Can Exercise-Induced Muscle Damage Be a Good Model for the Investigation of the Anti-Inflammatory Properties of Diet in Humans?
Abstract
1. Introduction
2. The Protective Anti-Inflammatory Role of Nutrition in Chronic Diseases
3. The Development of Inflammation Models in Humans Would Greatly Facilitate the Assessment of the Anti-Inflammatory Properties of Nutrition
4. Exercise-Induced Muscle Damage’s Prototypic Inflammatory Responses in Muscle Tissue
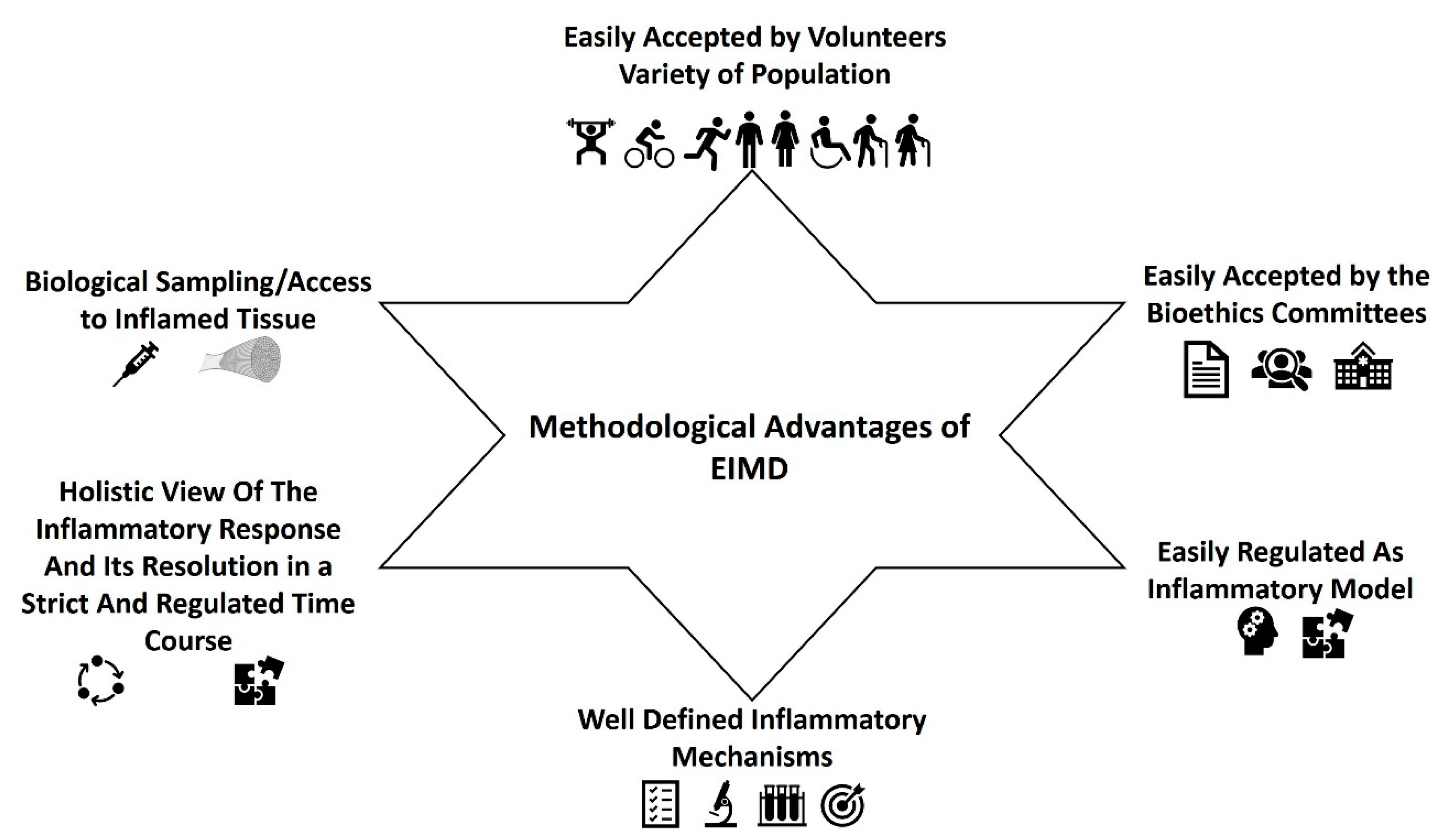
5. Exercise-Induced Muscle Damage and Its Methodological Advantages as an inflammatory Model in Humans
- EIMD can be easily accepted by the volunteers and the bioethics committees. It can be easily induced by different types of exercise/training either in the lower or the upper limbs (see below). The inflammatory response can be applied to all kinds of populations irrespective of their health and training status, age, gender, race, body composition etc. Most importantly, volunteers easily consent to this kind of intervention which is actually just about exercise for them. In addition, most bioethics committees would have no objections to this kind of experimentation in humans.
- EIMD can be easily applied to humans in a regulated manner. Exercise scientists can induce muscle damage by forcing muscles to lengthen while generating active tension, with various stimuli. As it has been discussed in the previous section, EIMD can be easily induced through eccentric training after 85 to 300 maximal eccentric contractions, while the magnitude and the extent of EIMD from eccentric exercise, seems to be higher and prolonged (lasting for 72 h until 1–2weeks) compared to other type of exercises [14,15,55,62,63,64,66,67,70,72,74,79,84,86,88,90,93,94,97,112,113,117,122,132,183,184,185,186,187,188,189,190,191,192,193,194,195,196,197,198,199,200]. It should be mentioned that eccentric EIMD can be used and recommended for the prevention and/or rehabilitation of many chronic health conditions [94,188,199,201,202,203,204,205,206,207,208,209,210,211,212,213,214,215,216,217,218,219,220,221] since eccentric exercise has about 2–4 times lower metabolic and cardiovascular demands compared to other types of exercises [96,222]. Therefore, it seems that the use of eccentric exercise is a safe, effective, and regulated way to induce EIMD in all population groups. However, other training stimuli could also be applied. For example, high volume drop jump sessions (≥100 jumps) [223,224,225,226,227,228], or in general exercises with an increased volume of stretch-lengthening cycle movements [229], prolonged moderate to high intensity running [196,230,231], cycling [232,233,234,235], and downhill running [236,237,238,239,240], have been repeatedly reported to induce significant EIMD, lasting for more than 72–96 h post-training. Although traditional resistance training (e.g., 60–80% of 1RM, 4–8 sets per exercise) can induce also EIMD [241,242,243] and trigger immune [244,245,246,247,248,249] and inflammation-related molecules (e.g., cytokines and chemokines) responses [245,246,247,248,249,250,251,252], the extent of EIMD is limited or significantly lower and with shorter duration than the above type of exercises, especially compared to eccentric training [74,117,202,253,254]. For most of these exercises, no special instrumentation is required. Independent of the exercise type, EIMD will be stronger and longer if the exercise used is characterized by very high mechanical and metabolic demands, e.g., with high volumes and intensities, fast contraction velocities, high contraction frequencies, short rest periods, and at long muscle lengths [14,15,55,62,63,64,65,66,67,70,71,72,73,74,75,79,84,86,88,90,93,97,112,113,117,122,132,183,184,185,186,187,188,189,190,191,192,193,194,195,196,197,198,199,200,201,202,203,204,205,206,207,208,209,210,211,212,213,214,215,216,217,218,219,220,221,222,223,224,225,226,227,228]. However, it seems that EIMD is even greater and with longer duration in untrained participants [255] and when a new/different training stimuli (unaccustomed exercise) that voluntaries are not familiar with it is applied [256].
- The inflammatory mechanisms underlying EIMD are well defined. The muscle microtrauma, induced by the different types of exercise, but mostly from eccentric exercise, can trigger a typical cascade of inflammatory events that resemble aseptic inflammation after tissue damage (see above). It is therefore easier for researchers to identify the crucial mechanistic points that each intervention could affect.
- One of the biggest advantages of EIMD, is that researchers could have the whole picture of the inflammatory response and its lysis in a strict and regulated time course. In contrast, when individuals with already established low-grade, chronic inflammation are recruited, the variability of the clinical and biochemical phenotypes, pharmacology, and medical history is usually large even in well-controlled studies. Thus, even in the best controlled cross sectional studies, the diversity between the participants would have a strong conflicting impact on research outcomes.
- Biological sampling. Apart from the classical blood or saliva samples, that are usually collected before and several time points after the exercise trial this type of experiments allow you to take samples of the inflamed tissue, namely muscle biopsies. This technique has been used in many studies, investigating either the training-induced adaptations on muscle fibers (for example [89,122,257,258,259,260,261,262,263]), or muscle damage-inflammation (for example [63,122,128,231,248,264,265,266,267,268,269,270,271]. Muscle samples for such type of studies are usually obtained with Bergstrom needles from vastus lateralis of lower extremities, under local anesthesia, easy and quite safe for the volunteers, while in the majority of the countries, this is well accepted from the bioethics committees. The main advantage of taking muscle samples is that researchers, can investigate inflammation straight on the inflamed tissue and its cells in contrast to the majority of the studies where the inflammatory mechanisms are inferred by the alteration of biochemical markers in the circulation. Muscle biopsies can provide important information on the extent of sarcomere damage, of intra-cell biochemical-molecular procedures and/or genetic background of EIMD.
- The kinetics of clinical phenotypes linked to the inflammatory response can be easily determined. Such phenotypes are delayed-onset muscle soreness, maximum isometric torque, range of motion, limb circumference, and several other types of ergometric tests according to the inflamed limb.
6. Applications of Exercise Induced Muscle Damage as a Model of Inflammation
- Acute and chronic inflammations. EIMD inflammatory response share similar pathophysiological and biochemical responses with acute and chronic inflammation. Thus, EIMD could find application in studies investigating the effect of nutrition and/or exercise, in acute and chronic inflammatory conditions such as those observed before and/or during the majority of chronic and non-communicable diseases [1,2,4,5,8,9,20,24,29,32,33,34,128,272]. For example, the inflammatory status of certain population groups (e.g., obese vs. normal weight) can be better assessed and compared under the dynamic conditions of EIMD. Taking into account that muscle biopsies can also be obtained and then the molecular mechanisms of autophagy, apoptosis and regeneration-adaptation could also be studied [75,103,136,141,144,146,148,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176,177,178,179,180,181,182].
- Considering the pathophysiology behind conditions such as muscle/neurogenic inflammation, atrophy, cachexia, sarcopenia, and chronic muscle protein degradation EIMD can serve as a very reliable and regulated model to investigate how nutrition and or exercise may affect the physiological and biochemical background of those conditions.
- Ischemic preconditioning (IPC). After an EIMD stimuli, the following exercise bouts induce lower muscle damage and inflammation, due to the specific muscle adaptations, that minimize the extent of muscle damage, a phenomenon that it is known as “repeated bout effect” (RBE [79,84,273,274,275,276]). IPC protective mechanisms are comparable to those of RBE. IPC attained after one to five cycles of intermittent bouts of Ischemia/reperfusion, provide protection against the possibility of subsequent ischemia with longer duration. It has been documented that ROS are the main cardioprotective factor of IPC. Just a single bout of IPC produces a significant amount of ROS from mitochondria which trigger the protective signaling cascade [139]. Almost the same mechanisms seem to induce the RBE after repeated bouts of training sessions [79,84,273,274,275,276], providing further support that EIMD is a very good model to investigate the IPC and the hormetic effects of diet on those mechanisms.
- Rhabdomyolysis. Rhabdomyolysis is a pathophysiological condition of extensive skeletal muscle cell damage which could be induced from many physical (trauma, strenuous muscle exercise, electrical current) and non-physical causes (metabolic syndrome, drugs, electrolyte imbalance) [277]. This is a frequent phenomenon in patients taking statins, which may lead to unfavorable effects ranging from myalgia or myopathy to rhabdomyolysis and sometimes to acute renal failure [277]. The initial metabolic hypothesis of statin myopathy is that mitochondrial function is reduced while neutral lipids are increased, and ubiquitin proteasome is activated. In this case the activation of ubiquitin induces acceleration of proteolysis leading to muscle break down, atrophy, and necrosis [278]. Again, drug- and non-drug-induced rhabdomyolysis, have the same mechanisms and responses as those founded during an EIMD situation, specifically of those that are observed during the first 24–48 h post-exercise [14,15,18,21,55,63,65,66,71,72,74,75,76,77,78,79,80,87,90,93,103,116,117,120,121,122,123,136,137,138,140,141,142,143,144,146,148,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176,177,178,179,180,181,182,196,197,198,200,279,280,281,282,283,284,285]. Therefore, EIMD can be applied in studies investigating the phenotypes that are more prone to rhabdomyolysis or in studies investigating the protective effects of dietary compounds to it.
- Fibromyalgia (FM). FM is a complex syndrome characterized by widespread pain that affects many tissues and the presence of allodynia and hyperalgesia [286,287]. Fatigue and functional disorders usually appear during the syndrome [286,287]. Pain is a common feature of EIMD and the physiological-metabolic mechanisms-responses observed in FM are similar to those found during EIMD [288].
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Donath, M.; Meier, D.; Böni-Schnetzler, M. Inflammation in the pathophysiology and therapy of cardiometabolic disease. Endocr. Rev. 2019, 40, 1080–1091. [Google Scholar] [CrossRef] [PubMed]
- Saltiel, A.; Olefsky, J. Inflammatory mechanisms linking obesity and metabolic disease. J. Clin. Investig. 2017, 127, 1–4. [Google Scholar] [CrossRef]
- Deng, F.; Shivappa, N.; Tang, Y.; Mann, J.; Hebert, J. Association between diet-related inflammation, all-cause, all-cancer, and cardiovascular disease mortality, with special focus on prediabetics: Findings from NHANES III. Eur. J. Nutr. 2017, 56, 1085–1093. [Google Scholar] [CrossRef] [PubMed]
- Fougère, B.; Boulanger, E.; Nourhashémi, F.; Guyonnet, S.; Cesari, M. Chronic inflammation: Accelerator of biological aging. J. Gerontol. A Biol. Sci. Med. Sci. 2016, 72, 1218–1225. [Google Scholar] [CrossRef]
- Draganidis, D.; Karagounis, L.; Athanailidis, I.; Chatzinikolaou, A.; Jamurtas, A.; Fatouros, I. Inflammaging and Skeletal Muscle: Can Protein Intake Make a Difference? J. Nutr. 2016, 146, 1940–1952. [Google Scholar] [CrossRef] [PubMed]
- Torres, S.; Fabersani, E.; Marquez, A.; Gauffin-Cano, P. Adipose tissue inflammation and metabolic syndrome. The proactive role of probiotics. Eur. J. Nutr. 2019, 58, 27–43. [Google Scholar] [CrossRef]
- Razquin, C.; Martinez-Gonzalez, M. A Traditional Mediterranean Diet Effectively Reduces Inflammation and Improves Cardiovascular Health. Nutrients 2019, 11, 1842. [Google Scholar] [CrossRef]
- Koloverou, E.; Panagiotakos, D. Inflammation: A new player in the link between Mediterranean diet and diabetes mellitus: A review. Curr. Nutr. Rep. 2017, 6, 247–256. [Google Scholar] [CrossRef]
- Calder, P.; Bosco, N.; Bourdet-Sicard, R.; Capuron, L.; Delzenne, N.; Doré, J.; Franceschi, C.; Lehtinen, M.; Recker, T.; Salvioli, S. Health relevance of the modification of low grade inflammation in ageing (inflammageing) and the role of nutrition. Ageing Res. Rev. 2017, 40, 95–119. [Google Scholar] [CrossRef]
- Calder, P.; Albers, R.; Antoine, J.; Blum, S.; Bourdet-Sicard, R.; Ferns, G.; Folkerts, G.; Friedmann, P.; Frost, G.; Guarner, F. Inflammatory disease processes and interactions with nutrition. Br. J. Nutr. 2009, 101, 1–45. [Google Scholar] [CrossRef]
- Lankinen, M.; Uusitupa, M.; Schwab, U. Nordic Diet and Inflammation-A Review of Observational and Intervention Studies. Nutrients 2019, 11, 1396. [Google Scholar] [CrossRef] [PubMed]
- Perel, P.; Roberts, I.; Sena, E.; Wheble, P.; Briscoe, C.; Sandercock, P.; Macleod, M.; Mignini, L.; Jayaram, P.; Khan, K. Comparison of treatment effects between animal experiments and clinical trials: Systematic review. BMJ 2007, 334, 197. [Google Scholar] [CrossRef] [PubMed]
- Laman, J.; Kooistra, S.; Clausen, B. Reproducibility issues: Avoiding pitfalls in animal inflammation models. In Inflammation. Methods in Molecular Biology; Clausen, B., Laman, J., Eds.; Humana Press: New York, NY, USA, 2017; Volume 1559, pp. 1–17. [Google Scholar]
- Margaritelis, N.V.; Paschalis, V.; Theodorou, A.A.; Kyparos, A.; Nikolaidis, M.G. Redox basis of exercise physiology. Redox Biol. 2020, 35, 101499. [Google Scholar] [CrossRef]
- Peake, J.; Neubauer, O.; Della Gatta, P.; Nosaka, K. Muscle damage and inflammation during recovery from exercise. J. Appl. Physiol. 2017, 122, 559–570. [Google Scholar] [CrossRef]
- Parker, L.; Shaw, C.; Stepto, N.; Levinger, I. Exercise and Glycemic Control: Focus on Redox Homeostasis and Redox-Sensitive Protein Signaling. Front. Endocrinol. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- González, N.; Santivañez, J.; Fuster, B.; Boira, E.; Martinez-Navarro, I.; Bartoll, Ó.; Domingo, C. Quick Recovery of Renal Alterations and Inflammatory Activation after a Marathon. Kidney Dis. 2019, 5, 259–265. [Google Scholar] [CrossRef]
- Koyama, K. Exercise-induced oxidative stress: A tool for “hormesis” and “adaptive response”. J. Sports Med. Phys. Fitness. 2014, 3, 115–120. [Google Scholar] [CrossRef]
- Hikida, R.; Staron, R.; Hagerman, F.; Sherman, W.; Costill, D. Muscle fiber necrosis associated with human marathon runners. J. Neurol. Sci. 1983, 59, 185–203. [Google Scholar] [CrossRef]
- Soehnlein, O.; Steffens, S.; Hidalgo, A.; Weber, C. Neutrophils as protagonists and targets in chronic inflammation. Nat. Rev. Immunol. 2017, 17, 248. [Google Scholar] [CrossRef]
- Kajal, H.; Stephen, C.; Elizabeth, D.; Prabha, C.; David, M. Macrophages and the Recovery from Acute and Chronic Inflammation. Annu. Rev. Physiol. 2017, 79, 567–592. [Google Scholar] [CrossRef]
- Salminen, A.; Huuskonen, J.; Ojala, J.; Kauppinen, A.; Kaarniranta, K.; Suuronen, T. Activation of innate immunity system during aging: NF-kB signaling is the molecular culprit of inflamm-aging. Ageing Res. Rev. 2008, 7, 83–105. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. Inflammatory mechanisms: The molecular basis of inflammation and disease. Nutr. Rev. 2007, 65, S140–S146. [Google Scholar] [CrossRef] [PubMed]
- Pietzner, M.; Kaul, A.; Henning, A.; Kastenmüller, G.; Artati, A.; Lerch, M.; Adamski, J.; Nauck, M.; Friedrich, N. Comprehensive metabolic profiling of chronic low-grade inflammation among generally healthy individuals. BMC Med. 2017, 15, 210. [Google Scholar] [CrossRef] [PubMed]
- Soysal, P.; Stubbs, B.; Lucato, P.; Luchini, C.; Solmi, M.; Peluso, R.; Sergi, G.; Isik, A.; Manzato, E.; Maggi, S. Inflammation and frailty in the elderly: A systematic review and meta-analysis. Ageing Res. Rev. 2016, 31, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lasselin, J.; Capuron, L. Chronic low-grade inflammation in metabolic disorders: Relevance for behavioral symptoms. Neuroimmunomodulation 2014, 21, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Chovatiya, R.; Medzhitov, R. Stress, inflammation, and defense of homeostasis. Mol. Cell. 2014, 54, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Beyer, I.; Mets, T.; Bautmans, I. Chronic low-grade inflammation and age-related sarcopenia. Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 12–22. [Google Scholar] [CrossRef]
- Monteiro, R.; Azevedo, I. Chronic inflammation in obesity and the metabolic syndrome. Mediat. Inflamm. 2010, 2010, 289645. [Google Scholar] [CrossRef]
- Minihane, A.; Vinoy, S.; Russell, W.; Baka, A.; Roche, H.; Tuohy, K.; Teeling, J.; Blaak, E.; Fenech, M.; Vauzour, D.; et al. Low-grade inflammation, diet composition and health: Current research evidence and its translation. Br. J. Nutr. 2015, 114, 999–1012. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, S.; Leng, S. Chronic Low-grade Inflammatory Phenotype (CLIP) and Senescent Immune Dysregulation. Clin. Ther. 2019, 41, 400–409. [Google Scholar] [CrossRef]
- Nishida, K.; Otsu, K. Inflammation and metabolic cardiomyopathy. Cardiovasc. Res. 2017, 113, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G. Inflammation, metaflammation and immunometabolic disorders. Nature 2017, 542, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Kotas, M.; Medzhitov, R. Homeostasis, inflammation, and disease susceptibility. Cell 2015, 160, 816–827. [Google Scholar] [CrossRef] [PubMed]
- Durham, W.J.; Dillon, E.L.; Sheffield-Moore, M. Inflammatory burden and amino acid metabolism in cancer cachexia. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 72–77. [Google Scholar] [CrossRef]
- Danesh, J.; Wheeler, J.; Hirschfield, G.; Eda, S.; Eiriksdottir, G.; Rumley, A.; Lowe, G.; Pepys, M.; Gudnason, V. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N. Engl. J. Med. 2004, 350, 1387–1397. [Google Scholar] [CrossRef]
- Mills, P.; Hong, S.; Redwine, L.; Carter, S.; Chiu, A.; Ziegler, M.; Dimsdale, J.; Maisel, A. Physical fitness attenuates leukocyte-endothelial adhesion in response to acute exercise. J. Appl. Physiol. 2006, 101, 785–788. [Google Scholar] [CrossRef]
- Petersen, A.M.; Pedersen, B.K. The anti-inflammatory effect of exercise. J. Appl. Physiol. 2005, 98, 1154–1162. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Hoffmann, G. Mediterranean dietary pattern, inflammation and endothelial function: A systematic review and meta-analysis of intervention trials. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 929–939. [Google Scholar] [CrossRef]
- Ricker, M.; Haas, W. Anti-inflammatory diet in clinical practice: A review. Nutr. Clin. Pract. 2017, 32, 318–325. [Google Scholar] [CrossRef]
- Sears, B. Anti-inflammatory diets. J. Am. Coll. Nutr. 2015, 34, 14–21. [Google Scholar] [CrossRef]
- Halliwell, B. Antioxidant and Anti-Inflammatory Components of Foods; ILSI Europe: Brussels, Belgium, 2015; p. 34. [Google Scholar]
- Galland, L. Diet and inflammation. Nutr. Clin. Pract. 2010, 25, 634–640. [Google Scholar] [CrossRef]
- Arouca, A.; Meirhaeghe, A.; Dallongeville, J.; Moreno, L.; Lourenço, G.; Marcos, A.; Huybrechts, I.; Manios, Y.; Lambrinou, C.; Gottrand, F. Interplay between the Mediterranean diet and C-reactive protein genetic polymorphisms towards inflammation in adolescents. Clin. Nutr. 2020, 39, 1919–1926. [Google Scholar] [CrossRef] [PubMed]
- Nomikos, T.; Fragopoulou, E.; Antonopoulou, S.; Panagiotakos, D. Mediterranean diet and platelet-activating factor; a systematic review. Clin. Biochem. 2018, 60, 1–10. [Google Scholar] [CrossRef]
- Seok, J.; Warren, S.; Cuenca, A.; Mindrinos, M.; Baker, H.; Xu, W.; Richards, D.; McDonald-Smith, G.; Gao, H.; Hennessy, L. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc. Natl. Acad. Sci. USA 2013, 110, 3507–3512. [Google Scholar] [CrossRef] [PubMed]
- Gambini, J.; Inglés, M.; Olaso, G.; Lopez-Grueso, R.; Bonet-Costa, V.; Gimeno-Mallench, L.; Mas-Bargues, C.; Abdelaziz, K.; Gomez-Cabrera, M.; Vina, J. Properties of resveratrol: In vitro and in vivo studies about metabolism, bioavailability, and biological effects in animal models and humans. Oxidative Med. Cell. Longev. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Webb, D. Animal models of human disease: Inflammation. Biochem. Pharmacol. 2014, 87, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Ahluwalia, N.; Andreeva, V.; Kesse-Guyot, E.; Hercberg, S. Dietary patterns, inflammation and the metabolic syndrome. Diabetes Metab. 2013, 39, 99–110. [Google Scholar] [CrossRef]
- Wang, X.; Ouyang, Y.; Liu, J.; Zhao, G. Flavonoid intake and risk of CVD: A systematic review and meta-analysis of prospective cohort studies. Br. J. Nutr. 2014, 111, 1–11. [Google Scholar] [CrossRef]
- Rangel-Huerta, O.; Aguilera, C.; Mesa, M.; Gil, A. Omega-3 long-chain polyunsaturated fatty acids supplementation on inflammatory biomakers: A systematic review of randomised clinical trials. Br. J. Nutr. 2012, 107, S159–S170. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Christoph, M.; Hoffmann, G. Effects of olive oil on markers of inflammation and endothelial function—a systematic review and meta-analysis. Nutrients 2015, 7, 7651–7675. [Google Scholar] [CrossRef]
- Buyken, A.; Goletzke, J.; Joslowski, G.; Felbick, A.; Cheng, G.; Herder, C.; Brand-Miller, J. Association between carbohydrate quality and inflammatory markers: Systematic review of observational and interventional studies. Am. J. Clin. Nutr. 2014, 99, 813–833. [Google Scholar] [CrossRef] [PubMed]
- Moradi, S.; Issah, A.; Mohammadi, H.; Mirzaei, K. Associations between dietary inflammatory index and incidence of breast and prostate cancer: A systematic review and meta-analysis. Nutrition 2018, 55, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Owens, D.; Twist, C.; Cobley, J.; Howatson, G.; Close, G. Exercise-induced muscle damage: What is it, what causes it and what are the nutritional solutions? Eur. J. Sport Sci. 2018, 19, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Fouré, A.; Bendahan, D. Is branched-chain amino acids supplementation an efficient nutritional strategy to alleviate skeletal muscle damage? A systematic review. Nutrients 2017, 9, 1047. [Google Scholar] [CrossRef]
- Pasiakos, S.; Lieberman, H.; McLellan, T. Effects of protein supplements on muscle damage, soreness and recovery of muscle function and physical performance: A systematic review. Sports Med. 2014, 44, 655–670. [Google Scholar] [CrossRef]
- Cockburn, E.; Bell, P.; Stevenson, E. Effect of milk on team sport performance after exercise-induced muscle damage. Med. Sci. Sports Exerc. 2013, 45, 1585–1592. [Google Scholar] [CrossRef]
- Cockburn, E.; Hayes, P.; French, D.; Stevenson, E.; St Clair Gibson, A. Acute milk-based protein–CHO supplementation attenuates exercise-induced muscle damage. Appl. Physiol. Nutr. Metab. 2008, 33, 775–783. [Google Scholar] [CrossRef]
- Harty, P.; Cottet, M.; Malloy, J.; Kerksick, C. Nutritional and Supplementation Strategies to Prevent and Attenuate Exercise-Induced Muscle Damage: A Brief Review. Sports Med. Open 2019, 5, 1. [Google Scholar] [CrossRef]
- Sousa, M.; Teixeira, V.; Soares, J. Dietary strategies to recover from exercise-induced muscle damage. Int. J. Food Sci. Nutr. 2014, 65, 151–163. [Google Scholar] [CrossRef]
- Margaritelis, N.; Paschalis, V.; Theodorou, A.; Kyparos, A.; Nikolaidis, M. Antioxidants in Personalized Nutrition and Exercise. Adv. Nutr. 2018, 9, 813–823. [Google Scholar] [CrossRef]
- Michailidis, Y.; Karagounis, L.; Terzis, G.; Jamurtas, A.; Spengos, K.; Tsoukas, D.; Chatzinikolaou, A.; Mandalidis, D.; Stefanetti, R.; Papassotiriou, I. Thiol-based antioxidant supplementation alters human skeletal muscle signaling and attenuates its inflammatory response and recovery after intense eccentric exercise. Am. J. Clin. Nutr. 2013, 98, 233–245. [Google Scholar] [CrossRef]
- Paschalis, V.; Theodorou, A.; Margaritelis, N.; Kyparos, A.; Nikolaidis, M. N-acetylcysteine supplementation increases exercise performance and reduces oxidative stress only in individuals with low levels of glutathione. Free Radic. Biol. Med. 2017, 115, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Margaritelis, N.; Theodorou, A.; Paschalis, V.; Veskoukis, A.; Dipla, K.; Zafeiridis, A.; Panayiotou, G.; Vrabas, I.S.; Kyparos, A.; Nikolaidis, M. Adaptations to endurance training depend on exercise-induced oxidative stress: Exploiting redox inter-individual variability. Acta Physiol. 2017, 222, e12972. [Google Scholar] [CrossRef] [PubMed]
- Kotsis, Y.; Mikellidi, A.; Aresti, C.; Persia, E.; Sotiropoulos, A.; Panagiotakos, D.; Antonopoulou, S.; Nomikos, T. A low-dose, 6-week bovine colostrum supplementation maintains performance and attenuates inflammatory indices following a Loughborough Intermittent Shuttle Test in soccer players. Eur. J. Nutr. 2018, 57, 1181–1195. [Google Scholar] [CrossRef] [PubMed]
- Kotsis, Y.; Methenitis, S.; Mikellidi, A.; Aresti, C.; Persia, E.; Antonopoulou, S.; Nomikos, T. Changes of rate of torque development in soccer players after a Loughborough Intermittent Shuttle Test: Effect of bovine colostrum supplementation. Isokinet. Exerc. Sci. 2020, 28, 59–72. [Google Scholar] [CrossRef]
- Bongiovanni, T.; Genovesi, F.; Nemmer, M.; Carling, C.; Alberti, G.; Howatson, G. Nutritional interventions for reducing the signs and symptoms of exercise-induced muscle damage and accelerate recovery in athletes: Current knowledge, practical application and future perspectives. Eur. J. Appl. Physiol. 2020, 120, 1965–1996. [Google Scholar] [CrossRef]
- Panza, V.P.; Diefenthaeler, F.; Da Silva, E. Benefits of dietary phytochemical supplementation on eccentric exercise-induced muscle damage: Is including antioxidants enough? Nutrition 2015, 31, 1072–1082. [Google Scholar] [CrossRef]
- Margaritelis, N.; Paschalis, V.; Theodorou, A.; Kyparos, A.; Nikolaidis, M. Antioxidant supplementation, redox deficiencies and exercise performance: A falsification design. Free Radic. Biol. Med. 2020, 158, 44–52. [Google Scholar] [CrossRef]
- Paulsen, G.; Mikkelsen, U.R.; Raastad, T.; Peake, J. Leucocytes, cytokines and satellite cells: What role do they play in muscle damage and regeneration following eccentric exercise? Exerc. Immunol. Rev. 2012, 18, 42–97. [Google Scholar]
- Jamurtas, A.; Fatouros, I. Eccentric Exercise, Muscle Damage and Oxidative Stress. In An International Perspective on Topics in Sports Med. and Sports Injury; Zaslav, K., Ed.; IntechOpen: London, UK, 2012; pp. 113–130. [Google Scholar] [CrossRef]
- Clarkson, P.M.; Hubal, M.J. Exercise-induced muscle damage in humans. Am. J. Phys. Med. Rehabil. 2002, 81, S52–S69. [Google Scholar] [CrossRef]
- Proske, U.; Morgan, D. Muscle damage from eccentric exercise: Mechanism, mechanical signs, adaptation and clinical applications. J. Physiol. 2001, 537, 333–345. [Google Scholar] [CrossRef] [PubMed]
- Methenitis, S. A Brief Review on Concurrent Training: From Laboratory to the Field. Sports 2018, 6, 127. [Google Scholar] [CrossRef] [PubMed]
- Chazaud, B. Inflammation during skeletal muscle regeneration and tissue remodeling: Application to exercise-induced muscle damage management. Immunol. Cell Biol. 2016, 94, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Malm, C. Exercise-induced muscle damage and inflammation: Fact or fiction? Acta Physiol. Scand. 2001, 171, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Tidball, J.; Villalta, A. Regulatory interactions between muscle and the immune system during muscle regeneration. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 298, R1173–R1187. [Google Scholar] [CrossRef]
- Margaritelis, N.; Theodorou, A.; Baltzopoulos, V.; Maganaris, C.; Paschalis, V.; Kyparos, A.; Nikolaidis, M. Muscle damage and inflammation after eccentric exercise: Can the repeated bout effect be removed? Physiol. Rep. 2015, 3, e12648. [Google Scholar] [CrossRef]
- Tokinoya, K.; Ishikura, K.; Ra, S.; Ebina, K.; Miyakawa, S.; Ohmori, H. Relationship between early-onset muscle soreness and indirect muscle damage markers and their dynamics after a full marathon. J. Exerc. Sci. Fit. 2020, 18, 115–121. [Google Scholar] [CrossRef]
- Carmona, G.; Roca, E.; Guerrero, M.; Cussó, R.; Bàrcena, C.; Mateu, M.; Cadefau, J. Fibre-type-specific and Mitochondrial Biomarkers of Muscle Damage after Mountain Races. Int. J. Sports Med. 2019, 40, 253–262. [Google Scholar] [CrossRef]
- Flann, K.L.; LaStayo, P.C.; McClain, D.A.; Hazel, M.; Lindstedt, S.L. Muscle damage and muscle remodeling: No pain, no gain? J. Exp. Biol. 2011, 214, 674–679. [Google Scholar] [CrossRef]
- Baumert, P.; Lake, M.J.; Stewart, C.E.; Drust, B.; Erskine, R.M. Genetic variation and exercise-induced muscle damage: Implications for athletic performance, injury and ageing. Eur. J. Appl. Physiol. 2016, 116, 1595–1625. [Google Scholar] [CrossRef]
- Hyldahl, R.; Hubal, M. Lengthening our perspective: Morphological, cellular, and molecular responses to eccentric exercise. Muscle Nerve. 2014, 49, 155–170. [Google Scholar] [CrossRef]
- Cheung, K.; Hume, P.; Maxwell, L. Delayed onset muscle soreness. Sports Med. 2003, 33, 145–164. [Google Scholar] [CrossRef] [PubMed]
- Paschalis, V.; Koutedakis, Y.; Jamurtas, A.; Mougios, V.; Baltzopoulos, V. Equal volumes of high and low intensity of eccentric exercise in relation to muscle damage and performance. J. Strength Cond. Res. 2005, 19, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Kerksick, C.M.; Willoughby, D.; Kouretas, D.; Tsatsakis, A. Intramuscular responses with muscle damaging exercise and the interplay between multiple intracellular networks: A human perspective. Food Chem. Toxicol. 2013, 61, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Peake, J.; Nosaka, K.; Suzuki, K. Characterization of inflammatory responses to eccentric exercise in humans. Exerc. Immunol. Rev. 2005, 11, 64–85. [Google Scholar] [PubMed]
- Methenitis, S.; Mpampoulis, T.; Spiliopoulou, P.; Papadimas, G.; Papadopoulos, C.; Chalari, E.; Evangelidou, E.; Stasinaki, A.N.; Nomikos, T.; Terzis, G. Muscle fiber composition, jumping performance and rate of force development adaptations induced by different power training volumes in females. Appl. Physiol. Nutr. Metab. 2020, 45, 996–1006. [Google Scholar] [CrossRef] [PubMed]
- Hody, S.; Croisier, J.; Bury, T.; Rogister, B.; Leprince, P. Eccentric muscle contractions: Risks and benefits. Front. Physiol. 2019, 10. [Google Scholar] [CrossRef]
- Bogdanis, G.; Tsoukos, A.; Brown, L.; Selima, E.; Veligekas, P.; Spengos, K.; Terzis, G. Muscle fiber and performance changes after fast eccentric complex training. Med. Sci. Sports Exerc. 2018, 50, 729–738. [Google Scholar] [CrossRef]
- Tinwala, F.; Cronin, J.; Haemmerle, E.; Ross, A. Eccentric Strength Training: A Review of the Available Technology. Strength Cond. J. 2017, 39, 32–47. [Google Scholar] [CrossRef]
- Douglas, J.; Pearson, S.; Ross, A.; McGuigan, M. Eccentric exercise: Physiological characteristics and acute responses. Sports Med. 2017, 47, 663–675. [Google Scholar] [CrossRef]
- Douglas, J.; Pearson, S.; Ross, A.; McGuigan, M. Chronic Adaptations to Eccentric Training: A Systematic Review. Sports Med. 2016, 47, 917–941. [Google Scholar] [CrossRef] [PubMed]
- Vogt, M.; Hoppeler, H. Eccentric exercise: Mechanisms and effects when used as training regime or training adjunct. J. Appl. Physiol. 2014, 116, 1446–1454. [Google Scholar] [CrossRef] [PubMed]
- Isner-Horobeti, M.E.; Dufour, S.P.; Vautravers, P.; Geny, B.; Coudeyre, E.; Richard, R. Eccentric exercise training: Modalities, applications and perspectives. Sports Med. 2013, 43, 483–512. [Google Scholar] [CrossRef] [PubMed]
- Proske, U.; Allen, T. Damage to skeletal muscle from eccentric exercise. Exerc. Sport Sci. Rev. 2005, 33, 98–104. [Google Scholar] [CrossRef]
- Nishikawa, K. Eccentric contraction: Unraveling mechanisms of force enhancement and energy conservation. J. Exp. Biol. 2016, 219, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Lieber, R. Biomechanical response of skeletal muscle to eccentric contractions. J. Sport Health Sci. 2018, 7, 294–309. [Google Scholar] [CrossRef]
- Gordon, A.; Huxley, A.; Julian, F. The variation in isometric tension with sarcomere length in vertebrate muscle fibres. J. Physiol. 1966, 184, 170–192. [Google Scholar] [CrossRef]
- Maganaris, C. Force–length characteristics of in vivo human skeletal muscle. Acta Physiol. Scand. 2001, 172, 279–285. [Google Scholar] [CrossRef]
- Leonard, T.; Herzog, W. Regulation of muscle force in the absence of actin-myosin-based cross-bridge interaction. Am. J. Physiol. Cell Physiol. 2010, 299, C14–C20. [Google Scholar] [CrossRef]
- Krüger, M.; Kötter, S. Titin, a Central Mediator for Hypertrophic Signaling, Exercise-Induced Mechanosignaling and Skeletal Muscle Remodeling. Front. Physiol. 2016, 7. [Google Scholar] [CrossRef]
- Bottinelli, R.; Canepari, M.; Pellegrino, M.A.; Reggiani, C. Force-velocity properties of human skeletal muscle fibres: Myosin heavy chain isoform and temperature dependence. J. Physiol. 1996, 495 Pt 2, 573–586. [Google Scholar] [CrossRef]
- Babault, N.; Pousson, M.; Ballay, Y.; Van Hoecke, J. Activation of human quadriceps femoris during isometric, concentric, and eccentric contractions. J. Appl. Physiol. 2001, 91, 2628–2634. [Google Scholar] [CrossRef] [PubMed]
- Enoka, R.M. Eccentric contractions require unique activation strategies by the nervous system. J. Appl. Physiol. 1996, 81, 2339–2346. [Google Scholar] [CrossRef] [PubMed]
- Aagaard, P. Spinal and supraspinal control of motor function during maximaleccentric muscle contraction: Effects of resistance training. J. Sport Health Sci. 2018, 7, 282–293. [Google Scholar] [CrossRef]
- Morgan, D.; Allen, D. Early events in stretch-induced muscle damage. J. Appl. Physiol. 1999, 87, 2007–2015. [Google Scholar] [CrossRef] [PubMed]
- Lieber, R.; Friden, J. Muscle damage is not a function of muscle force but active muscle strain. J. Appl. Physiol. 1993, 74, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Friden, J.; Lieber, R. Structural and mechanical basis of exercise-induced muscle injury. Med. Sci. Sports Exerc. 1992, 24, 521–530. [Google Scholar] [CrossRef]
- Lee, K.; Ochi, E.; Song, H.; Nakazato, K. Activation of AMP-activated protein kinase induce expression of FoxO1, FoxO3a, and myostatin after exercise-induced muscle damage. Biochem. Biophys. Res. Commun. 2015, 466, 289–294. [Google Scholar] [CrossRef]
- Jiménez-Jiménez, R.; Cuevas, M.; Almar, M.; Lima, E.; García-López, D.; De Paz, J.; González-Gallego, J. Eccentric training impairs NF-κB activation and over-expression of inflammation-related genes induced by acute eccentric exercise in the elderly. Mech. Ageing Dev. 2008, 129, 313–321. [Google Scholar] [CrossRef]
- Murphy, R.; Goodman, C.; McKenna, M.; Bennie, J.; Leikis, M.; Lamb, G. Calpain-3 is autolyzed and hence activated in human skeletal muscle 24 h following a single bout of eccentric exercise. J. Appl. Physiol. 2007, 103, 926–931. [Google Scholar] [CrossRef]
- Belcastro, A.; Shewchuk, L.; Raj, D. Exercise-induced muscle injury: A calpain hypothesis. Mol. Cell. Biochem. 1998, 179, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Charge, S.; Rudnicki, M. Cellular and molecular regulation of muscle regeneration. Physiol. Rev. 2004, 84, 209–238. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, J.; Kim, S.; Ryu, H.Y.; Cha, K.; Sung, D.J. Exercise-induced rhabdomyolysis mechanisms and prevention: A literature review. J. Sport Health Sci. 2016, 5, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Franchi, M.; Reeves, N.; Narici, M. Skeletal Muscle Remodeling in Response to Eccentric vs. Concentric Loading: Morphological, Molecular, and Metabolic Adaptations. Front. Physiol. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Ulbricht, A.; Gehlert, S.; Leciejewski, B.; Schiffer, T.; Bloch, W.; Höhfeld, J. Induction and adaptation of chaperone-assisted selective autophagy CASA in response to resistance exercise in human skeletal muscle. Autophagy 2015, 11, 538–546. [Google Scholar] [CrossRef]
- Philippou, A.; Bogdanis, G.; Maridaki, M.; Halapas, A.; Sourla, A.; Koutsilieris, M. Systemic cytokine response following exercise-induced muscle damage in humans. Clin. Chem. Lab. Med. 2009, 47, 777–782. [Google Scholar] [CrossRef][Green Version]
- Cheng, A.; Yamada, T.; Rassier, D.; Andersson, D.; Westerblad, H.; Lanner, J. Reactive oxygen/nitrogen species and contractile function in skeletal muscle during fatigue and recovery. J. Physiol. 2016, 594, 5149–5160. [Google Scholar] [CrossRef]
- Zuo, L.; Pannell, B. Redox Characterization of Functioning Skeletal Muscle. Front. Physiol. 2015, 6. [Google Scholar] [CrossRef]
- Wright, C.R.; Brown, E.L.; Della Gatta, P.A.; Fatouros, I.G.; Karagounis, L.G.; Terzis, G.; Mastorakos, G.; Michailidis, Y.; Mandalidis, D.; Spengos, K.; et al. Regulation of Granulocyte Colony-Stimulating Factor and Its Receptor in Skeletal Muscle Is Dependent Upon the Type of Inflammatory Stimulus. J. Interferon Cytokine Res. 2015, 35, 710–719. [Google Scholar] [CrossRef]
- Tidball, J. Inflammatory processes in muscle injury and repair. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 288, R345–R353. [Google Scholar] [CrossRef]
- Brunelli, S.; Rovere-Querini, P. The immune system and the repair of skeletal muscle. Pharmacol. Res. 2008, 58, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Toth, K.; McKay, B.; De Lisio, M.; Little, J.; Tarnopolsky, M.; Parise, G. IL-6 induced STAT3 signalling is associated with the proliferation of human muscle satellite cells following acute muscle damage. PLoS ONE 2011, 6, e17392. [Google Scholar] [CrossRef] [PubMed]
- Costamagna, D.; Costelli, P.; Sampaolesi, M.; Penna, F. Role of Inflammation in Muscle Homeostasis and Myogenesis. Mediat. Inflamm. 2015, 501, 805172. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, K.; Steensberg, A.; Keller, P.; Keller, C.; Fischer, C.; Hiscock, N.; Van Hall, G.; Plomgaard, P.; Febbraio, M. Muscle-derived interleukin-6: Lipolytic, anti-inflammatory and immune regulatory effects. Pflug. Arch. 2003, 446, 9–16. [Google Scholar] [CrossRef]
- Perandini, L.; Chimin, P.; Lutkemeyer, D.S.; Câmara, N. Chronic inflammation in skeletal muscle impairs satellite cells function during regeneration: Can physical exercise restore the satellite cell niche? FEBS J. 2018, 285, 1973–1984. [Google Scholar] [CrossRef]
- Hawke, T.; Garry, D. Myogenic satellite cells: Physiology to molecular biology. J. Appl. Physiol. 2001, 91, 534–551. [Google Scholar] [CrossRef] [PubMed]
- Serrano, A.; Baeza-Raja, B.; Perdiguero, E.; Jardí, M.; Muñoz-Cánoves, P. Interleukin-6 is an essential regulator of satellite cell-mediated skeletal muscle hypertrophy. Cell Metab. 2008, 7, 33–44. [Google Scholar] [CrossRef]
- Muñoz-Cánoves, P.; Scheele, C.; Pedersen, B.; Serrano, A. Interleukin-6 myokine signaling in skeletal muscle: A double-edged sword? FEBS J. 2013, 280, 4131–4148. [Google Scholar] [CrossRef] [PubMed]
- Cermak, N.; Snijders, T.; McKay, B.; Parise, G.; Verdijk, L.; Tarnopolsky, M.; Gibala, M.; Van Loon, L.J. Eccentric exercise increases satellite cell content in type II muscle fibers. Med. Sci. Sports Exerc. 2013, 45, 230–237. [Google Scholar] [CrossRef]
- Dumont, N.; Bentzinger, F.; Sincennes, M.C.; Rudnicki, M. Satellite cells and skeletal muscle regeneration. Compr. Physiol. 2015. [Google Scholar] [CrossRef]
- Furuichi, Y.; Fujii, N. Mechanism of satellite cell regulation by myokines. J. Sports Med. Phys. Fitness. 2017, 6, 311–316. [Google Scholar] [CrossRef][Green Version]
- Snijders, T.; Verdijk, L.; Beelen, M.; McKay, B.; Parise, G.; Kadi, F.; Van Loon, L. A single bout of exercise activates skeletal muscle satellite cells during subsequent overnight recovery. Exp. Physiol. 2012, 97, 762–773. [Google Scholar] [CrossRef] [PubMed]
- Morales-Alamo, D.; Calbet, J.A. AMPK signaling in skeletal muscle during exercise: Role of reactive oxygen and nitrogen species. Free Radic. Biol. Med. 2016, 98, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Thirupathi, A.; Pinho, R. Effects of reactive oxygen species and interplay of antioxidants during physical exercise in skeletal muscles. J. Physiol. Biochem. 2018, 74, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Aguiló, A.; Tauler, P.; Fuentespina, E.; Tur, J.; Córdova, A.; Pons, A. Antioxidant response to oxidative stress induced by exhaustive exercise. Physiol. Behav. 2005, 84, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Alleman, R.; Katunga, L.; Nelson, M.; Brown, D.; Anderson, E. The “Goldilocks Zone” from a redox perspective—Adaptive vs. deleterious responses to oxidative stress in striated muscle. Front. Physiol. 2014, 5. [Google Scholar] [CrossRef]
- Cooper, C.; Vollaard, N.B.; Choueiri, T.; Wilson, M. Exercise, free radicals and oxidative stress. Biochem. Soc. Trans. 2002, 30, 280–284. [Google Scholar] [CrossRef]
- Filomeni, G.; De Zio, D.; Cecconi, F. Oxidative stress and autophagy: The clash between damage and metabolic needs. Cell Death Differ. 2015, 22, 377–388. [Google Scholar] [CrossRef]
- Pittaluga, M.; Parisi, P.; Sabatini, S.; Ceci, R.; Caporossi, D.; Catani, M.V.; Savini, I.; Avigliano, L. Cellular and biochemical parameters of exercise-induced oxidative stress: Relationship with training levels. Free Radic. Res. 2006, 40, 607–614. [Google Scholar] [CrossRef]
- Steinbacher, P.; Eckl, P. Impact of oxidative stress on exercising skeletal muscle. Biomolecules 2015, 5, 356–377. [Google Scholar] [CrossRef]
- Ferraro, E.; Giammarioli, A.; Chiandotto, S.; Spoletini, I.; Rosano, G. Exercise-induced skeletal muscle remodeling and metabolic adaptation: Redox signaling and role of autophagy. Antioxid. Redox Signal. 2014, 21, 154–176. [Google Scholar] [CrossRef] [PubMed]
- Fyfe, J.J.; Bishop, D.; Stepto, N. Interference between Concurrent Resistance and Endurance Exercise: Molecular Bases and the Role of Individual Training Variables. Sports Med. 2014, 44, 743–762. [Google Scholar] [CrossRef] [PubMed]
- Fyfe, J.J.; Bishop, D.; Zacharewicz, E.; Russell, A.; Stepto, N. Concurrent exercise incorporating high-intensity interval or continuous training modulates mTORC1 signaling and microRNA expression in human skeletal muscle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 310, R1297–R1311. [Google Scholar] [CrossRef] [PubMed]
- Olesen, J.; Kiilerich, K.; Pilegaard, H. PGC-1α-mediated adaptations in skeletal muscle. Pflug. Arch. 2010, 460, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Joshua, D.; Rebecca, W.; Zhen, Y. Molecular mechanisms for mitochondrial adaptation to exercise training in skeletal muscle. FASEB J. 2016, 30, 13–22. [Google Scholar] [CrossRef]
- Lundby, C.; Jacobs, R. Adaptations of skeletal muscle mitochondria to exercise training. Exp. Physiol. 2016, 101, 17–22. [Google Scholar] [CrossRef]
- Seene, T.; Kaasik, P.; Seppet, E. Changes in Myofibrillar and Mitochondrial Compartments during Increased Activity: Dependance from Oxidative Capacity of Muscle. Health 2017, 9, 779. [Google Scholar] [CrossRef][Green Version]
- Yan, Z.; Okutsu, M.; Akhtar, Y.; Lira, V. Regulation of exercise-induced fiber type transformation, mitochondrial biogenesis, and angiogenesis in skeletal muscle. J. Appl. Physiol. 2011, 110, 264–274. [Google Scholar] [CrossRef]
- Rodney, G.; Pal, R.; Abo-Zahrah, R. Redox regulation of autophagy in skeletal muscle. Free Radic. Biol. Med. 2016, 98, 103–112. [Google Scholar] [CrossRef]
- Vainshtein, A.; Grumati, P.; Sandri, M.; Bonaldo, P. Skeletal muscle, autophagy, and physical activity: The ménage à trois of metabolic regulation in health and disease. J. Mol. Med. 2014, 92, 127–137. [Google Scholar] [CrossRef]
- Tam, B.; Siu, P. Autophagic Cellular Responses to Physical Exercise in Skeletal Muscle. Sports Med. 2014, 44, 625–640. [Google Scholar] [CrossRef] [PubMed]
- Martin-Rincon, M.; Morales-Alamo, D.; Calbet, J. Exercise-mediated modulation of autophagy in skeletal muscle. Scand. J. Med. Sci. Sports 2018, 28, 772–781. [Google Scholar] [CrossRef]
- Fritzen, A.; Madsen, A.; Kleinert, M.; Treebak, J.; Lundsgaard, A.; Jensen, T.; Richter, E.; Wojtaszewski, J.; Kiens, B.; Frøsig, C. Regulation of autophagy in human skeletal muscle: Effects of exercise, exercise training and insulin stimulation. J. Physiol. 2016, 594, 745–761. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Li, Y. Distinct signal transductions in fast- and slow- twitch muscles upon denervation. Physiol. Rep. 2018, 6, e13606. [Google Scholar] [CrossRef]
- Schwalm, C.; Jamart, C.; Benoit, N.; Naslain, D.; Prémont, C.; Prévet, J.; Van Thienen, R.; Deldicque, L.; Francaux, M. Activation of autophagy in human skeletal muscle is dependent on exercise intensity and AMPK activation. FASEB J. 2015, 29, 3515–3526. [Google Scholar] [CrossRef] [PubMed]
- Rocchi, A.; He, C. Regulation of Exercise-Induced Autophagy in Skeletal Muscle. Curr. Pathobiol. Rep. 2017, 5, 177–186. [Google Scholar] [CrossRef]
- Halling, J.; Pilegaard, H. Autophagy-Dependent Beneficial Effects of Exercise. Cold Spring Harb. Perspect. Med. 2017, 7. [Google Scholar] [CrossRef]
- Vainshtein, A.; Hood, D. The regulation of autophagy during exercise in skeletal muscle. J. Appl. Physiol. 2016, 120, 664–673. [Google Scholar] [CrossRef]
- Codina-Martínez, H.; Fernández-García, B.; Díez-Planelles, C.; Fernández, Á.; Higarza, S.; Fernández-Sanjurjo, M.; Díez-Robles, S.; Iglesias-Gutiérrez, E.; Tomás-Zapico, C. Autophagy is required for performance adaptive response to resistance training and exercise-induced adult neurogenesis. Scand. J. Med. Sci. Sports 2020, 30, 238–253. [Google Scholar] [CrossRef]
- Martin-Rincon, M.; Pérez-López, A.; Morales-Alamo, D.; Perez-Suarez, I.; De Pablos-Velasco, P.; Perez-Valera, M.; Perez-Regalado, S.; Martinez-Canton, M.; Gelabert-Rebato, M.; Juan-Habib, J. Exercise Mitigates the Loss of Muscle Mass by Attenuating the Activation of Autophagy during Severe Energy Deficit. Nutrients 2019, 11, 2824. [Google Scholar] [CrossRef]
- Brandt, N.; Gunnarsson, T.; Bangsbo, J.; Pilegaard, H. Exercise and exercise training-induced increase in autophagy markers in human skeletal muscle. Physiol. Rep. 2018, 6, e13651. [Google Scholar] [CrossRef] [PubMed]
- Moberg, M.; Hendo, G.; Jakobsson, M.; Mattsson, M.; Ekblom-Bak, E.; Flockhart, M.; Pontén, M.; Söderlund, K.; Ekblom, B. Increased autophagy signaling but not proteasome activity in human skeletal muscle after prolonged low-intensity exercise with negative energy balance. Physiol. Rep. 2017, 5, e13518. [Google Scholar] [CrossRef] [PubMed]
- Hentilä, J.; Ahtiainen, J.P.; Paulsen, G.; Raastad, T.; Häkkinen, K.; Mero, A.A.; Hulmi, J.J. Autophagy is induced by resistance exercise in young men, but unfolded protein response is induced regardless of age. Acta Physiol. 2018, 224, e13069. [Google Scholar] [CrossRef] [PubMed]
- Ahtiainen, J. Physiological and Molecular Adaptations to Strength Training. In Concurrent Aerobic and Strength Training: Scientific Basics and Practical Applications; Schumann, M., Rønnestad, B.R., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 51–73. [Google Scholar] [CrossRef]
- Anthony, S.; Henri, B.; Guillaume, P.; Robin, C. Autophagy is essential to support skeletal muscle plasticity in response to endurance exercise. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014, 307, R956–R969. [Google Scholar] [CrossRef]
- Saxton, R.; Sabatini, D. mTOR signaling in growth, metabolism, and disease. Cell 2017, 168, 960–976. [Google Scholar] [CrossRef]
- Haun, C.; Mumford, P.; Roberson, P.; Romero, M.; Mobley, C.; Kephart, W.; Anderson, R.; Colquhoun, R.; Muddle, T.; Luera, M. Molecular, neuromuscular, and recovery responses to light versus heavy resistance exercise in young men. Physiol. Rep. 2017, 5, e13457. [Google Scholar] [CrossRef]
- Smiles, W.; Areta, J.; Coffey, V.; Phillips, S.; Moore, D.; Stellingwerff, T.; Burke, L.; Hawley, J.; Camera, D. Modulation of autophagy signaling with resistance exercise and protein ingestion following short-term energy deficit. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 309, R603–R612. [Google Scholar] [CrossRef]
- Sakuma, K.; Yamaguchi, A. Molecular Mechanisms Controlling Skeletal Muscle Mass. In Muscle Cell and Tissue; IntechOpen: London, UK, 2015. [Google Scholar]
- Schiaffino, S.; Dyar, K.; Ciciliot, S.; Blaauw, B.; Sandri, M. Mechanisms regulating skeletal muscle growth and atrophy. FEBS J. 2013, 280, 4294–4314. [Google Scholar] [CrossRef]
- Fernandez-Gonzalo, R.; Lundberg, T.R.; Tesch, P.A. Acute molecular responses in untrained and trained muscle subjected to aerobic and resistance exercise training versus resistance training alone. Acta Physiol. 2013, 209, 283–294. [Google Scholar] [CrossRef]
- De Souza, E.O.; Tricoli, V.; Roschel, H.; Brum, P.C.; Bacurau, A.V.; Ferreira, J.C.; Aoki, M.S.; Neves-Jr, M.; Aihara, A.Y.; Da Rocha Correa Fernandes, A. Molecular adaptations to concurrent training. Int. J. Sports Med. 2013, 34, 207–213. [Google Scholar] [CrossRef]
- Powell, J.; Pollizzi, K.; Heikamp, E.; Horton, M. Regulation of immune responses by mTOR. Annu. Rev. Immunol. 2012, 30, 39–68. [Google Scholar] [CrossRef]
- Hulmi, J.; Walker, S.; Ahtiainen, J.P.; Nyman, K.; Kraemer, W.J.; Häkkinen, K. Molecular signaling in muscle is affected by the specificity of resistance exercise protocol. Scand. J. Med. Sci. Sports 2010, 22, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Laplante, M.; Sabatini, D.M. mTOR signaling at a glance. J. Cell Sci. 2009, 122, 3589–3594. [Google Scholar] [CrossRef] [PubMed]
- Weihrauch, M.; Handschin, C. Pharmacological targeting of exercise adaptations in skeletal muscle: Benefits and pitfalls. Biochem. Pharmacol. 2017, 147, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Herzig, S.; Shaw, R. AMPK: Guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell Biol. 2018, 19, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Hardie, G.; Schaffer, B.; Brunet, A. AMPK: An energy-sensing pathway with multiple inputs and outputs. Trends Cell Biol. 2016, 26, 190–201. [Google Scholar] [CrossRef]
- Combes, A.; Dekerle, J.; Webborn, N.; Watt, P.; Bougault, V.; Daussin, F. Exercise-induced metabolic fluctuations influence AMPK, p38-MAPK and CaMKII phosphorylation in human skeletal muscle. Physiol. Rep. 2015, 3, e12462. [Google Scholar] [CrossRef]
- Chalari, E.; Methenitis, S.; Arnaoutis, G.; Stergiou, I.; Kampouropoulou, C.; Karampelas, E.; Prousinoudi, N.; Argyropoulou, V.; Nomikos, T. Different Kinetics of Oxidative Stress and Inflammatory Markers after Eccentric Exercise in Upper and Lower Limbs. Proceedings 2019, 25, 17. [Google Scholar] [CrossRef]
- Franz, A.; Behringer, M.; Nosaka, K.; Buhren, B.; Schrumpf, H.; Mayer, C.; Zilkens, C.; Schumann, M. Mechanisms underpinning protection against eccentric exercise-induced muscle damage by ischemic preconditioning. Med. Hypotheses 2017, 98, 21–27. [Google Scholar] [CrossRef]
- Chen, T.; Tseng, W.; Huang, G.; Chen, H.; Tseng, K.; Nosaka, K. Superior Effects of Eccentric to Concentric Knee Extensor Resistance Training on Physical Fitness, Insulin Sensitivity and Lipid Profiles of Elderly Men. Front. Physiol. 2017, 8. [Google Scholar] [CrossRef]
- Tajra, V.; Tibana, R.; Vieira, D.; De Farias, D.; Teixeira, T.; Funghetto, S.; Silva, A.; De Sousa, N.; Willardson, J.; Karnikowski, M. Identification of high responders for interleukin-6 and creatine kinase following acute eccentric resistance exercise in elderly obese women. J. Sci. Med. Sport 2014, 17, 662–666. [Google Scholar] [CrossRef] [PubMed]
- Franchi, M.; Atherton, P.; Reeves, N.; Flück, M.; Williams, J.; Mitchell, W.; Selby, A.; Valls, R.B.; Narici, M. Architectural, functional and molecular responses to concentric and eccentric loading in human skeletal muscle. Acta Physiol. 2014, 210, 642–654. [Google Scholar] [CrossRef] [PubMed]
- Paschalis, V.; Nikolaidis, M.; Theodorou, A.; Panayiotou, G.; Fatouros, I.; Koutedakis, Y.; Jamurtas, A. A weekly bout of eccentric exercise is sufficient to induce health-promoting effects. Med. Sci. Sports Exerc. 2011, 43, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Lin, K.; Chen, H.; Lin, M.; Nosaka, K. Comparison in eccentric exercise-induced muscle damage among four limb muscles. Eur. J. Appl. Physiol. 2011, 111, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Paschalis, V.; Nikolaidis, M.; Theodorou, A.; Giakas, G.; Jamurtas, A.; Koutedakis, Y. Eccentric exercise affects the upper limbs more than the lower limbs in position sense and reaction angle. J. Sports Sci. 2010, 28, 33–43. [Google Scholar] [CrossRef]
- Paschalis, V.; Nikolaidis, M.; Giakas, G.; Theodorou, A.; Sakellariou, G.; Fatouros, I.; Koutedakis, Y.; Jamurtas, A. Beneficial changes in energy expenditure and lipid profile after eccentric exercise in overweight and lean women. Scand. J. Med. Sci. Sports 2010, 20, e103–e111. [Google Scholar] [CrossRef]
- Saka, T.; Akova, B.; Yazici, Z.; Sekir, U.; Gür, H.; Ozarda, Y. Difference in the magnitude of muscle damage between elbow flexors and knee extensors eccentric exercises. J. Sports Sci. Med. 2009, 8, 107–115. [Google Scholar]
- Mahoney, D.J.; Safdar, A.; Parise, G.; Melov, S.; Fu, M.; MacNeil, L.; Kaczor, J.; Payne, E.; Tarnopolsky, M. Gene expression profiling in human skeletal muscle during recovery from eccentric exercise. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 294, R1901–R1910. [Google Scholar] [CrossRef]
- Jamurtas, A.; Theocharis, V.; Tofas, T.; Tsiokanos, A.; Yfanti, C.; Paschalis, V.; Koutedakis, Y.; Nosaka, K. Comparison between leg and arm eccentric exercises of the same relative intensity on indices of muscle damage. Eur. J. Appl. Physiol. 2005, 95, 179–185. [Google Scholar] [CrossRef]
- Feasson, L.; Stockholm, D.; Freyssenet, D.; Richard, I.; Duguez, S.; Beckmann, J.; Denis, C. Molecular adaptations of neuromuscular disease-associated proteins in response to eccentric exercise in human skeletal muscle. J. Physiol. 2002, 543, 297–306. [Google Scholar] [CrossRef]
- Fatouros, I.; Jamurtas, A.; Nikolaidis, M.G.; Destouni, A.; Michailidis, Y.; Vrettou, C.; Douroudos, I.; Avloniti, A.; Chatzinikolaou, A.; Taxildaris, K. Time of sampling is crucial for measurement of cell-free plasma DNA following acute aseptic inflammation induced by exercise. Clin. Biochem. 2010, 43, 1368–1370. [Google Scholar] [CrossRef] [PubMed]
- Margaritelis, N.; Theodorou, A.; Paschalis, V.; Veskoukis, A.; Dipla, K.; Zafeiridis, A.; Panayiotou, G.; Vrabas, I.; Kyparos, A.; Nikolaidis, M. Experimental verification of regression to the mean in Redox Biol.: Differential responses to exercise. Free Radic. Res. 2016, 50, 1237–1244. [Google Scholar] [CrossRef] [PubMed]
- Nikolaidis, M.; Jamurtas, A.Z. Blood as a reactive species generator and redox status regulator during exercise. Arch. Biochem. Biophys. 2009, 490, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Nikolaidis, M.; Paschalis, V.; Giakas, G.; Fatouros, I.; Sakellariou, G.; Theodorou, A.; Koutedakis, Y.; Jamurtas, A. Favorable and prolonged changes in blood lipid profile after muscle-damaging exercise. Med. Sci. Sports Exerc. 2008, 40, 1483–1489. [Google Scholar] [CrossRef] [PubMed]
- Sakelliou, A.; Fatouros, I.; Athanailidis, I.; Tsoukas, D.; Chatzinikolaou, A.; Draganidis, D.; Jamurtas, A.; Liacos, C.; Papassotiriou, I.; Mandalidis, D. Evidence of a redox-dependent regulation of immune responses to exercise-induced inflammation. Oxidative Med. Cell. Longev. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Higbie, E.J.; Cureton, K.J.; Warren, G.L.; Prior, B.M. Effects of concentric and eccentric training on muscle strength, cross-sectional area, and neural activation. J. Appl. Physiol. 1996, 81, 2173–2181. [Google Scholar] [CrossRef]
- Vikne, H.; Refsnes, P.E.; Ekmark, M.; Medbø, J.I.; Gundersen, V.; Gundersen, K. Muscular performance after concentric and eccentric exercise in trained men. Med. Sci. Sports Exerc. 2006, 38, 1770–1781. [Google Scholar] [CrossRef]
- Roig, M.; O’Brien, K.; Kirk, G.; Murray, R.; McKinnon, P.; Shadgan, B.; Reid, D.W. The effects of eccentric versus concentric resistance training on muscle strength and mass in healthy adults: A systematic review with meta-analyses. Br. J. Sports Med. 2009, 43, 556–568. [Google Scholar] [CrossRef]
- Nickols-Richardson, S.M.; Miller, L.E.; Wootten, D.F.; Ramp, W.K.; Herbert, W.G. Concentric and eccentric isokinetic resistance training similarly increases muscular strength, fat-free soft tissue mass, and specific bone mineral measurements in young women. Osteoporos. Int. 2007, 18, 789–796. [Google Scholar] [CrossRef]
- Walker, S.; Blazevich, A.J.; Haff, G.G.; Tufano, J.J.; Newton, R.U.; Häkkinen, K. Greater Strength Gains after Training with Accentuated Eccentric than Traditional Isoinertial Loads in Already Strength-Trained Men. Front. Physiol. 2016, 7. [Google Scholar] [CrossRef]
- Mike, J.; Cole, N.; Herrera, C.; VanD’usseldorp, T.; Kravitz, L.; Kerksick, C. The Effects of Eccentric Contraction Duration on Muscle Strength, Power Production, Vertical Jump, and Soreness. J. Strength Cond. Res. 2017, 31, 773–786. [Google Scholar] [CrossRef] [PubMed]
- LaStayo, P.; Marcus, R.; Dibble, L.; Frajacomo, F.; Lindstedt, S. Eccentric exercise in rehabilitation: Safety, feasibility, and application. J. Appl. Physiol. 2014, 116, 1426–1434. [Google Scholar] [CrossRef] [PubMed]
- Andersen, L.L.; Zeeman, P.; Jørgensen, J.R.; Bech-Pedersen, D.T.; Sørensen, J.; Kjær, M.; Andersen, J.L. Effects of intensive physical rehabilitation on neuromuscular adaptations in adults with poststroke hemiparesis. J. Strength Cond. Res. 2011, 25, 2808–2817. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, K.; Taivassalo, T.; Narici, M.; Franchi, M. Eccentric Exercise and the Critically ILL Patient. Front. Physiol. 2017, 8, 120. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, T.; Masuhara, M.; Ikuta, K. Effects of eccentric and concentric resistance training on arterial stiffness. J. Hum. Hypertens. 2006, 20, 348–354. [Google Scholar] [CrossRef]
- Melo, R.C.; Quitério, R.J.; Takahashi, A.C.; Silva, E.; Martins, L.; Catai, A.M. High eccentric strength training reduces heart rate variability in healthy older men. Br. J. Sports Med. 2008, 42, 59–63. [Google Scholar] [CrossRef]
- Dos Santos, E.S.; Asano, R.Y.; Filho, G.I.; Lopes, N.L.; Panelli, P.; Nascimento, D.; Collier, S.R.; Prestes, J. Acute and chronic cardiovascular response to 16 weeks of combined eccentric or traditional resistance and aerobic training in elderly hypertensive women: A randomized controlled trial. J. Strength Cond. Res. 2014, 28, 3073–3084. [Google Scholar] [CrossRef]
- Takahashi, A.C.; Melo, R.C.; Quitério, R.J.; Silva, E.; Catai, A.M. The effect of eccentric strength training on heart rate and on its variability during isometric exercise in healthy older men. Eur. J. Appl. Physiol. 2009, 105, 315–323. [Google Scholar] [CrossRef]
- Hawkins, S.; Schroeder, T.; Wiswell, R.; Jaque, V.; Marcell, T.; Costa, K. Eccentric muscle action increases site-specific osteogenic response. Med. Sci. Sports Exerc. 1999, 31, 1287–1292. [Google Scholar] [CrossRef]
- Mueller, M.; Breil, F.A.; Vogt, M.; Steiner, R.; Lippuner, K.; Popp, A.; Klossner, S.; Hoppeler, H.; Däpp, C. Different response to eccentric and concentric training in older men and women. Eur. J. Appl. Physiol. 2009, 107, 145–153. [Google Scholar] [CrossRef]
- Leszczak, T.J.; Olson, J.M.; Stafford, J.; Di Brezzo, R. Early adaptations to eccentric and high-velocity training on strength and functional performance in community-dwelling older adults. J. Strength Cond. Res. 2013, 27, 442–448. [Google Scholar] [CrossRef]
- Selva Raj, I.; Bird, S.; Westfold, B.; Shield, A. Effects of eccentrically biased versus conventional weight training in older adults. Med. Sci. Sports Exerc. 2012, 44, 1167–1176. [Google Scholar] [CrossRef]
- Marcus, R.L.; Smith, S.; Morrell, G.; Addison, O.; Dibble, L.E.; Wahoff-Stice, D.; LaStayo, P.C. Comparison of combined aerobic and high-force eccentric resistance exercise with aerobic exercise only for people with type 2 diabetes mellitus. J. Phys. Ther. 2008, 88, 1345. [Google Scholar] [CrossRef] [PubMed]
- Casillas, J.M.; Besson, D.; Hannequin, A.; Gremeaux, V.; Morisset, C.; Tordi, N.; Laurent, Y.; Laroche, D. Effects of an eccentric training personalized by a low rate of perceived exertion on the maximal capacities in chronic heart failure. Eur. J. Phys. Rehabil. Med. 2015, 52, 159–168. [Google Scholar] [PubMed]
- Besson, D.; Joussain, C.; Gremeaux, V.; Morisset, C.; Laurent, Y.; Casillas, J.M.; Laroche, D. Eccentric training in chronic heart failure: Feasibility and functional effects. Results of a comparative study. Ann. Phys. Rehabil. Med. 2013, 56, 30–40. [Google Scholar] [CrossRef]
- Karagiannis, C.; Savva, C.; Mamais, I.; Efstathiou, M.; Monticone, M.; Xanthos, T. Eccentric exercise in ischemic cardiac patients and functional capacity: A systematic review and meta-analysis of randomized controlled trials. Ann. Phys. Rehabil. Med. 2016, 60, 58–64. [Google Scholar] [CrossRef]
- Lastayo, P.C.; Reich, T.E.; Urquhart, M.; Hoppeler, H.; Lindstedt, S.L. Chronic eccentric exercise: Improvements in muscle strength can occur with little demand for oxygen. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1999, 276, R611–R615. [Google Scholar] [CrossRef]
- Bridgeman, L.; Gill, N.; Dulson, D.; McGuigan, M. The Effect of Exercise-Induced Muscle Damage After a Bout of Accentuated Eccentric Load Drop Jumps and the Repeated Bout Effect. J. Strength Cond. Res. 2017, 31, 386–394. [Google Scholar] [CrossRef]
- Skurvydas, A.; Brazaitis, M.; Venckūnas, T.; Kamandulis, S. Predictive value of strength loss as an indicator of muscle damage across multiple drop jumps. Appl. Physiol. Nutr. Metab. 2011, 36, 353–360. [Google Scholar] [CrossRef]
- Howatson, G.; Hoad, M.; Goodall, S.; Tallent, J.; Bell, P.; French, D. Exercise-induced muscle damage is reduced in resistance-trained males by branched chain amino acids: A randomized, double-blind, placebo controlled study. J. Int. Soc. Sports Nutr. 2012, 9, 20. [Google Scholar] [CrossRef]
- Tee, J.; Bosch, A.; Lambert, M. Metabolic Consequences of Exercise-Induced Muscle Damage. Sports Med. 2007, 37, 827–836. [Google Scholar] [CrossRef] [PubMed]
- Tofas, T.; Jamurtas, A.; Fatouros, I.; Nikolaidis, M.; Koutedakis, Y.; Sinouris, E.; Papageorgakopoulou, N.; Theocharis, D. Plyometric Exercise Increases Serum Indices of Muscle Damage and Collagen Breakdown. J. Strength Cond. Res. 2008, 22, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Chatzinikolaou, A.; Fatouros, I.; Gourgoulis, V.; Avloniti, A.; Jamurtas, A.; Nikolaidis, M.; Douroudos, I.; Michailidis, Y.; Beneka, A.; Malliou, P. Time course of changes in performance and inflammatory responses after acute plyometric exercise. J. Strength Cond. Res. 2010, 24, 1389–1398. [Google Scholar] [CrossRef] [PubMed]
- Komi, P.V. Stretch-shortening cycle: A powerful model to study normal and fatigued muscle. J. Biomech. 2000, 33, 1197–1206. [Google Scholar] [CrossRef]
- Peake, J.; Suzuki, K.; Hordern, M.; Wilson, G.; Nosaka, K.; Coombes, J. Plasma cytokine changes in relation to exercise intensity and muscle damage. Eur. J. Appl. Physiol. 2005, 95, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Marklund, P.; Mattsson, M.; Wåhlin-Larsson, B.; Ponsot, E.; Lindvall, B.; Lindvall, L.; Ekblom, B.; Kadi, F. Extensive inflammatory cell infiltration in human skeletal muscle in response to an ultraendurance exercise bout in experienced athletes. J. Appl. Physiol. 2013, 114, 66–72. [Google Scholar] [CrossRef]
- Peñailillo, L.; Blazevich, A.; Numazawa, H.; Nosaka, K. Metabolic and Muscle Damage Profiles of Concentric versus Repeated Eccentric Cycling. Med. Sci. Sports Exerc. 2013, 45, 1773–1781. [Google Scholar] [CrossRef]
- Nieman, D.; Davis, M.; Henson, D.; Gross, S.; Dumke, C.; Utter, A.; Vinci, D.; Carson, J.; Brown, A.; Mcanulty, S.; et al. Muscle Cytokine mRNA Changes after 2.5 h of Cycling: Influence of Carbohydrate. Med. Sci. Sports Exerc. 2005, 37, 1283–1290. [Google Scholar] [CrossRef]
- González-Bartholin, R.; Mackay, K.; Valladares, D.; Zbinden-Foncea, H.; Nosaka, K.; Peñailillo, L. Changes in oxidative stress, inflammation and muscle damage markers following eccentric versus concentric cycling in older adults. Eur. J. Appl. Physiol. 2019, 119, 2301–2312. [Google Scholar] [CrossRef]
- Li, T.L.; Cheng, P.Y. Alterations of immunoendocrine responses during the recovery period after acute prolonged cycling. Eur. J. Appl. Physiol. 2007, 101, 539. [Google Scholar] [CrossRef]
- Kawanishi, N.; Kato, K.; Takahashi, M.; Mizokami, T.; Otsuka, Y.; Imaizumi, A.; Shiva, D.; Yano, H.; Suzuki, K. Curcumin attenuates oxidative stress following downhill running-induced muscle damage. Biochem. Biophys. Res. Commun. 2013, 441, 573–578. [Google Scholar] [CrossRef]
- Peake, J.; Suzuki, K.; Wilson, G.; Hordern, M.; Nosaka, K.; Mackinnon, L.; Coombes, J. Exercise-Induced Muscle Damage, Plasma Cytokines, and Markers of Neutrophil Activation. Med. Sci. Sports Exerc. 2005, 37, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Lee, M. Effects of unaccustomed downhill running on muscle damage, oxidative stress, and leukocyte apoptosis. J. Exerc. Nutr. Biochem. 2015, 19, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Maeo, S.; Ando, Y.; Kanehisa, H.; Kawakami, Y. Localization of damage in the human leg muscles induced by downhill running. Sci. Rep. 2017, 7, 5769. [Google Scholar] [CrossRef]
- Malm, C.; Sjödin, B.; Sjöberg, B.; Lenkei, R.; Renström, P.; Lundberg, I.; Ekblom, B. Leukocytes, cytokines, growth factors and hormones in human skeletal muscle and blood after uphill or downhill running. J. Physiol. 2004, 556, 983–1000. [Google Scholar] [CrossRef]
- Damas, F.; Libardi, C.; Ugrinowitsch, C. The development of skeletal muscle hypertrophy through resistance training: The role of muscle damage and muscle protein synthesis. Eur. J. Appl. Physiol. 2018, 118, 485–500. [Google Scholar] [CrossRef]
- Damas, F.; Phillips, S.; Libardi, C.; Vechin, F.; Lixandrão, M.; Jannig, P.; Costa, L.; Bacurau, A.; Snijders, T.; Parise, G. Resistance training-induced changes in integrated myofibrillar protein synthesis are related to hypertrophy only after attenuation of muscle damage. J. Physiol. 2016, 594, 5209–5222. [Google Scholar] [CrossRef]
- Damas, F.; Ugrinowitsch, C.; Libardi, C.; Jannig, P.; Hector, A.; McGlory, C.; Lixandrão, M.; Vechin, F.; Montenegro, H.; Tricoli, V. Resistance training in young men induces muscle transcriptome-wide changes associated with muscle structure and metabolism refining the response to exercise-induced stress. Eur. J. Appl. Physiol. 2018, 118, 2607–2616. [Google Scholar] [CrossRef]
- Prestes, J.; Pereira, G.; Tibana, R.; Navalta, J. The acute response of apoptosis and migration to resistance exercise is protocol-dependent. Int. J. Sports Med. 2014, 35, 1051–1056. [Google Scholar] [CrossRef]
- Brown, W.; Davison, G.; McClean, C.; Murphy, M. A Systematic Review of the Acute Effects of Exercise on Immune and Inflammatory Indices in Untrained Adults. Sports Med. Open 2015, 1, 35. [Google Scholar] [CrossRef]
- Calle, M.; Fernandez, M. Effects of resistance training on the inflammatory response. Nutr. Res. Pract. 2010, 4, 259–269. [Google Scholar] [CrossRef]
- Cerqueira, É.; Marinho, D.; Neiva, H.; Lourenço, O. Inflammatory Effects of High and Moderate Intensity Exercise—A Systematic Review. Front. Physiol. 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Della Gatta, P.; Cameron-Smith, D.; Peake, J. Acute resistance exercise increases the expression of chemotactic factors within skeletal muscle. Eur. J. Appl. Physiol. 2014, 114, 2157–2167. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Koh, T.; Pizza, F.X. Do inflammatory cells influence skeletal muscle hypertrophy? Front. Biosci. 2009, E1, 60–71. [Google Scholar]
- Ihalainen, J.; Walker, S.; Paulsen, G.; Häkkinen, K.; Kraemer, W.; Hämäläinen, M.; Vuolteenaho, K.; Moilanen, E.; Mero, A. Acute leukocyte, cytokine and adipocytokine responses to maximal and hypertrophic resistance exercise bouts. Eur. J. Appl. Physiol. 2014, 114, 2607–2616. [Google Scholar] [CrossRef] [PubMed]
- Miles, M.; Kraemer, W.; Nindl, B.; Grove, D.; Leach, S.; Dohi, K.; Marx, J.; Volek, J.; Mastro, A. Strength, workload, anaerobic intensity and the immune response to resistance exercise in women. Acta Physiol. 2003, 178, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, C.J.; Churchward-Venne, T.A.; Bellamy, L.; Parise, G.; Baker, S.K.; Phillips, S. Muscular and systemic correlates of resistance training-induced muscle hypertrophy. PLoS ONE 2013, 8, e78636. [Google Scholar] [CrossRef]
- Vissing, K.; Overgaard, K.; Nedergaard, A.; Fredsted, A.; Schjerling, P. Effects of concentric and repeated eccentric exercise on muscle damage and calpain–calpastatin gene expression in human skeletal muscle. Eur. J. Appl. Physiol. 2008, 103, 323–332. [Google Scholar] [CrossRef]
- Hyldahl, R.; Olson, T.; Welling, T.; Groscost, L.; Parcell, A. Satellite cell activity is differentially affected by contraction mode in human muscle following a work-matched bout of exercise. Front. Physiol. 2014, 5. [Google Scholar] [CrossRef]
- Newton, M.; Morgan, G.; Sacco, P.; Chapman, D.; Nosaka, K. Comparison of Responses to Strenuous Eccentric Exercise of the Elbow Flexors Between Resistance-Trained and Untrained Men. J. Strength Cond. Res. 2008, 22, 597–607. [Google Scholar] [CrossRef]
- Margaritelis, N.; Theodorou, A.; Chatzinikolaou, P.; Kyparos, A.; Nikolaidis, M.; Paschalis, V. Eccentric exercise per se does not affect muscle damage biomarkers: Early and late phase adaptations. Eur. J. Appl. Physiol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Methenitis, S.; Karandreas, N.; Spengos, K.; Zaras, N.; Stasinaki, A.N.; Terzis, G. Muscle Fiber Conduction Velocity, Muscle Fiber Composition, and Power Performance. Med. Sci. Sports Exerc. 2016, 48, 1761–1771. [Google Scholar] [CrossRef] [PubMed]
- Methenitis, S.; Spengos, K.; Zaras, N.; Stasinaki, A.N.; Papadimas, G.; Karampatsos, G.; Arnaoutis, G.; Terzis, G. Fiber Type Composition And Rate Of Force Development In Endurance And Resistance Trained Individuals. J. Strength Cond. Res. 2019, 33, 2388–2397. [Google Scholar] [CrossRef] [PubMed]
- Methenitis, S.; Zaras, N.; Spengos, K.; Stasinaki, A.N.; Karampatsos, G.; Georgiadis, G.; Terzis, G. Role of Muscle Morphology in Jumping, Sprinting, and Throwing Performance in Participants With Different Power Training Duration Experience. J. Strength Cond. Res. 2016, 30, 807–817. [Google Scholar] [CrossRef]
- Spiliopoulou, P.; Zaras, N.; Methenitis, S.; Papadimas, G.; Papadopoulos, C.; Bogdanis, G.; Terzis, G. The effect of concurrent power training and high intensity interval cycling on muscle morphology and performance. J. Strength Cond. Res. 2019. [Google Scholar] [CrossRef]
- Terzis, G.; Spengos, K.; Methenitis, S.; Aagaard, P.; Karandreas, N.; Bogdanis, G. Early phase interference between low-intensity running and power training in moderately trained females. Eur. J. Appl. Physiol. 2016, 116, 1063–1073. [Google Scholar] [CrossRef]
- Zacharia, E.; Spiliopoulou, P.; Methenitis, S.; Stasinaki, A.N.; Zaras, N.; Papadopoulos, C.; Papadimas, G.; Karampatsos, G.; Bogdanis, G.; Terzis, G. Changes in muscle power and muscle morphology with different volumes of fast eccentric half-squats. Sports 2019, 7, 164. [Google Scholar] [CrossRef]
- Zaras, N.; Spengos, K.; Methenitis, S.; Papadopoulos, C.; Karampatsos, G.; Georgiadis, G.; Stasinaki, A.N.; Manta, P.; Terzis, G. Effects of Strength vs. Ballistic-Power Training on Throwing Performance. J. Sports Sci. Med. 2013, 12, 130–137. [Google Scholar]
- Abramowitz, M.W.P.; Zhang, K.; Brightwell, K.; Newsom, J.; Kwon, K.; Custodio, M.; Buttar, R.; Farooq, H.; Zaidi, B. Skeletal muscle fibrosis is associated with decreased muscle inflammation and weakness in patients with chronic kidney disease. Am. J. Physiol. Ren. Physiol. 2018, 315, F1658–F1669. [Google Scholar] [CrossRef]
- Levinger, I.; Levinger, P.; Trenerry, M.; Feller, J.; Bartlett, J.; Bergman, N.; McKenna, M.; Cameron-Smith, D. Increased inflammatory cytokine expression in the vastus lateralis of patients with knee osteoarthritis. Arthritis Rheum. 2011, 63, 1343–1348. [Google Scholar] [CrossRef]
- Caldow, M.; Cameron-Smith, D.; Levinger, P.; McKenna, M.; Levinger, I. Inflammatory markers in skeletal muscle of older adults. Eur. J. Appl. Physiol. 2013, 113, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Deyhle, M.; Gier, A.; Evens, K.; Eggett, D.; Nelson, W.; Parcell, A.; Hyldahl, R. Skeletal muscle inflammation following repeated bouts of lengthening contractions in humans. Front. Physiol. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Vella, L.; Markworth, J.; Paulsen, G.; Raastad, T.; Peake, J.; Snow, R.; Cameron-Smith, D.; Russell, A. Ibuprofen Ingestion Does Not Affect Markers of Post-exercise Muscle Inflammation. Front. Physiol. 2016, 7. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stupka, N.; Tarnopolsky, M.; Yardley, N.; Phillips, S. Cellular adaptation to repeated eccentric exercise-induced muscle damage. J. Appl. Physiol. 2001, 91, 1669–1678. [Google Scholar] [CrossRef]
- Vella, L.; Markworth, J.; Farnfield, M.; Maddipati, K.; Russell, A.; Cameron-Smith, D. Intramuscular inflammatory and resolving lipid profile responses to an acute bout of resistance exercise in men. Physiol. Rep. 2019, 7, e14108. [Google Scholar] [CrossRef] [PubMed]
- Sag, E.; Kale, G.; Haliloglu, G.; Bilginer, Y.; Akcoren, Z.; Orhan, D.; Gucer, S.; Topaloglu, H.; Ozen, S.; Talim, B. Inflammatory milieu of muscle biopsies in juvenile dermatomyositis. Rheumatol. Int. 2020. [Google Scholar] [CrossRef] [PubMed]
- Singer, K.; Lumeng, C. The initiation of metabolic inflammation in childhood obesity. J. Clin. Investig. 2017, 127, 65–73. [Google Scholar] [CrossRef]
- Hyldahl, R.; Chen, T.; Nosaka, K. Mechanisms and Mediators of the Skeletal Muscle Repeated Bout Effect. Exerc. Sport Sci. Rev. 2017, 45, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Sieljacks, P.; Matzon, A.; Wernbom, M.; Ringgaard, S.; Vissing, K.; Overgaard, K. Muscle damage and repeated bout effect following blood flow restricted exercise. Eur. J. Appl. Physiol. 2016, 116, 513–525. [Google Scholar] [CrossRef]
- Falvo, M.; Schilling, B.; Bloomer, R.; Smith, W. Repeated bout effect is absent in resistance trained men: An electromyographic analysis. J. Electromyogr. Kinesiol. 2009, 19, e529–e535. [Google Scholar] [CrossRef] [PubMed]
- Mascher, H.; Tannerstedt, J.; Brink-Elfegoun, T.; Ekblom, B.; Gustafsson, T.; Blomstrand, E. Repeated resistance exercise training induces different changes in mRNA expression of MAFbx and MuRF-1 in human skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2008, 294, E43–E51. [Google Scholar] [CrossRef] [PubMed]
- O’Carroll, C.; Fenwick, R. Rhabdomyolysis: A case-based critical reflection on its causes and diagnosis. J. Emerg. Nurse 2020, 28. [Google Scholar] [CrossRef]
- Phillips, P.; Haas, R. Statin myopathy as a metabolic muscle disease. Expert Rev. Cardiovasc. Ther. 2008, 6, 971–978. [Google Scholar] [CrossRef] [PubMed]
- Camera, D.; Smiles, W.; Hawley, J. Exercise-induced skeletal muscle signaling pathways and human athletic performance. Free Radic. Biol. Med. 2016, 98, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Radak, Z.; Bori, Z.; Koltai, E.; Fatouros, I.; Jamurtas, A.; Douroudos, I.; Terzis, G.; Nikolaidis, M.; Chatzinikolaou, A.; Sovatzidis, A. Age-dependent changes in 8-oxoguanine-DNA glycosylase activity are modulated by adaptive responses to physical exercise in human skeletal muscle. Free Radic. Biol. Med. 2011, 51, 417–423. [Google Scholar] [CrossRef]
- Powers, S.; Nelson, B.; Hudson, M. Exercise-induced oxidative stress in humans: Cause and consequences. Free Radic. Biol. Med. 2011, 51, 942–950. [Google Scholar] [CrossRef]
- Finaud, J.; Lac, G.; Filaire, E. Oxidative stress. Sports Med. 2006, 36, 327–358. [Google Scholar] [CrossRef]
- Radak, Z. Free Radicals in exeRcise and Aging; Human Kinetics Europe: Leeds, UK, 2000. [Google Scholar]
- Fehrenbach, E.; Northoff, H. Free radicals, exercise, apoptosis, and heat shock proteins. Exerc. Immunol. Rev. 2000, 7, 66–89. [Google Scholar]
- Brancaccio, P.; Lippi, G.; Maffulli, N. Biochemical markers of muscular damage. Clin. Chem. Lab. Med. 2010, 48, 757–767. [Google Scholar] [CrossRef]
- Wolfe, F.; Clauw, D.; Fitzcharles, M.; Goldenberg, D.; Häuser, W.; Katz, R.; Mease, P.; Russell, A.; Russell, I.; Walitt, B. 2016 Revisions to the 2010/2011 fibromyalgia diagnostic criteria. Semin. Arthritis Rheum. 2016, 46, 319–329. [Google Scholar] [CrossRef]
- Atzeni, F.; Talotta, R.; Masala, I.; Giacomelli, C.; Conversano, C.; Nucera, V.; Lucchino, B.; Iannuccelli, C.; Di Franco, M.; Bazzichi, L. One year in review 2019: Fibromyalgia. Clin. Exp. Rheumatol. 2019, 37 (Suppl. 116), 3–10. [Google Scholar] [PubMed]
- Littlejohn, G.; Guymer, E. Neurogenic inflammation in fibromyalgia. Semin. Immunopathol. 2018, 40, 291–300. [Google Scholar] [CrossRef] [PubMed]

| Physiological Response | Marker |
|---|---|
| Pain/Delayed Muscle Soreness | Perceived Muscle Soreness by Visual Analog Scale or Algometers |
| Muscle Function | Rate of Force/Torque Development, Maximum Strength Power, Range of Motion, Muscular Work |
| Oedema | Limb Circumferences |
| Oxidative Stress | Protein Carbonyls, MDA, Isoprostanes, GSH/GSSG, Antioxidant Enzymes (Glutathione Peroxidases, Superoxide Dismutase, Catalase), Total Antioxidant Capacity, Antioxidant Vitamins |
| Muscle Damage | CK, LDH, Myoglobin, T-Troponin, Hydroxyproline, Hydroxylysine |
| Systemic Inflammation | WBC Count, IL-6, TNF-a, CRP, IL-10, IL-8, IL-1Ra, Lipid Mediators, INF-γ, MCP1, MIP-1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Methenitis, S.; Stergiou, I.; Antonopoulou, S.; Nomikos, T. Can Exercise-Induced Muscle Damage Be a Good Model for the Investigation of the Anti-Inflammatory Properties of Diet in Humans? Biomedicines 2021, 9, 36. https://doi.org/10.3390/biomedicines9010036
Methenitis S, Stergiou I, Antonopoulou S, Nomikos T. Can Exercise-Induced Muscle Damage Be a Good Model for the Investigation of the Anti-Inflammatory Properties of Diet in Humans? Biomedicines. 2021; 9(1):36. https://doi.org/10.3390/biomedicines9010036
Chicago/Turabian StyleMethenitis, Spyridon, Ioanna Stergiou, Smaragdi Antonopoulou, and Tzortzis Nomikos. 2021. "Can Exercise-Induced Muscle Damage Be a Good Model for the Investigation of the Anti-Inflammatory Properties of Diet in Humans?" Biomedicines 9, no. 1: 36. https://doi.org/10.3390/biomedicines9010036
APA StyleMethenitis, S., Stergiou, I., Antonopoulou, S., & Nomikos, T. (2021). Can Exercise-Induced Muscle Damage Be a Good Model for the Investigation of the Anti-Inflammatory Properties of Diet in Humans? Biomedicines, 9(1), 36. https://doi.org/10.3390/biomedicines9010036







