CrossRef]
- Chambers, N.E.; Meadows, S.M.; Taylor, A.; Sheena, E.; Lanza, K.; Conti, M.M.; Bishop, C. Effects of Muscarinic Acetylcholine m1 and m4 Receptor Blockade on Dyskinesia in the Hemi-Parkinsonian Rat. Neuroscience 2019. [Google Scholar] [CrossRef]
- Jeon, J.; Dencker, D.; Wörtwein, G.; Woldbye, D.P.D.; Cui, Y.; Davis, A.A.; Levey, A.I.; Schütz, G.; Sager, T.N.; Mørk, A.; et al. A Subpopulation of Neuronal M4 Muscarinic Acetylcholine Receptors Plays a Critical Role in Modulating Dopamine-Dependent Behaviors. J. Neurosci. 2010, 30, 2396–2405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nair, A.G.; Castro, L.R.V.; El Khoury, M.; Gorgievski, V.; Giros, B.; Tzavara, E.T.; Hellgren-Kotaleski, J.; Vincent, P. The high efficacy of muscarinic M4 receptor in D1 medium spiny neurons reverses striatal hyperdopaminergia. Neuropharmacology 2019, 146, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, M.; Woldbye, D.P.; Wortwein, G.; Fink-Jensen, A.; Wess, J.; Caine, S.B. Reduced cocaine self-administration in muscarinic M5 acetylcholine receptor-deficient mice. J. Neurosci. 2005, 25, 8141–8149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ztaou, S.; Maurice, N.; Camon, J.; Guiraudie-Capraz, G.; Kerkerian-Le Goff, L.; Beurrier, C.; Liberge, M.; Amalric, M. Involvement of Striatal Cholinergic Interneurons and M1 and M4 Muscarinic Receptors in Motor Symptoms of Parkinson’s Disease. J. Neurosci. 2016, 36, 9161–9172. [Google Scholar] [CrossRef]
- Narushima, M.; Uchigashima, M.; Fukaya, M.; Matsui, M.; Manabe, T.; Hashimoto, K.; Watanabe, M.; Kano, M. Tonic Enhancement of Endocannabinoid-Mediated Retrograde Suppression of Inhibition by Cholinergic Interneuron Activity in the Striatum. J. Neurosci. 2007, 27, 496–506. [Google Scholar] [CrossRef] [Green Version]
- Surmeier, D.J.; Mercer, J.N.; Chan, C.S. Autonomous pacemakers in the basal ganglia: Who needs excitatory synapses anyway? Curr. Opin. Neurobiol. 2005, 15, 312–318. [Google Scholar] [CrossRef]
- Kawaguchi, Y.; Wilson, C.; Emson, P. Projection subtypes of rat neostriatal matrix cells revealed by intracellular injection of biocytin. J. Neurosci. 1990, 10, 3421–3438. [Google Scholar] [CrossRef] [Green Version]
- Shin, J.H.; Adrover, M.F.; Wess, J.; Alvarez, V.A. Muscarinic regulation of dopamine and glutamate transmission in the nucleus accumbens. Proc. Natl. Acad. Sci. USA 2015. [Google Scholar] [CrossRef] [Green Version]
- Moehle, M.S.; Pancani, T.; Byun, N.; Yohn, S.E.; Wilson Iii, G.H.; Dickerson, J.W.; Remke, D.H.; Xiang, Z.; Niswender, C.M.; Wess, J.; et al. Cholinergic Projections to the Substantia Nigra Pars Reticulata Inhibit Dopamine Modulation of Basal Ganglia through the M4 Muscarinic Receptor. Neuron 2017, 96, 1358–1372.e1354. [Google Scholar] [CrossRef] [Green Version]
- Garção, P.; Szabó, E.C.; Wopereis, S.; Castro, A.A.; Tomé, Â.R.; Prediger, R.D.; Cunha, R.A.; Agostinho, P.; Köfalvi, A. Functional interaction between presynaptic α6β2-containing nicotinic and adenosine A2A receptors in the control of dopamine release in the rat striatum. Br. J. Pharm. 2013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marks, M.J.; Grady, S.R.; Salminen, O.; Paley, M.A.; Wageman, C.R.; McIntosh, J.M.; Whiteaker, P. α6β2*-subtype nicotinic acetylcholine receptors are more sensitive than α4β2*-subtype receptors to regulation by chronic nicotine administration. J. Neurochem. 2014, 130, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Soll, L.G.; Grady, S.R.; Salminen, O.; Marks, M.J.; Tapper, A.R. A role for α4(non-α6)* nicotinic acetylcholine receptors in motor behavior. Neuropharmacology 2013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikeda, E.; Matsunaga, N.; Kakimoto, K.; Hamamura, K.; Hayashi, A.; Koyanagi, S.; Ohdo, S. Molecular mechanism regulating 24-hour rhythm of dopamine D3 receptor expression in mouse ventral striatum. Mol. Pharm. 2013, 83, 959–967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coffey, K.R.; Nader, M.; Bawa, J.; West, M.O. Homogeneous processing in the striatal direct and indirect pathways: Single body part sensitive type IIb neurons may express either dopamine receptor D1 or D2. Eur. J. Neurosci. 2017, 46, 2380–2391. [Google Scholar] [CrossRef]
- Gatev, P.; Darbin, O.; Wichmann, T. Oscillations in the basal ganglia under normal conditions and in movement disorders. Mov. Disord. 2006, 21, 1566–1577. [Google Scholar] [CrossRef]
- Kravitz, A.V.; Freeze, B.S.; Parker, P.R.L.; Kay, K.; Thwin, M.T.; Deisseroth, K.; Kreitzer, A.C. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature 2010, 466, 622–626. [Google Scholar] [CrossRef] [Green Version]
- Moehle, M.S.; Conn, P.J. Roles of the M4 acetylcholine receptor in the basal ganglia and the treatment of movement disorders. Mov. Disord. 2019, 34, 1089–1099. [Google Scholar] [CrossRef]
- Suzuki, E.; Momiyama, T. M1 muscarinic acetylcholine receptor-mediated inhibition of GABA release from striatal medium spiny neurons onto cholinergic interneurons. Eur. J. Neurosci. 2021. [Google Scholar] [CrossRef]
- Koranda, J.L.; Cone, J.J.; McGehee, D.S.; Roitman, M.F.; Beeler, J.A.; Zhuang, X. Nicotinic Receptors Regulate the Dynamic Range of Dopamine Release in Vivo. J. Neurophysiol. 2014, 111, 103–111. [Google Scholar] [CrossRef] [Green Version]
- Kljakic, O.; Janickova, H.; Prado, V.F.; Prado, M.A.M. Cholinergic/glutamatergic co-transmission in striatal cholinergic interneurons: New mechanisms regulating striatal computation. J. Neurochem. 2017, 142, 90–102. [Google Scholar] [CrossRef] [PubMed]
- Klein, M.O.; Battagello, D.S.; Cardoso, A.R.; Hauser, D.N.; Bittencourt, J.C.; Correa, R.G. Dopamine: Functions, Signaling, and Association with Neurological Diseases. Cell. Mol. Neurobiol. 2019, 39, 31–59. [Google Scholar] [CrossRef] [PubMed]
- Ztaou, S.; Amalric, M. Contribution of cholinergic interneurons to striatal pathophysiology in Parkinson’s disease. Neurochem. Int. 2019, 126, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zoli, M.; Torri, C.; Ferrari, R.; Jansson, A.; Zini, I.; Fuxe, K.; Agnati, L.F. The emergence of the volume transmission concept. Brain Res. Rev. 1998, 26, 136–147. [Google Scholar] [CrossRef]
- Guzman, M.S.; De Jaeger, X.; Raulic, S.; Souza, I.A.; Li, A.X.; Schmid, S.; Menon, R.S.; Gainetdinov, R.R.; Caron, M.G.; Bartha, R.; et al. Elimination of the Vesicular Acetylcholine Transporter in the Striatum Reveals Regulation of Behaviour by Cholinergic-Glutamatergic Co-Transmission. PLoS Biol. 2011, 9, e1001194. [Google Scholar] [CrossRef]
- Villalba, R.M.; Smith, Y. Differential striatal spine pathology in Parkinson’s disease and cocaine addiction: A key role of dopamine? Neuroscience 2013, 251, 2–20. [Google Scholar] [CrossRef] [Green Version]
- Day, J.; Damsma, G.; Fibiger, H.C. Cholinergic activity in the rat hippocampus, cortex and striatum correlates with locomotor activity: An in vivo microdialysis study. Pharm. Biochem. Behav. 1991, 38, 723–729. [Google Scholar] [CrossRef]
- Sun, Z.; Jia, J.; Gong, X.; Jia, Y.; Deng, J.; Wang, X.; Wang, X. Inhibition of glutamate and acetylcholine release in behavioral improvement induced by electroacupuncture in parkinsonian rats. Neurosci. Lett. 2012, 520, 32–37. [Google Scholar] [CrossRef]
- Creed, R.B.; Menalled, L.; Casey, B.; Dave, K.D.; Janssens, H.B.; Veinbergs, I.; van der Hart, M.; Rassoulpour, A.; Goldberg, M.S. Basal and Evoked Neurotransmitter Levels in Parkin, DJ-1, PINK1 and LRRK2 Knockout Rat Striatum. Neuroscience 2019, 409, 169–179. [Google Scholar] [CrossRef]
- Jamwal, S.; Kumar, P. Insight Into the Emerging Role of Striatal Neurotransmitters in the Pathophysiology of Parkinson’s Disease and Huntington’s Disease: A Review. Curr. Neuropharmacol. 2019, 17, 165–175. [Google Scholar] [CrossRef]
- Avena, N.M.; Rada, P.V. Cholinergic modulation of food and drug satiety and withdrawal. Physiol. Behav. 2012, 106, 332–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brami-Cherrier, K.; Lewis, R.G.; Cervantes, M.; Liu, Y.; Tognini, P.; Baldi, P.; Sassone-Corsi, P.; Borrelli, E. Cocaine-mediated circadian reprogramming in the striatum through dopamine D2R and PPARγ activation. Nat. Commun. 2020, 11, 4448. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Custodio, R.J.; Botanas, C.J.; de la Pena, J.B.; Sayson, L.V.; Abiero, A.; Ryoo, Z.Y.; Cheong, J.H.; Kim, H.J. The circadian gene, Per2, influences methamphetamine sensitization and reward through the dopaminergic system in the striatum of mice. Addict. Biol. 2019, 24, 946–957. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, H.; Obata, T.; Takano, H.; Nogami, T.; Suhara, T.; Ito, H. Relation between dopamine synthesis capacity and cell-level structure in human striatum: A multi-modal study with positron emission tomography and diffusion tensor imaging. PLoS ONE 2014, 9, e87886. [Google Scholar] [CrossRef]
- Ruppert, M.C.; Greuel, A.; Tahmasian, M.; Schwartz, F.; Stürmer, S.; Maier, F.; Hammes, J.; Tittgemeyer, M.; Timmermann, L.; van Eimeren, T.; et al. Network degeneration in Parkinson’s disease: Multimodal imaging of nigro-striato-cortical dysfunction. Brain 2020, 143, 944–959. [Google Scholar] [CrossRef]
- Laverty, R.; Michaelson, I.A.; Sharman, D.F.; Whittaker, V.P. The subcellular localization of dopamine and acetylcholine in the dog caudate nucleus. Br. J. Pharm. Chemother. 1963, 21, 482–490. [Google Scholar] [CrossRef] [Green Version]
- Snyder, S.H.; Bennett, J.P., Jr. Neurotransmitter Receptors in the Brain: Biochemical Identification. Annu. Rev. Physiol. 1976, 38, 153–175. [Google Scholar] [CrossRef]
- Reisine, T.D.; Fields, J.Z.; Yamamura, H.I.; Bird, E.D.; Spokes, E.; Schreiner, P.S.; Enna, S.J. Neurotransmitter receptor alterations in Parkinson’s disease. Life Sci. 1977, 21, 335–343. [Google Scholar] [CrossRef]
- Weiner, D.M.; Levey, A.I.; Brann, M.R. Expression of muscarinic acetylcholine and dopamine receptor mRNAs in rat basal ganglia. Proc. Natl. Acad. Sci. USA 1990, 87, 7050–7054. [Google Scholar] [CrossRef] [Green Version]
- Bernard, V.; Normand, E.; Bloch, B. Phenotypical characterization of the rat striatal neurons expressing muscarinic receptor genes. J. Neurosci. Off. J. Soc. Neurosci. 1992, 12, 3591–3600. [Google Scholar] [CrossRef] [Green Version]
- Gomeza, J.; Zhang, L.; Kostenis, E.; Felder, C.; Bymaster, F.; Brodkin, J.; Shannon, H.; Xia, B.; Deng, C.-x.; Wess, J. Enhancement of D1 dopamine receptor-mediated locomotor stimulation in M4 muscarinic acetylcholine receptor knockout mice. Proc. Natl. Acad. Sci. USA 1999, 96, 10483–10488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayorga, A.J.; Gianutsos, G.; Salamone, J.D. Effects of striatal injections of 8-bromo-cyclic-AMP on pilocarpine-induced tremulous jaw movements in rats. Brain Res. 1999, 829, 180–184. [Google Scholar] [CrossRef]
- Gerber, D.J.; Sotnikova, T.D.; Gainetdinov, R.R.; Huang, S.Y.; Caron, M.G.; Tonegawa, S. Hyperactivity, elevated dopaminergic transmission, and response to amphetamine in M1 muscarinic acetylcholine receptor-deficient mice. Proc. Natl. Acad. Sci. USA 2001, 98, 15312–15317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Yamada, M.; Gomeza, J.; Basile, A.S.; Wess, J. Multiple muscarinic acetylcholine receptor subtypes modulate striatal dopamine release, as studied with M1-M5 muscarinic receptor knock-out mice. J. Neurosci. 2002, 22, 6347–6352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Surmeier, D.J.; Ding, J.; Day, M.; Wang, Z.; Shen, W. D1 and D2 dopamine-receptor modulation of striatal glutamatergic signaling in striatal medium spiny neurons. Trends Neurosci. 2007, 30, 228–235. [Google Scholar] [CrossRef]
- Threlfell, S.; Clements, M.A.; Khodai, T.; Pienaar, I.S.; Exley, R.; Wess, J.; Cragg, S.J. Striatal Muscarinic Receptors Promote Activity Dependence of Dopamine Transmission via Distinct Receptor Subtypes on Cholinergic Interneurons in Ventral versus Dorsal Striatum. J. Neurosci. 2010, 30, 3398–3408. [Google Scholar] [CrossRef]
- Yokoi, F.; Oleas, J.; Xing, H.; Liu, Y.; Dexter, K.M.; Misztal, C.; Gerard, M.; Efimenko, I.; Lynch, P.; Villanueva, M.; et al. Decreased number of striatal cholinergic interneurons and motor deficits in dopamine receptor 2-expressing-cell-specific Dyt1 conditional knockout mice. Neurobiol. Dis. 2020, 134, 104638. [Google Scholar] [CrossRef]
- Patel, J.C.; Rossignol, E.; Rice, M.E.; Machold, R.P. Opposing regulation of dopaminergic activity and exploratory motor behavior by forebrain and brainstem cholinergic circuits. Nat. Commun. 2012, 3, 1172. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Flores, T.; Hernández-González, O.; Pérez-Ramírez, M.B.; Lara-González, E.; Arias-García, M.A.; Duhne, M.; Pérez-Burgos, A.; Prieto, G.A.; Figueroa, A.; Galarraga, E.; et al. Modulation of direct pathway striatal projection neurons by muscarinic M4-type receptors. Neuropharmacology 2014. [Google Scholar] [CrossRef]
- Crittenden, J.R.; Lacey, C.J.; Lee, T.; Bowden, H.A.; Graybiel, A.M. Severe drug-induced repetitive behaviors and striatal overexpression of VAChT in ChAT-ChR2-EYFP BAC transgenic mice. Front. Neural Circuits 2014, 8. [Google Scholar] [CrossRef] [Green Version]
- Laplante, F.; Lappi, D.A.; Sullivan, R.M. Cholinergic depletion in the nucleus accumbens: Effects on amphetamine response and sensorimotor gating. Prog. Neuropsychopharmacol. Biol. Psychiatry 2011, 35, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Karasawa, H.; Taketo, M.M.; Matsui, M. Loss of anti-cataleptic effect of scopolamine in mice lacking muscarinic acetylcholine receptor subtype 4. Eur. J. Pharm. 2003, 468, 15–19. [Google Scholar] [CrossRef]
- Nestby, P.; Vanderschuren, L.J.M.J.; De Vries, T.J.; Hogenboom, F.; Wardeh, G.; Mulder, A.H.; Schoffelmeer, A.N.M. Ethanol, like psychostimulants and morphine, causes long-lasting hyperreactivity of dopamine and acetylcholine neurons of rat nucleus accumbens: Possible role in behavioural sensitization. Psychopharmacology 1997, 133, 69–76. [Google Scholar] [CrossRef]
- Hikida, T.; Kaneko, S.; Isobe, T.; Kitabatake, Y.; Watanabe, D.; Pastan, I.; Nakanishi, S. Increased sensitivity to cocaine by cholinergic cell ablation in nucleus accumbens. Proc. Natl. Acad. Sci. USA 2001, 98, 13351–13354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fink-Jensen, A.; Schmidt, L.S.; Dencker, D.; Schülein, C.; Wess, J.; Wörtwein, G.; Woldbye, D.P.D. Antipsychotic-induced catalepsy is attenuated in mice lacking the M4 muscarinic acetylcholine receptor. Eur. J. Pharm. 2011, 656, 39–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dencker, D.; Weikop, P.; Sorensen, G.; Woldbye, D.P.; Wortwein, G.; Wess, J.; Fink-Jensen, A. An allosteric enhancer of M(4) muscarinic acetylcholine receptor function inhibits behavioral and neurochemical effects of cocaine. Psychopharmacology 2012, 224, 277–287. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Darvas, M.; Storey, G.P.; Bamford, I.J.; Gibbs, J.T.; Palmiter, R.D.; Bamford, N.S. Acetylcholine encodes long-lasting presynaptic plasticity at glutamatergic synapses in the dorsal striatum after repeated amphetamine exposure. J. Neurosci. 2013, 33, 10405–10426. [Google Scholar] [CrossRef] [Green Version]
- Acevedo-Rodriguez, A.; Zhang, L.; Zhou, F.; Gong, S.; Gu, H.; De Biasi, M.; Zhou, F.-M.; Dani, J.A. Cocaine Inhibition of Nicotinic Acetylcholine Receptors Influences Dopamine Release. Front. Synaptic Neurosci. 2014, 6, 19. [Google Scholar] [CrossRef] [Green Version]
- de Kloet, S.F.; Mansvelder, H.D.; De Vries, T.J. Cholinergic modulation of dopamine pathways through nicotinic acetylcholine receptors. Biochem. Pharm. 2015, 97, 425–438. [Google Scholar] [CrossRef]
- Xu, W.; Jain, M.K.; Zhang, L. Molecular link between circadian clocks and cardiac function: A network of core clock, slave clock, and effectors. Curr. Opin. Pharm. 2021, 57, 28–40. [Google Scholar] [CrossRef]
- Chartove, J.A.K.; McCarthy, M.M.; Pittman-Polletta, B.R.; Kopell, N.J. A biophysical model of striatal microcircuits suggests gamma and beta oscillations interleaved at delta/theta frequencies mediate periodicity in motor control. PLoS Comput. Biol. 2020, 16, e1007300. [Google Scholar] [CrossRef] [PubMed]
- Ballesta, A.; Innominato, P.F.; Dallmann, R.; Rand, D.A.; Lévi, F.A. Systems Chronotherapeutics. Pharm. Rev. 2017, 69, 161–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dluzen, D.; Ramirez, V.D. In vitro dopamine release from the rat striatum: Diurnal rhythm and its modification by the estrous cycle. Neuroendocrinology 1985, 41, 97–100. [Google Scholar] [CrossRef] [PubMed]
- Nowak, J.Z.; Zurawska, E. Dopamine in the rabbit retina and striatum: Diurnal rhythm and effect of light stimulation. J. Neural Transm. 1989, 75, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Doi, M.; Yujnovsky, I.; Hirayama, J.; Malerba, M.; Tirotta, E.; Sassone-Corsi, P.; Borrelli, E. Impaired light masking in dopamine D2 receptor–null mice. Nat. Neurosci. 2006, 9, 732–734. [Google Scholar] [CrossRef] [PubMed]
- Imbesi, M.; Yildiz, S.; Dirim Arslan, A.; Sharma, R.; Manev, H.; Uz, T. Dopamine receptor-mediated regulation of neuronal “clock” gene expression. Neuroscience 2009, 158, 537–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hood, S.; Cassidy, P.; Cossette, M.P.; Weigl, Y.; Verwey, M.; Robinson, B.; Stewart, J.; Amir, S. Endogenous dopamine regulates the rhythm of expression of the clock protein PER2 in the rat dorsal striatum via daily activation of D2 dopamine receptors. J. Neurosci. 2010, 30, 14046–14058. [Google Scholar] [CrossRef]
- Naber, D.; Wirz-Justice, A.; Kafka, M.S.; Wehr, T.A. Dopamine receptor binding in rat striatum: Ultradian rhythm and its modification by chronic imipramine. Psychopharmacology 1980, 68, 1–5. [Google Scholar] [CrossRef]
- Wirz-Justice, A.; Tobler, I.; Kafka, M.S.; Naber, D.; Marangos, P.J.; Borbély, A.A.; Wehr, T.A. Sleep deprivation: Effects on circadian rhythms of rat brain neurotransmitter receptors. Psychiatry Res. 1981, 5, 67–76. [Google Scholar] [CrossRef]
- Naber, D.; Wirz-Justice, A.; Kafka, M.S.; Tobler, I.; Borbély, A.A. Seasonal variations in the endogenous rhythm of dopamine receptor binding in rat striatum. Biol. Psychiatry 1981, 16, 831–835. [Google Scholar]
- Byrne, J.E.M.; Tremain, H.; Leitan, N.D.; Keating, C.; Johnson, S.L.; Murray, G. Circadian modulation of human reward function: Is there an evidentiary signal in existing neuroimaging studies? Neurosci. Biobehav. Rev. 2019, 99, 251–274. [Google Scholar] [CrossRef] [PubMed]
- Requejo, C.; López-de-Ipiña, K.; Ruiz-Ortega, J.Á.; Fernández, E.; Calvo, P.M.; Morera-Herreras, T.; Miguelez, C.; Cardona-Grifoll, L.; Cepeda, H.; Ugedo, L.; et al. Changes in Day/Night Activity in the 6-OHDA-Induced Experimental Model of Parkinson’s Disease: Exploring Prodromal Biomarkers. Front. Neurosci. 2020, 14. [Google Scholar] [CrossRef] [PubMed]
- Friedman, A.H.; Walker, C.A. The acute toxicity of drugs acting at cholinoceptive sites and twenty-four hour rhythms in brain acetylcholine. Arch. Toxikol. 1972, 29, 39–49. [Google Scholar] [CrossRef]
- Jabourian, M.; Bourgoin, S.; Pérez, S.; Godeheu, G.; Glowinski, J.; Kemel, M.L. μ opioid control of the N-methyl-d-aspartate-evoked release of [3h]-acetylcholine in the limbic territory of the rat striatum in vitro: Diurnal variations and implication of a dopamine link. Neuroscience 2004, 123, 733–742. [Google Scholar] [CrossRef] [PubMed]
- Jabourian, M.; Pérez, S.; Ezan, P.; Glowinski, J.; Deniau, J.-M.; Kemel, M.-L. Impact of 6-hydroxydopamine lesions and cocaine exposure on µ-opioid receptor expression and regulation of cholinergic transmission in the limbic–prefrontal territory of the rat dorsal striatum. Eur. J. Neurosci. 2007, 25, 1546–1556. [Google Scholar] [CrossRef]
- Divito, C.B.; Steece-Collier, K.; Case, D.T.; Williams, S.-P.G.; Stancati, J.A.; Zhi, L.; Rubio, M.E.; Sortwell, C.E.; Collier, T.J.; Sulzer, D.; et al. Loss of VGLUT3 Produces Circadian-Dependent Hyperdopaminergia and Ameliorates Motor Dysfunction and l-Dopa-Mediated Dyskinesias in a Model of Parkinson’s Disease. J. Neurosci. 2015, 35, 14983–14999. [Google Scholar] [CrossRef] [Green Version]
- Kondabolu, K.; Roberts, E.A.; Bucklin, M.; McCarthy, M.M.; Kopell, N.; Han, X. Striatal cholinergic interneurons generate beta and gamma oscillations in the corticostriatal circuit and produce motor deficits. Proc. Natl. Acad. Sci. USA 2016, 113, E3159–E3168. [Google Scholar] [CrossRef] [Green Version]
- Por, S.; Bondy, S. Regional circadian variation of acetylcholine muscarinic receptors in the rat brain. J. Neurosci. Res. 1981, 6, 315–318. [Google Scholar] [CrossRef]
- Pan, S.Y. Circadian effects of scopolamine on memory, exploratory behavior, and muscarinic receptors in mouse brain. Zhongguo Yao Li Xue Bao 1992, 13, 323–326. [Google Scholar]
- Riljak, V.; Janisova, K.; Myslivecek, J. Lack of M4 muscarinic receptors in the striatum, thalamus and intergeniculate leaflet alters the biological rhythm of locomotor activity in mice. Brain Struct. Funct. 2020, 225, 1615–1629. [Google Scholar] [CrossRef]
- Valuskova, P.; Riljak, V.; Forczek, S.T.; Farar, V.; Myslivecek, J. Variability in the Drug Response of M4 Muscarinic Receptor Knockout Mice during Day and Night Time. Front. Pharm. 2019, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, A.; Lee, S.; Kim, H.; Yoon, H.; Ha, S.; Kang, S.U. Molecular Crosstalk between Circadian Rhythmicity and the Development of Neurodegenerative Disorders. Front. Neurosci. 2020, 14. [Google Scholar] [CrossRef] [PubMed]
- Gillman, A.G.; Rebec, G.V.; Pecoraro, N.C.; Kosobud, A.E.K. Circadian entrainment by food and drugs of abuse. Behav. Process. 2019, 165, 23–28. [Google Scholar] [CrossRef] [PubMed]

| Neuron Type | Principal Neurotransmitter | Main Dopamine Receptor Subtype Expressed | Main Pathway/Efferent Structure (or Neurons) Activated |
|---|---|---|---|
| Medium spiny neurons (type I) | GABA | D2 | Indirect pathway/GP |
| Medium spiny neurons (type IIa) | GABA | D1 | Direct pathway/GP, EP, SN |
| Medium spiny neurons (type IIb) | GABA | D1 | Direct pathway/GP, SN |
| Interneurons | Acetylcholine | D1, D5 | Medium spiny neurons |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Myslivecek, J. Two Players in the Field: Hierarchical Model of Interaction between the Dopamine and Acetylcholine Signaling Systems in the Striatum. Biomedicines 2021, 9, 25. https://doi.org/10.3390/biomedicines9010025
Myslivecek J. Two Players in the Field: Hierarchical Model of Interaction between the Dopamine and Acetylcholine Signaling Systems in the Striatum. Biomedicines. 2021; 9(1):25. https://doi.org/10.3390/biomedicines9010025
Chicago/Turabian StyleMyslivecek, Jaromir. 2021. "Two Players in the Field: Hierarchical Model of Interaction between the Dopamine and Acetylcholine Signaling Systems in the Striatum" Biomedicines 9, no. 1: 25. https://doi.org/10.3390/biomedicines9010025





