Sudden Cell Death Induced by Ca2+ Delivery via Microbubble Cavitation
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Line
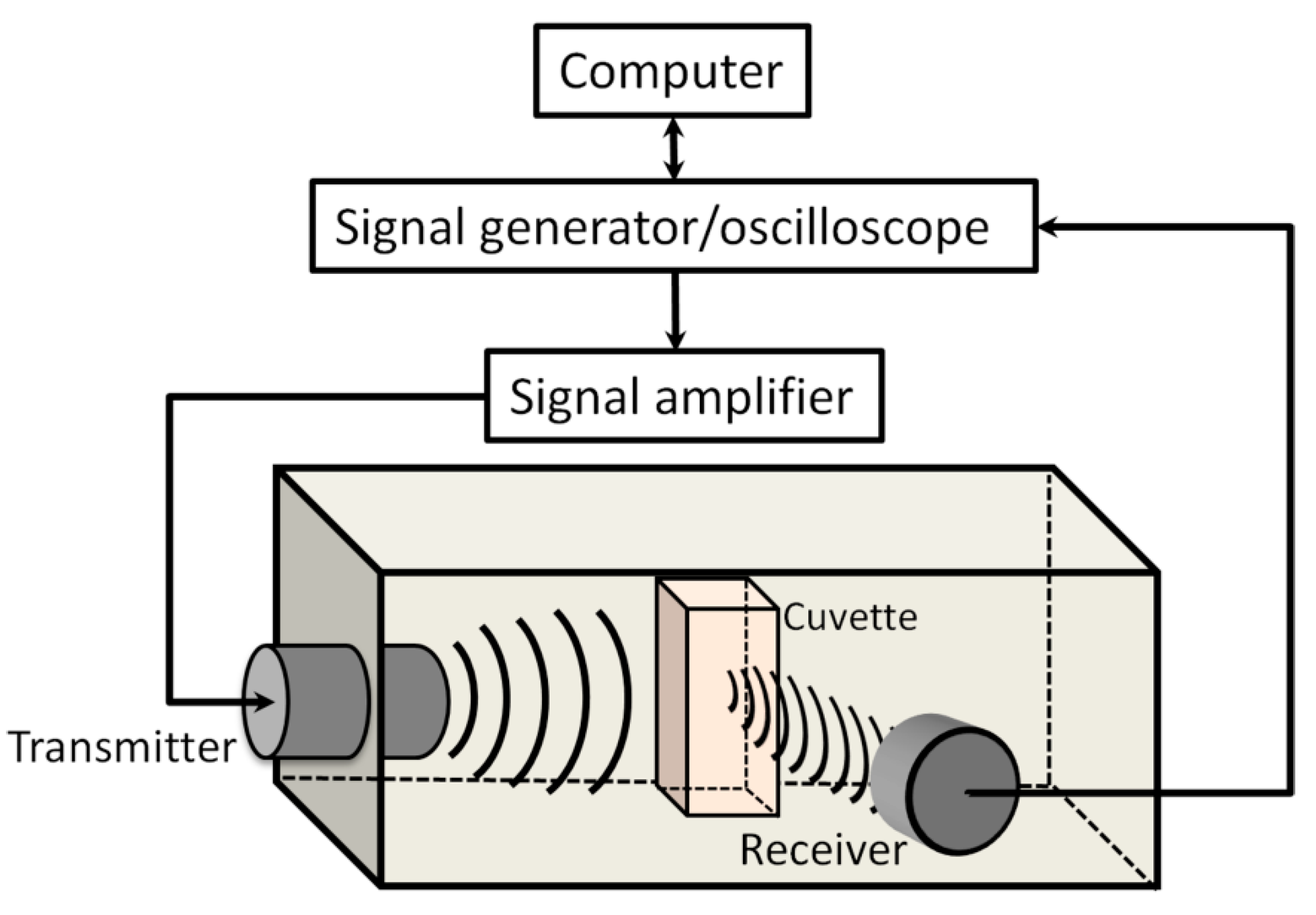
2.2. Experimental Setup
2.3. Experimental Procedure
2.4. Propidium Iodide (PI) Assay
2.5. MTT Assay
2.6. Cell Clonogenic Assay
2.7. Cavitation Signal Analysis
2.8. Statistical Analysis
3. Results
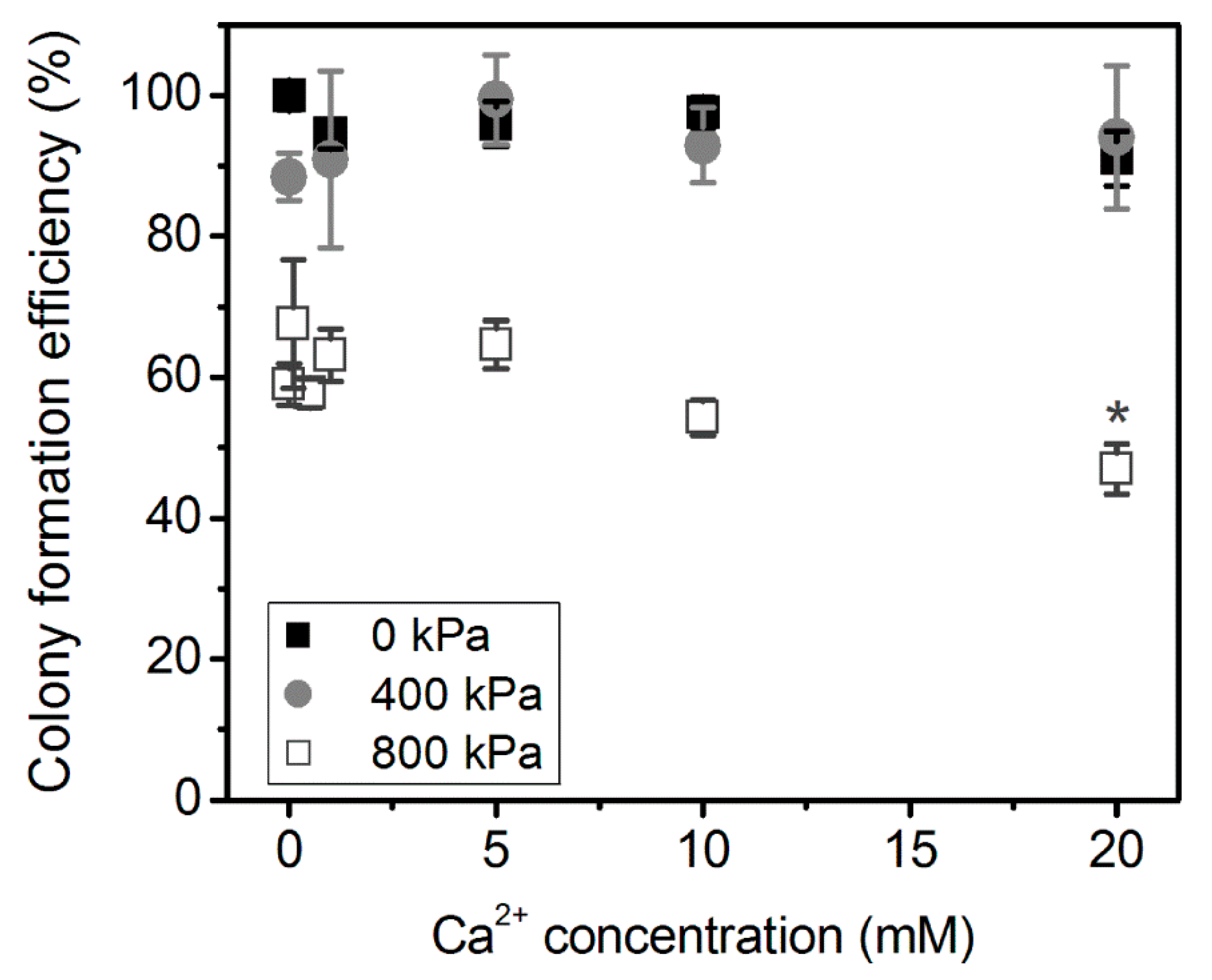
3.1. Ca2+ Delivery Using US Alone
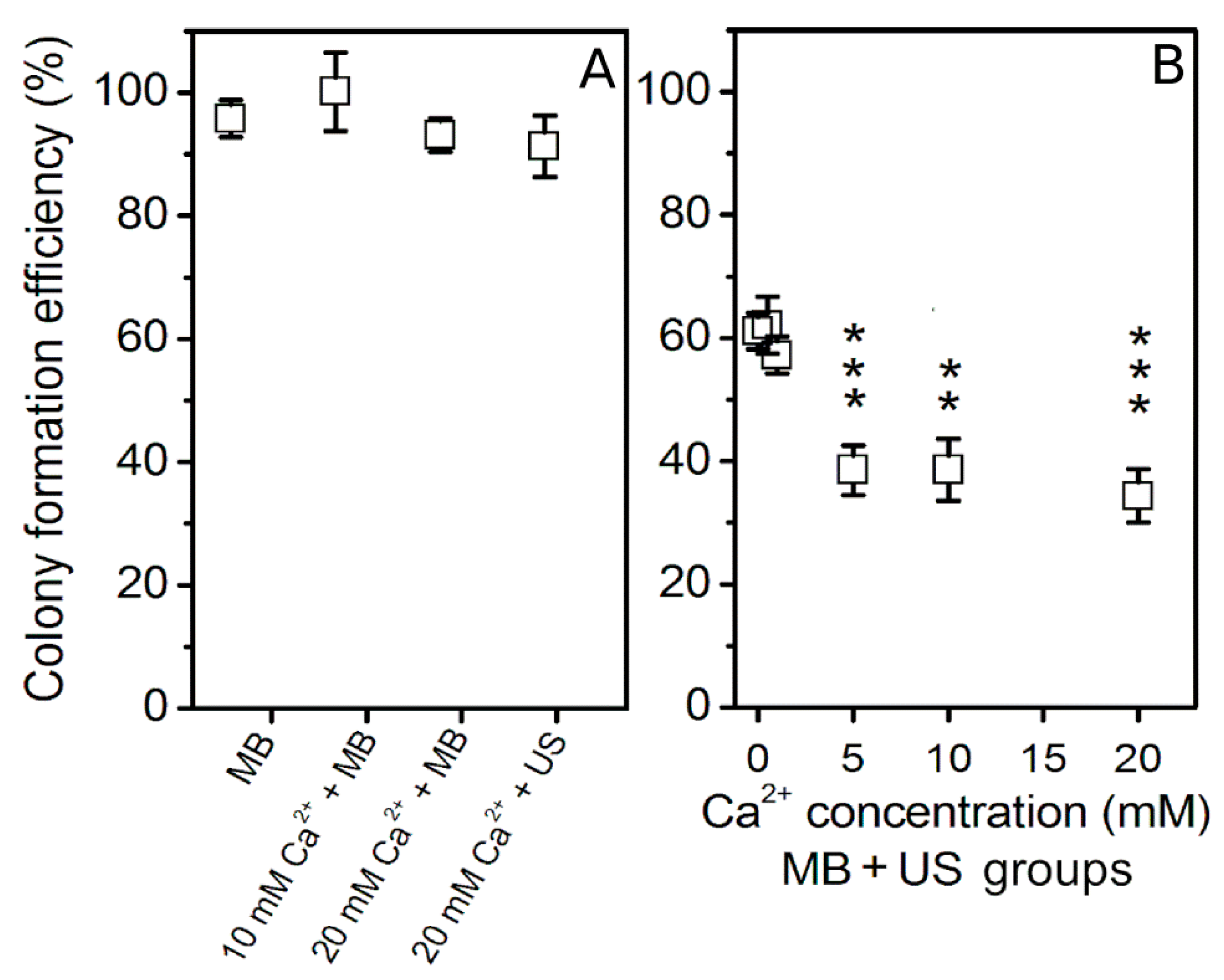
3.2. Ca2+ Delivery Using US and MB Combination
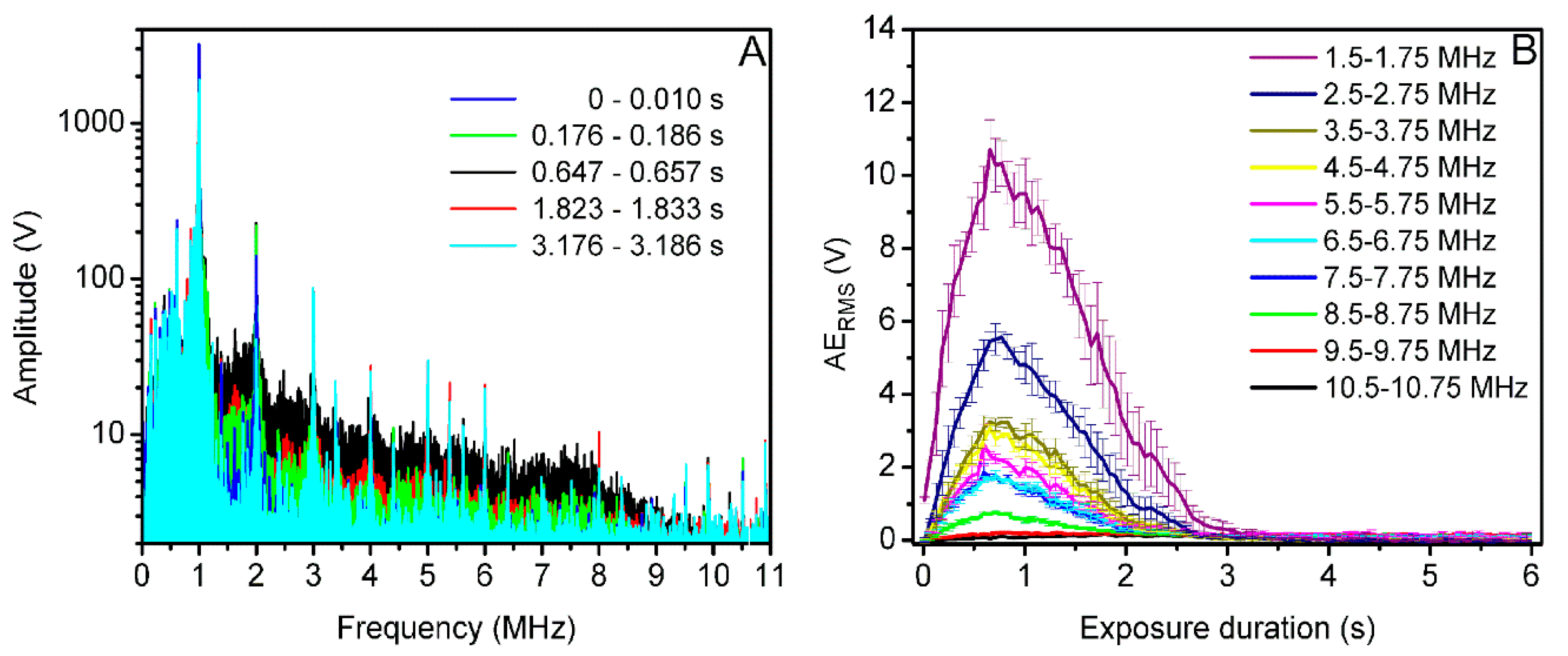
3.3. Acoustic Emissions at 400 kPa Acoustic Pressure
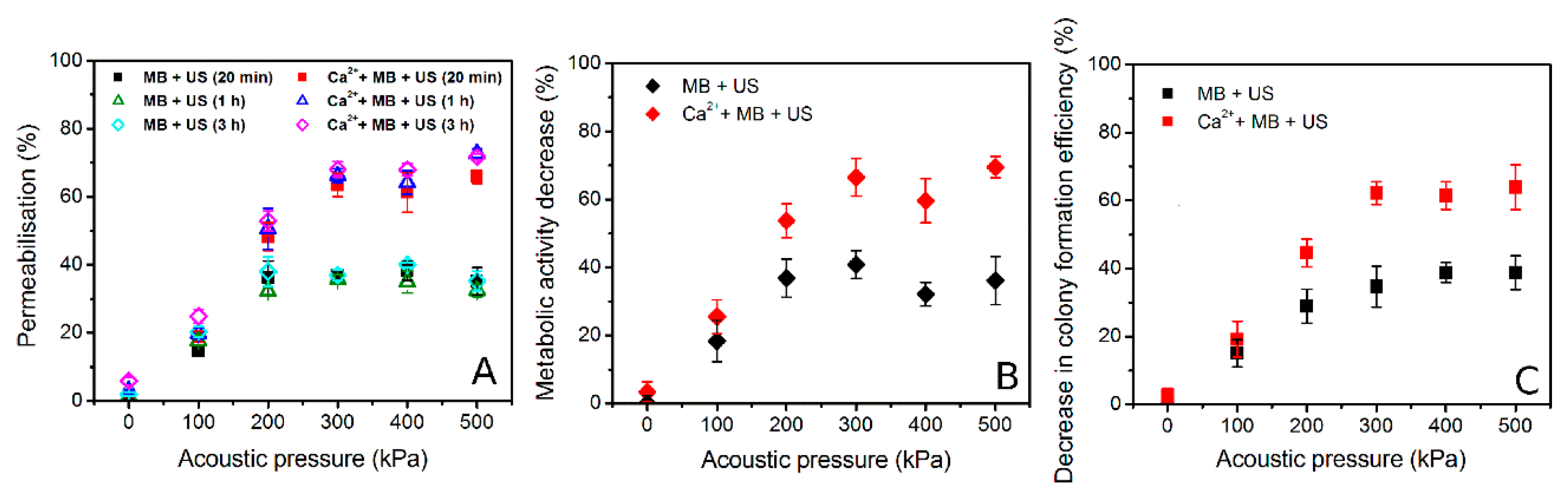
3.4. Sonoporation Results in Acoustic Pressure Scale
3.5. Acoustic Emission Quantification in Acoustic Pressure Scale
3.6. ICD and Biological Effect Correlation
4. Discussion
5. Conclusions
- The increase in Ca2+ concentration during sonoporation negatively affects the fundamental characteristics of cell viability: (i) cell membrane integrity, (ii) cell metabolic activity, and (iii) cell colony formation efficiency, which were independently evaluated using different cell viability assays.
- Ca2+ delivery via sonoporation induces instantly occurring cell death—within 20 min after treatment, with no additional cell death observed within the 2-d to 6-d period.
- High correlation (R2 > 0.85, p < 0.01) of inertial cavitation dose with the results of different-criterion-based cell viability assays implies inertial cavitation activities to be directly involved in calcium delivery via sonoporation.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qin, J.; Wang, T.Y.; Willmann, J.K. Sonoporation: Applications for cancer therapy. Adv. Exp. Med. Biol. 2016, 880, 263–291. [Google Scholar]
- Hoejholt, K.L.; Mužić, T.; Jensen, S.D.; Dalgaard, L.T.; Bilgin, M.; Nylandsted, J.; Heimburg, T.; Frandsen, S.K.; Gehl, J. Calcium electroporation and electrochemotherapy for cancer treatment: Importance of cell membrane composition investigated by lipidomics, calorimetry and in vitro efficacy. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.X.; Sieling, F.; Pan, H.; Cui, J. Ultrasound-induced cell membrane porosity. Ultrasound Med. Biol. 2004, 30, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Shi, J.; Cui, J.; Deng, C.X. Effects of extracellular calcium on cell membrane resealing in sonoporation. J. Control. Release 2008, 126, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Ueno, Y.; Sonoda, S.; Suzuki, R.; Yokouchi, M.; Kawasoe, Y.; Tachibana, K.; Maruyama, K.; Sakamoto, T.; Komiya, S. Combination of ultrasound and bubble liposome enhance the effect of doxorubicin and inhibit murine osteosarcoma growth. Cancer Biol. Ther. 2011, 12, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Piron, J.; Kaddur, K.; Bouakaz, A. Enhancement of doxorubicin effect on cancer cell mortality with ultrasound and microbubbles. AIP Conf. Proc. 2010, 1215, 161–163. [Google Scholar]
- Lee, N.G.; Berry, J.L.; Lee, T.C.; Wang, A.T.; Honowitz, S.; Linn Murphree, A.; Varshney, N.; Hinton, D.R.; Fawzi, A.A. Sonoporation enhances chemotherapeutic efficacy in retinoblastoma cells in vitro. Investig. Ophthalmol. Vis. Sci. 2011, 52, 3868–3873. [Google Scholar] [CrossRef]
- Wang, Y.; Bai, W.K.; Shen, E.; Hu, B. Sonoporation by low-frequency and low-power ultrasound enhances chemotherapeutic efficacy in prostate cancer cells in vitro. Oncol. Lett. 2013, 6, 495–498. [Google Scholar] [CrossRef]
- Jakštys, B.; Ruzgys, P.; Tamošiūnas, M.; Šatkauskas, S. Different Cell Viability Assays Reveal Inconsistent Results After Bleomycin Electrotransfer In Vitro. J. Membr. Biol. 2015, 248, 857–863. [Google Scholar] [CrossRef]
- Sonoda, S.; Tachibana, K.; Uchino, E.; Yamashita, T.; Sakoda, K.; Sonoda, K.H.; Hisatomi, T.; Izumi, Y.; Sakamoto, T. Inhibition of melanoma by ultrasound-microbubble-aided drug delivery suggests membrane permeabilization. Cancer Biol. Ther. 2007, 6, 1282–1289. [Google Scholar] [CrossRef]
- Fix, S.M.; Novell, A.; Escoffre, J.M.; Tsuruta, J.K.; Dayton, P.A.; Bouakaz, A. In-vitro delivery of BLM into resistant cancer cell line using sonoporation with low-boiling point phase change ultrasound contrast agents. In Proceedings of the 2017 IEEE International Ultrasonics Symposium (IUS), Washington, DC, USA, 6–9 September 2017; pp. 3–6. [Google Scholar]
- Lentacker, I.; Geers, B.; Demeester, J.; De Smedt, S.C.; Sanders, N.N. Design and evaluation of doxorubicin-containing microbubbles for ultrasound-triggered doxorubicin delivery: Cytotoxicity and mechanisms involved. Mol. Ther. 2010, 18, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Escoffre, J.M.; Piron, J.; Novell, A.; Bouakaz, A. Doxorubicin delivery into tumor cells with ultrasound and microbubbles. Mol. Pharm. 2011, 8, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Kumon, R.E.; Deng, C.X. Mechanisms of microbubble-facilitated sonoporation for drug and gene delivery. Ther. Deliv. 2014, 5, 467–486. [Google Scholar] [CrossRef] [PubMed]
- Mehier-Humbert, S.; Bettinger, T.; Yan, F.; Guy, R.H. Ultrasound-mediated gene delivery: Kinetics of plasmid internalization and gene expression. J. Control. Release 2005, 104, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Ohta, S.; Suzuki, K.; Tachibana, K.; Yamada, G. Microbubble-Enhanced Sonoporation: Efficient Gene Transduction Technique for Chick Embryos. Genesis 2003, 37, 91–101. [Google Scholar] [CrossRef]
- Tlaxca, J.L.; Anderson, C.R.; Klibanov, A.L.; Lowrey, B.; Hossack, J.A.; Alexander, J.S.; Lawrence, M.B.; Rychak, J.J. Analysis of in vitro transfection by sonoporation using cationic and neutral microbubbles. Ultrasound Med. Biol. 2010, 36, 1907–1918. [Google Scholar] [CrossRef]
- Manta, S.; Renault, G.; Delalande, A.; Couture, O.; Lagoutte, I.; Seguin, J.; Lager, F.; Houzé, P.; Midoux, P.; Bessodes, M.; et al. Cationic microbubbles and antibiotic-free miniplasmid for sustained ultrasound–mediated transgene expression in liver. J. Control. Release 2017, 262, 170–181. [Google Scholar] [CrossRef]
- Pislaru, S.V.; Pislaru, C.; Kinnick, R.R.; Singh, R.; Gulati, R.; Greenleaf, J.F.; Simari, R.D. Optimization of ultrasound-mediated gene transfer: Comparison of contrast agents and ultrasound modalities. Eur. Heart J. 2003, 24, 1690–1698. [Google Scholar] [CrossRef]
- Lentacker, I.; De Cock, I.; Deckers, R.; De Smedt, S.C.; Moonen, C.T.W. Understanding ultrasound induced sonoporation: Definitions and underlying mechanisms. Adv. Drug Deliv. Rev. 2014, 72, 49–64. [Google Scholar] [CrossRef]
- Chen, W.S.; Brayman, A.A.; Matula, T.J.; Crum, L.A. Inertial cavitation dose and hemolysis produced in vitro with or without Optison®. Ultrasound Med. Biol. 2003, 29, 725–737. [Google Scholar] [CrossRef]
- Hallow, D.M.; Mahajan, A.D.; McCutchen, T.E.; Prausnitz, M.R. Measurement and correlation of acoustic cavitation with cellular bioeffects. Ultrasound Med. Biol. 2006, 32, 1111–1122. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Luo, Y.; Zhang, Y.; Cui, W.; Zhang, D.; Wu, J.; Zhang, J.; Tu, J. The correlation between acoustic cavitation and sonoporation involved in ultrasound-mediated DNA transfection with polyethylenimine (PEI) in vitro. J. Control. Release 2010, 145, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.Y.; Wu, C.H.; Chen, C.C.; Li, P.C. Quantitative relations of acoustic inertial cavitation with sonoporation and cell viability. Ultrasound Med. Biol. 2006, 32, 1931–1941. [Google Scholar] [CrossRef] [PubMed]
- Ammi, A.Y.; Cleveland, R.O.; Mamou, J.; Wang, G.I.; Bridal, S.L.; O’Brien, W.D. Ultrasonic contrast agent shell rupture detected by inertial cavitation and rebound signals. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2006, 53, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Tamošiunas, M.; Jurkonis, R.; Mir, L.M.; Lukoševičius, A.; Venslauskas, M.S.; Šatkauskas, S. Microbubble sonodestruction rate as a metric to evaluate sonoporation efficiency. J. Ultrasound Med. 2012, 31, 1993–2000. [Google Scholar] [CrossRef]
- Tamošiunas, M.; Jurkonis, R.; Mir, L.M.; Lukoševičius, A.; Venslauskas, M.S.; Šatkauskas, S. Adjustment of ultrasound exposure duration to microbubble sonodestruction kinetics for optimal cell sonoporation in vitro. Technol. Cancer Res. Treat. 2012, 11, 375–388. [Google Scholar] [CrossRef]
- Sundaram, J.; Mellein, B.R.; Mitragotri, S. An experimental and theoretical analysis of ultrasound-induced permeabilization of cell membranes. Biophys. J. 2003, 84, 3087–3101. [Google Scholar] [CrossRef]
- Tezel, A.; Sens, A.; Mitragotri, S. Investigations of the role of cavitation in low-frequency sonophoresis using acoustic spectroscopy. J. Pharm. Sci. 2002, 91, 444–453. [Google Scholar] [CrossRef]
- Maciulevicius, M.; Tamosiunas, M.; Jurkonis, R.; Venslauskas, M.S.; Satkauskas, S. Analysis of metrics for molecular sonotransfer in vitro. Mol. Pharm. 2015, 12, 3620–3627. [Google Scholar] [CrossRef]
- Kooiman, K.; Foppen-Harteveld, M.; Der Steen, A.F.W.V.; De Jong, N. Sonoporation of endothelial cells by vibrating targeted microbubbles. J. Control. Release 2011, 154, 35–41. [Google Scholar] [CrossRef]
- Van Wamel, A.; Bouakaz, A.; Versluis, M.; De Jong, N. Micromanipulation of endothelial cells: Ultrasound-microbubble-cell interaction. Ultrasound Med. Biol. 2004, 30, 1255–1258. [Google Scholar] [CrossRef] [PubMed]
- Forbes, M.M.; Steinberg, R.L.; O’Brien, W.D. Examination of Inertial Cavitation of Optison in Producing Sonoporation of Chinese Hamster Ovary Cells. Ultrasound Med. Biol. 2008, 34, 2009–2018. [Google Scholar] [CrossRef] [PubMed]
- Forbes, M.M.; Steinberg, R.L.; O’Brien, W.D. Frequency-dependent evaluation of the role of definity in producing sonoporatior of Chinese hamster ovary cells. J. Ultrasound Med. 2011, 30, 61–69. [Google Scholar] [CrossRef] [PubMed]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maciulevičius, M.; Navickaitė, D.; Chopra, S.; Jakštys, B.; Šatkauskas, S. Sudden Cell Death Induced by Ca2+ Delivery via Microbubble Cavitation. Biomedicines 2021, 9, 32. https://doi.org/10.3390/biomedicines9010032
Maciulevičius M, Navickaitė D, Chopra S, Jakštys B, Šatkauskas S. Sudden Cell Death Induced by Ca2+ Delivery via Microbubble Cavitation. Biomedicines. 2021; 9(1):32. https://doi.org/10.3390/biomedicines9010032
Chicago/Turabian StyleMaciulevičius, Martynas, Diana Navickaitė, Sonam Chopra, Baltramiejus Jakštys, and Saulius Šatkauskas. 2021. "Sudden Cell Death Induced by Ca2+ Delivery via Microbubble Cavitation" Biomedicines 9, no. 1: 32. https://doi.org/10.3390/biomedicines9010032
APA StyleMaciulevičius, M., Navickaitė, D., Chopra, S., Jakštys, B., & Šatkauskas, S. (2021). Sudden Cell Death Induced by Ca2+ Delivery via Microbubble Cavitation. Biomedicines, 9(1), 32. https://doi.org/10.3390/biomedicines9010032





