The Role of Vitamins in Neurodegenerative Disease: An Update
Abstract
1. Introduction
2. Water-Soluble Vitamins
3. Fat-Soluble Vitamins
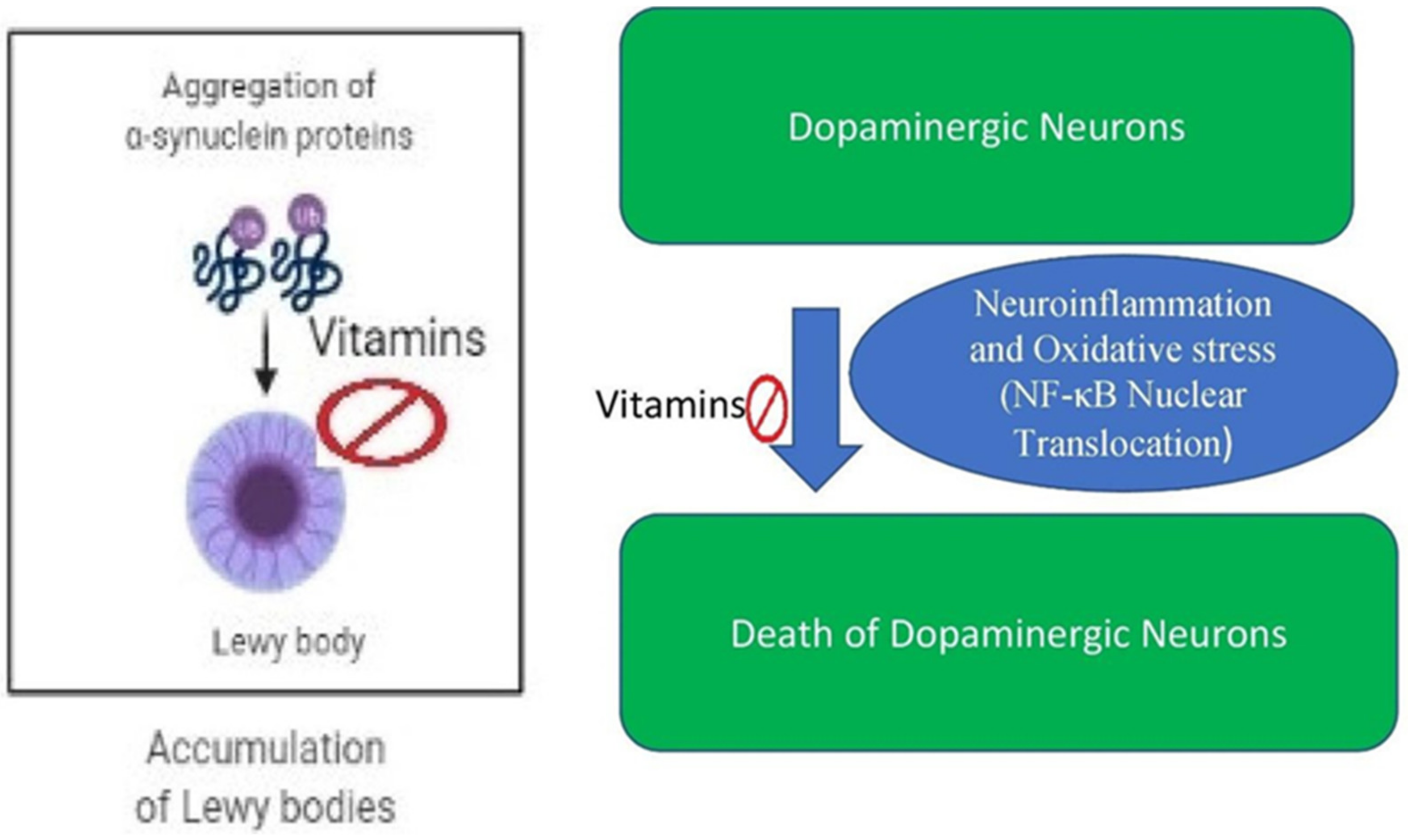
4. Vitamins in Parkinson’s Disease
4.1. Vitamins Based Clinical Studies in Parkinson’s Disease
4.2. Vitamin Based Animal Studies in Parkinson’s Disease
4.3. Vitamins in Cell-Based Parkinson’s Disease
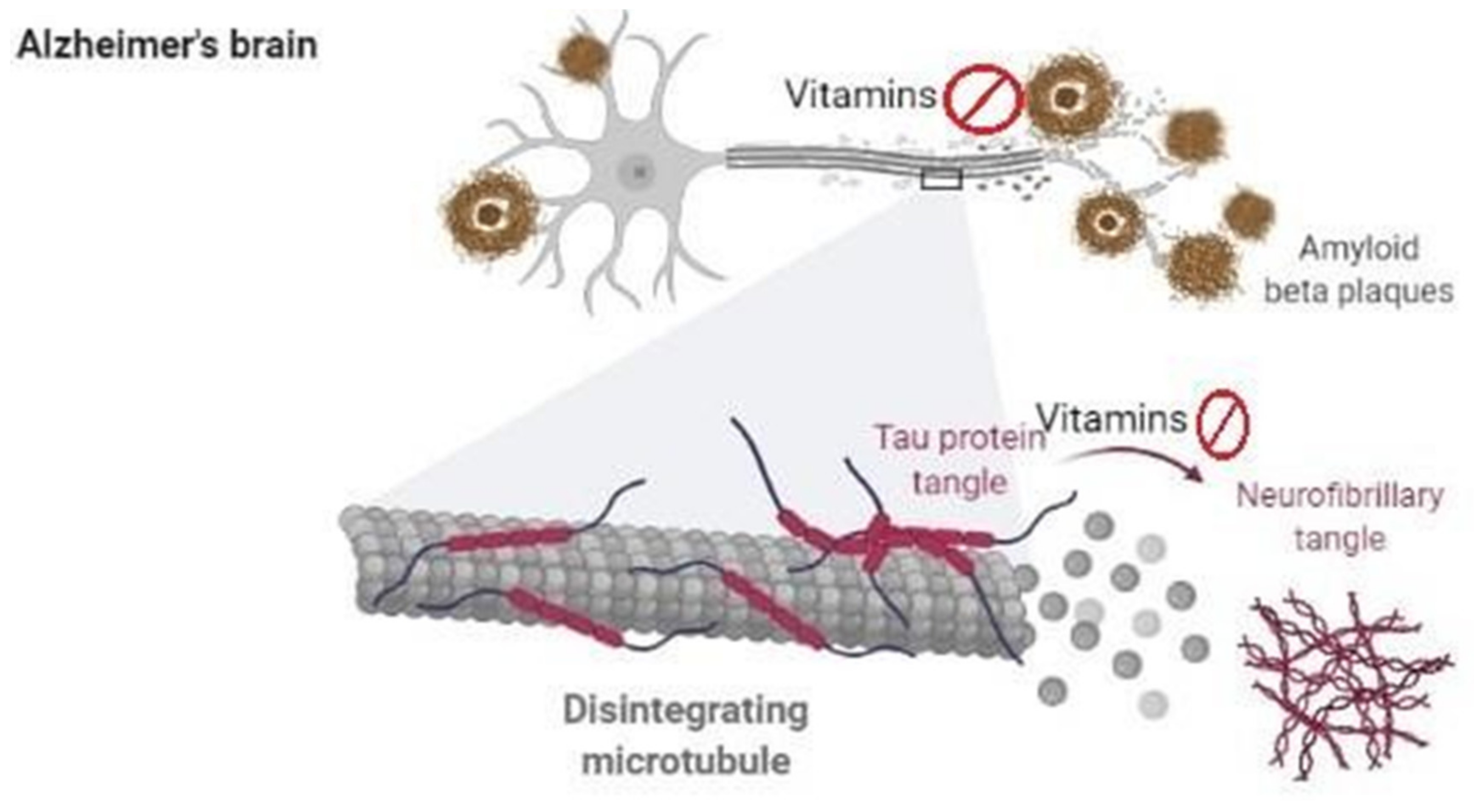
5. Vitamins in Alzheimer’s Disease
5.1. Vitamins Based Clinical Studies in Alzheimer’s Disease
5.2. Vitamins Based Animal and Cellular Studies in Alzheimer’s Disease
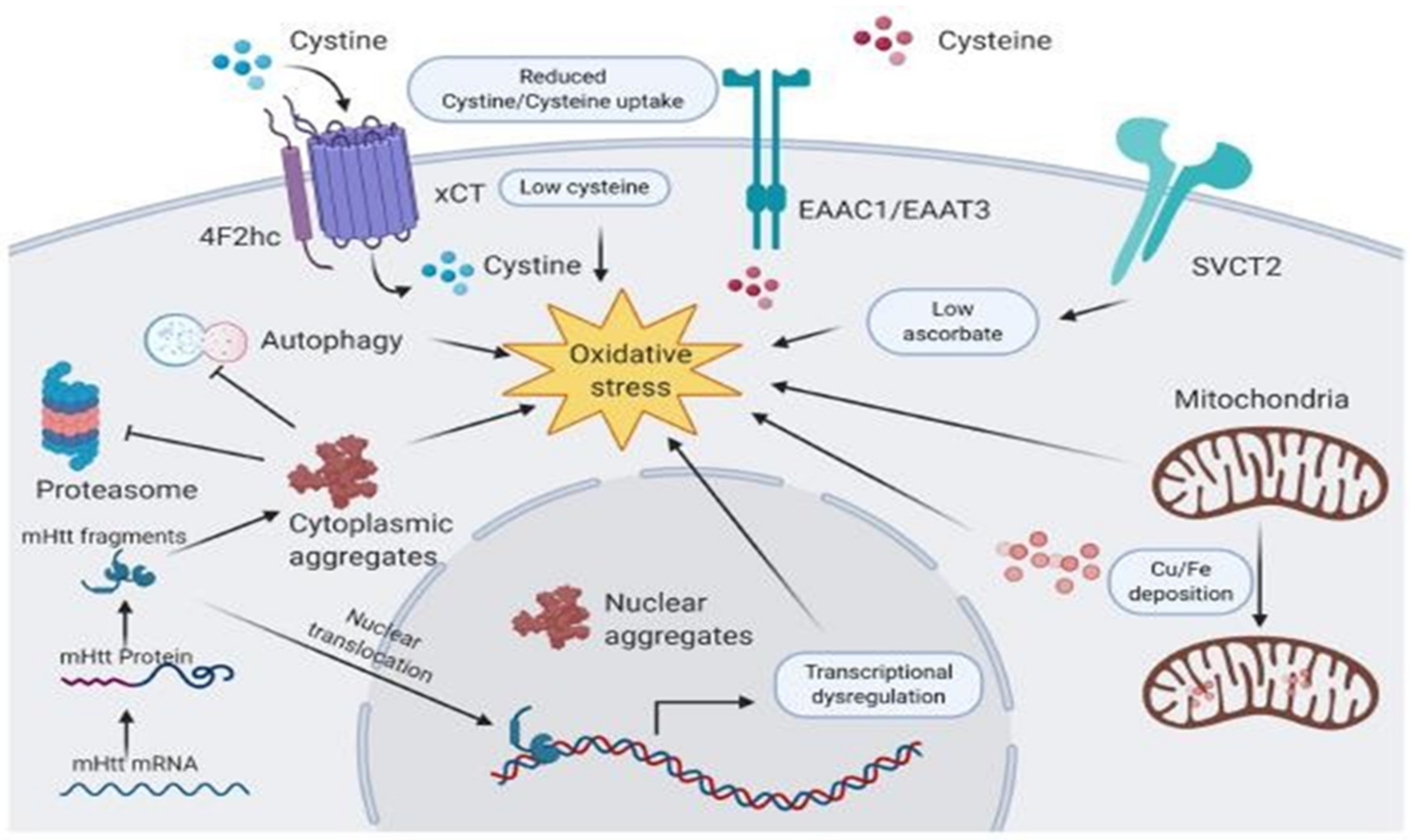
6. Vitamins in Huntington’s Disease
7. Vitamins in Multiple Sclerosis
8. Vitamins in Amyotrophic Lateral Sclerosis
9. Vitamins in Prion Disease
10. Vitamins in Age-Related Macular Degeneration
11. Conclusions and Future Prospective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Darnton-Hill, I. Public health aspects in the prevention and control of vitamin deficiencies. Curr. Dev. Nutr. 2019, 3, nzz075. [Google Scholar] [CrossRef]
- Shenkin, A. Micronutrients in health and disease. Postgrad. Med. J. 2006, 82, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Woteki, C.E.; Thomas, P.R. In eat for life: The food and nutrition board’s guide to reducing your risk of chronic disease. Clin. Nutr. Insight 1993, 19, 7. [Google Scholar] [CrossRef]
- Heaney, R.P. The nutrient problem. Nutr. Rev. 2012, 70, 165–169. [Google Scholar] [CrossRef]
- Shao, A.; Drewnowski, A.; Willcox, D.C.; Kramer, L.; Lausted, C.; Eggersdorfer, M.; Mathers, J.; Bell, J.D.; Randolph, R.K.; Witkamp, R.; et al. Optimal nutrition and the ever- changing dietary landscape: A conference report. Eur. J. Nutr. 2017, 56, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Kurutas, E.B. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: Current state. Nutr. J. 2015, 15, 71. [Google Scholar] [CrossRef]
- Sies, H.; Stahl, W.; Sundquist, A.R. Antioxidant functions of vitamins. Vitamins E and C, beta-carotene, and other carotenoids. Ann. N. Y. Acad. Sci. 1992, 669, 7–20. [Google Scholar] [CrossRef]
- Albahrani, A.A.; Greaves, R.F. Fat-soluble vitamins: Clinical indications and current challenges for chromatographic measurement. Clin. Biochem. Rev. 2016, 37, 27. [Google Scholar] [PubMed]
- Bruno, E.J.; Ziegenfuss, T.N. Water-soluble vitamins: Research update. Curr. Sports Med. Rep. 2005, 4, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Said, H.M.; Mohammed, Z.M. Intestinal absorption of water-soluble vitamins: An update. Curr. Opin. Gastroenterol. 2006, 22, 140–146. [Google Scholar] [CrossRef]
- Lykstad, J.; Sharma, S. Biochemistry, Water Soluble Vitamins; StatPearls: Treasure Island, FL, USA, 2021. [Google Scholar]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 1130, Thiamine. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Thiamine (accessed on 17 September 2021).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 493570, Riboflavin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Riboflavin (accessed on 17 September 2021).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 938, Nicotinic Acid. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Nicotinic-acid (accessed on 17 September 2021).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 6613, Pantothenic Acid. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Pantothenic-acid (accessed on 17 September 2021).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 1054, Pyridoxine. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Pyridoxine (accessed on 17 September 2021).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 171548, Biotin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Biotin (accessed on 17 September 2021).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 135398658, Folic Acid. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Folic-acid (accessed on 17 September 2021).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 5311498, Cyanocobalamin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Cyanocobalamin (accessed on 17 September 2021).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 54670067, Ascorbic Acid. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Ascorbic-acid (accessed on 17 September 2021).
- Zhao, X.; Zhang, M.; Li, C.; Jiang, X.; Su, Y.; Zhang, Y. Benefits of vitamins in the treatment of parkinson’s disease. Oxid. Med. Cell. Long. 2019, 2019, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.O. B vitamins and the brain: Mechanisms, dose and efficacy—A review. Nutrients 2016, 8, 68. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, A.; Roi, S.; Nowicki, M.; Dhaussy, A.; Huertas, A.; Amiot, M.J.; Reboul, E. Fat-soluble vitamin intestinal absorption: Absorption sites in the intestine and interactions for absorption. Food Chem. 2015, 172, 155–160. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 445354, Retinol. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Retinol (accessed on 17 September 2021).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 5280795, Cholecalciferol. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Cholecalciferol (accessed on 17 September 2021).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 14985, Vitamin E. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Vitamin-E (accessed on 17 September 2021).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 5280483, Vitamin K. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Vitamin-K (accessed on 17 September 2021).
- Paes-Silva, R.P.; Tomiya, M.T.O.; Maio, R.; De Castro, C.M.M.B.; De Arruda, I.K.G.; da Silva Diniz, A. Prevalence and factors associated with fat-soluble vitamin deficiency in adolescents. Nutr. Hosp. Organo Of. Soc. Esp. Nutr. Parenter. Y Enter. 2018, 35, 1153–1162. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am. J. Clin. Nutr. 2004, 80, 1678S–1688S. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, Y.; Qi, G.; Brand, D.; Zheng, S.G. Role of vitamin A in the immune system. J. Clin. Med. 2018, 7, 258. [Google Scholar] [CrossRef] [PubMed]
- Stephensen, C.B. Vitamin A, infection, and immune function. Annu. Rev. Nutr. 2001, 21, 167–192. [Google Scholar] [CrossRef] [PubMed]
- Committee on Diet and Health; National Research Council. Diet and Health: Implications for Reducing Chronic Disease Risk; National Academies Press: Washington, DC, USA, 1989. [Google Scholar] [CrossRef]
- Carlberg, C. Lipid soluble vitamins in gene regulation. Biofactors 1999, 10, 91–97. [Google Scholar] [CrossRef]
- Wu-Wong, J. Potential for vitamin D receptor agonists in the treatment of cardiovascular disease. Br. J. Pharmacol. 2009, 158, 395–412. [Google Scholar] [CrossRef] [PubMed]
- Koduah, P.; Paul, F.; Dorr, J.M. Vitamin D in the prevention, prediction and treatment of neurodegenerative and neuroinflammatory diseases. EPMA J. 2017, 8, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Moretti, R.; Caruso, P.; Dal Ben, M.; Conti, C.; Gazzin, S.; Tiribelli, C. Vitamin D, Homocysteine, and folate in subcortical vascular dementia and alzheimer dementia. Front. Aging Neurosci. 2017, 9, 169. [Google Scholar] [CrossRef]
- Royal, W.; Gartner, S.; Gajewski, C.D. Retinol measurements and retinoid receptor gene expression in patients with multiple sclerosis. Mult. Scler. J. 2002, 8, 452–458. [Google Scholar] [CrossRef]
- Szutowicz, A.; Bielarczyk, H.; Jankowska-Kulawy, A.; Ronowska, A.; Pawelczyk, T. Retinoic acid as a therapeutic option in Alzheimer’s disease: A focus on cholinergic restoration. Expert Rev. Neurother. 2015, 15, 239–249. [Google Scholar] [CrossRef]
- Haar, C.V.; Peterson, T.C.; Martens, K.M.; Hoane, M.R. Vitamins and nutrients as primary treatments in experimental brain injury: Clinical implications for nutraceutical therapies. Brain Res. 2016, 1640, 114–129. [Google Scholar] [CrossRef]
- Rai, S.N.; Birla, H.; Singh, S.S.; Zahra, W.; Patil, R.R.; Jadhav, J.P.; Gedda, M.R.; Singh, S.P. Mucuna pruriens protects against MPTP Intoxicated neuroinflammation in Parkinson’s disease through NF-κB/pAKT signaling pathways. Front. Aging Neurosci. 2017, 9, 421. [Google Scholar] [CrossRef] [PubMed]
- Rai, S.N.; Birla, H.; Zahra, W.; Singh, S.S.; Singh, S.P. Immunomodulation of Parkinson’s disease using Mucuna pruriens (Mp). J. Chem. Neuroanat. 2017, 85, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Rai, S.N.; Zahra, W.; Singh, S.S.; Birla, H.; Keswani, C.; Dilnashin, H.; Rathore, A.S.; Singh, R.; Singh, R.K.; Singh, S.P. Anti-inflammatory activity of ursolic acid in MPTP-induced parkinsonian mouse model. Neurotox. Res. 2019, 36, 452–462. [Google Scholar] [CrossRef] [PubMed]
- Rai, S.N.; Chaturvedi, V.K.; Singh, P.; Singh, B.K.; Singh, M.P. Mucuna pruriens in Parkinson’s and in some other diseases: Recent advancement and future prospective. 3 Biotech 2020, 10, 522. [Google Scholar] [CrossRef] [PubMed]
- Rai, S.N.; Singh, P.; Varshney, R.; Chaturvedi, V.K.; Vamanu, E.; Singh, M.P.; Singh, B.K. Promising drug targets and associated therapeutic interventions in Parkinson’s disease. Neural Regen. Res. 2021, 16, 1730–1739. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, M.; Rodriguez-Sabate, C.; Morales, I.; Sanchez, A.; Sabate, M. Parkinson’s disease as a result of aging. Aging Cell 2015, 14, 293–308. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, M.M. Medical management of Parkinson’s disease. Pharm. Ther. 2008, 33, 590. [Google Scholar]
- Elkouzi, A.; Vedam-Mai, V.; Eisinger, R.S.; Okun, M.S. Emerging therapies in Parkinson disease—Repurposed drugs and new approaches. Nat. Rev. Neurol. 2019, 15, 204–223. [Google Scholar] [CrossRef]
- Mischley, L.K.; Lau, R.C.; Bennett, R.D. Role of diet and nutritional supplements in Parkinson’s disease progression. Oxid. Med. Cell. Longev. 2017, 2017, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ciulla, M.; Marinelli, L.; Cacciatore, I.; Stefano, A.D. Role of dietary supplements in the management of Parkinson’s disease. Biomolecules 2019, 9, 271. [Google Scholar] [CrossRef] [PubMed]
- Dias, V.; Junn, E.; Mouradian, M. The role of oxidative stress in Parkinson’s disease. J. Park. Dis. 2013, 3, 461–491. [Google Scholar] [CrossRef]
- Gilbert, C. What is vitamin A and why do we need it? Community Eye Health 2013, 26, 65. [Google Scholar]
- Burri, B.J.; Chang, J.S.; Neidlinger, T.R. beta-Cryptoxanthin- and alpha-carotene-rich foods have greater apparent bioavailability than beta-carotene-rich foods in Western diets. Br. J. Nutr. 2011, 105, 212–219. [Google Scholar] [CrossRef]
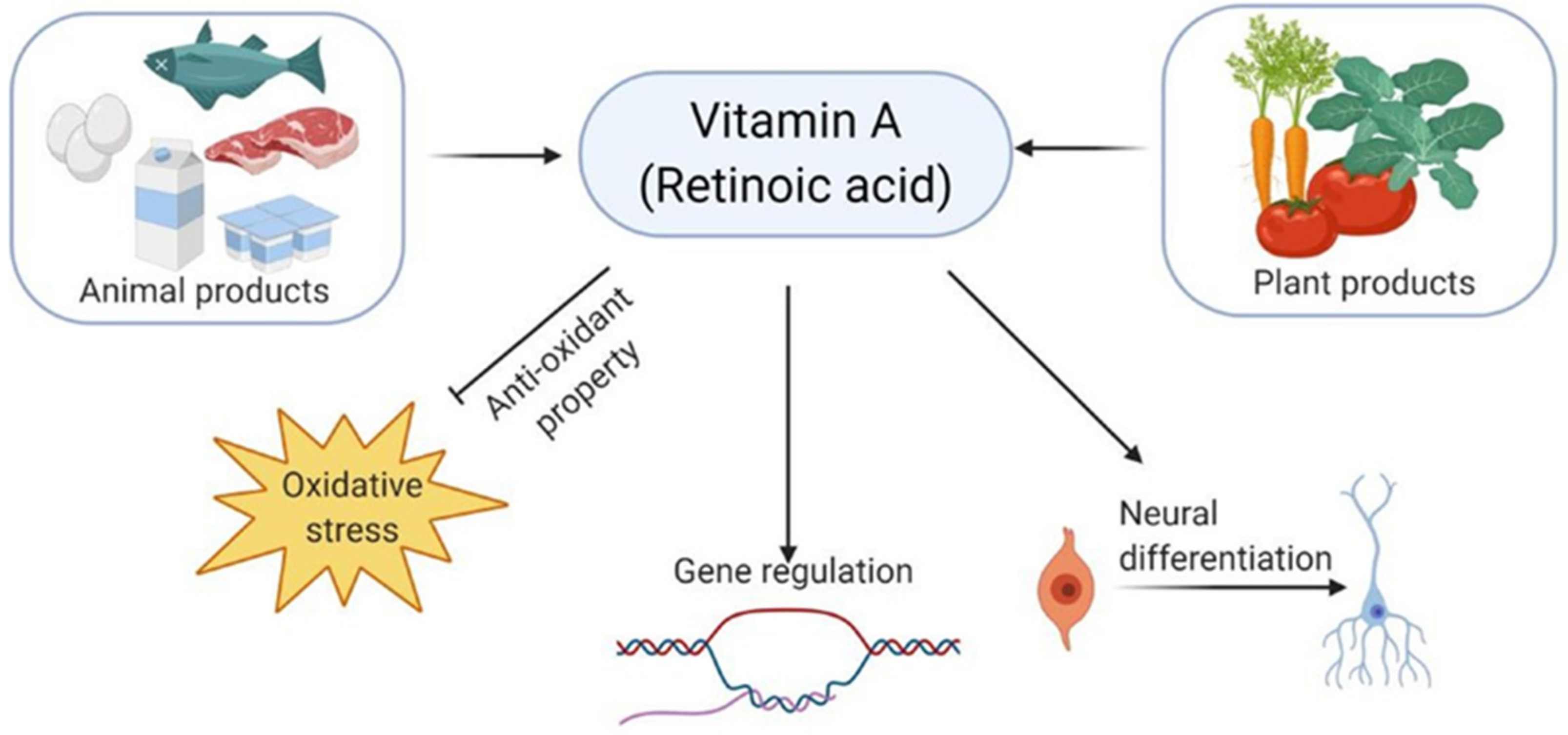
- Al Tanoury, Z.; Piskunov, A.; Rochette-Egly, C. Vitamin A and retinoid signaling: Genomic and nongenomic effects. J. Lipid Res. 2013, 54, 1761–1775. [Google Scholar] [CrossRef] [PubMed]
- Janesick, A.; Wu, S.C.; Blumberg, B. Retinoic acid signaling and neuronal differentiation. Cell. Mol. Life Sci. 2015, 72, 1559–1576. [Google Scholar] [CrossRef]
- Tafti, M.; Ghyselinck, N.B. Functional implication of the vitamin A signaling pathway in the brain. Arch. Neurol. 2007, 64, 1706–1711. [Google Scholar] [CrossRef]
- Craft, N.E.; Haitema, T.B.; Garnett, K.M.; Fitch, K.A.; Dorey, C.K. Carotenoid, tocopherol, and retinol concentrations in elderly human brain. J. Nutr. Health Aging 2004, 8, 156–162. [Google Scholar]
- Ying, A.F.; Khan, S.; Wu, Y.; Jin, A.; Wong, A.S.Y.; Tan, E.K.; Yuan, J.M.; Koh, W.P.; Tan, L.C.S. Dietary Antioxidants and Risk of Parkinson’s Disease in the Singapore Chinese Health Study. Mov. Disord. 2020, 35, 1765–1773. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.M.; Hernán, M.A.; Chen, H.; Spiegelman, D.; Willett, W.C.; Ascherio, A. Intakes of vitamins E and C, carotenoids, vitamin supplements, and PD risk. Neurology 2002, 59, 1161–1169. [Google Scholar] [CrossRef]
- Moretti, R.; Caruso, P. The controversial role of homocysteine in neurology: From labs to clinical practice. Int. J. Mol. Sci. 2019, 20, 231. [Google Scholar] [CrossRef]
- Müller, T. Role of homocysteine in the treatment of Parkinson’s disease. Exp. Rev. Neurotherap. 2008, 8, 957–967. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Oroz, M.C.; Lage, P.M.; Sanchez-Mut, J.; Lamet, I.; Pagonabarraga, J.; Toledo, J.B.; García-Garcia, D.; Clavero, P.; Samaranch, L.; Irurzun, C. Homocysteine and cognitive impairment in Parkinson’s disease: A biochemical, neuroimaging, and genetic study. Mov. Disord. 2009, 24, 1437–1444. [Google Scholar] [CrossRef]
- Saadat, P.; Ahmadi Ahangar, A.; Samaei, S.E.; Firozjaie, A.; Abbaspour, F.; Khafri, S.; Khoddami, A. Serum homocysteine level in parkinson’s disease and its association with duration, cardinal manifestation, and severity of disease. Park. Dis. 2018, 2018, 1–6. [Google Scholar] [CrossRef]
- Martignoni, E.; Tassorelli, C.; Nappi, G.; Zangaglia, R.; Pacchetti, C.; Blandini, F. Homocysteine and Parkinson’s disease: A dangerous liaison? J. Neurol. Sci. 2007, 257, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Rozycka, A.; P Jagodzinski, P.; Kozubski, W.; Lianeri, M.; Dorszewska, J. Homocysteine level and mechanisms of injury in Parkinson’s disease as related to MTHFR, MTR, and MTHFD1 genes polymorphisms and Ldopa treatment. Curr. Gen. 2013, 14, 534–542. [Google Scholar] [CrossRef]
- Markišić, M.; Pavlović, A.M.; Pavlović, D.M. The impact of homocysteine, vitamin b12, and vitamin d levels on functional outcome after first-ever ischaemic stroke. BioMed Res. Int. 2017, 2017, 5489057. [Google Scholar] [CrossRef]
- Kumar, A.; Palfrey, H.A.; Pathak, R.; Kadowitz, P.J.; Gettys, T.W.; Murthy, S.N. The metabolism and significance of homocysteine in nutrition and health. Nutr. Metab. 2017, 14, 78. [Google Scholar] [CrossRef]
- Maruyama, K.; Eshak, E.S.; Kinuta, M.; Nagao, M.; Cui, R.; Imano, H.; Ohira, T.; Iso, H. Association between vitamin B group supplementation with changes in % flow-mediated dilatation and plasma homocysteine levels: A randomized controlled trial. J. Clin. Biochem. Nutr. 2019, 64, 243–249. [Google Scholar] [CrossRef]
- De Lau, L.M.; Koudstaal, P.J.; Witteman, J.C.; Hofman, A.; Breteler, M.M. Dietary folate, vitamin B12, and vitamin B6 and the risk of Parkinson disease. Neurology 2006, 67, 315–318. [Google Scholar] [CrossRef] [PubMed]
- Shen, L. Associations between B Vitamins and Parkinson’s Disease. Nutrients 2015, 7, 7197–7208. [Google Scholar] [CrossRef]
- Dietiker, C.; Kim, S.; Zhang, Y.; Christine, C.W.; Investigators, N.N.-P. Characterization of Vitamin B12 Supplementation and correlation with clinical outcomes in a large longitudinal study of early Parkinson’s disease. J. Mov. Dis. 2019, 12, 91. [Google Scholar] [CrossRef]
- Kennedy, D.O. Review: Power foods for the brain. Cerebrum 2015, 2015, 5. [Google Scholar] [PubMed]
- Luong, K.V.; Nguyễn, L.T. The beneficial role of thiamine in Parkinson disease. CNS Neurosci. Ther. 2013, 19, 461–468. [Google Scholar] [CrossRef]
- Costantini, A.; Fancellu, R. An open-label pilot study with high-dose thiamine in Parkinson’s disease. Neural Regen. Res. 2016, 11, 406–407. [Google Scholar] [CrossRef]
- Håglin, L.; Johansson, I.; Forsgren, L.; Bäckman, L. Intake of vitamin B before onset of Parkinson’s disease and atypical parkinsonism and olfactory function at the time of diagnosis. Eur. J. Clin. Nutr. 2017, 71, 97–102. [Google Scholar] [CrossRef]
- Drouin, G.; Godin, J.R.; Page, B. The genetics of vitamin C loss in vertebrates. Curr. Genom. 2011, 12, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Alisky, J.M. Niacin improved rigidity and bradykinesia in a Parkinson’s disease patient but also caused unacceptable nightmares and skin rash—A case report. Nutr. Neurosci. 2005, 8, 327–329. [Google Scholar] [CrossRef] [PubMed]
- McAllister, C.J.; Scowden, E.B.; Dewberry, F.L.; Richman, A. Renal failure secondary to massive infusion of vitamin C. JAMA 1984, 252, 1684. [Google Scholar] [CrossRef]
- Wong, K.; Thomson, C.; Bailey, R.R.; McDiarmid, S.; Gardner, J. Acute oxalate nephropathy after a massive intravenous dose of vitamin C. Aust. N. Z. J. Med. 1994, 24, 410–411. [Google Scholar] [CrossRef]
- Grosso, G.; Bei, R.; Mistretta, A.; Marventano, S.; Calabrese, G.; Masuelli, L.; Giganti, M.G.; Modesti, A.; Galvano, F.; Gazzolo, D. Effects of vitamin C on health: A review of evidence. Front. Biosci. 2013, 18, 1017–1029. [Google Scholar] [CrossRef]
- Nagayama, H.; Hamamoto, M.; Ueda, M.; Nito, C.; Yamaguchi, H.; Katayama, Y. The effect of ascorbic acid on the pharmacokinetics of levodopa in elderly patients with Parkinson disease. Clin. Neuropharmacol. 2004, 27, 270–273. [Google Scholar] [CrossRef]
- Luong, K.V.Q.; Nguyễn, L.T.H. Roles of vitamin D in amyotrophic lateral sclerosis: Possible genetic and cellular signaling mechanisms. Mol. Brain 2013, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- Wacker, M.; Holick, M.F. Sunlight and vitamin D: A global perspective for health. Dermato-Endocrinology 2013, 5, 51–108. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.Y.; Bettany-Saltikov, J.; Cheung, I.Y.K.; Chan, K.K.Y. The Role of vitamin D in the pathogenesis of adolescent idiopathic scoliosis. Asian Spine J. 2018, 12, 1127–1145. [Google Scholar] [CrossRef]
- Engelsen, O. The relationship between ultraviolet radiation exposure and vitamin D status. Nutrients 2010, 2, 482–495. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, S.; Lutsey, P.L.; Alonso, A.; Huang, X.; Mosley, T.H., Jr.; Chen, H. Serum 25- hydroxyvitamin D concentrations in Mid-adulthood and Parkinson’s disease risk. Mov. Disord. 2016, 31, 972–978. [Google Scholar] [CrossRef]
- Sleeman, I.; Aspray, T.; Lawson, R.; Coleman, S.; Duncan, G.; Khoo, T.K.; Schoenmakers, I.; Rochester, L.; Burn, D.; Yarnall, A. The role of vitamin D in disease progression in early Parkinson’s disease. J. Park. Dis. 2017, 7, 669–675. [Google Scholar] [CrossRef]
- Brandi, M. Indications on the use of vitamin D and vitamin D metabolites in clinical phenotypes. Clin. Cases Miner. Bone Metab. 2010, 7, 243. [Google Scholar] [PubMed]
- Sunyecz, J.A. The use of calcium and vitamin D in the management of osteoporosis. Therap. Clin. Risk Manag. 2008, 4, 827. [Google Scholar] [CrossRef]
- Ozturk, E.A.; Gundogdu, I.; Tonuk, B.; Kocer, B.G.; Tombak, Y.; Comoglu, S.; Cakci, A. Bone mass and vitamin D levels in Parkinson’s disease: Is there any difference between genders? J. Phys. Ther. Sci. 2016, 28, 2204–2209. [Google Scholar] [CrossRef] [PubMed]
- Rimmelzwaan, L.M.; van Schoor, N.M.; Lips, P.; Berendse, H.W.; Eekhoff, E.M. Systematic review of the relationship between vitamin D and Parkinson’s disease. J. Park. Dis. 2016, 6, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Anjum, I.; Jaffery, S.S.; Fayyaz, M.; Samoo, Z.; Anjum, S. The role of vitamin D in brain health: A mini literature review. Cureus 2018, 10, e2960. [Google Scholar] [CrossRef]
- Fullard, M.E.; Duda, J.E. A Review of the Relationship Between Vitamin D and Parkinson Disease Symptoms. Front. Neurol. 2020, 11, 454. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, R.; Chatterjee, K.; Sen, C. Reversible Parkinsonism Due to Vitamin D Toxicity. J. Neurosci. Rural Pract. 2017, 8, 305–306. [Google Scholar] [CrossRef]
- Luo, X.; Ou, R.; Dutta, R.; Tian, Y.; Xiong, H.; Shang, H. Association between serum vitamin D levels and Parkinson’s disease: A systematic review and meta-analysis. Front. Neurol. 2018, 9, 909. [Google Scholar] [CrossRef]
- Luthra, N.S.; Kim, S.; Zhang, Y.; Christine, C.W.; NINDS NET-PD Investigators. Characterization of vitamin D supplementation and clinical outcomes in a large cohort of early Parkinson’s disease. J. Clin. Mov. Disord. 2018, 5, 7. [Google Scholar] [CrossRef]
- Rizvi, S.; Raza, S.T.; Faizal Ahmed, A.A.; Abbas, S.; Mahdi, F. The role of vitamin E in human health and some diseases. Sultan Qaboos Univ. Med. J. 2014, 14, e157. [Google Scholar]
- Schirinzi, T.; Martella, G.; Imbriani, P.; Di Lazzaro, G.; Franco, D.; Colona, V.L.; Alwardat, M.; Salimei, P.S.; Mercuri, N.B.; Pierantozzi, M. Dietary vitamin E as a protective factor for Parkinson’s disease: Clinical and experimental evidence. Front. Neurol. 2019, 10, 148. [Google Scholar] [CrossRef] [PubMed]
- Etminan, M.; Gill, S.S.; Samii, A. Intake of vitamin E, vitamin C, and carotenoids and the risk of Parkinson’s disease: A meta-analysis. Lancet Neurol. 2005, 4, 362–365. [Google Scholar] [CrossRef]
- Ricciarelli, R.; Argellati, F.; Pronzato, M.A.; Domenicotti, C. Vitamin E and neurodegenerative diseases. Mol. Aspects Med. 2007, 28, 591–606. [Google Scholar] [CrossRef] [PubMed]
- Fahn, S. A pilot trial of high-dose alpha-tocopherol and ascorbate in early Parkinson’s disease. Ann. Neurol. 1992, 32, 28–32. [Google Scholar] [CrossRef]
- Vatassery, G.T.; Fahn, S.; Kuskowski, M.A. Alpha tocopherol in CSF of subjects taking high-dose vitamin E in the DATATOP study. Parkinson Study Group. Neurology 1998, 50, 1900–1902. [Google Scholar] [CrossRef] [PubMed]
- Molina, J.A.; de Bustos, F.; Jiménez-Jiménez, F.J.; Benito-León, J.; Ortí-Pareja, M.; Gasalla, T.; Tallón-Barranco, A.; Navarro, J.A.; Arenas, J.; Enríquez-de-Salamanca, R. Cerebrospinal fluid levels of alpha-tocopherol (vitamin E) in Parkinson’s disease. J. Neural Transm. 1997, 104, 1287–1293. [Google Scholar] [CrossRef]
- Pham, D.Q.; Plakogiannis, R. Vitamin E supplementation in Alzheimer’s disease, Parkinson’s disease, tardive dyskinesia, and cataract: Part 2. Ann. Pharmacother. 2005, 39, 2065–2072. [Google Scholar] [CrossRef] [PubMed]
- Conrad, G.D. Is Ginkgo biloba and/or a Multivitamin-multimineral supplement a therapeutic option for Parkinson’s disease? A case report. Global Adv. Health Med. 2014, 3, 43–44. [Google Scholar] [CrossRef]
- Dong, R.; Wang, H.; Ye, J.; Wang, M.; Bi, Y. Publication trends for Alzheimer’s disease worldwide and in China: A 30-year bibliometric analysis. Front. Human Neurosci. 2019, 13, 259. [Google Scholar] [CrossRef]
- MacDonald, P.N.; Bok, D.; Ong, D.E. Localization of cellular retinol-binding protein and retinol-binding protein in cells comprising the blood-brain barrier of rat and human. Proc. Natl. Acad. Sci. USA 1990, 87, 4265–4269. [Google Scholar] [CrossRef]
- Maden, M. Retinoic acid in the development, regeneration and maintenance of the nervous system. Nat. Rev. Neurosci. 2007, 8, 755–765. [Google Scholar] [CrossRef] [PubMed]
- Levesque, D.; Rouillard, C. Nur77 and retinoid X receptors: Crucial factors in dopamine-related neuroadaptation. Trends Neurosci. 2007, 30, 22–30. [Google Scholar] [CrossRef]
- Samad, T.A.; Krezel, W.; Chambon, P.; Borrelli, E. Regulation of dopaminergic pathways by retinoids: Activation of the D2 receptor promoter by members of the retinoic acid receptor– retinoid X receptor family. Proc. Nat. Acad. Sci. USA 1997, 94, 14349–14354. [Google Scholar] [CrossRef] [PubMed]
- Esteves, M.; Cristóvão, A.C.; Saraiva, T.; Rocha, S.M.; Baltazar, G.; Ferreira, L.; Bernardino, L. Retinoic acid-loaded polymeric nanoparticles induce neuroprotection in a mouse model for Parkinson’s disease. Front. Aging Neurosci. 2015, 7, 20. [Google Scholar] [CrossRef]
- Yin, L.-H.; Shen, H.; Diaz-Ruiz, O.; Bäckman, C.M.; Bae, E.; Yu, S.-J.; Wang, Y. Early post-treatment with 9-cis retinoic acid reduces neurodegeneration of dopaminergic neurons in a rat model of Parkinson’s disease. BMC Neurosci. 2012, 13, 120. [Google Scholar] [CrossRef]
- Kunzler, A.; Ribeiro, C.T.; Gasparotto, J.; Petiz, L.L.; da Rosa Silva, H.T.; da Silva, J.D., Jr.; Bortolin, R.; de Souza, P.O.; Barreto, F.; Espitia-Perez, P.; et al. The effects of retinol oral supplementation in 6-hydroxydopamine dopaminergic denervation model in Wistar rats. Neurochem. Int. 2019, 125, 25–34. [Google Scholar] [CrossRef]
- Clark, J.N.; Whiting, A.; McCaffery, P. Retinoic acid receptor-targeted drugs in neurodegenerative disease. Expert Opin. Drug Metab. Toxicol. 2020, 16, 1097–1108. [Google Scholar] [CrossRef]
- Prema, A.; Janakiraman, U.; Manivasagam, T.; Thenmozhi, A.J. Neuroprotective effect of lycopene against MPTP induced experimental Parkinson’s disease in mice. Neurosci. Lett. 2015, 599, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Chauhan, S.; Sandhir, R. Protective effect of lycopene on oxidative stress and cognitive decline in rotenone induced model of Parkinson’s disease. Neurochem. Res. 2011, 36, 1435–1443. [Google Scholar] [CrossRef]
- Naidu, K.A. Vitamin C in human health and disease is still a mystery? An Overview. Nutr. J. 2003, 2, 7. [Google Scholar] [CrossRef]
- Tran, H.H.; Dang, S.N.A.; Nguyen, T.T.; Huynh, A.M.; Dao, L.M.; Kamei, K.; Yamaguchi, M.; Dang, T.T.P. Drosophila Ubiquitin C-Terminal Hydrolase Knockdown Model of Parkinson’s Disease. Sci. Rep. 2018, 8, 4468. [Google Scholar] [CrossRef] [PubMed]
- Man Anh, H.; Linh, D.M.; My Dung, V.; Thi Phuong Thao, D. Evaluating dose-and time- dependent effects of vitamin c treatment on a parkinson’s disease fly model. Parkinson’s Dis. 2019, 2019. [Google Scholar] [CrossRef] [PubMed]
- Sershen, H.; Reith, M.E.; Hashim, A.; Lajtha, A. Protection against 1-methyl-4-phenyl-1,2,3,6- tetrahydropyridine neurotoxicity by the antioxidant ascorbic acid. Neuropharmacology 1985, 24, 1257–1259. [Google Scholar] [CrossRef]
- Calvello, R.; Cianciulli, A.; Nicolardi, G.; De Nuccio, F.; Giannotti, L.; Salvatore, R.; Porro, C.; Trotta, T.; Panaro, M.A.; Lofrumento, D.D. Vitamin D Treatment Attenuates Neuroinflammation and Dopaminergic Neurodegeneration in an Animal Model of Parkinson’s Disease, Shifting M1 to M2 Microglia Responses. J. Neuroimmune Pharmacol. 2017, 12, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Lima, L.A.R.; Lopes, M.J.P.; Costa, R.O.; Lima, F.A.V.; Neves, K.R.T.; Calou, I.B.F.; Andrade, G.M.; Viana, G.S.B. Vitamin D protects dopaminergic neurons against neuroinflammation and oxidative stress in hemiparkinsonian rats. J. Neuroinflam. 2018, 15, 249. [Google Scholar] [CrossRef]
- Ueda, S.; Sakakibara, S.; Nakadate, K.; Noda, T.; Shinoda, M.; Joyce, J.N. Degeneration of dopaminergic neurons in the substantia nigra of zitter mutant rat and protection by chronic intake of Vitamin E. Neurosci. Lett. 2005, 380, 252–256. [Google Scholar] [CrossRef]
- Olanow, C.W. Dietary vitamin E and Parkinson’s disease: Something to chew on. Lancet Neurol. 2003, 2, 74. [Google Scholar] [CrossRef]
- Nakaso, K.; Tajima, N.; Horikoshi, Y.; Nakasone, M.; Hanaki, T.; Kamizaki, K.; Matsura, T. The estrogen receptor beta-PI3K/Akt pathway mediates the cytoprotective effects of tocotrienol in a cellular Parkinson’s disease model. Biochim. Biophys. Acta 2014, 1842, 1303–1312. [Google Scholar] [CrossRef]
- Cadet, J.L.; Katz, M.; Jackson-Lewis, V.; Fahn, S. Vitamin E attenuates the toxic effects of intrastriatal injection of 6-hydroxydopamine (6-OHDA) in rats: Behavioral and biochemical evidence. Brain Res. 1989, 476, 10–15. [Google Scholar] [CrossRef]
- Sharma, N.; Nehru, B. Beneficial Effect of Vitamin E in Rotenone Induced Model of PD: Behavioural, Neurochemical and Biochemical Study. Exp. Neurobiol. 2013, 22, 214. [Google Scholar] [CrossRef]
- Roghani, M.; Behzadi, G. Neuroprotective effect of vitamin E on the early model of Parkinson’s disease in rat: Behavioral and histochemical evidence. Brain Res. 2001, 892, 211–217. [Google Scholar] [CrossRef]
- Heim, C.; Kolasiewicz, W.; Kurz, T.; Sontag, K.H. Behavioral alterations after unilateral 6- hydroxydopamine lesions of the striatum. Effect of alpha-tocopherol. Pol. J. Pharmacol. 2001, 53, 435–448. [Google Scholar] [PubMed]
- Abdin, A.A.; Hamouda, H.E. Mechanism of the neuroprotective role of coenzyme Q10 with or without L-dopa in rotenone-induced parkinsonism. Neuropharmacology 2008, 55, 1340–1346. [Google Scholar] [CrossRef]
- Podleśny-Drabiniok, A.; Sobska, J.; de Lera, A.R.; Gołembiowska, K.; Kamińska, K.; Dollé, P.; Cebrat, M.; Krężel, W. Distinct retinoic acid receptor (RAR) isotypes control differentiation of embryonal carcinoma cells to dopaminergic or striatopallidal medium spiny neurons. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef]
- Giri, B.; Belanger, K.; Seamon, M.; Bradley, E.; Purohit, S.; Chong, R.; Morgan, J.C.; Baban, B.; Wakade, C. Niacin Ameliorates Neuro-Inflammation in Parkinson’s Disease via GPR109A. Int. J. Mol. Sci. 2019, 20, 4559. [Google Scholar] [CrossRef]
- Pearl, S.M.; Antion, M.D.; Stanwood, G.D.; Jaumotte, J.D.; Kapatos, G.; Zigmond, M.J. Effects of NADH on dopamine release in rat striatum. Synapse 2000, 36, 95–101. [Google Scholar] [CrossRef]
- Seitz, G.; Gebhardt, S.; Beck, J.F.; Böhm, W.; Lode, H.N.; Niethammer, D.; Bruchelt, G. Ascorbic acid stimulates DOPA synthesis and tyrosine hydroxylase gene expression in the human neuroblastoma cell line SK-N-SH. Neurosci. Lett. 1998, 244, 33–36. [Google Scholar] [CrossRef]
- Pardo, B.; Mena, M.A.; Fahn, S.; de Yébenes, J.G. Ascorbic acid protects against levodopa- induced neurotoxicity on a catecholamine-rich human neuroblastoma cell line. Mov. Disord. 1993, 8, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Tang, L.; Wei, W.; Hong, Y.; Chen, H.; Ying, W.; Chen, S. Nicotinamide mononucleotide improves energy activity and survival rate in an in vitro model of Parkinson’s disease. Exp. Ther. Med. 2014, 8, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Y.; Wu, J.N.; Cherng, T.L.; Hoffer, B.J.; Chen, H.H.; Borlongan, C.V.; Wang, Y. Vitamin D(3) attenuates 6-hydroxydopamine-induced neurotoxicity in rats. Brain Res. 2001, 904, 67–75. [Google Scholar] [CrossRef]
- Kim, J.S.; Ryu, S.Y.; Yun, I.; Kim, W.J.; Lee, K.S.; Park, J.W.; Kim, Y.I. 1alpha,25-Dihydroxyvitamin D(3) Protects Dopaminergic Neurons in Rodent Models of Parkinson’s Disease through Inhibition of Microglial Activation. J. Clin. Neurol. 2006, 2, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Pozo, A.; Frosch, M.P.; Masliah, E.; Hyman, B.T. Neuropathological alterations in Alzheimer disease. Cold Spring Harb. Perspect. Med. 2011, 1, a006189. [Google Scholar] [CrossRef] [PubMed]
- DeTure, M.A.; Dickson, D.W. The neuropathological diagnosis of Alzheimer’s disease. Mol. Neurodegen. 2019, 14, 1–18. [Google Scholar] [CrossRef]
- Lacosta, A.-M.; Insua, D.; Badi, H.; Pesini, P.; Sarasa, M. Neurofibrillary tangles of Aβ x- 40 in Alzheimer’s disease brains. J. Alzheimers Dis. 2017, 58, 661–667. [Google Scholar] [CrossRef] [PubMed]
- Castellani, R.J.; Lee, H.-G.; Zhu, X.; Nunomura, A.; Perry, G.; Smith, M.A. Neuropathology of Alzheimer disease: Pathognomonic but not pathogenic. Acta Neuropathol. 2006, 111, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Tönnies, E.; Trushina, E. Oxidative stress, synaptic dysfunction, and Alzheimer’s disease. J. Alz. Dis. 2017, 57, 1105–1121. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.L.; Norhaizan, M.E.; Liew, W.P.; Sulaiman Rahman, H. Antioxidant and oxidative stress: A mutual interplay in age-related diseases. Front. Pharmacol. 2018, 9, 1162. [Google Scholar] [CrossRef] [PubMed]
- Salech, F.; Ponce, D.P.; Paula-Lima, A.C.; SanMartin, C.D.; Behrens, M.I. Nicotinamide, a Poly [ADP-Ribose] polymerase 1 (PARP-1) inhibitor, as an adjunctive therapy for the treatment of Alzheimer’s disease. Front. Aging Neurosci. 2020, 12, 255. [Google Scholar] [CrossRef] [PubMed]
- De Wilde, M.C.; Vellas, B.; Girault, E.; Yavuz, A.C.; Sijben, J.W. Lower brain and blood nutrient status in Alzheimer’s disease: Results from meta-analyses. Alz. Dement. 2017, 3, 416–431. [Google Scholar] [CrossRef]
- Bhatti, A.B.; Usman, M.; Ali, F.; Satti, S.A. Vitamin supplementation as an adjuvant treatment for Alzheimer’s disease. J. Clin. Diag. Res. 2016, 10, OE07–OE11. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, S.L.; Vellas, B.; Elemans, S.; Luchsinger, J.; Kamphuis, P.; Yaffe, K.; Sijben, J.; Groenendijk, M.; Stijnen, T. Plasma nutrient status of patients with Alzheimer’s disease: Systematic review and meta-analysis. Alz. Dement. 2014, 10, 485–502. [Google Scholar] [CrossRef]
- Foy, C.; Passmore, A.; Vahidassr, M.; Young, I.; Lawson, J. Plasma chain-breaking antioxidants in Alzheimer’s disease, vascular dementia and Parkinson’s disease. QJM 1999, 92, 39–45. [Google Scholar] [CrossRef]
- Lloret, A.; Esteve, D.; Monllor, P.; Cervera-Ferri, A.; Lloret, A. The effectiveness of vitamin E treatment in Alzheimer’s disease. Int. J. Mol. Sci. 2019, 20, 879. [Google Scholar] [CrossRef]
- Zandi, P.P.; Anthony, J.C.; Khachaturian, A.S.; Stone, S.V.; Gustafson, D.; Tschanz, J.T.; Norton, M.C.; Welsh-Bohmer, K.A.; Breitner, J.C.S.; Cache County Study Group. Reduced risk of Alzheimer disease in users of antioxidant vitamin supplements: The cache county study. Arch. Neurol. 2004, 61, 82–88. [Google Scholar] [CrossRef]
- Yuan, C.; Fondell, E.; Ascherio, A.; Okereke, O.I.; Grodstein, F.; Hofman, A.; Willett, W.C. Long-term intake of dietary carotenoids is positively associated with late-life subjective cognitive function in a prospective study in US women. J. Nutr. 2020, 150, 1871–1879. [Google Scholar] [CrossRef]
- Endres, K. Retinoic Acid and the Gut Microbiota in Alzheimer’s Disease: Fighting Back-to-Back? Curr. Alzheimer Res. 2019, 16, 405–417. [Google Scholar] [CrossRef] [PubMed]
- Ishii, M.; Kamel, H.; Iadecola, C. Retinol Binding Protein 4 Levels Are Not Altered in Preclinical Alzheimer’s Disease and Not Associated with Cognitive Decline or Incident Dementia. J. Alzheimers Dis. 2019, 67, 257–263. [Google Scholar] [CrossRef]
- Wołoszynowska-Fraser, M.U.; Kouchmeshky, A.; McCaffery, P. Vitamin A and Retinoic Acid in Cognition and Cognitive Disease. Annu. Rev. Nutr. 2020, 40, 247–272. [Google Scholar] [CrossRef] [PubMed]
- Refsum, H.; Ueland, P.M. Clinical significance of pharmacological modulation of homocysteine metabolism. Trends Pharmacol. Sci. 1990, 11, 411–416. [Google Scholar] [CrossRef]
- Douaud, G.; Refsum, H.; de Jager, C.A.; Jacoby, R.; Nichols, T.E.; Smith, S.M.; Smith, A.D. Preventing Alzheimer’s disease-related gray matter atrophy by B-vitamin treatment. Proc. Natl. Acad. Sci. USA 2013, 110, 9523–9528. [Google Scholar] [CrossRef]
- Price, B.R.; Wilcock, D.M.; Weekman, E.M. Hyperhomocysteinemia as a risk factor for vascular contributions to cognitive impairment and dementia. Front. Aging Neurosci. 2018, 10, 350. [Google Scholar] [CrossRef] [PubMed]
- Durga, J.; van Boxtel, M.P.; Schouten, E.G.; Kok, F.J.; Jolles, J.; Katan, M.B.; Verhoef, P. Effect of 3-year folic acid supplementation on cognitive function in older adults in the FACIT trial: A randomised, double blind, controlled trial. Lancet 2007, 369, 208–216. [Google Scholar] [CrossRef]
- Morris, M.C.; Schneider, J.A.; Tangney, C.C. Thoughts on B-vitamins and dementia. J. Alzheimers Dis. 2006, 9, 429–433. [Google Scholar] [CrossRef]
- De Jager, C.A.; Oulhaj, A.; Jacoby, R.; Refsum, H.; Smith, A.D. Cognitive and clinical outcomes of homocysteine- lowering B- vitamin treatment in mild cognitive impairment: A randomized controlled trial. Int. J. Geriatr. Psychiatry 2012, 27, 592–600. [Google Scholar] [CrossRef] [PubMed]
- Ford, A.H.; Almeida, O.P. Effect of homocysteine lowering treatment on cognitive function: A systematic review and meta-analysis of randomized controlled trials. J. Alzheimers Dis. 2012, 29, 133–149. [Google Scholar] [CrossRef] [PubMed]
- Ford, A.H.; Almeida, O.P. Effect of vitamin B supplementation on cognitive function in the elderly: A systematic review and meta-analysis. Drugs Aging 2019, 36, 419–434. [Google Scholar] [CrossRef]
- Wald, D.S.; Kasturiratne, A.; Simmonds, M. Effect of folic acid, with or without other B vitamins, on cognitive decline: Meta-analysis of randomized trials. Am. J. Med. 2010, 123, 522–527. [Google Scholar] [CrossRef]
- Sun, Y.; Lu, C.J.; Chien, K.L.; Chen, S.T.; Chen, R.C. Efficacy of multivitamin supplementation containing vitamins B6 and B12 and folic acid as adjunctive treatment with a cholinesterase inhibitor in Alzheimer’s disease: A 26-week, randomized, double-blind, placebo-controlled study in Taiwanese patients. Clin. Ther. 2007, 29, 2204–2214. [Google Scholar] [CrossRef]
- Aisen, P.S.; Schneider, L.S.; Sano, M.; Diaz-Arrastia, R.; van Dyck, C.H.; Weiner, M.F.; Bottiglieri, T.; Jin, S.; Stokes, K.T.; Thomas, R.G.; et al. High-dose B vitamin supplementation and cognitive decline in Alzheimer disease: A randomized controlled trial. JAMA 2008, 300, 1774–1783. [Google Scholar] [CrossRef]
- Kwok, T.; Wu, Y.; Lee, J.; Lee, R.; Yung, C.Y.; Choi, G.; Lee, V.; Harrison, J.; Lam, L.; Mok, V. Randomized placebo-controlled trial of using B vitamins to prevent cognitive decline in older mild cognitive impairment patients. Clin. Nutr. 2020, 39, 2399–2405. [Google Scholar] [CrossRef] [PubMed]
- Van der Zwaluw, N.L.; Dhonukshe-Rutten, R.A.; van Wijngaarden, J.; Brouwer-Brolsma, E.M.; van de Rest, O.; In’t Veld, P.H.; Enneman, A.W.; van Dijk, S.C.; Ham, A.C.; Swart, K.M.; et al. Results of 2-year vitamin B treatment on cognitive performance: Secondary data from an RCT. Neurology 2014, 83, 2158–2166. [Google Scholar] [CrossRef]
- Meng, H.; Li, Y.; Zhang, W.; Zhao, Y.; Niu, X.; Guo, J. The relationship between cognitive impairment and homocysteine in a B12 and folate deficient population in China: A cross-sectional study. Medicine 2019, 47, e17970. [Google Scholar] [CrossRef]
- Ulusu, N.N.; Yilmaz, G.; Erbayraktar, Z.; Evlice, A.T.; Aras, S.; Yener, G.; Avci, A.A. Turkish 3-center study evaluation of serum folic acid and vitamin B12 levels in Alzheimer disease. Turk. J. Med. Sci. 2015, 45, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
- Lu’o’ng, K.V.Q.; Nguyễn, L.T.H. Role of thiamine in Alzheimer’s disease. Am. J. Alzheimer’s Dis. Other Dement. 2011, 26, 588–598. [Google Scholar] [CrossRef] [PubMed]
- Osimani, A.; Berger, A.; Friedman, J.; Porat-Katz, B.S.; Abarbanel, J.M. Neuropsychology of vitamin B12 deficiency in elderly dementia patients and control subjects. J. Geriatr. Psychiatry Neurol. 2005, 18, 33–38. [Google Scholar] [CrossRef]
- Rafiee, S.; Asadollahi, K.; Riazi, G.; Ahmadian, S.; Saboury, A.A. Vitamin B12 inhibits tau fibrillization via binding to cysteine residues of tau. ACS Chem. Neurosci. 2017, 8, 2676–2682. [Google Scholar] [CrossRef]
- Lanyau-Domínguez, Y.; Macías-Matos, C.; Jesús, J.; Pita-Rodríguez, G.M.; Suárez-Medina, R.; Quintero-Alejo, M.E.; Díaz-Domínguez, M. Levels of vitamins and homocysteine in older adults with Alzheimer disease or mild cognitive impairment in cuba. MEDICC Rev. 2020, 2, 40–47. [Google Scholar] [CrossRef]
- Hama, Y.; Hamano, T.; Shirafuji, N.; Hayashi, K.; Ueno, A.; Enomoto, S.; Nagata, M.; Kimura, H.; Matsunaga, A.; Ikawa, M.; et al. Influences of folate supplementation on homocysteine and cognition in patients with folate deficiency and cognitive impairment. Nutrients 2020, 12, 3138. [Google Scholar] [CrossRef]
- Chen, H.; Liu, S.; Ji, L.; Wu, T.; Ji, Y.; Zhou, Y.; Zheng, M.; Zhang, M.; Xu, W.; Huang, G. Folic acid supplementation mitigates Alzheimer’s disease by reducing inflammation: A randomized controlled trial. Mediat. Inflamm. 2016, 2016, 5912146. [Google Scholar] [CrossRef]
- Littlejohns, T.J.; Henley, W.E.; Lang, I.A.; Annweiler, C.; Beauchet, O.; Chaves, P.H.; Fried, L.; Kestenbaum, B.R.; Kuller, L.H.; Langa, K.M. Vitamin D and the risk of dementia and Alzheimer disease. Neurology 2014, 83, 920–928. [Google Scholar] [CrossRef]
- Banerjee, A.; Khemka, V.K.; Ganguly, A.; Roy, D.; Ganguly, U.; Chakrabarti, S. Vitamin D and Alzheimer’s disease: Neurocognition to therapeutics. Int. J. Alzheimers Dis. 2015, 2015, 192747. [Google Scholar] [CrossRef] [PubMed]
- Diesel, B.; Radermacher, J.; Bureik, M.; Bernhardt, R.; Seifert, M.; Reichrath, J.; Fischer, U.; Meese, E. Vitamin D3 metabolism in human glioblastoma multiforme: Functionality of CYP27B1 splice variants, metabolism of calcidiol, and effect of calcitriol. Clin. Cancer Res. 2005, 11, 5370–5380. [Google Scholar] [CrossRef] [PubMed]
- Holick, M. Vitamin D and brain health: The need for vitamin D supplementation and sensible sun exposure. J. Int. Med. 2015, 277, 90–93. [Google Scholar] [CrossRef] [PubMed]
- Neveu, I.; Naveilhan, P.; Menaa, C.; Wion, D.; Brachet, P.; Garabedian, M. Synthesis of 1, 25- dihydroxyvitamin D3 by rat brain macrophages in vitro. J. Neurosci. Res. 1994, 38, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.; Bianco, J.I.; McGrath, J.J.; Eyles, D.W. 1, 25-dihydroxyvitamin D3 induces nerve growth factor, promotes neurite outgrowth and inhibits mitosis in embryonic rat hippocampal neurons. Neurosci. Lett. 2003, 343, 139–143. [Google Scholar] [CrossRef]
- Orme, R.P.; Bhangal, M.S.; Fricker, R.A. Calcitriol imparts neuroprotection in vitro to midbrain dopaminergic neurons by upregulating GDNF expression. PLoS ONE 2013, 8, e62040. [Google Scholar] [CrossRef]
- Budni, J.; Bellettini-Santos, T.; Mina, F.; Garcez, M.L.; Zugno, A.I. The involvement of BDNF, NGF and GDNF in aging and Alzheimer’s disease. Aging Dis. 2015, 6, 331. [Google Scholar] [CrossRef]
- Allen, S.J.; Watson, J.J.; Dawbarn, D. The neurotrophins and their role in Alzheimer’s disease. Curr. Neuropharmacol. 2011, 9, 559–573. [Google Scholar] [CrossRef] [PubMed]
- Calissano, P.; Matrone, C.; Amadoro, G. Nerve growth factor as a paradigm of neurotrophins related to Alzheimer’s disease. Dev. Neurobiol. 2010, 70, 372–383. [Google Scholar] [CrossRef] [PubMed]
- Veenstra, T.D.; Fahnestock, M.; Kumar, R. An AP-1 site in the nerve growth factor promoter is essential for 1, 25-dihydroxyvitamin D3-mediated nerve growth factor expression in osteoblasts. Biochemistry 1998, 37, 5988–5994. [Google Scholar] [CrossRef]
- Soh, Y.; Lee, D.H.; Won, C.W. Association between Vitamin B12 levels and cognitive function in the elderly Korean population. Medicine 2020, 99, e21371. [Google Scholar] [CrossRef]
- La Fata, G.; Weber, P.; Mohajeri, M.H. Effects of vitamin E on cognitive performance during ageing and in Alzheimer’s disease. Nutrients 2014, 6, 5453–5472. [Google Scholar] [CrossRef] [PubMed]
- Browne, D.; McGuinness, B.; Woodside, J.V.; McKay, G.J. Vitamin E and Alzheimer’s disease: What do we know so far? Clin. Interv. Aging 2019, 14, 1303. [Google Scholar] [CrossRef] [PubMed]
- Gugliandolo, A.; Bramanti, P.; Mazzon, E. Role of vitamin E in the treatment of Alzheimer’s disease: Evidence from animal models. Int. J. Mol. Sci. 2017, 18, 2504. [Google Scholar] [CrossRef]
- Baroni, L.; Bonetto, C.; Rizzo, G.; Bertola, C.; Caberlotto, L.; Bazzerla, G. Association between cognitive impairment and vitamin B12, folate, and homocysteine status in elderly adults: A retrospective study. J. Alzheimers Dis. 2019, 70, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Petersen, R.C.; Thomas, R.G.; Grundman, M.; Bennett, D.; Doody, R.; Ferris, S.; Galasko, D.; Jin, S.; Kaye, J.; Levey, A. Vitamin E and donepezil for the treatment of mild cognitive impairment. N. Engl. J. Med. 2005, 352, 2379–2388. [Google Scholar] [CrossRef]
- Dysken, M.W.; Sano, M.; Asthana, S.; Vertrees, J.E.; Pallaki, M.; Llorente, M.; Love, S.; Schellenberg, G.D.; McCarten, J.R.; Malphurs, J.; et al. Effect of vitamin E and memantine on functional decline in Alzheimer disease: The TEAM-AD VA cooperative randomized trial. JAMA 2014, 311, 33–44. [Google Scholar] [CrossRef]
- Hira, S.; Saleem, U.; Anwar, F.; Sohail, M.F.; Raza, Z.; Ahmad, B. β-Carotene: A Natural Compound Improves Cognitive Impairment and Oxidative Stress in a Mouse Model of Streptozotocin-Induced Alzheimer’s Disease. Biomolecules 2019, 9, 441. [Google Scholar] [CrossRef]
- Ono, K.; Yamada, M. Vitamin A and Alzheimer’s disease. Ger. Gerontol. Int. 2012, 12, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Takasaki, J.; Ono, K.; Yoshiike, Y.; Hirohata, M.; Ikeda, T.; Morinaga, A.; Takashima, A.; Yamada, M. Vitamin A has anti-oligomerization effects on amyloid-beta in vitro. J. Alzheimers Dis. 2011, 27, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Murakami, K.; Murata, N.; Ozawa, Y.; Kinoshita, N.; Irie, K.; Shirasawa, T.; Shimizu, T. Vitamin C restores behavioral deficits and amyloid-β oligomerization without affecting plaque formation in a mouse model of Alzheimer’s disease. J. Alzheimers Dis. 2011, 26, 7–18. [Google Scholar] [CrossRef]
- Mehta, V.; Desai, N.; Perwez, A.; Nemade, D.; Dawoodi, S.; Zaman, S.B. ACE Alzheimer’s: The role of vitamin A, C and E (ACE) in oxidative stress induced Alzheimer’s disease. J. Med. Res. Innov. 2018, 2, e000086. [Google Scholar] [CrossRef]
- Zhao, H.; Li, S.; Li, Z.; Yang, S.; Li, D.; Zheng, J.; Gao, H.; Yun, L.; Gu, Y.; Li, L.; et al. Intranasal delivery of 9-cis retinoic acid reduces beta-amyloid deposition via inhibiting astrocyte-mediated inflammation. Aging 2020, 12, 5469–5478. [Google Scholar] [CrossRef]
- Das, B.C.; Dasgupta, S.; Ray, S.K. Potential therapeutic roles of retinoids for prevention of neuroinflammation and neurodegeneration in Alzheimer’s disease. Neural Regen. Res. 2019, 14, 1880–1892. [Google Scholar] [PubMed]
- Duong, S.; Patel, T.; Chang, F. Dementia: What pharmacists need to know. Can. Pharm. J./Rev. Pharm. Can. 2017, 150, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, E.L.; McGuinness, B.; Herron, B.; Passmore, A.P. Dementia. Ulster Med. J. 2015, 84, 79–87. [Google Scholar]
- Gibson, G.E.; Hirsch, J.A.; Fonzetti, P.; Jordon, B.D.; Cirio, R.T.; Elder, J. Vitamin B1 (thiamine) and dementia. Ann. N. Y. Acad. Sci. 2016, 1367, 21. [Google Scholar] [CrossRef] [PubMed]
- Blundo, C.; Marin, D.; Ricci, M. Vitamin B12 deficiency associated with symptoms of frontotemporal dementia. Neurol. Sci. 2011, 32, 101–105. [Google Scholar] [CrossRef]
- Malaguarnera, M.; Ferri, R.; Bella, R.; Alagona, G.; Carnemolla, A.; Pennisi, G. Homocysteine, vitamin B12 and folate in vascular dementia and in Alzheimer disease. Clin. Chem. Lab. Med. 2004, 42, 1032–1035. [Google Scholar] [CrossRef]
- Hegyi, J.; Schwartz, R.A.; Hegyi, V. Pellagra: Dermatitis, dementia, and diarrhea. Int. J. Dermatol. 2004, 43, 1–5. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Xu, Z.P.; Wang, W.; Cao, J.B.; Fu, Q.; Zhao, W.X.; Li, Y.; Huo, X.L.; Zhang, L.M.; Li, Y.F.; et al. Vitamin C alleviates LPS-induced cognitive impairment in mice by suppressing neuroinflammation and oxidative stress. Int. Immunopharmacol. 2018, 65, 438–447. [Google Scholar] [CrossRef]
- Olajide, O.J.; Yawson, E.O.; Gbadamosi, I.T.; Arogundade, T.T.; Lambe, E.; Obasi, K.; Lawal, I.T.; Ibrahim, A.; Ogunrinola, K.Y. Ascorbic acid ameliorates behavioural deficits and neuropathological alterations in rat model of Alzheimer’s disease. Environ. Toxicol. Pharmacol. 2017, 50, 200–211. [Google Scholar] [CrossRef]
- Sil, S.; Ghosh, T.; Gupta, P.; Ghosh, R.; Kabir, S.N.; Roy, A. Dual Role of Vitamin C on the Neuroinflammation Mediated Neurodegeneration and Memory Impairments in Colchicine Induced Rat Model of Alzheimer Disease. J. Mol. Neurosci. 2016, 60, 421–435. [Google Scholar] [CrossRef] [PubMed]
- Yamini, P.; Ray, R.S.; Chopra, K. Vitamin D3 attenuates cognitive deficits and neuroinflammatory responses in ICV-STZ induced sporadic Alzheimer’s disease. Inflammopharmacology 2018, 26, 39–55. [Google Scholar] [CrossRef]
- Briones, T.L.; Darwish, H. Vitamin D mitigates age-related cognitive decline through the modulation of pro-inflammatory state and decrease in amyloid burden. J. Neuroinflam. 2012, 9, 244. [Google Scholar] [CrossRef] [PubMed]
- El-Din, S.S.; Rashed, L.; Medhat, E.; Aboulhoda, B.E.; Badawy, A.D.; ShamsEldeen, A.M.; Abdelgwad, M. Active form of vitamin D analogue mitigates neurodegenerative changes in Alzheimer’s disease in rats by targeting Keap1/Nrf2 and MAPK-38p/ERK signaling pathways. Steroids 2020, 156, 108586. [Google Scholar] [CrossRef] [PubMed]
- Ertilav, E.; Barcin, N.E.; Ozdem, S. Comparison of Serum Free and Bioavailable 25- Hydroxyvitamin D Levels in Alzheimer’s Disease and Healthy Control Patients. Lab. Med. 2020, 52, 219–225. [Google Scholar] [CrossRef]
- Ali, A.; Shah, S.A.; Zaman, N.; Uddin, M.N.; Khan, W.; Ali, A.; Riaz, M.; Kamil, A. Vitamin D Exerts Neuroprotection via SIRT1/Nrf-2/ NF-kB Signaling Pathways against D-Galactose-induced Memory Impairment in Adult Mice. Neurochem. Int. 2020, 4, 104893. [Google Scholar] [CrossRef]
- Fan, Y.G.; Pang, Z.Q.; Wu, T.Y.; Zhang, Y.H.; Xuan, W.Q.; Wang, Z.; Yu, X.; Li, Y.C.; Guo, C.; Wang, Z.Y. Vitamin D deficiency exacerbates Alzheimer-like pathologies by reducing antioxidant capacity. Free Radic. Biol. Med. 2020, 161, 139–149. [Google Scholar] [CrossRef]
- Boccardi, V.; Baroni, M.; Mangialasche, F.; Mecocci, P. Vitamin E family: Role in the pathogenesis and treatment of Alzheimer’s disease. Alzheimers Dement. 2016, 2, 182–191. [Google Scholar] [CrossRef]
- Yu, L.; Chen, Y.; Wang, W.; Xiao, Z.; Hong, Y. Multi-vitamin B supplementation reverses hypoxia-induced tau hyperphosphorylation and improves memory function in adult mice. J. Alzheimers Dis. 2016, 54, 297–306. [Google Scholar] [CrossRef]
- Huang, J.K.; Jarjour, A.A.; Oumesmar, B.N.; Kerninon, C.; Williams, A.; Krezel, W.; Kagechika, H.; Bauer, J.; Zhao, C.; Baron-Van Evercooren, A. Retinoid X receptor gamma signaling accelerates CNS remyelination. Nat. Neurosci. 2011, 14, 45. [Google Scholar] [CrossRef]
- Caron, N.S.; Wright, G.E.B.; Hayden, M.R. Huntington Disease; Adam, M.P., Ardinger, H.H., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Stephens, K., Amemiya, A., Eds.; GeneReviews: Seattle, DC, USA, 1993. Available online: http://www.ncbi.nlm.nih.gov/books/nbk1305/ (accessed on 17 September 2021).
- Dayalu, P.; Albin, R.L. Huntington disease: Pathogenesis and treatment. Neurol. Clin. 2015, 33, 101–114. [Google Scholar] [CrossRef]
- Chel, V.G.; Ooms, M.E.; van der Bent, J.; Veldkamp, F.; Roos, R.A.; Achterberg, W.P.; Lips, P. High prevalence of vitamin D deficiency and insufficiency in patients with manifest Huntington disease: An explorative study. Dermato Endocrinol. 2013, 5, 348–351. [Google Scholar] [CrossRef][Green Version]
- Roos, R. Huntington’s disease: A clinical review. Orphanet J. Rare Dis. 2010, 5, 40. [Google Scholar] [CrossRef]
- Patassini, S.; Begley, P.; Xu, J.; Church, S.J.; Kureishy, N.; Reid, S.J.; Waldvogel, H.J.; Faull, R.L.; Snell, R.G.; Unwin, R.D. Cerebral vitamin B5 (D-Pantothenic Acid) deficiency as a potential cause of metabolic perturbation and neurodegeneration in Huntington’s disease. Metabolites 2019, 9, 113. [Google Scholar] [CrossRef] [PubMed]
- Uttara, B.; Singh, A.V.; Zamboni, P.; Mahajan, R.T. Oxidative stress and neurodegenerative diseases: A review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 2009, 7, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Johri, A.; Beal, M.F. Antioxidants in Huntington’s disease. Biochim. Biophys. Acta 2012, 1822, 664–674. [Google Scholar] [CrossRef]
- Maden, M.; Holder, N. The involvement of retinoic acid in the development of the vertebrate central nervous system. Dev. Suppl. 1991, 113, 87–94. [Google Scholar]
- Chakrabarti, M.; McDonald, A.J.; Will Reed, J.; Moss, M.A.; Das, B.C.; Ray, S.K. Molecular signaling mechanisms of natural and synthetic retinoids for inhibition of pathogenesis in Alzheimer’s disease. J. Alzheimers Dis. 2016, 50, 335–352. [Google Scholar] [CrossRef] [PubMed]
- Lane, M.A.; Bailey, S.J. Role of retinoid signalling in the adult brain. Prog. Neurobiol. 2005, 75, 275–293. [Google Scholar] [CrossRef]
- Lee, H.-P.; Casadesus, G.; Zhu, X.; Lee, H.-G.; Perry, G.; Smith, M.A.; Gustaw-Rothenberg, K.; Lerner, A. All-trans retinoic acid as a novel therapeutic strategy for Alzheimer’s disease. Exp. Rev. Neurotherap. 2009, 9, 1615–1621. [Google Scholar] [CrossRef]
- Niewiadomska-Cimicka, A.; Krzyżosiak, A.; Ye, T.; Podleśny-Drabiniok, A.; Dembélé, D.; Dollé, P.; Krężel, W. Genome-wide analysis of RARβ transcriptional targets in mouse striatum links retinoic acid signaling with Huntington’s disease and other neurodegenerative disorders. Mol. Neurobiol. 2017, 54, 3859–3878. [Google Scholar] [CrossRef] [PubMed]
- Rataj-Baniowska, M.; Niewiadomska-Cimicka, A.; Paschaki, M.; Szyszka-Niagolov, M.; Carramolino, L.; Torres, M.; Dollé, P.; Krężel, W. Retinoic acid receptor β controls development of striatonigral projection neurons through FGF-dependent and Meis1- dependent mechanisms. J. Neurosci. 2015, 35, 14467–14475. [Google Scholar] [CrossRef]
- Moutinho, M.; Codocedo, J.F.; Puntambekar, S.S.; Landreth, G.E. Nuclear Receptors as Therapeutic Targets for Neurodegenerative Diseases: Lost in Translation. Ann. Rev. Pharmacol. Toxicol. 2019, 59, 237–261. [Google Scholar] [CrossRef]
- Li, J.Y.; Popovic, N.; Brundin, P. The use of the R6 transgenic mouse models of Huntington’s disease in attempts to develop novel therapeutic strategies. NeuroRx 2005, 2, 447–464. [Google Scholar] [CrossRef]
- Liu, D.; Ke, Z.; Luo, J. Thiamine deficiency and neurodegeneration: The interplay among oxidative stress, endoplasmic reticulum stress, and autophagy. Mol. Neurobiol. 2017, 54, 5440–5448. [Google Scholar] [CrossRef]
- Lonsdale, D. Thiamin and protein folding. Med. Hypotheses 2019, 129, 109252. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xu, M.; Frank, J.A.; Ke, Z.J.; Luo, J. Thiamine deficiency induces endoplasmic reticulum stress and oxidative stress in human neurons derived from induced pluripotent stem cells. Toxicol. Appl. Pharmacol. 2017, 320, 26–31. [Google Scholar] [CrossRef]
- Gruber-Bzura, B.M.; Krzysztoń-Russjan, J.; Bubko, I.; Syska, J.; Jaworska, M.; Zmysłowski, A.; Rosłon, M.; Drozd, J.; Drozd, E.; Majorczyk, E. Role of thiamine in Huntington’s disease pathogenesis: In vitro studies. Adv. Clin. Exp. Med. 2017, 26, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, A.; Diwan, V.; Kaur, H.; Bhateja, D.; Singh, C.K.; Sharma, S.; Padi, S.S. Nicotinamide reverses behavioral impairments and provides neuroprotection in 3-nitropropionic acid induced animal model ofHuntington’s disease: Implication of oxidative stress- poly (ADP- ribose) polymerase pathway. Metab. Brain Dis. 2018, 33, 1911–1921. [Google Scholar] [CrossRef]
- Blanchet, M.; Prince, F.; Chouinard, S.; Messier, J. Postural stability limits in manifest and premanifest Huntington’s disease under different sensory conditions. Neuroscience 2014, 279, 102–112. [Google Scholar] [CrossRef]
- Montero-Odasso, M.; Pieruccini-Faria, F.; Bartha, R.; Black, S.E.; Finger, E.; Freedman, M.; Greenberg, B.; Grimes, D.A.; Hegele, R.A.; Hudson, C.; et al. Motor phenotype in neurodegenerative disorders: Gait and balance platform study design protocol for the ontario neurodegenerative research initiative (ONDRI). J. Alzheimers Dis. 2017, 59, 707–721. [Google Scholar] [CrossRef]
- Wilczynski, J.; Pedrycz, A.; Mucha, D.; Ambrozy, T.; Mucha, D. Body posture, postural stability, and metabolic age in patients with Parkinson’s disease. BioMed Res. Int. 2017, 2017, 3975417. [Google Scholar] [CrossRef]
- Aghajanian, P.; Hall, S.; Wongworawat, M.D.; Mohan, S. The roles and mechanisms of actions of vitamin C in bone: New developments. J. Bone Min. Res. 2015, 30, 1945–1955. [Google Scholar] [CrossRef]
- Rebec, G.V.; Barton, S.J.; Marseilles, A.M.; Collins, K. Ascorbate treatment attenuates the Huntington behavioral phenotype in mice. Neuroreport 2003, 14, 1263–1265. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, S.; Gharagozloo, M.; Simard, C.; Gris, D. Astrocytes maintain glutamate homeostasis in the CNS by controlling the balance between glutamate uptake and release. Cells 2019, 8, 184. [Google Scholar] [CrossRef]
- Rebec, G.V. Dysregulation of corticostriatal ascorbate release and glutamate uptake in transgenic models of Huntington’s disease. Antiox. Redox Sig. 2013, 19, 2115–2128. [Google Scholar] [CrossRef]
- Dorner, J.L.; Miller, B.R.; Klein, E.L.; Murphy-Nakhnikian, A.; Andrews, R.L.; Barton, S.J.; Rebec, G.V. Corticostriatal dysfunction underlies diminished striatal ascorbate release in the R6/2 mouse model of Huntington’s disease. Brain Res. 2009, 1290, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Sahay, M.; Sahay, R. Rickets–vitamin D deficiency and dependency. Indian J. Endocrinol. Metab. 2012, 16, 164. [Google Scholar] [CrossRef]
- Rejnmark, L. Effects of vitamin d on muscle function and performance: A review of evidence from randomized controlled trials. Ther. Adv. Chronic. Dis. 2011, 2, 25–37. [Google Scholar] [CrossRef]
- Molnar, M.F.; Torok, R.; Szalardy, L.; Sumegi, E.; Vecsei, L.; Klivenyi, P. High-dose 1,25-dihydroxyvitamin D supplementation elongates the lifespan of Huntington’s disease transgenic mice. Acta Neurobiol. Exp. 2016, 76, 176–181. [Google Scholar] [CrossRef][Green Version]
- Peyser, C.E.; Folstein, M.; Chase, G.A.; Starkstein, S.; Brandt, J.; Cockrell, J.R.; Bylsma, F.; Coyle, J.T.; McHugh, P.R.; Folstein, S.E. Trial of d-!a-tocopherol in Huntington’s disease. Am. J. Psychiatry. 1995, 152, 1771–1775. [Google Scholar] [CrossRef] [PubMed]
- Kašparová, S.; Sumbalová, Z.; Bystrický, P.; Kucharská, J.; Liptaj, T.; Mlynárik, V.; Gvozdjáková, A. Effect of coenzyme Q10 and vitamin E on brain energy metabolism in the animal model of Huntington’s disease. Neurochem. Int. 2006, 48, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Fitzner, D.; Simons, M. Chronic progressive multiple sclerosis-pathogenesis of neurodegeneration and therapeutic strategies. Curr. Neuropharmacol. 2010, 8, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Correale, J.; Marrodan, M.; Ysrraelit, M.C. Mechanisms of neurodegeneration and axonal dysfunction in progressive multiple sclerosis. Biomedicines 2019, 7, 14. [Google Scholar] [CrossRef]
- Miljković, D.; Spasojević, I. Multiple sclerosis: Molecular mechanisms and therapeutic opportunities. Antioxid. Redox Signal. 2013, 19, 2286–2334. [Google Scholar] [CrossRef]
- Serra, A.; Chisari, C.G.; Matta, M. Eye movement abnormalities in multiple sclerosis: Pathogenesis, modeling, and treatment. Front. Neurol. 2018, 9, 31. [Google Scholar] [CrossRef] [PubMed]
- Dobson, R.; Giovannoni, G. Multiple sclerosis—A review. Eur. J. Neurol. 2019, 26, 27–40. [Google Scholar] [CrossRef]
- Adzemovic, M.Z.; Zeitelhofer, M.; Hochmeister, S.; Gustafsson, S.A.; Jagodic, M. Efficacy of vitamin D in treating multiple sclerosis-like neuroinflammation depends on developmental stage. Exp. Neurol. 2013, 249, 39–48. [Google Scholar] [CrossRef]
- Munger, K.L.; Åivo, J.; Hongell, K.; Soilu-Hänninen, M.; Surcel, H.-M.; Ascherio, A. Vitamin D status during pregnancy and risk of multiple sclerosis in offspring of women in the Finnish maternity cohort. JAMA Neurol. 2016, 73, 515–519. [Google Scholar] [CrossRef]
- Smolders, J.; Damoiseaux, J.; Menheere, P.; Hupperts, R. Vitamin D as an immune modulator in multiple sclerosis, a review. J. Neuroimmun. 2008, 194, 7–17. [Google Scholar] [CrossRef]
- Sintzel, M.B.; Rametta, M.; Reder, A.T. Vitamin D and multiple sclerosis: A comprehensive review. Neurol. Ther. 2018, 7, 59–85. [Google Scholar] [CrossRef]
- Ascherio, A.; Weisskopf, M.G.; O’Reilly, E.J.; Jacobs, E.J.; McCullough, M.L.; Calle, E.E.; Cudkowicz, M.; Thun, M.J. Vitamin E intake and risk of amyotrophic lateral sclerosis. Ann. Neurol. 2005, 57, 104–110. [Google Scholar] [CrossRef]
- Niino, M.; Fukazawa, T.; Kikuchi, S.; Sasaki, H. Therapeutic potential of vitamin D for multiple sclerosis. Curr. Med. Chem. 2008, 15, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Ramagopalan, S.V.; Dobson, R.; Meier, U.C.; Giovannoni, G. Multiple sclerosis: Risk factors, prodromes, and potential causal pathways. Lancet Neurol. 2010, 9, 727–739. [Google Scholar] [CrossRef]
- Sandberg, L.; Biström, M.; Salzer, J.; Vågberg, M.; Svenningsson, A.; Sundström, P. Vitamin D and axonal injury in multiple sclerosis. Mult. Scler. J. 2016, 22, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Muller, T.; Lohse, L.; Blodau, A.; Frommholz, K. Vitamin D rise enhances blood perfusion in patients with multiple sclerosis. J. Neural Transm. 2019, 126, 1631–1636. [Google Scholar] [CrossRef] [PubMed]
- Perga, S.; Albo, A.G.; Lis, K.; Minari, N.; Falvo, S.; Marnetto, F.; Caldano, M.; Reviglione, R.; Berchialla, P.; Capobianco, M.A. Vitamin D binding protein isoforms and apolipoprotein E in cerebrospinal fluid as prognostic biomarkers of multiple sclerosis. PLoS ONE 2015, 10, e0129291. [Google Scholar] [CrossRef]
- Disanto, G.; Ramagopalan, S.V.; Para, A.E.; Handunnetthi, L. The emerging role of vitamin D binding protein in multiple sclerosis. J. Neurol. 2011, 258, 353–358. [Google Scholar] [CrossRef]
- Smolders, J.; Peelen, E.; Thewissen, M.; Menheere, P.; Damoiseaux, J.; Hupperts, R. Circulating vitamin D binding protein levels are not associated with relapses or with vitamin D status in multiple sclerosis. Mult. Scler. J. 2014, 20, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Fragoso, Y.D.; Stoney, P.N.; McCaffery, P.J. The evidence for a beneficial role of vitamin A in multiple sclerosis. CNS Drugs 2014, 28, 291–299. [Google Scholar] [CrossRef]
- Khosravi-Largani, M.; Pourvali-Talatappeh, P.; Rousta, A.M.; Karimi-Kivi, M.; Noroozi, E.; Mahjoob, A.; Asaadi, Y.; Shahmohammadi, A.; Sadeghi, S.; Shakeri, S.; et al. A review on potential roles of vitamins in incidence, progression, and improvement of multiple sclerosis. eNeurologicalsci 2018, 10, 37–44. [Google Scholar] [CrossRef]
- Torkildsen, O.; Loken-Amsrud, K.I.; Wergeland, S.; Myhr, K.M.; Holmoy, T. Fat-soluble vitamins as disease modulators in multiple sclerosis. Acta Neurol. Scand. 2013, 127, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Warren, T.R. The increased prevalence of multiple sclerosis among people who were born and bred in areas where goitre is endemic. Med. Hypotheses 1984, 14, 111–114. [Google Scholar] [CrossRef]
- Miller, E.D.; Dziedzic, A.; Saluk-Bijak, J.; Bijak, M. A review of various antioxidant compounds and their potential utility as complementary therapy in multiple sclerosis. Nutrients 2019, 11, 1528. [Google Scholar] [CrossRef]
- Jafarirad, S.; Siassi, F.; Harirchian, M.-H.; Sahraian, M.-A.; Eshraghian, M.-R.; Shokri, F.; Amani, R.; Bitarafan, S.; Mozafari, S.; Saboor-Yaraghi, A. The effect of vitamin A supplementation on stimulated T-cell proliferation with myelin oligodendrocyte glycoprotein in patients with multiple sclerosis. J. Neurosci. Rural Pract. 2012, 3, 294–298. [Google Scholar] [CrossRef] [PubMed]
- Løken-Amsrud, K.I.; Myhr, K.-M.; Bakke, S.J.; Beiske, A.G.; Bjerve, K.S.; Bjørnarå, B.T.; Hovdal, H.; Lilleås, F.; Midgard, R.; Pedersen, T. Retinol levels are associated with magnetic resonance imaging outcomes in multiple sclerosis. Mult. Scler. J. 2013, 19, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Mizee, M.R.; Nijland, P.G.; van der Pol, S.M.; Drexhage, J.A.; van het Hof, B.; Mebius, R.; van der Valk, P.; van Horssen, J.; Reijerkerk, A.; de Vries, H.E. Astrocyte-derived retinoic acid: A novel regulator of blood–brain barrier function in multiple sclerosis. Acta Neuropathol. 2014, 128, 691–703. [Google Scholar] [CrossRef]
- Salzer, J.; Hallmans, G.; Nyström, M.; Stenlund, H.; Wadell, G.; Sundström, P. Vitamin A and systemic inflammation as protective factors in multiple sclerosis. Mult. Scler. J. 2013, 19, 1046–1051. [Google Scholar] [CrossRef] [PubMed]
- Runia, T.; Hop, W.; De Rijke, Y.; Hintzen, R. Vitamin A is not associated with exacerbations in multiple sclerosis. Mult. Scler. Rel. Dis. 2014, 3, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Evans, E.; Piccio, L.; Cross, A.H. Use of vitamins and dietary supplements by patients with multiple sclerosis: A review. JAMA Neurol. 2018, 75, 1013–1021. [Google Scholar] [CrossRef]
- Besler, H.T.; Comoglu, S.; Okcu, Z. Serum levels of antioxidant vitamins and lipid peroxidation in multiple sclerosis. Nutr. Neurosci. 2002, 5, 215–220. [Google Scholar] [CrossRef]
- Polachini, C.R.; Spanevello, R.M.; Zanini, D.; Baldissarelli, J.; Pereira, L.B.; Schetinger, M.R.; da Cruz, I.B.; Assmann, C.E.; Bagatini, M.D.; Morsch, V.M. Evaluation of delta-aminolevulinic dehydratase activity, oxidative stress biomarkers, and vitamin D levels in patients with multiple sclerosis. Neurotox. Res. 2016, 29, 230–242. [Google Scholar] [CrossRef]
- Tavazzi, B.; Batocchi, A.P.; Amorini, A.M.; Nociti, V.; D’Urso, S.; Longo, S.; Gullotta, S.; Picardi, M.; Lazzarino, G. Serum metabolic profile in multiple sclerosis patients. Mult. Scler. Int. 2011, 2011, 167156. [Google Scholar] [CrossRef]
- Babri, S.; Mehrvash, F.; Mohaddes, G.; Hatami, H.; Mirzaie, F. Effect of intrahippocampal administration of vitamin C and progesterone on learning in a model of multiple sclerosis in rats. Adv. Pharm. Bull. 2015, 5, 83. [Google Scholar] [CrossRef]
- Hernández-Pedro, N.Y.; Espinosa-Ramirez, G.; De La Cruz, V.P.; Pineda, B.; Sotelo, J. Initial immunopathogenesis of multiple sclerosis: Innate immune response. Clin. Dev. Immunol. 2013, 2013, 413465. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.M.; Hernan, M.A.; Olek, M.J.; Spiegelman, D.; Willett, W.C.; Ascherio, A. Intakes of carotenoids, vitamin C, and vitamin E and MS risk among two large cohorts of women. Neurology 2001, 57, 75–80. [Google Scholar] [CrossRef]
- Najafi, M.R.; Shaygannajad, V.; Mirpourian, M.; Gholamrezaei, A. Vitamin B12 deficiency and multiple sclerosis; is there any association? Int. J. Prev. Med. 2012, 3, 286. [Google Scholar] [PubMed]
- Kruman, I.I.; Culmsee, C.; Chan, S.L.; Kruman, Y.; Guo, Z.; Penix, L.; Mattson, M.P. Homocysteine elicits a DNA damage response in neurons that promotes apoptosis and hypersensitivity to excitotoxicity. J. Neurosci. 2000, 20, 6920–6926. [Google Scholar] [CrossRef] [PubMed]
- Moghaddasi, M.; Mamarabadi, M.; Mohebi, N.; Razjouyan, H.; Aghaei, M. Homocysteine, vitamin B12 and folate levels in Iranian patients with multiple sclerosis: A case control study. Clin. Neurol. Neurosurg. 2013, 115, 1802–1805. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, E.; Bottiglieri, T.; Laundy, M.; Crellin, R.; Kirker, S. Vitamin B12 metabolism in multiple sclerosis. Arch. Neurol. 1992, 49, 649–652. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, E.; Linnell, J.; Faludy, J. Multiple sclerosis associated with vitamin B12 deficiency. Arch. Neurol. 1991, 48, 808–811. [Google Scholar] [CrossRef]
- Weinstein, S.J.; Hartman, T.J.; Stolzenberg-Solomon, R.; Pietinen, P.; Barrett, M.J.; Taylor, P.R.; Virtamo, J.; Albanes, D. Null association between prostate cancer and serum folate, vitamin B6, vitamin B12, and homocysteine. Cancer Epid. Prev. Biomark. 2003, 12, 1271–1272. [Google Scholar]
- Lempriere, S. Vitamin B3 promotes remyelination. Nat. Rev. Neurol. 2020, 16, 184–185. [Google Scholar] [CrossRef]
- Costantini, A.; Nappo, A.; Pala, M.I.; Zappone, A. High dose thiamine improves fatigue in multiple sclerosis. BMJ Case Rep. 2013, 2013, bcr2013009144. [Google Scholar] [CrossRef]
- Bitarafan, S.; Saboor-Yaraghi, A.; Sahraian, M.A.; Nafissi, S.; Togha, M.; Moghadam, N.B.; Roostaei, T.; Siassi, F.; Eshraghian, M.R.; Ghanaati, H.; et al. Impact of vitamin A supplementation on disease progression in patients with multiple sclerosis. Arch. Iran Med. 2015, 18, 435–440. [Google Scholar] [CrossRef]
- Loken-Amsrud, K.I.; Myhr, K.M.; Bakke, S.J.; Beiske, A.G.; Bjerve, K.S.; Bjornara, B.T.; Hovdal, H.; Lilleas, F.; Midgard, R.; Pedersen, T.; et al. Alpha-tocopherol and MRI outcomes in multiple sclerosis—Association and prediction. PLoS ONE 2013, 8, e54417. [Google Scholar] [CrossRef]
- Talbot, K. Motor neuron disease: The bare essentials. Pract. Neurol. 2009, 9, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.; Al Khleifat, A.; Al-Chalabi, A. What causes amyotrophic lateral sclerosis? F1000Research 2017, 6, 371. [Google Scholar] [CrossRef]
- Zarei, S.; Carr, K.; Reiley, L.; Diaz, K.; Guerra, O.; Altamirano, P.F.; Pagani, W.; Lodin, D.; Orozco, G.; Chinea, A. A comprehensive review of amyotrophic lateral sclerosis. Surg. Neurol. Int. 2015, 6, 171. [Google Scholar] [CrossRef]
- Cortese, R.; D’Errico, E.; Introna, A.; Schirosi, G.; Scarafino, A.; Distaso, E.; Nazzaro, P.; Zoccolella, S.; Simone, I. Vitamin D levels in serum of amyotrophic lateral sclerosis patients. (P2. 069). AAN Enterp. 2015, 84, 14S. [Google Scholar]
- Camu, W.; Tremblier, B.; Plassot, C.; Alphandery, S.; Salsac, C.; Pageot, N.; Juntas-Morales, R.; Scamps, F.; Daures, J.P.; Raoul, C. Vitamin D confers protection to motoneurons and is a prognostic factor of amyotrophic lateral sclerosis. Neurobiol. Aging 2014, 35, 1198–1205. [Google Scholar] [CrossRef]
- Gianforcaro, A.; Hamadeh, M.J. Dietary vitamin D3 supplementation at 10× the adequate intake improves functional capacity in the G93A transgenic mouse model of ALS, a pilot study. CNS Neurosci. Ther. 2012, 18, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Gianforcaro, A.; Solomon, J.A.; Hamadeh, M.J. Vitamin D(3) at 50× AI attenuates the decline in paw grip endurance, but not disease outcomes, in the G93A mouse model of ALS, and is toxic in females. PLoS ONE 2013, 8, e30243. [Google Scholar] [CrossRef]
- Solomon, J.A.; Gianforcaro, A.; Hamadeh, M.J. Vitamin D3 deficiency differentially affects functional and disease outcomes in the G93A mouse model of amyotrophic lateral sclerosis. PLoS ONE 2011, 6, e29354. [Google Scholar] [CrossRef]
- Libonati, L.; Onesti, E.; Gori, M.C.; Ceccanti, M.; Cambieri, C.; Fabbri, A.; Frasca, V.; Inghilleri, M. Vitamin D in amyotrophic lateral sclerosis. Funct. Neurol. 2017, 32, 35. [Google Scholar] [CrossRef]
- Ascherio, A.; Munger, K.L.; Simon, K.C. Vitamin D and multiple sclerosis. Lancet Neurol. 2010, 9, 599–612. [Google Scholar] [CrossRef]
- Chiricosta, L.; Gugliandolo, A.; Tardiolo, G.; Bramanti, P.; Mazzon, E. Transcriptomic analysis of MAPK signaling in NSC-34 motor neurons treated with vitamin E. Nutrients 2019, 11, 1081. [Google Scholar] [CrossRef] [PubMed]
- Soto, C.; Satani, N. The intricate mechanisms of neurodegeneration in prion diseases. Trends Mol. Med. 2011, 17, 14–24. [Google Scholar] [CrossRef]
- Kupfer, L.; Hinrichs, W.; Groschup, M. Prion protein misfolding. Curr. Mol. Med. 2009, 9, 826–835. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, C.J.; Zhang, K.; Munn, A.L.; Wiegmans, A.; Wei, M.Q. Prion protein scrapie and the normal cellular prion protein. Prion 2016, 10, 63–82. [Google Scholar] [CrossRef]
- Terry, C.; Wadsworth, J.D. Recent Advances in Understanding Mammalian Prion Structure: A Mini Review. Front. Mol. Neurosci. 2019, 12, 169. [Google Scholar] [CrossRef] [PubMed]
- Geschwind, M.D. Prion diseases. Continuum 2015, 21, 1612–1638. [Google Scholar] [CrossRef] [PubMed]
- Benetti, F.; Biarnes, X.; Attanasio, F.; Giachin, G.; Rizzarelli, E.; Legname, G. Structural determinants in prion protein folding and stability. J. Mol. Biol. 2014, 426, 3796–3810. [Google Scholar] [CrossRef]
- Prasad, K.N.; Bondy, S.C. Oxidative and inflammatory events in prion diseases: Can they be therapeutic targets? Curr. Aging Sci. 2019, 11, 216–225. [Google Scholar] [CrossRef]
- Singh, N.; Singh, A.; Das, D.; Mohan, M.L. Redox control of prion and disease pathogenesis. Antioxid. Redox Signal. 2010, 12, 1271–1294. [Google Scholar] [CrossRef] [PubMed]
- Briani, C.; Dalla Torre, C.; Citton, V.; Manara, R.; Pompanin, S.; Binotto, G.; Adami, F. Cobalamin deficiency: Clinical picture and radiological findings. Nutrients 2013, 5, 4521–4539. [Google Scholar] [CrossRef]
- Calderon-Ospina, C.A.; Nava-Mesa, M.O. B Vitamins in the nervous system: Current knowledge of the biochemical modes of action and synergies of thiamine, pyridoxine, and cobalamin. CNS Neurosci. Ther. 2020, 26, 5–13. [Google Scholar] [CrossRef]
- Rzepka, Z.; Respondek, M.; Pawlik, J.; Beberok, A.; Gryko, D.; Wrześniok, D. Cobalamin deficiency: Effect on homeostasis of cultured human astrocytes. Cells 2019, 8, 1505. [Google Scholar] [CrossRef]
- Scalabrino, G. The multi-faceted basis of vitamin B12 (cobalamin) neurotrophism in adult central nervous system: Lessons learned from its deficiency. Prog. Neurobiol. 2009, 88, 203–220. [Google Scholar] [CrossRef] [PubMed]
- Scalabrino, G.; Nicolini, G.; Buccellato, F.R.; Peracchi, M.; Tredici, G.; Manfridi, A.; Pravettoni, G. Epidermal growth factor as a local mediator of the neurotrophic action of vitamin B(12) (cobalamin) in the rat central nervous system. FASEB J. 1999, 13, 2083–2090. [Google Scholar] [CrossRef]
- Scalabrino, G.; Veber, D. Cobalamin and normal prions: A new horizon for cobalamin neurotrophism. Biochimie 2013, 95, 1041–1046. [Google Scholar] [CrossRef] [PubMed]
- Suenaga, M.; Hiramoto, Y.; Matsunaga, Y. Vitamin D2 interacts with Human PrPc (90–231) and breaks PrPc oligomerization in vitro. Prion 2013, 7, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Kozlowski, H.; Janicka-Klos, A.; Brasun, J.; Gaggelli, E.; Valensin, D.; Valensin, G. Copper, iron, and zinc ions homeostasis and their role in neurodegenerative disorders (metal uptake, transport, distribution and regulation). Coord. Chem. Rev. 2009, 253, 2665–2685. [Google Scholar] [CrossRef]
- Toni, M.; Massimino, M.L.; De Mario, A.; Angiulli, E.; Spisni, E. Metal dyshomeostasis and their pathological role in prion and prion-like diseases: The basis for a nutritional approach. Front. Neurosci. 2017, 11, 3. [Google Scholar] [CrossRef]
- Aridi, Y.S.; Walker, J.L.; Wright, O.R. The association between the Mediterranean dietary pattern and cognitive health: A systematic review. Nutrients 2017, 9, 674. [Google Scholar] [CrossRef]
- Broadhead, G.K.; Grigg, J.R.; Chang, A.A.; McCluskey, P. Dietary modification and supplementation for the treatment of age-related macular degeneration. Nutr. Rev. 2015, 73, 448–462. [Google Scholar] [CrossRef]
- Chew, E.Y.; Clemons, T.E.; Sangiovanni, J.P.; Danis, R.P.; Ferris, F.L., 3rd; Elman, M.J.; Antoszyk, A.N.; Ruby, A.J.; Orth, D.; Bressler, S.B.; et al. Secondary analyses of the effects of lute-in/zeaxanthin on age-related macular degeneration progression: AREDS2 report No. 3. JAMA Ophthalmol. 2014, 132, 142–149. [Google Scholar] [CrossRef]
- Tohari, A.M.; Zhou, X.; Shu, X. Protection against oxidative stress by vitamin D in cone cells. Cell Biochem. Funct. 2016, 34, 82–94. [Google Scholar] [CrossRef]
- Kim, E.C.; Han, K.; Jee, D. Inverse relationship between high blood 25-hydroxyvitamin D and late stage of age-related macular degeneration in a repre-sentative Korean population. Investig. Ophthalmol. Vis. Sci. 2014, 55, 4823–4831. [Google Scholar] [CrossRef] [PubMed]
- Di Somma, C.; Scarano, E.; Barrea, L.; Zhukouskaya, V.V.; Savastano, S.; Mele, C.; Scacchi, M.; Aimaretti, G.; Colao, A.; Marzullo, P. Vitamin D and neurological diseases: An endocrine view. Int. J. Mol. Sci. 2017, 18, 2482. [Google Scholar] [CrossRef] [PubMed]
- Fricker, R.A.; Green, E.L.; Jenkins, S.I.; Griffin, S.M. The influence of nicotinamide on health and disease in the central nervous system. Int. J. Trypt. Res. 2018, 11, 1178646918776658. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rai, S.N.; Singh, P.; Steinbusch, H.W.M.; Vamanu, E.; Ashraf, G.; Singh, M.P. The Role of Vitamins in Neurodegenerative Disease: An Update. Biomedicines 2021, 9, 1284. https://doi.org/10.3390/biomedicines9101284
Rai SN, Singh P, Steinbusch HWM, Vamanu E, Ashraf G, Singh MP. The Role of Vitamins in Neurodegenerative Disease: An Update. Biomedicines. 2021; 9(10):1284. https://doi.org/10.3390/biomedicines9101284
Chicago/Turabian StyleRai, Sachchida Nand, Payal Singh, Harry W.M. Steinbusch, Emanuel Vamanu, Ghulam Ashraf, and Mohan Prasad Singh. 2021. "The Role of Vitamins in Neurodegenerative Disease: An Update" Biomedicines 9, no. 10: 1284. https://doi.org/10.3390/biomedicines9101284
APA StyleRai, S. N., Singh, P., Steinbusch, H. W. M., Vamanu, E., Ashraf, G., & Singh, M. P. (2021). The Role of Vitamins in Neurodegenerative Disease: An Update. Biomedicines, 9(10), 1284. https://doi.org/10.3390/biomedicines9101284









