Cardioluminescence in Transgenic Zebrafish Larvae: A Calcium Imaging Tool to Study Drug Effects and Pathological Modeling
Abstract
:1. Introduction
2. Materials and Methods
2.1. Zebrafish Husbandry
2.2. Generation of Tg(myl7:GA) Zebrafish Line
2.3. Synthesis of Diacetyl CTZ-h
2.4. Aequorin Reconstitution with Coelenterazine
2.5. Bioluminescence Imaging
2.6. GCaMP Fluorescence Imaging
2.7. Reagents
2.8. Heart Failure Induced by Treatment with Terfenadine
2.9. Data Analysis
2.10. Statistics
3. Results
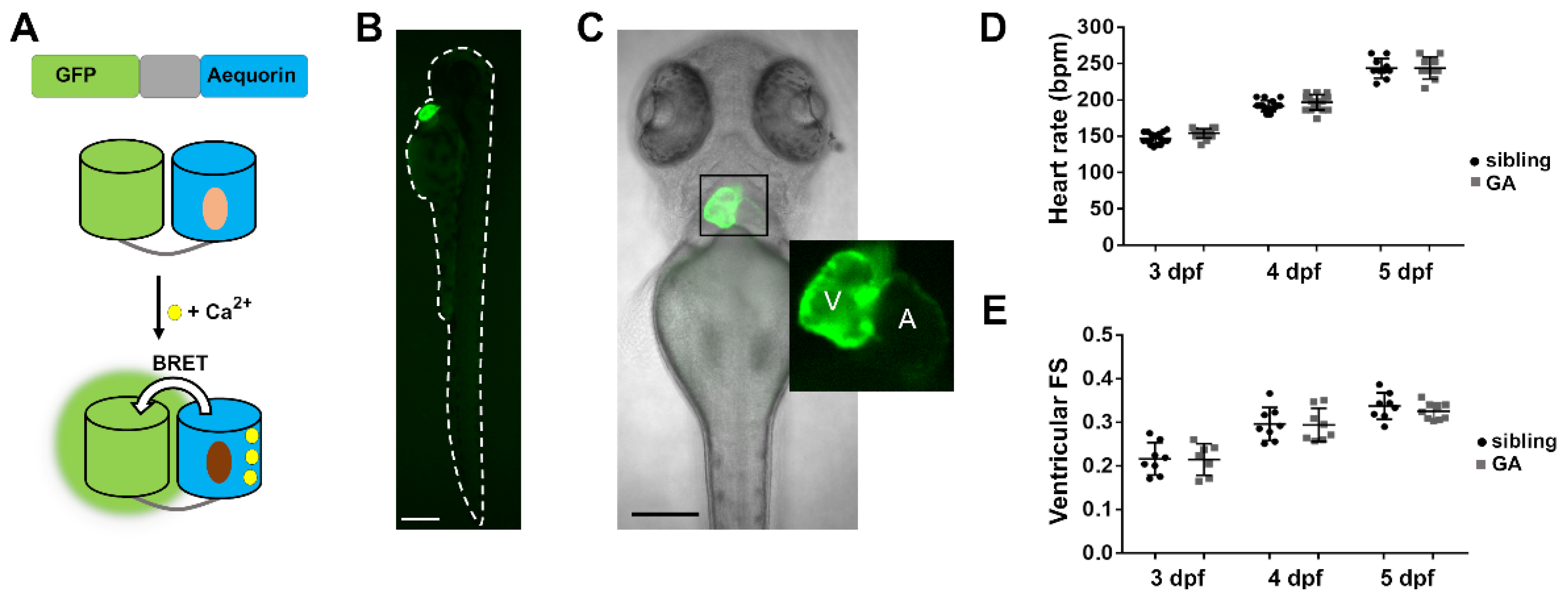
3.1. Tg(myl7:GA) Zebrafish Line Generation
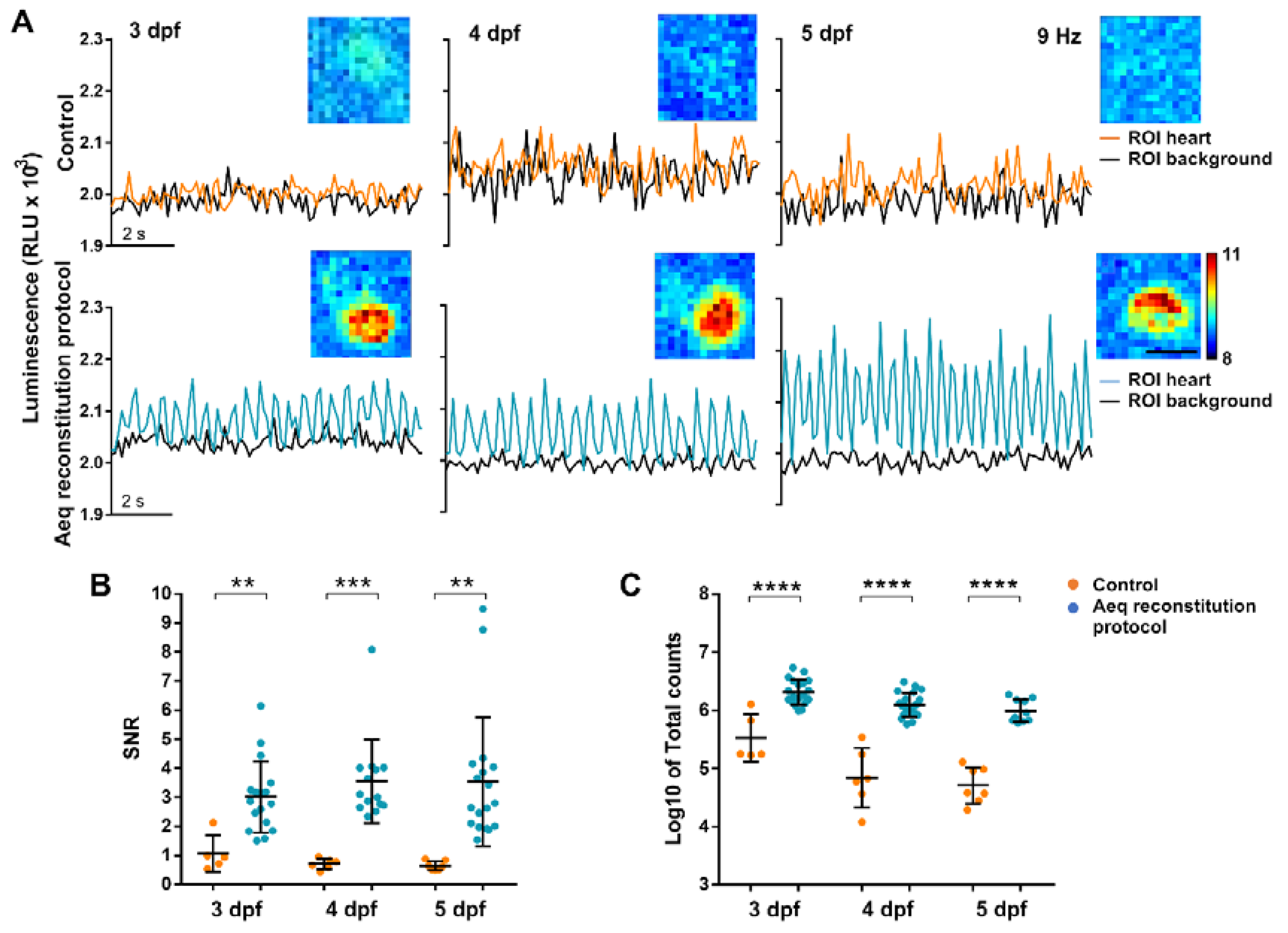
3.2. Aequorin Reconstitution Protocol
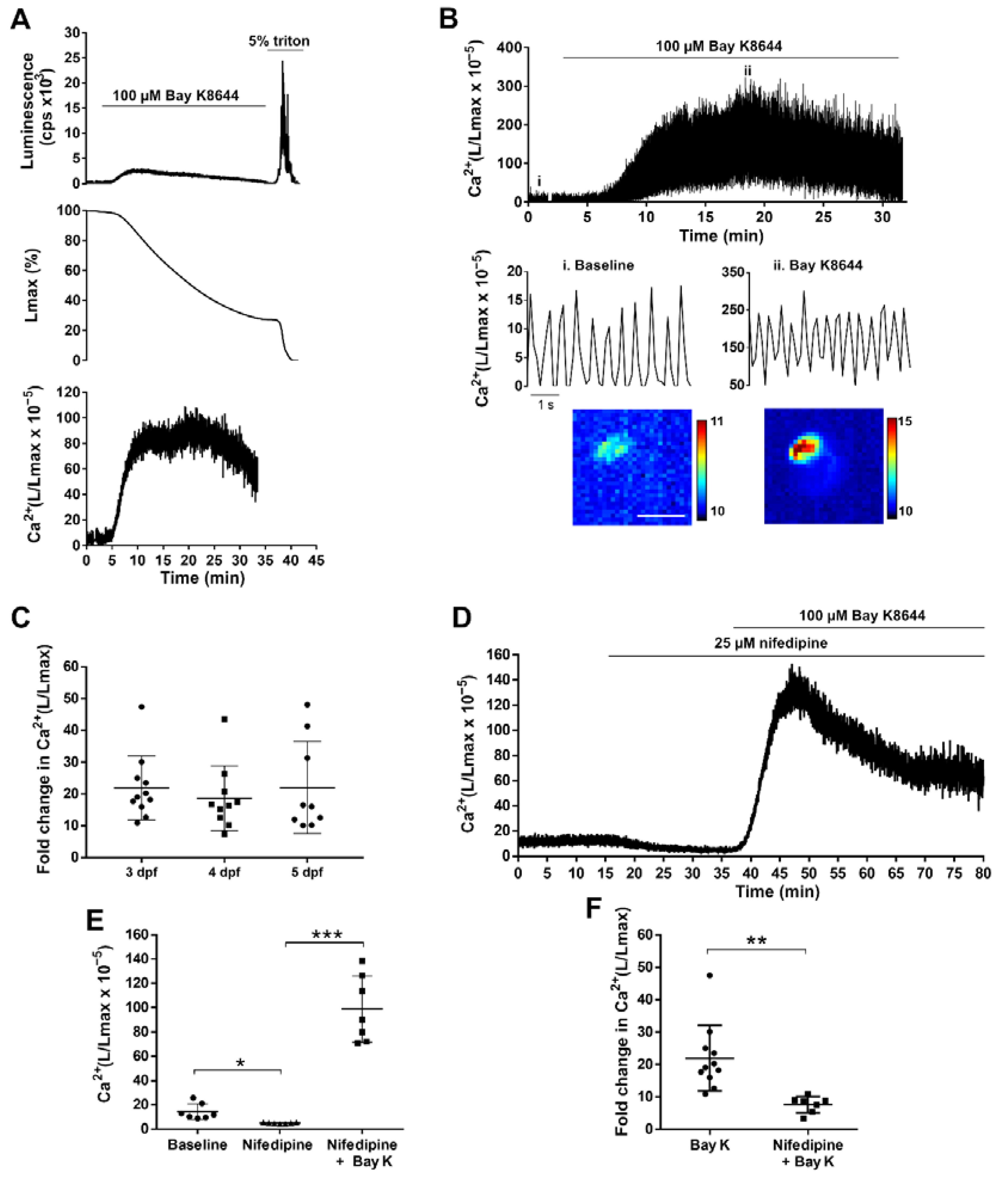
3.2.1. Suppressing Ca2+ Rise in the Heart with an LTCC Blocker
3.2.2. Larvae Treated with the Aequorin Reconstitution Protocol Recovered Heart Function after Restoring Ca2+ into the Medium
3.2.3. Imaging Individual Ventricular Ca2+ Transients in the Heart
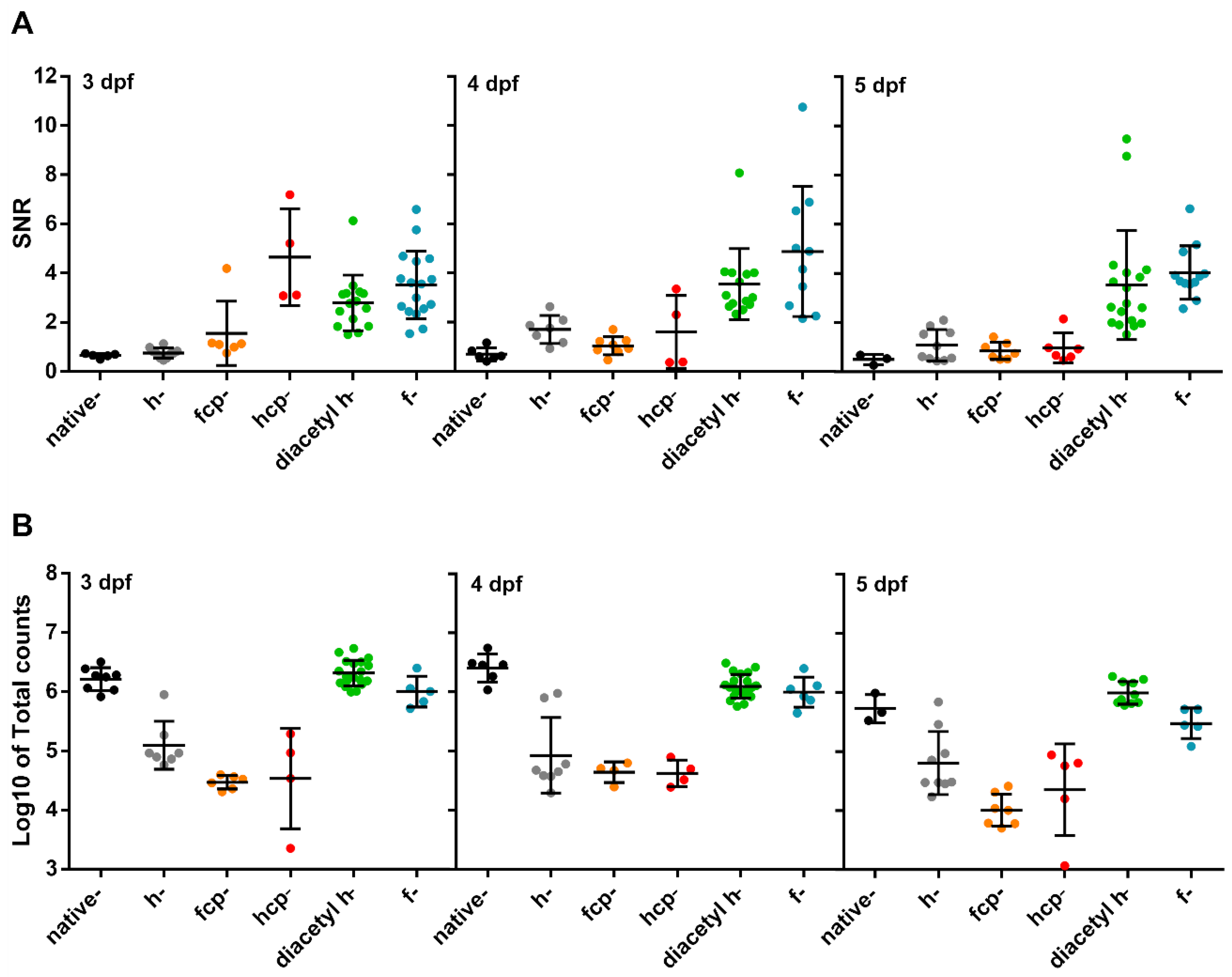
3.3. Testing Different CTZ Analogues
3.4. GA Reports the Effect of Drugs Acting on LTCC in the Zebrafish Ventricle
3.5. Decreasing Adrenergic Tone with Propranolol
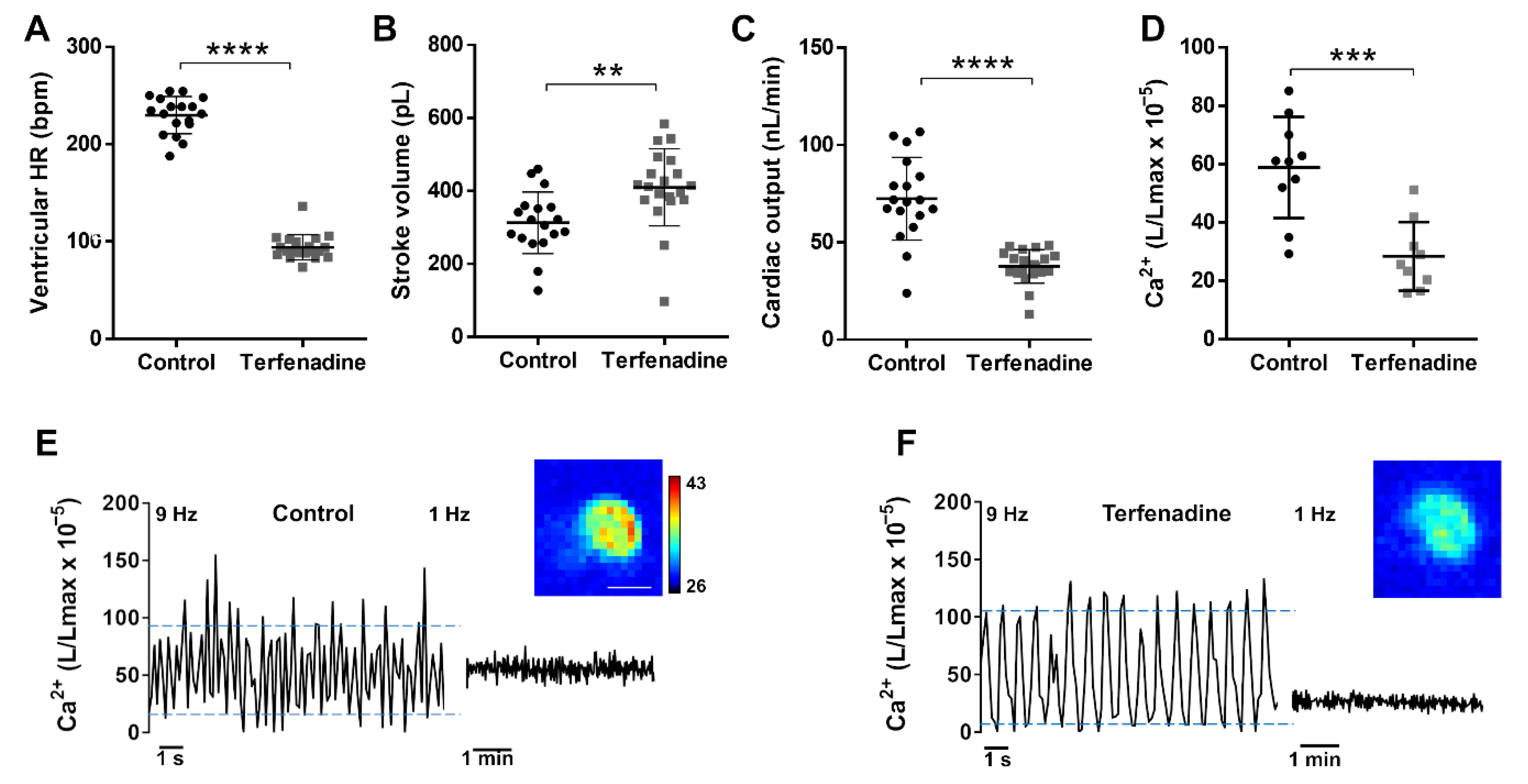
3.6. Ca2+ Levels in a Terfenadine-Induced Heart Failure Model
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Eisner, D.A. Ups and downs of calcium in the heart. J. Physiol. 2018, 596, 19–30. [Google Scholar] [CrossRef] [Green Version]
- Eisner, D.A.; Caldwell, J.L.; Trafford, A.W.; Hutchings, D.C. The control of diastolic calcium in the heart: Basic mechanisms and functional implications. Circ. Res. 2020, 126, 395–412. [Google Scholar] [CrossRef] [PubMed]
- Trafford, A.W.; Díaz, M.E.; Eisner, D.A. Coordinated control of cell Ca2+ loading and triggered release from the sarcoplasmic reticulum underlies the rapid inotropic response to increased L-Type Ca2+ current. Circ. Res. 2001, 88, 195–201. [Google Scholar] [CrossRef] [Green Version]
- Shinnawi, R.; Huber, I.; Maizels, L.; Shaheen, N.; Gepstein, A.; Arbel, G.; Tijsen, A.J.; Gepstein, L. Monitoring Human-induced pluripotent stem cell-derived cardiomyocytes with genetically encoded calcium and voltage fluorescent reporters. Stem. Cell Rep. 2015, 5, 582–596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bedut, S.; Seminatore-Nole, C.; Lamamy, V.; Caignard, S.; Boutin, J.A.; Nosjean, O.; Stephan, J.-P.; Coge, F. High-throughput drug profiling with voltage- and calcium-sensitive fluorescent probes in human IPSC-derived cardiomyocytes. Am. J. Physiol. Heart Circ. Physiol. 2016, 311, H44–H53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, P.-C.; Llach, A.; Sheng, X.Y.; Hove-Madsen, L.; Tibbits, G.F. Calcium handling in zebrafish ventricular myocytes. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 300, R56–R66. [Google Scholar] [CrossRef] [Green Version]
- Brown, D.R.; Samsa, L.A.; Qian, L.; Liu, J. Advances in the study of heart development and disease using zebrafish. J. Cardiovasc. Dev. Dis. 2016, 3, 13. [Google Scholar] [CrossRef]
- Burns, C.G.; Milan, D.J.; Grande, E.J.; Rottbauer, W.; MacRae, C.A.; Fishman, M.C. High-throughput assay for small molecules that modulate zebrafish embryonic heart rate. Nat. Chem. Biol. 2005, 1, 263–264. [Google Scholar] [CrossRef]
- Panáková, D.; Werdich, A.A.; Macrae, C.A. Wnt11 patterns a myocardial electrical gradient through regulation of the L-type Ca(2+) channel. Nature 2010, 466, 874–878. [Google Scholar] [CrossRef] [Green Version]
- Driever, W.; Solnica-Krezel, L.; Schier, A.F.; Neuhauss, S.C.; Malicki, J.; Stemple, D.L.; Stainier, D.Y.; Zwartkruis, F.; Abdelilah, S.; Rangini, Z.; et al. A genetic screen for mutations affecting embryogenesis in zebrafish. Dev. Camb. Engl. 1996, 123, 37–46. [Google Scholar]
- Chi, N.C.; Shaw, R.M.; Jungblut, B.; Huisken, J.; Ferrer, T.; Arnaout, R.; Scott, I.; Beis, D.; Xiao, T.; Baier, H.; et al. Genetic and physiologic dissection of the vertebrate cardiac conduction system. PLoS Biol. 2008, 6, e109. [Google Scholar] [CrossRef] [Green Version]
- Parker, T.; Libourel, P.-A.; Hetheridge, M.J.; Cumming, R.I.; Sutcliffe, T.P.; Goonesinghe, A.C.; Ball, J.S.; Owen, S.F.; Chomis, Y.; Winter, M.J. A multi-endpoint in vivo larval zebrafish (Danio Rerio) model for the assessment of integrated cardiovascular function. J. Pharmacol. Toxicol. Methods 2014, 69, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.M.; Ahlberg, G.; Hansen, C.V.; Guenther, S.; Marín-Juez, R.; Sokol, A.M.; El-Sammak, H.; Piesker, J.; Hellsten, Y.; Olesen, M.S.; et al. Early sarcomere and metabolic defects in a zebrafish Pitx2c cardiac arrhythmia model. Proc. Natl. Acad. Sci. USA 2019, 116, 24115–24121. [Google Scholar] [CrossRef] [PubMed]
- Alday, A.; Alonso, H.; Gallego, M.; Urrutia, J.; Letamendia, A.; Callol, C.; Casis, O. Ionic channels underlying the ventricular action potential in zebrafish embryo. Pharmacol. Res. 2014, 84, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, P.; Ireland, J.; Grunow, B. Fish, the better model in human heart research? Zebrafish heart aggregates as a 3D spontaneously cardiomyogenic in vitro model system. Prog. Biophys. Mol. Biol. 2018, 138, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Nemtsas, P.; Wettwer, E.; Christ, T.; Weidinger, G.; Ravens, U. Adult zebrafish heart as a model for human heart? An electrophysiological study. J. Mol. Cell. Cardiol. 2010, 48, 161–171. [Google Scholar] [CrossRef]
- Haverinen, J.; Hassinen, M.; Dash, S.N.; Vornanen, M. Expression of calcium channel transcripts in the zebrafish heart: Dominance of T-Type channels. J. Exp. Biol. 2018, 221, jeb179226. [Google Scholar] [CrossRef] [Green Version]
- Van Opbergen, C.J.M.; van der Voorn, S.M.; Vos, M.A.; de Boer, T.P.; van Veen, T.A.B. Cardiac Ca2+ signalling in zebrafish: Translation of findings to man. Prog. Biophys. Mol. Biol. 2018, 138, 45–58. [Google Scholar] [CrossRef]
- Van Opbergen, C.J.M.; Koopman, C.D.; Kok, B.J.M.; Knöpfel, T.; Renninger, S.L.; Orger, M.B.; Vos, M.A.; van Veen, T.A.B.; Bakkers, J.; de Boer, T.P. Optogenetic sensors in the zebrafish heart: A novel in vivo electrophysiological tool to study cardiac arrhythmogenesis. Theranostics 2018, 8, 4750–4764. [Google Scholar] [CrossRef]
- Weber, M.; Scherf, N.; Meyer, A.M.; Panáková, D.; Kohl, P.; Huisken, J. Cell-accurate optical mapping across the entire developing heart. eLife 2017, 6, e28307. [Google Scholar] [CrossRef]
- Kamel, S.M.; Koopman, C.D.; Kruse, F.; Willekers, S.; Chocron, S.; Bakkers, J. A Heterozygous mutation in cardiac troponin T Promotes Ca2+ dysregulation and adult cardiomyopathy in zebrafish. J. Cardiovasc. Dev. Dis. 2021, 8, 46. [Google Scholar] [CrossRef] [PubMed]
- Sehnert, A.J.; Huq, A.; Weinstein, B.M.; Walker, C.; Fishman, M.; Stainier, D.Y.R. Cardiac troponin T is essential in sarcomere assembly and cardiac contractility. Nat. Genet. 2002, 31, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Várkuti, B.H.; Képiró, M.; Horváth, I.Á.; Végner, L.; Ráti, S.; Zsigmond, Á.; Hegyi, G.; Lenkei, Z.; Varga, M.; Málnási-Csizmadia, A. A highly soluble, non-phototoxic, non-fluorescent blebbistatin derivative. Sci. Rep. 2016, 6, 26141. [Google Scholar] [CrossRef] [Green Version]
- Salgado-Almario, J.; Vicente, M.; Vincent, P.; Domingo, B.; Llopis, J. Mapping calcium dynamics in the heart of zebrafish embryos with ratiometric genetically encoded calcium indicators. Int. J. Mol. Sci. 2020, 21, 6610. [Google Scholar] [CrossRef]
- Kiepas, A.; Voorand, E.; Mubaid, F.; Siegel, P.M.; Brown, C.M. Optimizing live-cell fluorescence imaging conditions to minimize phototoxicity. J. Cell Sci. 2020, 133, jcs242834. [Google Scholar] [CrossRef] [PubMed]
- Brini, M. Calcium-sensitive photoproteins. Methods San Diego Calif. 2008, 46, 160–166. [Google Scholar] [CrossRef]
- Bakayan, A.; Domingo, B.; Vaquero, C.F.; Peyriéras, N.; Llopis, J. Fluorescent protein–photoprotein fusions and their applications in calcium imaging. Photochem. Photobiol. 2017, 93, 448–465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alvarez, J.; Montero, M. Measuring [Ca2+] in the endoplasmic reticulum with aequorin. Cell Calcium 2002, 32, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Webb, S.E.; Cheung, C.C.Y.; Chan, C.M.; Love, D.R.; Miller, A.L. Application of complementary luminescent and fluorescent imaging techniques to visualize nuclear and cytoplasmic Ca2+ signalling during the in vivo differentiation of slow muscle cells in zebrafish embryos under normal and dystrophic conditions. Clin. Exp. Pharmacol. Physiol. 2012, 39, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Naumann, E.A.; Kampff, A.R.; Prober, D.A.; Schier, A.F.; Engert, F. Monitoring neural activity with bioluminescence during natural behavior. Nat. Neurosci. 2010, 13, 513–520. [Google Scholar] [CrossRef] [Green Version]
- Bakayan, A.; Domingo, B.; Miyawaki, A.; Llopis, J. Imaging Ca(2+) activity in mammalian cells and zebrafish with a novel red-emitting aequorin variant. Pflugers Arch. 2015, 467, 2031–2042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vicente, M.; Salgado-Almario, J.; Soriano, J.; Burgos, M.; Domingo, B.; Llopis, J. Visualization of mitochondrial Ca2+ signals in skeletal muscle of zebrafish embryos with bioluminescent indicators. Int. J. Mol. Sci. 2019, 20, 5409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baubet, V.; Mouellic, H.L.; Campbell, A.K.; Lucas-Meunier, E.; Fossier, P.; Brûlet, P. Chimeric green fluorescent protein-aequorin as bioluminescent Ca2+ reporters at the single-cell level. Proc. Natl. Acad. Sci. USA 2000, 97, 7260–7265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adamczyk, M.; Akireddy, S.R.; Johnson, D.D.; Mattingly, P.G.; Pan, Y.; Reddy, R.E. Synthesis of 3,7-dihydroimidazo [1,2a]Pyrazine-3-ones and their chemiluminescent properties. Tetrahedron 2003, 59, 8129–8142. [Google Scholar] [CrossRef]
- Kim, T.J.; Türkcan, S.; Pratx, G. Modular low-light microscope for imaging cellular bioluminescence and radioluminescence. Nat. Protoc. 2017, 12, 1055–1076. [Google Scholar] [CrossRef] [Green Version]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH image to ImageJ: 25 Years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Kurnia, K.A.; Saputra, F.; Roldan, M.J.M.; Castillo, A.L.; Huang, J.-C.; Chen, K.H.-C.; Lai, H.-T.; Hsiao, C.-D. Measurement of multiple cardiac performance endpoints in daphnia and zebrafish by kymograph. Inventions 2021, 6, 8. [Google Scholar] [CrossRef]
- Manjarrés, I.M.; Chamero, P.; Domingo, B.; Molina, F.; Llopis, J.; Alonso, M.T.; García-Sancho, J. Red and green aequorins for simultaneous monitoring of Ca2+ signals from two different organelles. Pflüg. Arch. Eur. J. Physiol. 2008, 455, 961–970. [Google Scholar] [CrossRef] [Green Version]
- Shimomura, O.; Johnson, F.H.; Saiga, Y. Extraction, purification and properties of aequorin, a bioluminescent protein from the luminous hydromedusan, aequorea. J. Cell. Comp. Physiol. 1962, 59, 223–239. [Google Scholar] [CrossRef]
- Shimomura, O.; Musicki, B.; Kishi, Y.; Inouye, S. Light-emitting properties of recombinant semi-synthetic aequorins and recombinant fluorescein-conjugated aequorin for measuring cellular calcium. Cell Calcium 1993, 14, 373–378. [Google Scholar] [CrossRef]
- Shimomura, O.; Kishi, Y.; Inouye, S. The relative rate of aequorin regeneration from apoaequorin and coelenterazine analogues. Biochem. J. 1993, 296, 549–551. [Google Scholar] [CrossRef] [Green Version]
- Shimomura, O. Membrane permeability of coelenterazine analogues measured with fish eggs. Biochem. J. 1997, 326, 297–298. [Google Scholar] [CrossRef] [Green Version]
- Takai, A.; Nakano, M.; Saito, K.; Haruno, R.; Watanabe, T.M.; Ohyanagi, T.; Jin, T.; Okada, Y.; Nagai, T. Expanded Palette of nano-lanterns for real-time multicolor luminescence imaging. Proc. Natl. Acad. Sci. USA 2015, 112, 4352–4356. [Google Scholar] [CrossRef] [Green Version]
- Gu, G.; Na, Y.; Chung, H.; Seok, S.H.; Lee, H.-Y. Zebrafish larvae model of dilated cardiomyopathy induced by terfenadine. Korean Circ. J. 2017, 47, 960–969. [Google Scholar] [CrossRef] [Green Version]
- Quan, H.; Oh, G.C.; Seok, S.H.; Lee, H.-Y. Fimasartan, an angiotensin II Receptor antagonist, ameliorates an in vivo zebrafish model of heart failure. Korean J. Intern. Med. 2020, 35, 1400–1410. [Google Scholar] [CrossRef]
- Lu, H.R.; Hermans, A.N.; Gallacher, D.J. Does terfenadine-induced ventricular tachycardia/fibrillation directly relate to its QT prolongation and torsades de pointes? Br. J. Pharmacol. 2012, 166, 1490–1502. [Google Scholar] [CrossRef] [Green Version]
- Webb, S.E.; Fluck, R.A.; Miller, A.L. Calcium signaling during the early development of medaka and zebrafish. Biochimie 2011, 93, 2112–2125. [Google Scholar] [CrossRef]
- Hove-Madsen, L.; Llach, A.; Molina, C.E.; Prat-Vidal, C.; Farré, J.; Roura, S.; Cinca, J. The proarrhythmic antihistaminic drug terfenadine increases spontaneous calcium release in human atrial myocytes. Eur. J. Pharmacol. 2006, 553, 215–221. [Google Scholar] [CrossRef]
- Jou, C.J.; Barnett, S.M.; Bian, J.-T.; Weng, H.C.; Sheng, X.; Tristani-Firouzi, M. An in vivo cardiac assay to determine the functional consequences of putative long QT syndrome mutations. Circ. Res. 2013, 112, 826–830. [Google Scholar] [CrossRef] [Green Version]
- Deng, L.; Vysotski, E.S.; Markova, S.V.; Liu, Z.-J.; Lee, J.; Rose, J.; Wang, B.-C. All three Ca2+-binding loops of photoproteins bind calcium ions: The crystal structures of calcium-loaded apo-aequorin and apo-obelin. Protein Sci. Publ. Protein Soc. 2005, 14, 663–675. [Google Scholar] [CrossRef] [Green Version]
- Rizzuto, R.; Pozzan, T. Microdomains of intracellular Ca2+: Molecular determinants and functional consequences. Physiol. Rev. 2006, 86, 369–408. [Google Scholar] [CrossRef] [PubMed]

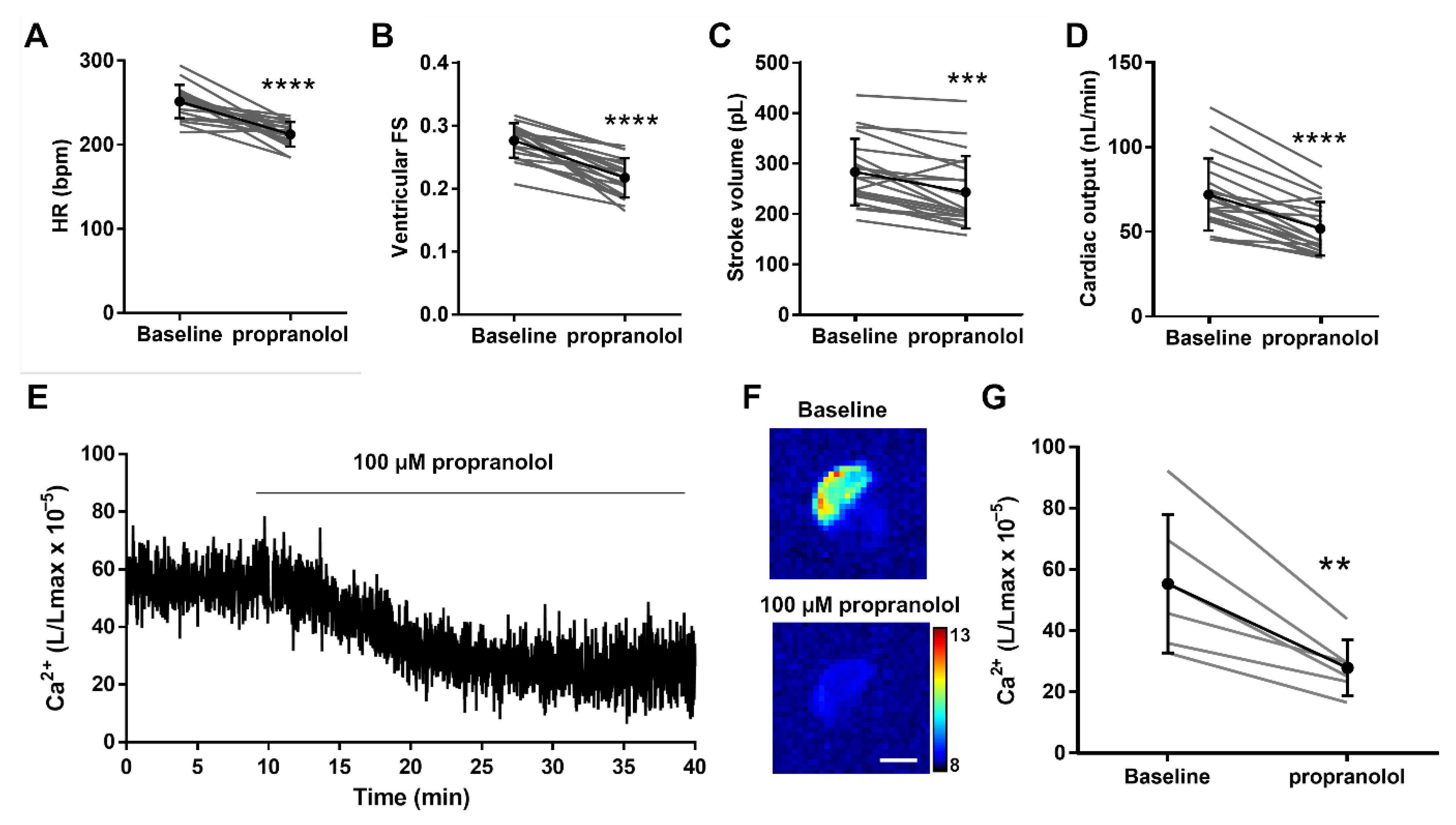
| FS | FS | FAC | EDV | ESV | SV | HR | CO | EF | A/V HR | Aver.Ca | CaT amp | Ca syst | Ca diast | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| major diam. | minor diam. | pL | pL | pL/beat | bpm | nL/min | L/Lmax | L/Lmax | L/Lmax | L/Lmax | |||||
| Control | mean | 0.28 | 0.33 | 0.39 | 420.9 | 137.5 | 283.3 | 251 | 72.0 | 0.67 | 1.00 | 56.8 | n.d. | n.d. | n.d. |
| SD | 0.03 | 0.06 | 0.05 | 89.6 | 39.7 | 66.0 | 20 | 21.3 | 0.07 | 0.00 | 24.5 | ||||
| n | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 6 | ||||
| Propranolol | mean | 0.22 | 0.27 | 0.34 | 420.7 | 177.2 | 243.5 | 212 | 51.8 | 0.58 | 1.00 | 26.1 | n.d. | n.d. | n.d. |
| SD | 0.03 | 0.06 | 0.05 | 94.7 | 45.2 | 71.9 | 15 | 15.8 | 0.08 | 0.00 | 5.7 | ||||
| n | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 6 | ||||
| p value | <0.0001 | <0.0001 | 0.001 | 0.979 | <0.0001 | 0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.0139 | |||||
| Fold change | Propranolol/ control | 0.79 | 0.81 | 0.87 | 1.00 | 1.29 | 0.86 | 0.84 | 0.72 | 0.86 | 1.00 | 0.46 | |||
| Control | mean | 0.36 | 0.37 | 0.39 | 422.1 | 109.3 | 312.8 | 229.9 | 72.4 | 0.74 | 1.00 | 58.9 | 61.0 | 71.6 | 10.6 |
| SD | 0.04 | 0.05 | 0.04 | 115.3 | 39.6 | 84.2 | 19.1 | 21.2 | 0.05 | 0.00 | 17.3 | 28.3 | 36.0 | 12.0 | |
| n | 18 | 18 | 18 | 18 | 18 | 18 | 18 | 18 | 18 | 18 | 10 | 16 | 16 | 16 | |
| Terfenadine | mean | 0.27 | 0.46 | 0.47 | 518.7 | 108.8 | 409.9 | 94.1 | 37.7 | 0.78 | 0.51 | 28.4 | 121.5 | 125.1 | 4.0 |
| SD | 0.06 | 0.05 | 0.08 | 127.6 | 31.7 | 105.7 | 12.9 | 8.7 | 0.06 | 0.11 | 11.8 | 21.4 | 21.1 | 2.4 | |
| n | 19 | 19 | 19 | 19 | 19 | 19 | 19 | 19 | 19 | 19 | 9 | 9 | 9 | 9 | |
| p value | <0.0001 | <0.0001 | 0.0009 | 0.019 | 0.96 | 0.0037 | <0.0001 | <0.0001 | 0.38 | <0.0001 | 0.0004 | <0.0001 | 0.0005 | 0.12 | |
| Fold change | Terfenadine/ control | 0.75 | 1.25 | 1.19 | 1.23 | 1.00 | 1.31 | 0.41 | 0.52 | 1.06 | 0.51 | 0.48 | 1.99 | 1.75 | 0.38 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vicente, M.; Salgado-Almario, J.; Collins, M.M.; Martínez-Sielva, A.; Minoshima, M.; Kikuchi, K.; Domingo, B.; Llopis, J. Cardioluminescence in Transgenic Zebrafish Larvae: A Calcium Imaging Tool to Study Drug Effects and Pathological Modeling. Biomedicines 2021, 9, 1294. https://doi.org/10.3390/biomedicines9101294
Vicente M, Salgado-Almario J, Collins MM, Martínez-Sielva A, Minoshima M, Kikuchi K, Domingo B, Llopis J. Cardioluminescence in Transgenic Zebrafish Larvae: A Calcium Imaging Tool to Study Drug Effects and Pathological Modeling. Biomedicines. 2021; 9(10):1294. https://doi.org/10.3390/biomedicines9101294
Chicago/Turabian StyleVicente, Manuel, Jussep Salgado-Almario, Michelle M. Collins, Antonio Martínez-Sielva, Masafumi Minoshima, Kazuya Kikuchi, Beatriz Domingo, and Juan Llopis. 2021. "Cardioluminescence in Transgenic Zebrafish Larvae: A Calcium Imaging Tool to Study Drug Effects and Pathological Modeling" Biomedicines 9, no. 10: 1294. https://doi.org/10.3390/biomedicines9101294
APA StyleVicente, M., Salgado-Almario, J., Collins, M. M., Martínez-Sielva, A., Minoshima, M., Kikuchi, K., Domingo, B., & Llopis, J. (2021). Cardioluminescence in Transgenic Zebrafish Larvae: A Calcium Imaging Tool to Study Drug Effects and Pathological Modeling. Biomedicines, 9(10), 1294. https://doi.org/10.3390/biomedicines9101294






