Recent Trends in Photoacoustic Imaging Techniques for 2D Nanomaterial-Based Phototherapy
Abstract
:1. Introduction
2. Photoacoustic Imaging System
2.1. Photoacoustic Microscopy
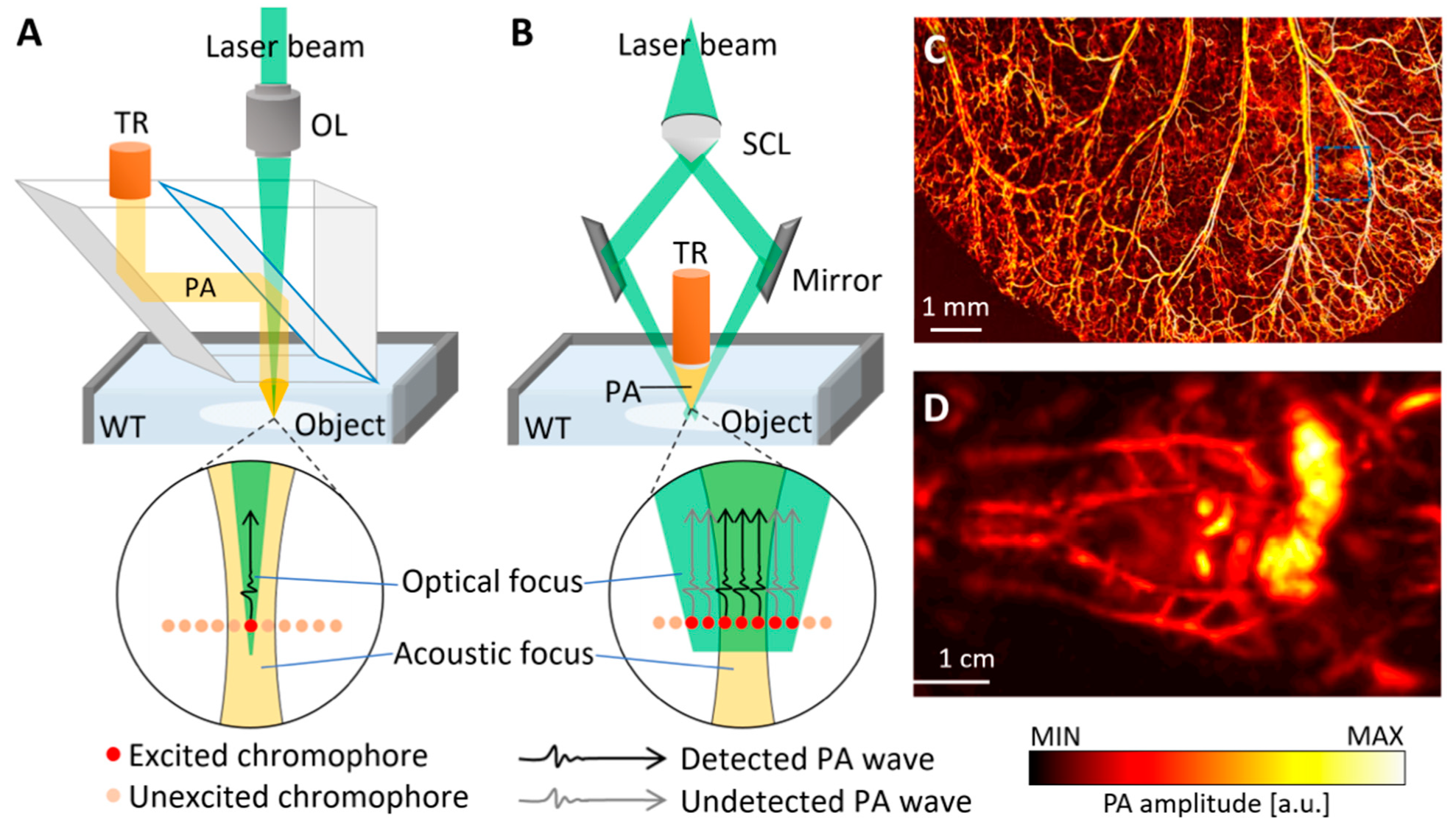
2.2. Photoacoustic Computed Tomography
2.3. Performance Benchmarks of Photoacoustic Imaging Systems
3. Phototherapy Using 2D Nanomaterials
3.1. Types and Characteristics
3.2. Phototherapy Using 2D Nanomaterials

| 2D Nanomaterials | Photothermal Conversion | Therapy | Applied Forms | Ref. |
|---|---|---|---|---|
| Graphene derivatives | 63% (G), 35% (GO) [59] | PTT, PDT | GO-UCNPs-ZnPc | [61] |
| PTT | GO/MnFe2O4/DOX | [62] | ||
| TiO2 | 40.8% [86] | PTT | Ag@TiO2 | [82] |
| PDT | N-TiO2 | [81] | ||
| MoS2 | 0.84% [67] | PTT | MoS2-HA-DTPA-Gd | [71] |
| PTT, PDT | AuNBPs@MoS2 | [72] | ||
| PTT | MoS2-Gd-BSA | [73] | ||
| BP | 30.84% [97] | PTT | BP-Au NSs | [98] |
| PTT | BP-PEG-FA/Cy7 NSs | [99] | ||
| PTT, PDT | BP@PEG/Ce6 NSs | [100] | ||
| Mxene(Ti3C2) | ≒100% [92] | PTT | Ti3C2@Au | [93] |
| PTT, PDT | Ti3C2-SP | [94] | ||
| PTT, PDT | Ti3C2-DOX | [95] | ||
| WS2 | 35% [68] | PTT, PDT | BSA-WS2@MB | [75] |
| PTT | WS2-PEG | [76] | ||
| PTT | WS2-IO/S@MO-PEG | [77] | ||
| MoSe2 | 54.3% [69] | PTT, PDT | MoSe2/Fe3O4 | [79] |
| PTT, PDT | MoSe2@PEG-Dox | [80] | ||
| 2D Boron | 42.5% [104] | PTT, PDT | B@Ce6–PAH–PAA | [105] |
| MnO2 | 62.4% [87] | PTT | MnO2-PEG-FA/DOX | [84] |
| PTT | BSA-MnO2 NPs | [85] |
4. Photoacoustic Image-Guided Phototherapy
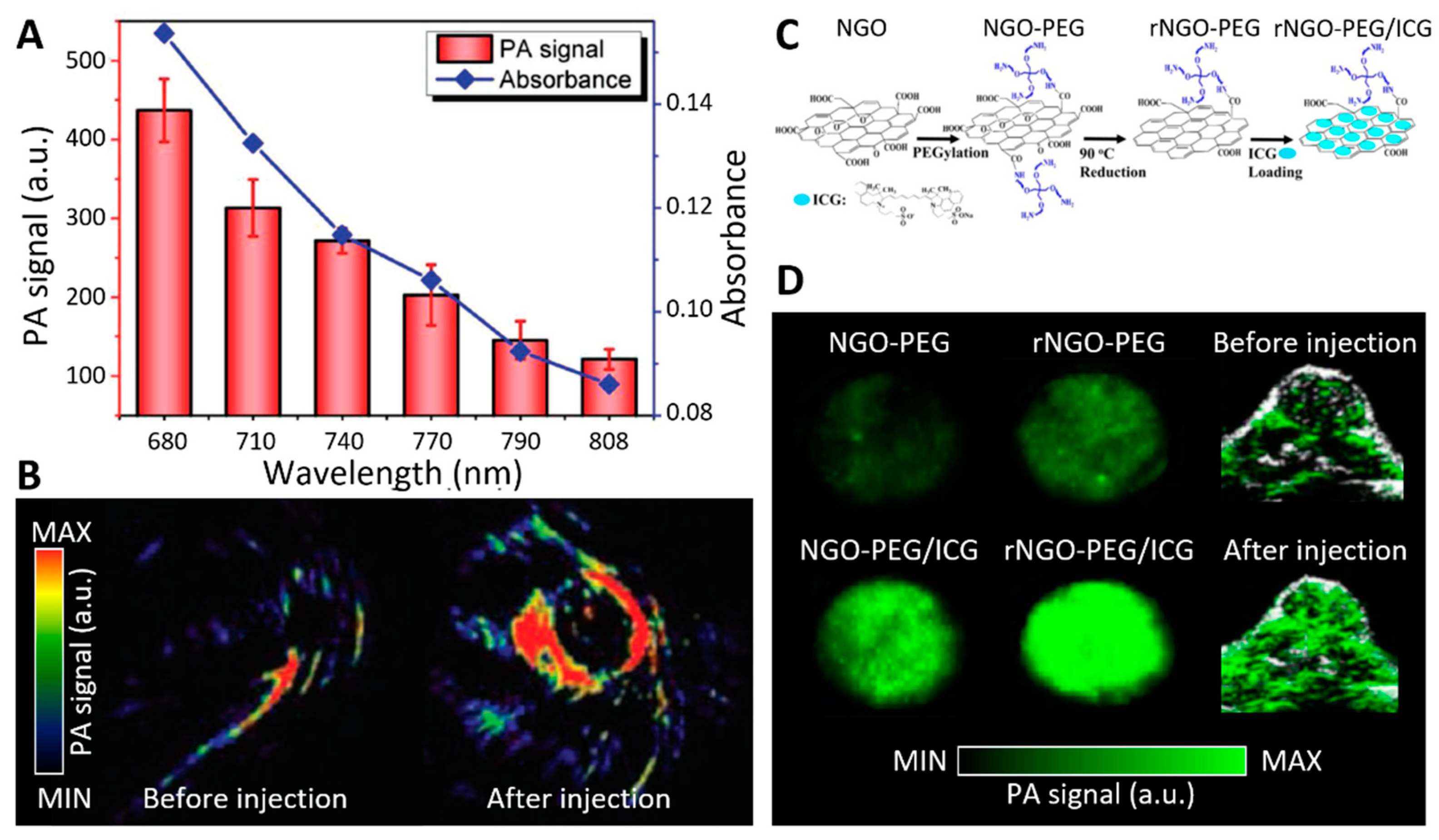
4.1. Contrast-Enhanced Photoacoustic Imaging Using 2D Nanomaterials
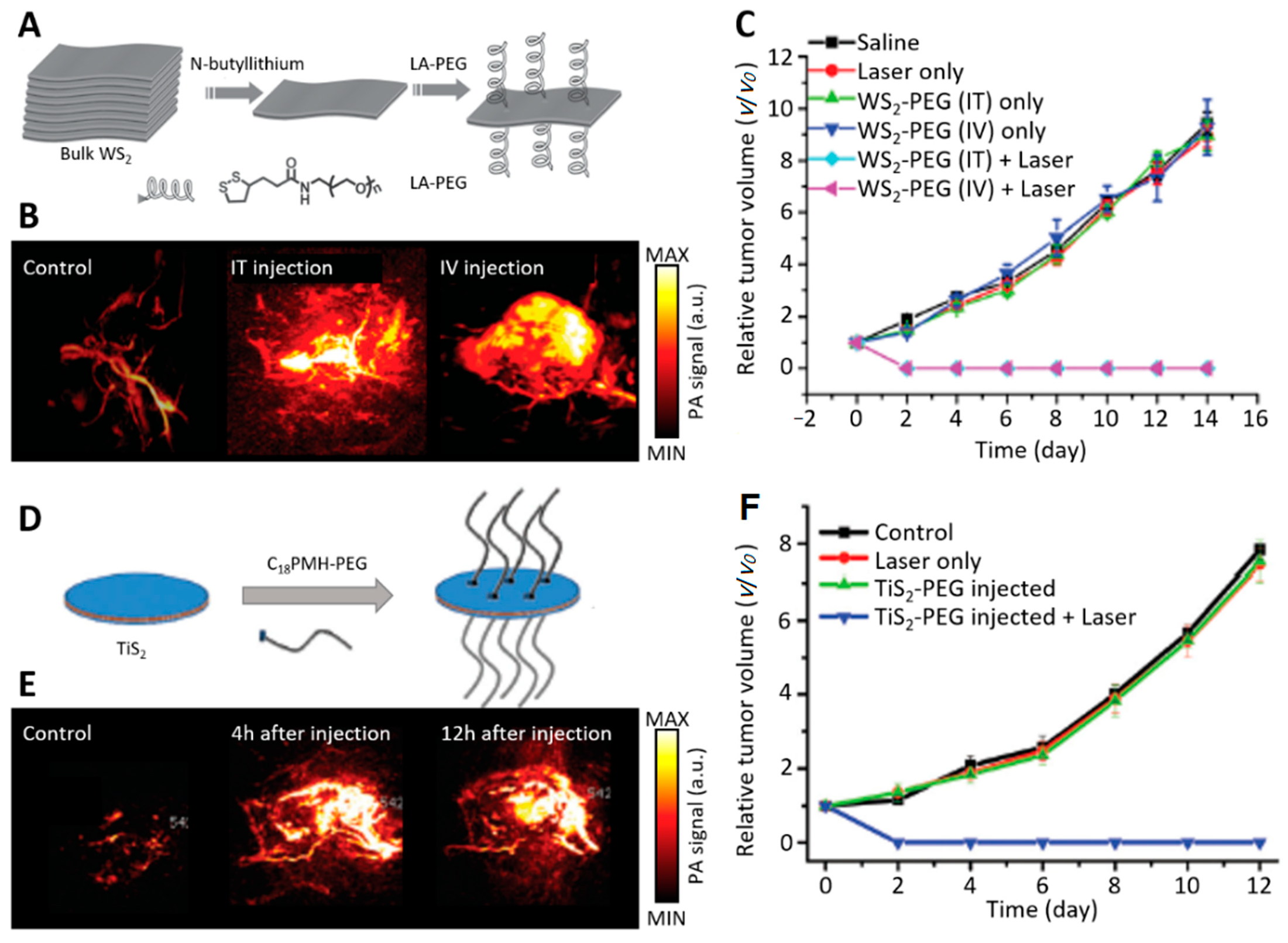
4.2. Photoacoustic Imaging for Monitoring Phototherapy
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cao, X.; Halder, A.; Tang, Y.; Hou, C.; Wang, H.; Duus, J.Ø.; Chi, Q. Engineering two-dimensional layered nanomaterials for wearable biomedical sensors and power devices. Mater. Chem. Front. 2018, 2, 1944–1986. [Google Scholar] [CrossRef]
- Hu, T.; Mei, X.; Wang, Y.; Weng, X.; Liang, R.; Wei, M. Two-dimensional nanomaterials: Fascinating materials in biomedical field. Sci. Bull. 2019, 64, 1707–1727. [Google Scholar] [CrossRef] [Green Version]
- Guan, G.; Han, M.Y. Functionalized Hybridization of 2D Nanomaterials. Adv. Sci. 2019, 6, 1901837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, F.; Song, P.; Ruan, M.; Xu, W. Recent progress in two-dimensional nanomaterials: Synthesis, engineering, and applications. FlatChem 2019, 18, 100133. [Google Scholar] [CrossRef]
- Fusco, L.; Gazzi, A.; Peng, G.; Shin, Y.; Vranic, S.; Bedognetti, D.; Vitale, F.; Yilmazer, A.; Feng, X.; Fadeel, B. Graphene and other 2D materials: A multidisciplinary analysis to uncover the hidden potential as cancer theranostics. Theranostics 2020, 10, 5435. [Google Scholar] [CrossRef]
- Chimene, D.; Alge, D.L.; Gaharwar, A.K. Two-dimensional nanomaterials for biomedical applications: Emerging trends and future prospects. Adv. Mater. 2015, 27, 7261–7284. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, P.; Abrahamse, H. Phototherapy Combined with Carbon Nanomaterials (1D and 2D) and their Applications in Cancer Therapy. Mater. Chem. Front. 2020, 13, 4830. [Google Scholar]
- Lin, C.; Hao, H.; Mei, L.; Wu, M. Metal-free two-dimensional nanomaterial-mediated photothermal tumor therapy. Smart Mater. Med. 2020, 1, 150–167. [Google Scholar] [CrossRef]
- Liu, S.; Pan, X.; Liu, H. Two-Dimensional Nanomaterials for Photothermal Therapy. Angew. Chem. 2020, 132, 5943–5953. [Google Scholar] [CrossRef]
- Gazzi, A.; Fusco, L.; Khan, A.; Bedognetti, D.; Zavan, B.; Vitale, F.; Yilmazer, A.; Delogu, L.G. Photodynamic therapy based on graphene and MXene in cancer theranostics. Front. Bioeng. Biotechnol. 2019, 7, 295. [Google Scholar] [CrossRef] [Green Version]
- Ritman, E.L. Current status of developments and applications of micro-CT. Annu. Rev. Biomed. Eng. 2011, 13, 531–552. [Google Scholar] [CrossRef] [PubMed]
- Judenhofer, M.S.; Cherry, S.R. Applications for preclinical PET/MRI. In Seminars in Nuclear Medicine; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Xie, T.; Zaidi, H. Development of computational small animal models and their applications in preclinical imaging and therapy research. Med. Phys. 2016, 43, 111–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wehrl, H.F.; Wiehr, S.; Divine, M.R.; Gatidis, S.; Gullberg, G.T.; Maier, F.C.; Rolle, A.-M.; Schwenck, J.; Thaiss, W.M.; Pichler, B.J. Preclinical and translational PET/MR imaging. J. Nucl. Med. 2014, 55, 11S–18S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pirovano, G.; Roberts, S.; Kossatz, S.; Reiner, T. Optical imaging modalities: Principles and applications in preclinical research and clinical settings. J. Nucl. Med. 2020, 61, 1419–1427. [Google Scholar] [CrossRef]
- Grashin, P.S.; Karabutov, A.A.; Oraevsky, A.A.; Pelivanov, I.M.; Podymova, N.B.; Savateeva, E.V.; Solomatin, V.S. Distribution of the laser radiation intensity in turbid media: Monte Carlo simulations, theoretical analysis, and results of optoacoustic measurements. Quantum Electron. 2002, 32, 868. [Google Scholar] [CrossRef]
- Bell, A.G. The photophone. Science 1880, 1, 130–131. [Google Scholar] [CrossRef]
- Kim, C.; Favazza, C.; Wang, L.V. In Vivo Photoacoustic Tomography of Chemicals: High-Resolution Functional and Molecular Optical Imaging at New Depths. Chem. Rev. 2010, 110, 2756–2782. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Lee, D.; Jung, U.; Kim, C. Photoacoustic imaging platforms for multimodal imaging. Ultrasonography 2015, 34, 88. [Google Scholar] [CrossRef]
- Kim, J.; Park, S.; Lee, C.; Kim, J.Y.; Kim, C. Organic Nanostructures for Photoacoustic Imaging. ChemNanoMat 2015, 2, 156–166. [Google Scholar] [CrossRef]
- Lee, C.; Kim, J.; Zhang, Y.; Jeon, M.; Liu, C.; Song, L.; Lovell, J.F.; Kim, C. Dual-color photoacoustic lymph node imaging using nanoformulated naphthalocyanines. Biomaterials 2015, 73, 142–148. [Google Scholar] [CrossRef]
- Jeon, M.; Song, W.; Huynh, E.; Kim, J.; Kim, J.; Helfield, B.L.; Leung, B.Y.; Goertz, D.E.; Zheng, G.; Oh, J. Methylene blue microbubbles as a model dual-modality contrast agent for ultrasound and activatable photoacoustic imaging. J. Biomed. Opt. 2014, 19, 016005. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kim, J.; Jeon, M.; Song, J.; Kim, C. In Vivo Photoacoustic and Fluorescence Cystography Using Clinically Relevant Dual Modal Indocyanine Green. Sensors 2014, 14, 19660–19668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, M.Y.; Lee, C.; Jung, H.S.; Jeon, M.; Kim, K.S.; Yun, S.H.; Kim, C.; Hahn, S.K. Biodegradable Photonic Melanoidin for Theranostic Applications. ACS Nano 2015, 10, 822–831. [Google Scholar] [CrossRef] [PubMed]
- De La Zerda, A.; Zavaleta, C.; Keren, S.; Vaithilingam, S.; Bodapati, S.; Liu, Z.; Levi, J.; Smith, B.R.; Ma, T.-J.; Oralkan, O. Carbon nanotubes as photoacoustic molecular imaging agents in living mice. Nat. Nanotechnol. 2008, 3, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jeon, M.; Rich, L.J.; Hong, H.; Geng, J.; Zhang, Y.; Shi, S.; Barnhart, T.E.; Alexandridis, P.; Huizinga, J.D. Non-Invasive Multimodal Functional Imaging of the Intestine with Frozen Micellar Naphthalocyanines. Nat. Nanotechnol. 2014, 9, 631–638. [Google Scholar] [CrossRef]
- Kim, J.; Park, S.; Jung, Y.; Chang, S.; Park, J.; Zhang, Y.; Lovell, J.F.; Kim, C. Programmable Real-time Clinical Photoacoustic and Ultrasound Imaging System. Sci. Rep. 2016, 6, 35137. [Google Scholar] [CrossRef] [Green Version]
- Jeon, S.; Park, E.-Y.; Choi, W.; Managuli, R.; jong Lee, K.; Kim, C. Real-time delay-multiply-and-sum beamforming with coherence factor for in vivo clinical photoacoustic imaging of humans. Photoacoustics 2019, 15, 100136. [Google Scholar] [CrossRef]
- Park, J.; Jeon, S.; Meng, J.; Song, L.; Lee, J.S.; Kim, C. Delay-multiply-and-sum-based synthetic aperture focusing in photoacoustic microscopy. J. Biomed. Opt. 2016, 21, 036010. [Google Scholar] [CrossRef]
- Yapici, M.K.; Kim, C.; Chang, C.-C.; Jeon, M.; Guo, Z.; Cai, X.; Zou, J.; Wang, L.V. Parallel acoustic delay lines for photoacoustic tomography. J. Biomed. Opt. 2012, 17, 116019. [Google Scholar] [CrossRef] [Green Version]
- Hu, S.; Maslov, K.; Wang, L.V. Second-generation optical-resolution photoacoustic microscopy with improved sensitivity and speed. Opt. Lett. 2011, 36, 1134–1136. [Google Scholar] [CrossRef] [Green Version]
- Bi, R.; Dinish, U.; Goh, C.C.; Imai, T.; Moothanchery, M.; Li, X.; Kim, J.Y.; Jeon, S.; Pu, Y.; Kim, C.; et al. In vivo label-free functional photoacoustic monitoring of ischemic reperfusion. J. Biophotonics 2019, 12, e201800454. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Wang, L.V. Photoacoustic Microscopy. Laser Photonics Rev. 2013, 7, 758–778. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Lee, C.; Park, K.; Lim, G.; Kim, C. Fast optical-resolution photoacoustic microscopy using a 2-axis water-proofing MEMS scanner. Sci. Rep. 2015, 5, srep07932. [Google Scholar] [CrossRef] [PubMed]
- Ning, B.; Sun, N.; Cao, R.; Chen, R.; Shung, K.K.; Hossack, J.A.; Lee, J.-M.; Zhou, Q.; Hu, S. Ultrasound-aided multi-parametric photoacoustic microscopy of the mouse brain. Sci. Rep. 2015, 5, 18775. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.; Beack, S.; Yoo, J.; Kim, S.K.; Lee, C.; Kwon, W.; Hahn, S.K.; Kim, C. In Vivo Photoacoustic Imaging of Livers Using Biodegradable Hyaluronic Acid-Conjugated Silica Nanoparticles. Adv. Funct. Mater. 2018, 28, 1800941. [Google Scholar] [CrossRef]
- Jung, H.; Park, S.; Gunassekaran, G.R.; Jeon, M.; Cho, Y.-E.; Baek, M.-C.; Park, J.Y.; Shim, G.; Oh, Y.-K.; Kim, I.-S.; et al. A Peptide Probe Enables Pphotoacoustic-Guided Imaging and Drug Delivery to Lung Tumors in K-rasLA2 Mutant Mice. Cancer Res. 2019, 79, 4271–4282. [Google Scholar] [CrossRef] [Green Version]
- Noh, I.; Kim, M.; Kim, J.; Lee, D.; Oh, D.; Kim, J.; Kim, C.; Jon, S.; Kim, Y.-C. Structure-inherent near-infrared bilayer nanovesicles for use as photoacoustic image-guided chemo-thermotherapy. J. Control. Release 2020, 320, 283–292. [Google Scholar] [CrossRef]
- Jeon, M.; Kim, J.; Kim, C. Multiplane spectroscopic whole-body photoacoustic imaging of small animals in vivo. Med. Biol. Eng. Comput. 2014, 54, 1–12. [Google Scholar] [CrossRef]
- Moothanchery, M.; Pramanik, M. Performance characterization of a switchable acoustic resolution and optical resolution photoacoustic microscopy system. Sensors 2017, 17, 357. [Google Scholar] [CrossRef]
- Baik, J.W.; Kim, J.Y.; Cho, S.; Choi, S.; Kim, J.; Kim, C. Super Wide-field Photoacoustic Microscopy of Animals and Humans In Vivo. IEEE Trans. Med. Imaging 2019, 39, 975–984. [Google Scholar] [CrossRef]
- Lin, L.; Xia, J.; Wong, T.T.; Li, L.; Wang, L.V. In vivo deep brain imaging of rats using oral-cavity illuminated photoacoustic computed tomography. J. Biomed. Opt. 2015, 20, 016019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chatni, M.R.; Xia, J.; Sohn, R.; Maslov, K.; Guo, Z.; Zhang, Y.; Wang, K.; Xia, Y.; Anastasio, M.; Arbeit, J.; et al. Tumor glucose metabolism imaged in vivo in small animals with whole-body photoacoustic computed tomography. J. Biomed. Opt. 2012, 17, 076012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.; Wang, Y.; Wang, W.; Luo, D.; Chitgupi, U.; Geng, J.; Zhou, Y.; Wang, L.; Lovell, J.F.; Xia, J. Deep tissue photoacoustic computed tomography with a fast and compact laser system. Biomed. Opt. Express 2017, 8, 112–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Zhu, L.; Ma, C.; Lin, L.; Yao, J.; Wang, L.; Maslov, K.; Zhang, R.; Chen, W.; Shi, J. Single-impulse panoramic photoacoustic computed tomography of small-animal whole-body dynamics at high spatiotemporal resolution. Nat. Biomed. Eng. 2017, 1, 1–11. [Google Scholar] [CrossRef]
- Heijblom, M.; Steenbergen, W.; Manohar, S. Clinical Photoacoustic Breast Imaging: The Twente experience. Pulse IEEE 2015, 6, 42–46. [Google Scholar] [CrossRef]
- Luís Deán-Ben, X.; Razansky, D. Adding fifth dimension to optoacoustic imaging: Volumetric time-resolved spectrally enriched tomography. Light Sci. Appl. 2014, 3, e137. [Google Scholar] [CrossRef] [Green Version]
- Park, S.; Park, G.; Kim, J.; Choi, W.; Jeong, U.; Kim, C. Bi2Se3 nanoplates for contrast-enhanced photoacoustic imaging at 1064 nm. Nanoscale 2018, 10, 20548–20558. [Google Scholar] [CrossRef]
- Chitgupi, U.; Nyayapathi, N.; Kim, J.; Wang, D.; Sun, B.; Li, C.; Carter, K.; Huang, W.C.; Kim, C.; Xia, J. Surfactant-Stripped Micelles for NIR-II Photoacoustic Imaging through 12 cm of Breast Tissue and Whole Human Breasts. Adv. Mater. 2019, 31, 1902279. [Google Scholar] [CrossRef]
- Choi, W.; Park, E.-Y.; Jeon, S.; Kim, C. Clinical photoacoustic imaging platforms. Biomed. Eng. Lett. 2018, 8, 139–155. [Google Scholar] [CrossRef]
- Kim, C.; Erpelding, T.N.; Jankovic, L.; Wang, L.V. Performance benchmarks of an array-based hand-held photoacoustic probe adapted from a clinical ultrasound system for non-invasive sentinel lymph node imaging. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2011, 369, 4644–4650. [Google Scholar] [CrossRef] [Green Version]
- Park, B.; Lee, K.M.; Park, S.; Yun, M.; Choi, H.-J.; Kim, J.; Lee, C.; Kim, H.; Kim, C. Deep tissue photoacoustic imaging of nickel (II) dithiolene-containing polymeric nanoparticles in the second near-infrared window. Theranostics 2020, 10, 2509. [Google Scholar] [CrossRef] [PubMed]
- Toi, M.; Asao, Y.; Matsumoto, Y.; Sekiguchi, H.; Yoshikawa, A.; Takada, M.; Kataoka, M.; Endo, T.; Kawaguchi-Sakita, N.; Kawashima, M. Visualization of tumor-related blood vessels in human breast by photoacoustic imaging system with a hemispherical detector array. Sci. Rep. 2017, 7, 41970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Kim, Y.H.; Park, B.; Seo, H.M.; Bang, C.H.; Park, G.S.; Park, Y.M.; Rhie, J.W.; Lee, J.H.; Kim, C. Multispectral Ex Vivo Photoacoustic Imaging of Cutaneous Melanoma for Better Selection of the Excision Margin. Br. J. Dermatol. 2018, 179, 780–782. [Google Scholar] [CrossRef] [PubMed]
- Neuschler, E.I.; Butler, R.; Young, C.A.; Barke, L.D.; Bertrand, M.L.; Böhm-Vélez, M.; Destounis, S.; Donlan, P.; Grobmyer, S.R.; Katzen, J. A pivotal study of optoacoustic imaging to diagnose benign and malignant breast masses: A new evaluation tool for radiologists. Radiology 2017, 287, 398–412. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, J.; Schwarz, M.; Garzorz, N.; Omar, M.; Buehler, A.; Eyerich, K.; Ntziachristos, V. Precision assessment of label-free psoriasis biomarkers with ultra-broadband optoacoustic mesoscopy. Nat. Biomed. Eng. 2017, 1, 1–8. [Google Scholar] [CrossRef]
- Lin, J.; Huang, Y.; Huang, P. Graphene-based nanomaterials in bioimaging. In Biomedical Applications of Functionalized Nanomaterials; Elsevier: Amsterdam, The Netherlands, 2018; pp. 247–287. [Google Scholar]
- Tang, Y.; Zhao, Z.; Hu, H.; Liu, Y.; Wang, X.; Zhou, S.; Qiu, J. Highly stretchable and ultrasensitive strain sensor based on reduced graphene oxide microtubes–elastomer composite. ACS Appl. Mater. Interfaces 2015, 7, 27432–27439. [Google Scholar] [CrossRef]
- Savchuk, O.A.; Carvajal, J.; Massons, J.; Aguiló, M.; Díaz, F. Determination of photothermal conversion efficiency of graphene and graphene oxide through an integrating sphere method. Carbon 2016, 103, 134–141. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, Y.; Li, P.; Nie, Z.; Li, J. Applications of graphene and its derivatives in intracellular biosensing and bioimaging. Analyst 2016, 141, 4541–4553. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.; Liu, D.; Song, S.; Wang, X.; Zhang, H. Graphene oxide covalently grafted upconversion nanoparticles for combined NIR mediated imaging and photothermal/photodynamic cancer therapy. Biomaterials 2013, 34, 7715–7724. [Google Scholar] [CrossRef]
- Yang, Y.; Shi, H.; Wang, Y.; Shi, B.; Guo, L.; Wu, D.; Yang, S.; Wu, H. Graphene oxide/manganese ferrite nanohybrids for magnetic resonance imaging, photothermal therapy and drug delivery. J. Biomater. Appl. 2016, 30, 810–822. [Google Scholar] [CrossRef]
- Rives, V.; del Arco, M.; Martín, C. Intercalation of drugs in layered double hydroxides and their controlled release: A review. Appl. Clay Sci. 2014, 88, 239–269. [Google Scholar] [CrossRef]
- Khan, S.B.; Alamry, K.A.; Alyahyawi, N.A.; Asiri, A.M.; Arshad, M.N.; Marwani, H.M. Nanohybrid based on antibiotic encapsulated layered double hydroxide as a drug delivery system. Appl. Biochem. Biotechnol. 2015, 175, 1412–1428. [Google Scholar] [CrossRef] [PubMed]
- Bi, X.; Fan, T.; Zhang, H. Novel morphology-controlled hierarchical core@ shell structural organo-layered double hydroxides magnetic nanovehicles for drug release. ACS Appl. Mater. Interfaces 2014, 6, 20498–20509. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Xiang, B. 2D hetero-structures based on transition metal dichalcogenides: Fabrication, properties and applications. Sci. Bull. 2017, 62, 1148–1161. [Google Scholar] [CrossRef] [Green Version]
- Fu, C.; Tan, L.; Ren, X.; Wu, Q.; Shao, H.; Ren, J.; Zhao, Y.; Meng, X. Interlayer expansion of 2D MoS2 nanosheets for highly improved photothermal therapy of tumors in vitro and in vivo. Chem. Commun. 2018, 54, 13989–13992. [Google Scholar] [CrossRef]
- Cui, X.-Z.; Zhou, Z.-G.; Yang, Y.; Wei, J.; Wang, J.; Wang, M.-W.; Yang, H.; Zhang, Y.-J.; Yang, S.-P. PEGylated WS2 nanosheets for X-ray computed tomography imaging and photothermal therapy. Chin. Chem. Lett. 2015, 26, 749–754. [Google Scholar] [CrossRef]
- He, L.; Nie, T.; Xia, X.; Liu, T.; Huang, Y.; Wang, X.; Chen, T. Designing Bioinspired 2D MoSe2 Nanosheet for Efficient Photothermal-Triggered Cancer Immunotherapy with Reprogramming Tumor-Associated Macrophages. Adv. Funct. Mater. 2019, 29, 1901240. [Google Scholar] [CrossRef]
- Pumera, M.; Loo, A.H. Layered transition-metal dichalcogenides (MoS2 and WS2) for sensing and biosensing. Trac Trends Anal. Chem. 2014, 61, 49–53. [Google Scholar] [CrossRef]
- Liu, J.; Zheng, J.; Nie, H.; Zhang, D.; Cao, D.; Xing, Z.; Li, B.; Jia, L. Molybdenum disulfide-based hyaluronic acid-guided multifunctional theranostic nanoplatform for magnetic resonance imaging and synergetic chemo-photothermal therapy. J. Colloid Interface Sci. 2019, 548, 131–144. [Google Scholar] [CrossRef]
- Maji, S.K.; Yu, S.; Chung, K.; Sekkarapatti Ramasamy, M.; Lim, J.W.; Wang, J.; Lee, H.; Kim, D.H. Synergistic Nanozymetic Activity of Hybrid Gold Bipyramid–Molybdenum Disulfide Core@ Shell Nanostructures for Two-Photon Imaging and Anticancer Therapy. ACS Appl. Mater. Interfaces 2018, 10, 42068–42076. [Google Scholar] [CrossRef]
- Chen, L.; Zhou, X.; Nie, W.; Feng, W.; Zhang, Q.; Wang, W.; Zhang, Y.; Chen, Z.; Huang, P.; He, C. Marriage of Albumin–Gadolinium Complexes and MoS2 Nanoflakes as Cancer Theranostics for Dual-Modality Magnetic Resonance/Photoacoustic Imaging and Photothermal Therapy. ACS Appl. Mater. Interfaces 2017, 9, 17786–17798. [Google Scholar] [CrossRef] [PubMed]
- Chia, X.; Eng, A.Y.S.; Ambrosi, A.; Tan, S.M.; Pumera, M. Electrochemistry of nanostructured layered transition-metal dichalcogenides. Chem. Rev. 2015, 115, 11941–11966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yong, Y.; Zhou, L.; Gu, Z.; Yan, L.; Tian, G.; Zheng, X.; Liu, X.; Zhang, X.; Shi, J.; Cong, W. WS2 nanosheet as a new photosensitizer carrier for combined photodynamic and photothermal therapy of cancer cells. Nanoscale 2014, 6, 10394–10403. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Liu, J.; Gu, X.; Gong, H.; Shi, X.; Liu, T.; Wang, C.; Wang, X.; Liu, G.; Xing, H. PEGylated WS2 nanosheets as a multifunctional theranostic agent for in vivo dual-modal CT/photoacoustic imaging guided photothermal therapy. Adv. Mater. 2014, 26, 1886–1893. [Google Scholar] [CrossRef]
- Yang, G.; Zhang, R.; Liang, C.; Zhao, H.; Yi, X.; Shen, S.; Yang, K.; Cheng, L.; Liu, Z. Manganese Dioxide Coated WS2@ Fe3O4/sSiO2 Nanocomposites for pH-Responsive MR Imaging and Oxygen-Elevated Synergetic Therapy. Small 2018, 14, 1702664. [Google Scholar] [CrossRef]
- Zhou, X.; Sun, H.; Bai, X. Two-Dimensional Transition Metal Dichalcogenides: Synthesis, Biomedical Applications and Biosafety Evaluation. Front. Bioeng. Biotechnol. 2020, 8, 8. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, F.; Lin, H.; Qu, F. Biodegradable Hollow MoSe2/Fe3O4 Nanospheres as the Photodynamic Therapy-Enhanced Agent for Multimode CT/MR/IR Imaging and Synergistic Antitumor Therapy. ACS Appl. Mater. Interfaces 2019, 11, 43964–43975. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, F.; Wang, Q.; Yang, P.; Lin, H.; Qu, F. Hierarchical MoSe2 nanoflowers as novel nanocarriers for NIR-light-mediated synergistic photo-thermal/dynamic and chemo-therapy. Nanoscale 2018, 10, 14534–14545. [Google Scholar] [CrossRef]
- Moosavi, M.A.; Sharifi, M.; Ghafary, S.M.; Mohammadalipour, Z.; Khataee, A.; Rahmati, M.; Hajjaran, S.; Łos, M.J.; Klonisch, T.; Ghavami, S. Photodynamic N-TiO 2 nanoparticle treatment induces controlled ROS-mediated autophagy and terminal differentiation of leukemia cells. Sci. Rep. 2016, 6, 1–16. [Google Scholar] [CrossRef]
- Nie, C.; Du, P.; Zhao, H.; Xie, H.; Li, Y.; Yao, L.; Shi, Y.; Hu, L.; Si, S.; Zhang, M. Ag@ TiO2 Nanoprisms with Highly Efficient Near-Infrared Photothermal Conversion for Melanoma Therapy. Chem. Asian J. 2020, 15, 148–155. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Ye, D.; Wu, M.; Chen, H.; Zhang, L.; Shi, J.; Wang, L. Break-up of Two-Dimensional MnO2 Nanosheets Promotes Ultrasensitive pH-Triggered Theranostics of Cancer. Adv. Mater. 2014, 26, 7019–7026. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Wang, L.; Zhang, B.; Zhao, H.; Niu, M.; Hu, Y.; Zheng, C.; Zhang, H.; Chang, J.; Zhang, Z. Multifunctional nanosheets based on folic acid modified manganese oxide for tumor-targeting theranostic application. Nanotechnology 2015, 27, 025101. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Song, Y.; Zhu, G.; Zhang, D.; Liu, X. Highly biocompatible BSA-MnO2 nanoparticles as an efficient near-infrared photothermal agent for cancer therapy. Chin. Chem. Lett. 2018, 29, 1685–1688. [Google Scholar] [CrossRef]
- Li, W.; Elzatahry, A.; Aldhayan, D.; Zhao, D. Core–shell structured titanium dioxide nanomaterials for solar energy utilization. Chem. Soc. Rev. 2018, 47, 8203–8237. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Guan, S.; Weng, Y.; Xu, S.-M.; Lu, H.; Meng, X.; Zhou, S. Highly efficient vacancy-driven photothermal therapy mediated by ultrathin MnO2 nanosheets. ACS Appl. Mater. Interfaces 2019, 11, 6267–6275. [Google Scholar] [CrossRef]
- Ma, R.; Sasaki, T. Nanosheets of oxides and hydroxides: Ultimate 2D charge-bearing functional crystallites. Adv. Mater. 2010, 22, 5082–5104. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-zadeh, K.; Ou, J.Z.; Daeneke, T.; Mitchell, A.; Sasaki, T.; Fuhrer, M.S. Two dimensional and layered transition metal oxides. Appl. Mater. Today 2016, 5, 73–89. [Google Scholar] [CrossRef]
- Azadmanjiri, J.; Kumar, P.; Srivastava, V.K.; Sofer, Z. Surface Functionalization of 2D Transition Metal Oxides and Dichalcogenides via Covalent and Non-covalent Bonding for Sustainable Energy and Biomedical Applications. ACS Appl. Nano Mater. 2020, 3, 3116–3143. [Google Scholar] [CrossRef]
- Lin, H.; Wang, X.; Yu, L.; Chen, Y.; Shi, J. Two-dimensional ultrathin MXene ceramic nanosheets for photothermal conversion. Nano Lett. 2017, 17, 384–391. [Google Scholar] [CrossRef]
- Li, R.; Zhang, L.; Shi, L.; Wang, P. MXene Ti3C2: An effective 2D light-to-heat conversion material. ACS Nano 2017, 11, 3752–3759. [Google Scholar] [CrossRef] [Green Version]
- Tang, W.; Dong, Z.; Zhang, R.; Yi, X.; Yang, K.; Jin, M.; Yuan, C.; Xiao, Z.; Liu, Z.; Cheng, L. Multifunctional two-dimensional core–shell mxene@ gold nanocomposites for enhanced photo–radio combined therapy in the second biological window. ACS Nano 2018, 13, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Huang, J.; Lin, H.; Wang, Z.; Li, P.; Chen, Y. 2D ultrathin MXene-based drug-delivery nanoplatform for synergistic photothermal ablation and chemotherapy of cancer. Adv. Healthc. Mater. 2018, 7, 1701394. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zou, J.; Tang, Q.; Yang, X.; Zhang, Y.; Zhang, Q.; Huang, W.; Chen, P.; Shao, J.; Dong, X. Surface modified Ti3C2 MXene nanosheets for tumor targeting photothermal/photodynamic/chemo synergistic therapy. ACS Appl. Mater. Interfaces 2017, 9, 40077–40086. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.R.; Yong, K.W.; Choi, J.Y.; Nilghaz, A.; Lin, Y.; Xu, J.; Lu, X. Black phosphorus and its biomedical applications. Theranostics 2018, 8, 1005. [Google Scholar] [CrossRef]
- Xu, D.; Liu, J.; Wang, Y.; Jian, Y.; Wu, W.; Lv, R. Black Phosphorus Nanosheet with High Thermal Conversion Efficiency for Photodynamic/Photothermal/Immunotherapy. ACS Biomater. Sci. Eng. 2020, 6, 4940–4948. [Google Scholar] [CrossRef]
- Yang, G.; Liu, Z.; Li, Y.; Hou, Y.; Fei, X.; Su, C.; Wang, S.; Zhuang, Z.; Guo, Z. Facile synthesis of black phosphorus–Au nanocomposites for enhanced photothermal cancer therapy and surface-enhanced Raman scattering analysis. Biomater. Sci. 2017, 5, 2048–2055. [Google Scholar] [CrossRef]
- Tao, W.; Zhu, X.; Yu, X.; Zeng, X.; Xiao, Q.; Zhang, X.; Ji, X.; Wang, X.; Shi, J.; Zhang, H. Black phosphorus nanosheets as a robust delivery platform for cancer theranostics. Adv. Mater. 2017, 29, 1603276. [Google Scholar] [CrossRef]
- Yang, X.; Wang, D.; Shi, Y.; Zou, J.; Zhao, Q.; Zhang, Q.; Huang, W.; Shao, J.; Xie, X.; Dong, X. Black phosphorus nanosheets immobilizing Ce6 for imaging-guided photothermal/photodynamic cancer therapy. ACS Appl. Mater. Interfaces 2018, 10, 12431–12440. [Google Scholar] [CrossRef]
- Xiong, S.; Chen, X.; Liu, Y.; Fan, T.; Wang, Q.; Zhang, H.; Chen, T. Black phosphorus as a versatile nanoplatform: From unique properties to biomedical applications. J. Innov. Opt. Health Sci. 2020. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Wang, C.; Gu, X.; Gong, H.; Cheng, L.; Shi, X.; Feng, L.; Sun, B.; Liu, Z. Drug delivery with PEGylated MoS2 nano-sheets for combined photothermal and chemotherapy of cancer. Adv. Mater. 2014, 26, 3433–3440. [Google Scholar] [CrossRef]
- Zeng, D.; Wang, L.; Tian, L.; Zhao, S.; Zhang, X.; Li, H. Synergistic photothermal/photodynamic suppression of prostatic carcinoma by targeted biodegradable MnO2 nanosheets. Drug Deliv. 2019, 26, 661–672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, X.; Kong, N.; Wang, J.; Li, W.; Xiao, Y.; Gan, S.T.; Zhang, Y.; Li, Y.; Song, X.; Xiong, Q. A novel top-down synthesis of ultrathin 2D boron nanosheets for multimodal imaging-guided cancer therapy. Adv. Mater. 2018, 30, 1803031. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Ji, X.; Li, Z.; Su, Z.; Zhang, S. Boron-based nanosheets for combined cancer photothermal and photodynamic therapy. J. Mater. Chem. B 2020, 8, 4609–4619. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Wang, Y.; Hu, T.; Mei, X.; Zhao, X.; Bian, Y.; Jin, L.; Liang, R.; Weng, X.; Wei, M. Layered double hydroxide nanosheets: Towards ultrasensitive tumor microenvironment responsive synergistic therapy. J. Mater. Chem. B 2020, 8, 1445–1455. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, C.; Zeng, G.; You, Y.; Wang, H.; Gong, X.; Zheng, R.; Kim, J.; Kim, C.; Song, L. Indocyanine green loaded reduced graphene oxide for in vivo photoacoustic/fluorescence dual-modality tumor imaging. Nanoscale Res. Lett. 2016, 11, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, J.; Wang, X.H.; Feng, J.; Meng, X.; Bu, X.; Li, Y.; Zhang, N.; Wang, P. Antimonene Nanoflakes: Extraordinary Photoacoustic Performance for High-Contrast Imaging of Small Volume Tumors. Adv. Healthc. Mater. 2019, 8, 1900378. [Google Scholar] [CrossRef]
- Zhou, Y.; Feng, W.; Qian, X.; Yu, L.; Han, X.; Fan, G.; Chen, Y.; Zhu, J. Construction of 2D Antimony (III) Selenide Nanosheets for Highly Efficient Photonic Cancer Theranostics. ACS Appl. Mater. Interfaces 2019, 11, 19712–19723. [Google Scholar] [CrossRef]
- Lin, H.; Wang, Y.; Gao, S.; Chen, Y.; Shi, J. Theranostic 2D tantalum carbide (MXene). Adv. Mater. 2018, 30, 1703284. [Google Scholar] [CrossRef]
- Dai, C.; Chen, Y.; Jing, X.; Xiang, L.; Yang, D.; Lin, H.; Liu, Z.; Han, X.; Wu, R. Two-dimensional tantalum carbide (MXenes) composite nanosheets for multiple imaging-guided photothermal tumor ablation. ACS Nano 2017, 11, 12696–12712. [Google Scholar] [CrossRef]
- Xie, H.; Li, Z.; Sun, Z.; Shao, J.; Yu, X.F.; Guo, Z.; Wang, J.; Xiao, Q.; Wang, H.; Wang, Q.Q. Metabolizable ultrathin Bi2Se3 nanosheets in imaging-guided photothermal therapy. Small 2016, 12, 4136–4145. [Google Scholar] [CrossRef]
- Xie, H.; Liu, M.; You, B.; Luo, G.; Chen, Y.; Liu, B.; Jiang, Z.; Chu, P.K.; Shao, J.; Yu, X.F. Biodegradable Bi2O2Se Quantum Dots for Photoacoustic Imaging-Guided Cancer Photothermal Therapy. Small 2020, 16, 1905208. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Wang, J.; Liu, L.; Sun, Q.; You, Q.; Cheng, Y.; Wang, Y.; Wang, S.; Tan, F.; Li, N. One-pot synthesis of a bismuth selenide hexagon nanodish complex for multimodal imaging-guided combined antitumor phototherapy. Mol. Pharm. 2018, 15, 1941–1953. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.H.; Park, E.Y.; Han, S.; Jung, H.S.; Keum, D.H.; Lee, G.H.; Kim, T.; Kim, C.; Kim, K.S.; Yun, S.H. Multimodal cancer theranosis using hyaluronate-conjugated molybdenum disulfide. Adv. Healthc. Mater. 2019, 8, 1801036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, T.; Shi, S.; Liang, C.; Shen, S.; Cheng, L.; Wang, C.; Song, X.; Goel, S.; Barnhart, T.E.; Cai, W. Iron oxide decorated MoS2 nanosheets with double PEGylation for chelator-free radiolabeling and multimodal imaging guided photothermal therapy. ACS Nano 2015, 9, 950–960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Li, X.; Chen, Y.; Cai, X.; Yao, H.; Gao, W.; Zheng, Y.; An, X.; Shi, J.; Chen, H. A facile one-pot synthesis of a two-dimensional MoS2/Bi2S3 composite theranostic nanosystem for multi-modality tumor imaging and therapy. Adv. Mater. 2015, 27, 2775–2782. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Chen, M.; Liu, Y.; Tian, Y.; Song, Z.; Song, G.; Zhang, X. A dual factor activated metal–organic framework hybrid nanoplatform for photoacoustic imaging and synergetic photo-chemotherapy. Nanoscale 2019, 11, 20630–20637. [Google Scholar] [CrossRef]
- Chen, M.; Tang, S.; Guo, Z.; Wang, X.; Mo, S.; Huang, X.; Liu, G.; Zheng, N. Core–Shell Pd@ Au Nanoplates as Theranostic Agents for In-Vivo Photoacoustic Imaging, CT Imaging, and Photothermal Therapy. Adv. Mater. 2014, 26, 8210–8216. [Google Scholar] [CrossRef]
- Xie, Z.; Chen, S.; Duo, Y.; Zhu, Y.; Fan, T.; Zou, Q.; Qu, M.; Lin, Z.; Zhao, J.; Li, Y. Biocompatible two-dimensional titanium nanosheets for multimodal imaging-guided cancer theranostics. ACS Appl. Mater. Interfaces 2019, 11, 22129–22140. [Google Scholar] [CrossRef]
- Ouyang, J.; Feng, C.; Ji, X.; Li, L.; Gutti, H.K.; Kim, N.Y.; Artzi, D.; Xie, A.; Kong, N.; Liu, Y.N. 2D monoelemental germanene quantum dots: Synthesis as robust photothermal agents for photonic cancer nanomedicine. Angew. Chem. 2019, 131, 13539–13544. [Google Scholar] [CrossRef]
- Jin, Z.; Chen, D.; Zhao, P.; Wen, Y.; Fan, M.; Zhou, G.; Wang, Y.; He, Q. Coordination-induced exfoliation to monolayer Bi-anchored MnB2 nanosheets for multimodal imaging-guided photothermal therapy of cancer. Theranostics 2020, 10, 1861. [Google Scholar] [CrossRef]
- Tang, W.; Fan, W.; Zhang, W.; Yang, Z.; Li, L.; Wang, Z.; Chiang, Y.L.; Liu, Y.; Deng, L.; He, L. Wet/sono-chemical synthesis of enzymatic two-dimensional MnO2 nanosheets for synergistic catalysis-enhanced phototheranostics. Adv. Mater. 2019, 31, 1900401. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhao, J.; Yang, H.; Wu, C.; Hu, F.; Chang, H.; Li, G.; Ma, D.; Zou, D.; Huang, M. Bottom-up synthesis of WS2 nanosheets with synchronous surface modification for imaging guided tumor regression. Acta Biomater. 2017, 58, 442–454. [Google Scholar] [CrossRef] [PubMed]
- Song, X.R.; Wang, X.; Yu, S.X.; Cao, J.; Li, S.H.; Li, J.; Liu, G.; Yang, H.H.; Chen, X. Co9Se8 nanoplates as a new theranostic platform for photoacoustic/magnetic resonance Dual-Modal-Imaging-Guided Chemo-Photothermal combination therapy. Adv. Mater. 2015, 27, 3285–3291. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Wang, Z.; Liu, Y.; Zhu, G.; Jacobson, O.; Fu, X.; Bai, R.; Lin, X.; Lu, N.; Yang, X. Suppressing nanoparticle-mononuclear phagocyte system interactions of two-dimensional gold nanorings for improved tumor accumulation and photothermal ablation of tumors. ACS Nano 2017, 11, 10539–10548. [Google Scholar] [CrossRef] [PubMed]
- Ke, K.; Yang, W.; Xie, X.; Liu, R.; Wang, L.-L.; Lin, W.-W.; Huang, G.; Lu, C.-H.; Yang, H.-H. Copper manganese sulfide nanoplates: A new two-dimensional theranostic nanoplatform for MRI/MSOT dual-modal imaging-guided photothermal therapy in the second near-infrared window. Theranostics 2017, 7, 4763. [Google Scholar] [CrossRef]
- Qian, X.; Shen, S.; Liu, T.; Cheng, L.; Liu, Z.J.N. Two-dimensional TiS 2 nanosheets for in vivo photoacoustic imaging and photothermal cancer therapy. Nanoscale 2015, 7, 6380–6387. [Google Scholar] [CrossRef]
- Huang, P.; Lin, J.; Li, W.; Rong, P.; Wang, Z.; Wang, S.; Wang, X.; Sun, X.; Aronova, M.; Niu, G. Biodegradable gold nanovesicles with an ultrastrong plasmonic coupling effect for photoacoustic imaging and photothermal therapy. Angew. Chem. 2013, 125, 14208–14214. [Google Scholar] [CrossRef]
- Lyu, Y.; Fang, Y.; Miao, Q.; Zhen, X.; Ding, D.; Pu, K. Intraparticle molecular orbital engineering of semiconducting polymer nanoparticles as amplified theranostics for in vivo photoacoustic imaging and photothermal therapy. ACS Nano 2016, 10, 4472–4481. [Google Scholar] [CrossRef]
- Ares, P.; Aguilar-Galindo, F.; Rodríguez-San-Miguel, D.; Aldave, D.A.; Díaz-Tendero, S.; Alcamí, M.; Martín, F.; Gómez-Herrero, J.; Zamora, F. Mechanical isolation of highly stable antimonene under ambient conditions. Adv. Mater. 2016, 28, 6332–6336. [Google Scholar] [CrossRef]
- Evans, P.M. Anatomical imaging for radiotherapy. Phys. Med. Biol. 2008, 53, R151. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Liang, X.; Li, G.; Liu, H.; Zhang, H.; Guo, J.; Chen, J.; Shen, K.; San, X.; Yu, W. 9.2%-efficient core-shell structured antimony selenide nanorod array solar cells. Nat. Commun. 2019, 10, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mashtalir, O.; Naguib, M.; Mochalin, V.N.; Dall’Agnese, Y.; Heon, M.; Barsoum, M.W.; Gogotsi, Y. Intercalation and delamination of layered carbides and carbonitrides. Nat. Commun. 2013, 4, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, C.-X.; Qi, X.-L.; Dai, X.; Fang, Z.; Zhang, S.-C. Topological insulators in Bi2Se3, Bi2Te3 and Sb2Te3 with a single Dirac cone on the surface. Nat. Phys. 2009, 5, 438–442. [Google Scholar] [CrossRef]
- Wang, M.-X.; Liu, C.; Xu, J.-P.; Yang, F.; Miao, L.; Yao, M.-Y.; Gao, C.; Shen, C.; Ma, X.; Chen, X. The coexistence of superconductivity and topological order in the Bi2Se3 thin films. Science 2012, 336, 52–55. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Dang, W.; Cao, J.; Chen, Y.; Wu, D.; Zheng, W.; Li, H.; Shen, Z.-X.; Liu, Z. Topological insulator nanostructures for near-infrared transparent flexible electrodes. Nat. Chem. 2012, 4, 281–286. [Google Scholar] [CrossRef]
- Wang, S.; Li, K.; Chen, Y.; Chen, H.; Ma, M.; Feng, J.; Zhao, Q.; Shi, J. Biocompatible PEGylated MoS2 nanosheets: Controllable bottom-up synthesis and highly efficient photothermal regression of tumor. Biomaterials 2015, 39, 206–217. [Google Scholar] [CrossRef]
- Yin, W.; Yan, L.; Yu, J.; Tian, G.; Zhou, L.; Zheng, X.; Zhang, X.; Yong, Y.; Li, J.; Gu, Z. High-throughput synthesis of single-layer MoS2 nanosheets as a near-infrared photothermal-triggered drug delivery for effective cancer therapy. ACS Nano 2014, 8, 6922–6933. [Google Scholar] [CrossRef]
- Oh, E.J.; Park, K.; Kim, K.S.; Kim, J.; Yang, J.-A.; Kong, J.-H.; Lee, M.Y.; Hoffman, A.S.; Hahn, S.K. Target specific and long-acting delivery of protein, peptide, and nucleotide therapeutics using hyaluronic acid derivatives. J. Control. Release 2010, 141, 2–12. [Google Scholar] [CrossRef]
- Johnson, L.A.; Banerji, S.; Lawrance, W.; Gileadi, U.; Prota, G.; Holder, K.A.; Roshorm, Y.M.; Hanke, T.; Cerundolo, V.; Gale, N.W. Dendritic cells enter lymph vessels by hyaluronan-mediated docking to the endothelial receptor LYVE-1. Nat. Immunol. 2017, 18, 762. [Google Scholar] [CrossRef]
- Guo, Z.; Chen, M.; Peng, C.; Mo, S.; Shi, C.; Fu, G.; Wen, X.; Zhuang, R.; Su, X.; Liu, T. pH-sensitive radiolabeled and superfluorinated ultra-small palladium nanosheet as a high-performance multimodal platform for tumor theranostics. Biomaterials 2018, 179, 134–143. [Google Scholar] [CrossRef]
- Syverud, M.; Dahl, J.; Herø, H.; Morisbak, E. Corrosion and biocompatibility testing of palladium alloy castings. Dent. Mater. 2001, 17, 7–13. [Google Scholar] [CrossRef]
- Tang, S.; Chen, M.; Zheng, N. Multifunctional ultrasmall Pd nanosheets for enhanced near-infrared photothermal therapy and chemotherapy of cancer. Nano Res. 2015, 8, 165–174. [Google Scholar] [CrossRef]




| Type | Lateral Resolution | Axial Resolution | Imaging Depth | Imaging Time | FOV | Ref. |
|---|---|---|---|---|---|---|
| OR-PAM | 2.56 μm | - | 1.2 mm | 70 min | 7.8 × 10 mm2 | [31] |
| 3.6 μm | 27.7 μm | 1 mm | 7 s | 9 × 4 mm2 | [34] | |
| 2.7 μm | 46.4 μm | 400 μm | 4 min | 6 × 8 mm2 | [35] | |
| AR-PAM | 590 μm | 150 μm | 25 mm | 20 min | 60 × 32 mm2 | [39] |
| 45 μm | 33 μm | 7.6 mm | 10 min | 9 × 7 mm2 | [40] | |
| 84 μm | 38 μm | 2.3 mm | 224 s | 36 × 80 mm2 | [41] | |
| PACT | 250 μm | 100 μm | 13 mm | 16 s | 25 × 30 mm2 | [42] |
| 1.5 mm | - | 10 mm | 0.1 s | 20 × 20 mm2 | [44] | |
| 1.2 mm | 205 μm | 30 mm | 0.2 s | 40 × 60 mm2 | [27] |
| PT-Multimodality | Key Material | Formulation | Modification/Functionalization/Hybridization | Experimental | Ref. |
|---|---|---|---|---|---|
| x | Black phosphorus (BP) | Quantum dot | Sulfonic ester of the titanium ligand (TiL4) | MCF-7, 293T cells, and MCF-7 tumor-bearing Balb/c nude mice | [103] |
| Layered double hydroxide (LDH) | Nanosheet | CoMn and chlorin e6 (Ce6) | HeLa, U87mg, HepG2, 4T1 cells, and HeLa tumor-bearing Balb/c nude mice | [106] | |
| Reduced graphene oxide (rGO) | Nanocomposite | Polyethylene glycol (PEG), indocyanine green (ICG) | Hela cells, Balb/c nude mice | [107] | |
| o | Antimonene (AM) | Liquid phase nanoflake | PEG | 293T, MCF-7, SK-BR3, T47D cells, and MCF-7 tumor-bearing mice | [108] |
| Antimony (III) Selenide (Sb2Se3) | Nanosheet | Poly(vinyl pyrrolidone) (PVP) | 4T1, MBA-MD-231 cells, Balb/c nude mice | [109] | |
| Tantalum carbide (Ti3C2 MXene) | Nanosheet | SP (soybean phospholipid) and doxorubicin (DOX) | 4T1 cells, 4T1-inoculated mice | [94] | |
| Tantalum carbide (Ta4C3 Mxene) | Nanosheet | MnO, SP | 4T1 cells, Kunming mouse, 4T1 tumor-bearing mice, and Balb/c nude mice | [110] | |
| MXene (Ta4C3) | Nanosheet | Manganese oxide nanoparticles (MnOx) and SP | 4T1 cells and 4T1 tumor-bearing nude mice | [111] | |
| Bismuth selenide (Bi2Se3) | Nanosheet | None | MCF-7 cells and MCF-7 tumor-bearing Balb/c nude mice | [112] | |
| Bi2O2Se | Quantum dot | None | A549, MCF-7 cells, and MCF-7 tumor-bearing Balb/c nude mice | [113] | |
| Bi2Se3 | Nanodish | HA, polypyrrole (PPy), and zinc phthalocyanine (ZnPc) | 4T1 cells and 4T1 tumor-bearing Balb/c nude mice | [114] | |
| Molybdenum disulfide (MoS2) | Nanoconjugate | Hyaluronic acid (HA) | HCT116 cells and HCT116-inoculated mice | [115] | |
| MoS2 | Nanosheet | Iron oxide nanoparticle (IONP) and PEG | Balb/c mice and 4T1 tumor-bearing mice | [116] | |
| MoS2 and Bi2S3 | Nanosheet | PEG | L929 cells and 4T1 tumor-bearing Balb/c nude mice | [117] | |
| Palladium (Pd) | Nanosheet | DOX, Zeolitic imidazolate frameworks (ZIF-8), Polydopamine (PDA) | WBCs (white blood cells) from mice, 4T1 cells, and 4T1 tumor-bearing mice | [118] | |
| Pd | Nanoplate | Au and PEG | 4T1 tumor-bearing Balb/c mice | [119] | |
| Titanium (Ti) | Nanosheet | PEG | SMMC-7721, B16, J774A.1 cells, and Balb/c nude mice | [120] | |
| Germanene | Quantum dot | PEG | MCF-7, 4T1, H1299, HeLa cells, and Balb/c mice | [121] | |
| Boron | Nanosheet | PEG | HeLa, PC3, MCF-7, A549 cells, and MCF-7 tumor-bearing mice | [104] | |
| Manganese boride | Nanosheet | Bi | 4T1 cells and 4T1 tumor-bearing mice | [122] | |
| MnO2 | Nanosheet | None | U87MG cells and U87MG tumor-bearing mice | [123] | |
| Tungsten disulfide (WS2) | Nanosheet | PVP | L929, HT29 cells, KM mice, and HT29 tumor-bearing KM mice | [124] | |
| Co9Se8 | Nanoplate | Poly(acrylic acid) (PAA), DOX | HepG2 cells, HepG2 tumor-bearing Balb/c-nude mice | [125] | |
| Au | Nanoring | None | Raw 264.7 cells, U87MG tumor-bearing nude mice | [126] | |
| Cu2MnS2 | Nanoplate | Monomethoxycarboxyl polyethylene glycol (mPEG-COOH) | HeLa, S180 cells, and S180 tumor-bearing Balb/c nude mice | [127] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, W.Y.; Kang, M.S.; Lee, H.; Lee, J.H.; Kim, J.; Han, D.-W.; Kim, K.S. Recent Trends in Photoacoustic Imaging Techniques for 2D Nanomaterial-Based Phototherapy. Biomedicines 2021, 9, 80. https://doi.org/10.3390/biomedicines9010080
Jeong WY, Kang MS, Lee H, Lee JH, Kim J, Han D-W, Kim KS. Recent Trends in Photoacoustic Imaging Techniques for 2D Nanomaterial-Based Phototherapy. Biomedicines. 2021; 9(1):80. https://doi.org/10.3390/biomedicines9010080
Chicago/Turabian StyleJeong, Woo Yeup, Moon Sung Kang, Haeni Lee, Jong Hun Lee, Jeesu Kim, Dong-Wook Han, and Ki Su Kim. 2021. "Recent Trends in Photoacoustic Imaging Techniques for 2D Nanomaterial-Based Phototherapy" Biomedicines 9, no. 1: 80. https://doi.org/10.3390/biomedicines9010080
APA StyleJeong, W. Y., Kang, M. S., Lee, H., Lee, J. H., Kim, J., Han, D.-W., & Kim, K. S. (2021). Recent Trends in Photoacoustic Imaging Techniques for 2D Nanomaterial-Based Phototherapy. Biomedicines, 9(1), 80. https://doi.org/10.3390/biomedicines9010080