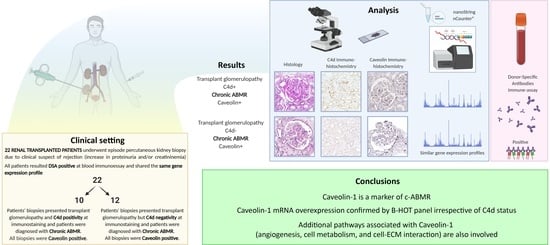
Caveolin-1 in Kidney Chronic Antibody-Mediated Rejection: An Integrated Immunohistochemical and Transcriptomic Analysis Based on the Banff Human Organ Transplant (B-HOT) Gene Panel
Abstract
:1. Introduction
- 1
- Establish Cav-1 as a reliable IHC marker of c-ABMR, irrespectively of C4d status, through transcriptomic profiling.
- 2
- Assess the molecular profile of c-ABMR cases and improve its transcriptomic characterization using the B-HOT-derived nCounter® Human Organ Transplant Panel.
- 3
- Investigate genes related to Cav-1 expression to elucidate its role in c-ABMR.
2. Materials and Methods
2.1. Case Selection and Database Construction
2.2. NanoString® Gene-Expression Profiling
2.3. Statistical Analysis
3. Results
3.1. Clinical and Histopathological Data
3.2. C4d Immunohistochemical Assessment
3.3. Cav-1 Immunohistochemical Assessment
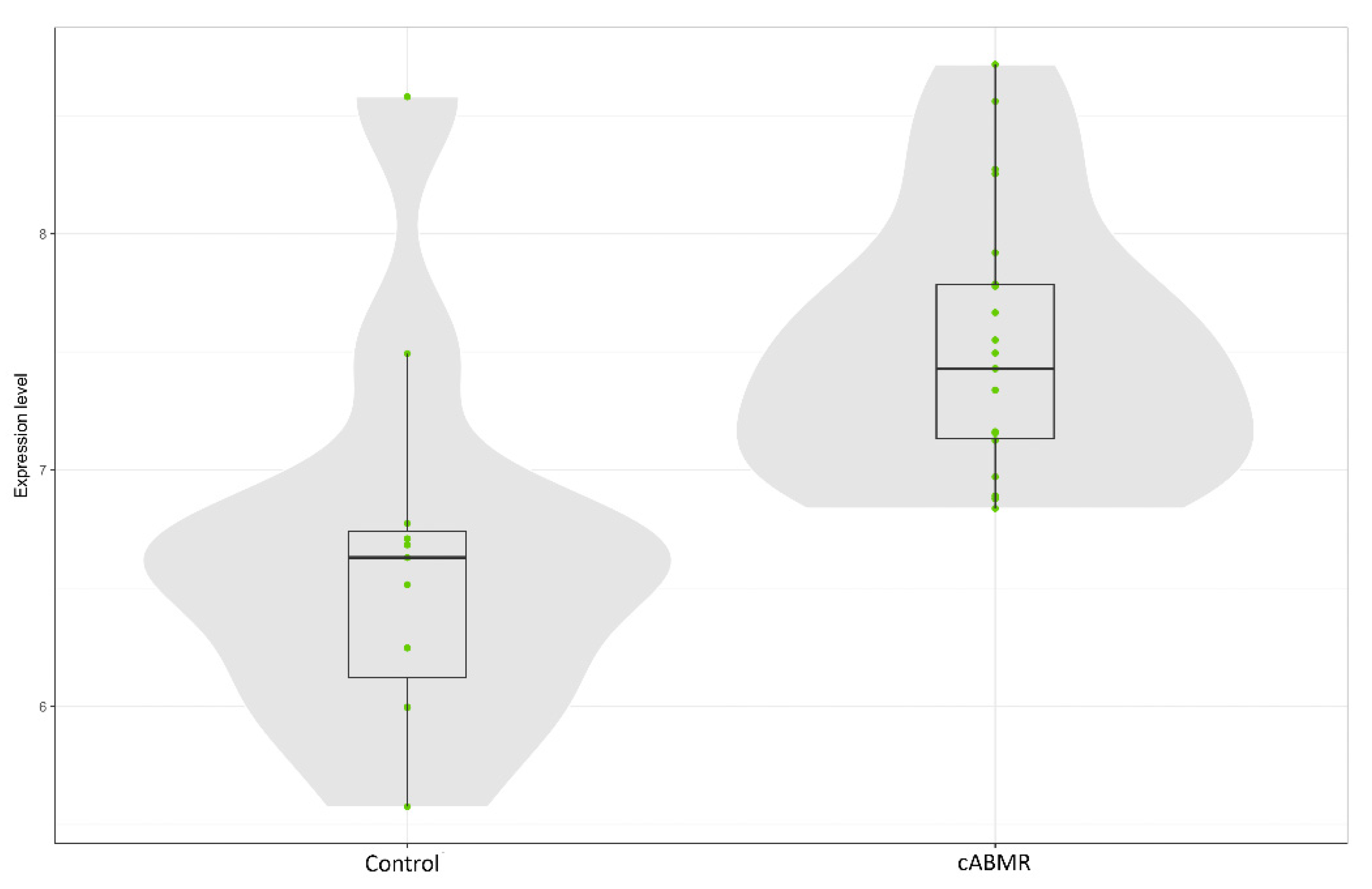
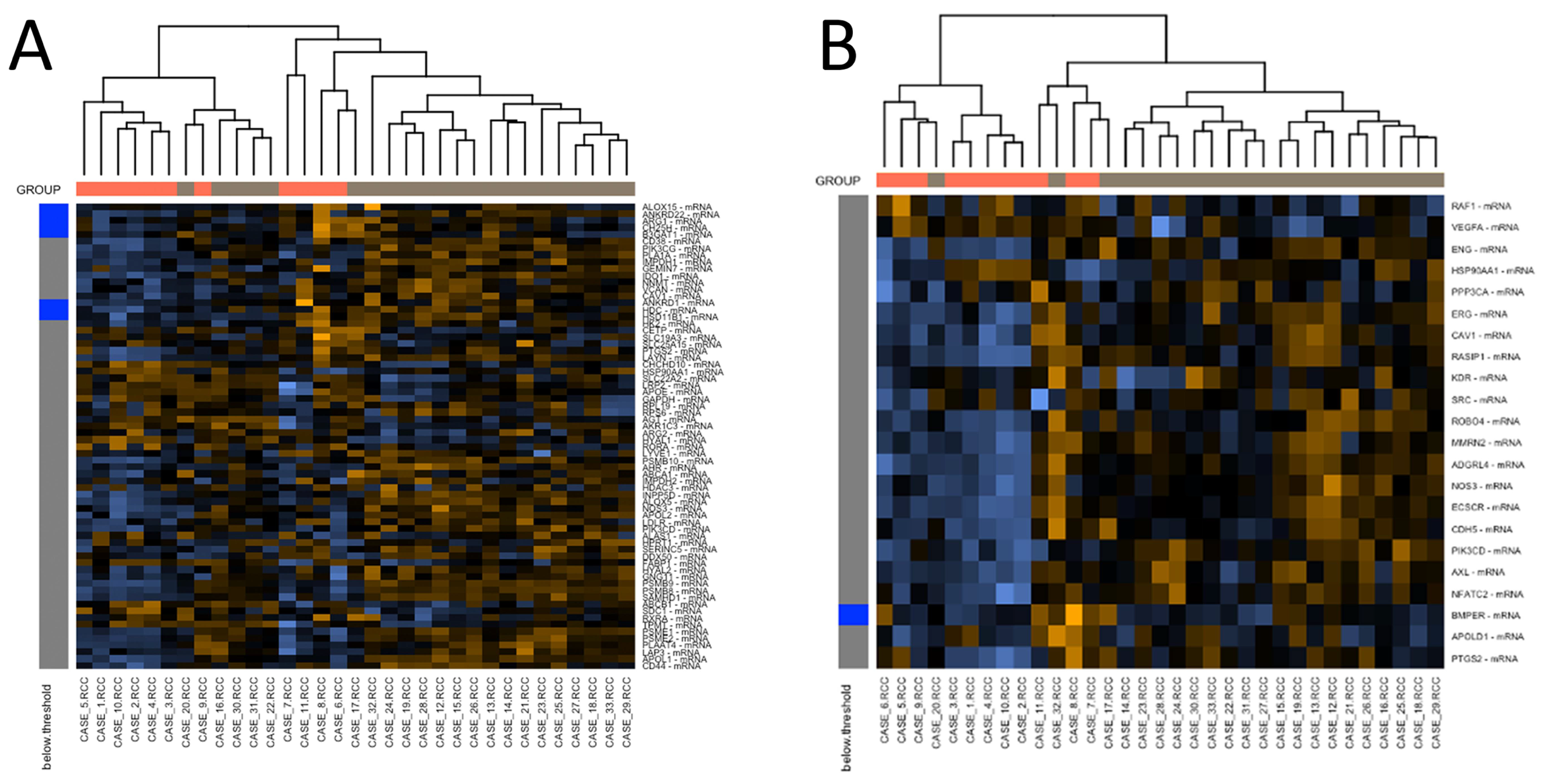
3.4. Gene Expression Profiling: c-ABMR versus Control Group
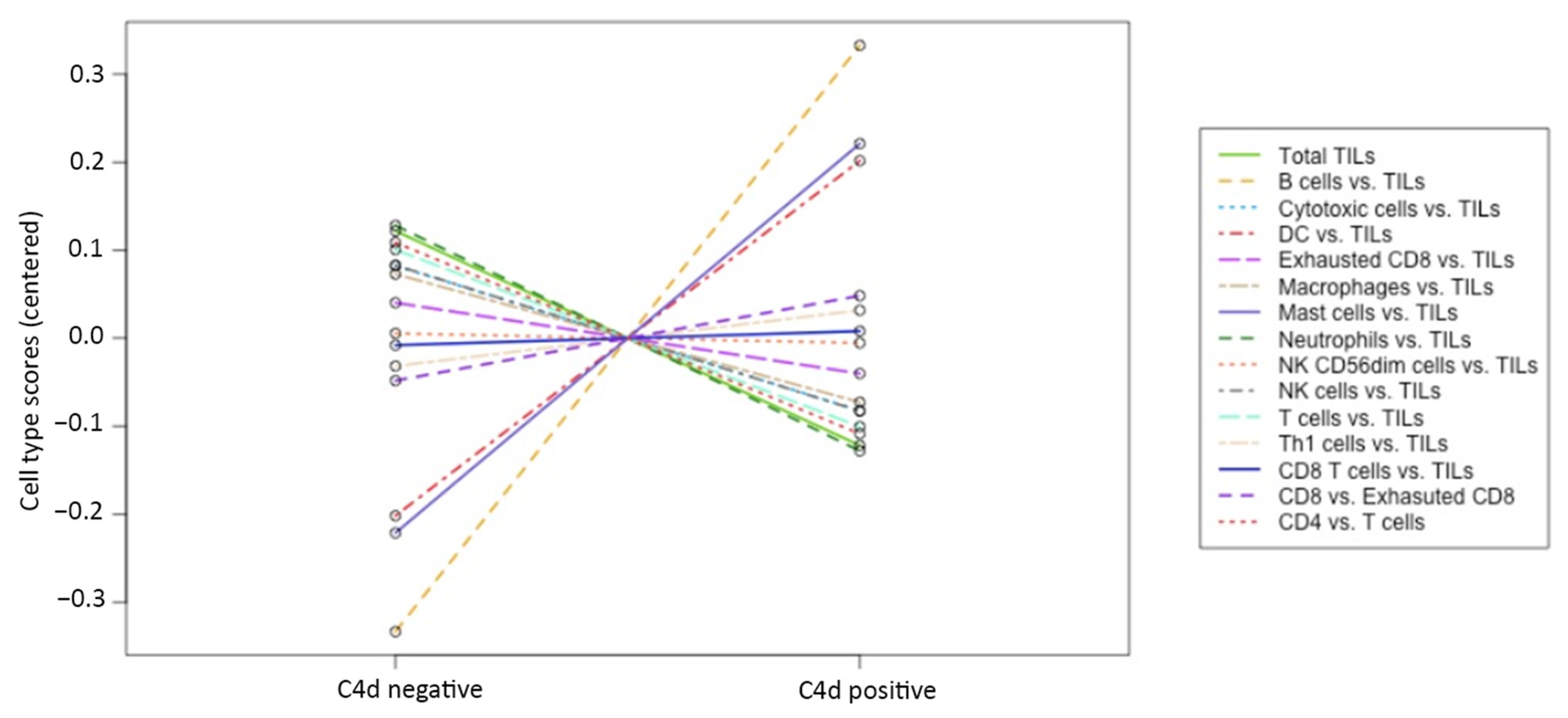
3.5. Gene Expression Profiling: C4d Positive versus C4d Negative c-ABMR Cases
3.6. Gene Expression Profiling: Analysis of Caveolin-1 Expression and Significance in c-ABMR
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saran, R.; Robinson, B.; Abbott, K.C.; Agodoa, L.Y.; Bragg-Gresham, J.; Balkrishnan, R.; Bhave, N.; Dietrich, X.; Ding, Z.; Eggers, P.W.; et al. US renal data system 2018 annual data report: Epidemiology of kidney disease in the United States. Am. J. Kidney Dis. 2019, 73, A7–A8. [Google Scholar] [CrossRef] [Green Version]
- Mayer, K.A.; Doberer, K.; Eskandary, F.; Halloran, P.F.; Böhmig, G.A. New concepts in chronic antibody-mediated kidney allograft rejection: Prevention and treatment. Curr. Opin. Organ Transpl. 2020, 26, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Loupy, A.; Lefaucheur, C. Antibody-mediated rejection of solid-organ allografts. N. Engl. J. Med. 2018, 379, 1150–1160. [Google Scholar] [CrossRef] [PubMed]
- Hart, A.; Singh, D.; Brown, S.J.; Wang, J.H.; Kasiske, B.L. Incidence, risk factors, treatment, and consequences of antibody-mediated kidney transplant rejection: A systematic review. Clin. Transpl. 2021, 35, e14320. [Google Scholar] [CrossRef] [PubMed]
- Hart, A.; Schladt, D.P.; Matas, A.J.; Itzler, R.; Israni, A.K.; Kasiske, B.L. Incidence, risk factors, and long-term outcomes associated with antibody-mediated rejection—The long-term deterioration of kidney allograft function (DeKAF) prospective cohort study. Clin. Transpl. 2021, 35, e14337. [Google Scholar] [CrossRef]
- Loupy, A.; Haas, M.; Roufosse, C.; Naesens, M.; Adam, B.; Afrouzian, M.; Akalin, E.; Alachkar, N.; Bagnasco, S.; Becker, J.U.; et al. The Banff 2019 kidney meeting report (I): Updates on and clarification of criteria for T cell—And antibody-mediated rejection. Am. J. Transpl. 2020, 20, 2318–2331. [Google Scholar] [CrossRef] [PubMed]
- Djamali, A.; Kaufman, D.B.; Ellis, T.M.; Zhong, W.; Matas, A.; Samaniego, M. Diagnosis and management of antibody-mediated rejection: Current status and novel approaches. Am. J. Transpl. 2014, 14, 255–271. [Google Scholar] [CrossRef]
- Haas, M. The relationship between pathologic lesions of active and chronic antibody-mediated rejection in renal allografts. Am. J. Transpl. 2018, 18, 2849–2856. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R. Donor-specific antibodies in kidney transplant recipients. Clin. J. Am. Soc. Nephrol. 2017, 13, 182–192. [Google Scholar] [CrossRef]
- Viklicky, O.; Novotny, M.; Hruba, P. Future developments in kidney transplantation. Curr. Opin. Organ Transpl. 2020, 25, 92–98. [Google Scholar] [CrossRef]
- Feucht, H.E.; Felber, E.; Gokel, M.J.; Hillebrand, G.; Nattermann, U.; Brockmeyer, C.; Held, E.; Riethmüller, G.; Land, W.; Albert, E. Vascular deposition of complement-split products in kidney allografts with cell-mediated rejection. Clin. Exp. Immunol. 1991, 86, 464–470. [Google Scholar] [CrossRef]
- Feucht, H.E.; Schneeberger, H.; Hillebrand, G.; Burkhardt, K.; Weiss, M.; Riethmüller, G.; Land, W.G.; Albert, E. Capillary deposition of C4d complement fragment and early renal graft loss. Kidney Int. 1993, 43, 1333–1338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mauiyyedi, S.; DELLA Pelle, P.; Saidman, S.; Collins, A.B.; Pascual, M.; Tolkoff-Rubin, N.E.; Williams, W.W.; Cosimi, A.B.; Schneeberger, E.E.; Colvin, R.B. Chronic humoral rejection: Identification of antibody-mediated chronic renal allograft rejection by C4d deposits in peritubular capillaries. J. Am. Soc. Nephrol. 2001, 12, 574–582. [Google Scholar] [CrossRef] [PubMed]
- Regele, H.; Bohmig, G.A.; Habicht, A.; Gollowitzer, D.; Schillinger, M.; Rockenschaub, S.; Watschinger, B.; Kerjaschki, D.; Exner, M. Capillary deposition of complement split product C4d in renal allografts is associated with basement membrane injury in peritubular and glomerular capillaries: A contribution of humoral immunity to chronic allograft rejection. J. Am. Soc. Nephrol. 2002, 13, 2371–2380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gloor, J.M.; Sethi, S.; Stegall, M.D.; Park, W.; Moore, S.B.; DeGoey, S.; Griffin, M.; Larson, T.S.; Cosio, F.G. Transplant glomerulopathy: Subclinical incidence and association with alloantibody. Am. J. Transpl. 2007, 7, 2124–2132. [Google Scholar] [CrossRef] [PubMed]
- Sis, B.; Halloran, P. Endothelial transcripts uncover a previously unknown phenotype: C4d-negative antibody-mediated rejection. Curr. Opin. Organ Transpl. 2010, 15, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Haas, M. C4d-negative antibody-mediated rejection in renal allografts: Evidence for its existence and effect on graft survival. Clin. Nephrol. 2011, 75, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Sis, B.; Campbell, P.M.; Mueller, T.; Hunter, C.; Cockfield, S.M.; Cruz, J.; Meng, C.; Wishart, D.S.; Solez, K.; Halloran, P.F. Transplant glomerulopathy, late antibody-mediated rejection and the ABCD tetrad in kidney allograft biopsies for cause. Am. J. Transpl. 2007, 7, 1743–1752. [Google Scholar] [CrossRef]
- Takeda, A.; Otsuka, Y.; Horike, K.; Inaguma, D.; Hiramitsu, T.; Yamamoto, T.; Nanmoku, K.; Goto, N.; Watarai, Y.; Uchida, K.; et al. Significance of C4d deposition in antibody-mediated rejection. Clin. Transpl. 2012, 26, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Gasim, A.H.; Chua, J.S.; Wolterbeek, R.; Schmitz, J.; Weimer, E.; Singh, H.K.; Nickeleit, V. Glomerular C4d deposits can mark structural capillary wall remodelling in thrombotic microangiopathy and transplant glomerulopathy: C4d beyond active antibody-mediated injury: A retrospective study. Transpl. Int. 2017, 30, 519–532. [Google Scholar] [CrossRef] [Green Version]
- Haas, M.; Sis, B.; Racusen, L.C.; Solez, K.; Glotz, D.; Colvin, R.B.; Castro, M.C.; David, D.S.; David-Neto, E.; Bagnasco, S.M.; et al. Banff 2013 meeting report: Inclusion of c4d-negative antibody-mediated rejection and antibody-associated arterial lesions. Am. J. Transpl. 2014, 14, 272–283. [Google Scholar] [CrossRef]
- Cornell, L.D.; Smith, R.N.; Colvin, R.B. Kidney transplantation: Mechanisms of rejection and acceptance. Annu. Rev. Pathol. 2008, 3, 189–220. [Google Scholar] [CrossRef]
- Akiyoshi, T.; Hirohashi, T.; Alessandrini, A.; Chase, C.M.; Farkash, E.A.; Smith, R.N.; Madsen, J.C.; Russell, P.S.; Colvin, R.B. Role of complement and NK cells in antibody mediated rejection. Hum. Immunol. 2012, 73, 1226–1232. [Google Scholar] [CrossRef] [Green Version]
- Haas, M.; Rahman, M.H.; Racusen, L.C.; Kraus, E.S.; Bagnasco, S.M.; Segev, D.L.; Simpkins, C.E.; Warren, D.S.; King, K.E.; Zachary, A.A.; et al. C4d and C3d staining in biopsies of ABO- and HLA-incompatible renal allografts: Correlation with histologic findings. Am. J. Transpl. 2006, 6, 1829–1840. [Google Scholar] [CrossRef]
- Dickenmann, M.; Steiger, J.; Descœudres, B.; Mihatsch, M.; Nickeleit, V. The fate of C4d positive kidney allografts lacking histological signs of acute rejection. Clin. Nephrol. 2006, 65, 173–179. [Google Scholar] [CrossRef]
- Setoguchi, K.; Ishida, H.; Shimmura, H.; Shimizu, T.; Shirakawa, H.; Omoto, K.; Toki, D.; Iida, S.; Setoguchi, S.; Tokumoto, T.; et al. Analysis of renal transplant protocol biopsies in ABO-incompatible kidney transplantation. Am. J. Transpl. 2008, 8, 86–94. [Google Scholar] [CrossRef]
- Mengel, M.; Loupy, A.; Haas, M.; Roufosse, C.; Naesens, M.; Akalin, E.; Clahsen-van Groningen, M.C.; Dagobert, J.; Demetris, A.J.; Duong van Huyen, J.P.; et al. Banff 2019 meeting report: Molecular diagnostics in solid organ transplantation-consensus for the Banff human organ transplant (B-HOT) gene panel and open source multicenter validation. Am. J. Transpl. 2020, 20, 2305–2317. [Google Scholar] [CrossRef]
- Yamamoto, I.; Horita, S.; Takahashi, T.; Kobayashi, A.; Toki, D.; Tanabe, K.; Hattori, M.; Teraoka, S.; Aita, K.; Nagata, M.; et al. Caveolin-1 expression is a distinct feature of chronic rejection-induced transplant capillaropathy. Am. J. Transpl. 2008, 8, 2627–2635. [Google Scholar] [CrossRef] [PubMed]
- Palanisamy, A.; Reeves-Daniel, A.M.; Freedman, B.I. The impact of APOL1, CAV1, and ABCB1 gene variants on outcomes in kidney transplantation: Donor and recipient effects. Pediatr. Nephrol. 2013, 29, 1485–1492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakada, Y.; Yamamoto, I.; Horita, S.; Kobayashi, A.; Mafune, A.; Katsumata, H.; Yamakawa, T.; Katsuma, A.; Kawabe, M.; Tanno, Y.; et al. The prognostic values of caveolin-1 immunoreactivity in peritubular capillaries in patients with kidney transplantation. Clin. Transpl. 2016, 30, 1417–1424. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, K.; Oka, K.; Imanaka, T.; Taniguchi, A.; Nakazawa, S.; Yoshida, T.; Kishikawa, H.; Nishimura, K. Immunoenzymatic staining of caveolin-1 in formalin-fixed renal graft showing chronic antibody mediated rejection. Transpl. Proc. 2019, 51, 1387–1391. [Google Scholar] [CrossRef]
- Teixeira, A.C.; Távora, F.; Silva, M.L.F.D.D.E.; Prado, R.M.G.; Esmeraldo, R.D.M.; de Sandes-Freitas, T.V. The immunohistochemical expression of von Willebrand factor, T-cadherin, and caveolin-1 is increased in kidney allograft biopsies with antibody-mediated injury. Clin. Exp. Nephrol. 2020, 25, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.; McKnight, A.J.; Simmonds, M.J.; Courtney, A.E.; Hanvesakul, R.; Brand, O.J.; Briggs, D.; Ball, S.; Cockwell, P.; Patterson, C.C.; et al. Association of caveolin-1 gene polymorphism with kidney transplant fibrosis and allograft failure. JAMA 2010, 303, 1282–1287. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, I.; Horita, S.; Takahashi, T.; Tanabe, K.; Fuchinoue, S.; Teraoka, S.; Hattori, M.; Yamaguchi, Y. Glomerular expression of plasmalemmal vesicle-associated protein-1 in patients with transplant glomerulopathy. Am. J. Transpl. 2007, 7, 1954–1960. [Google Scholar] [CrossRef] [PubMed]
- Moriyama, T.; Tsuruta, Y.; Shimizu, A.; Itabashi, M.; Takei, T.; Horita, S.; Uchida, K.; Nitta, K. The significance of caveolae in the glomeruli in glomerular disease. J. Clin. Pathol. 2011, 64, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Haas, M.; Loupy, A.; Lefaucheur, C.; Roufosse, C.; Glotz, D.; Seron, D.; Nankivell, B.J.; Halloran, P.F.; Colvin, R.B.; Akalin, E.; et al. The Banff 2017 kidney meeting report: Revised diagnostic criteria for chronic active T cell-mediated rejection, antibody-mediated rejection, and prospects for integrative endpoints for next-generation clinical trials. Am. J. Transpl. 2018, 18, 293–307. [Google Scholar] [CrossRef] [Green Version]
- Bukosza, E.N.; Kratochwill, K.; Kornauth, C.; Schachner, H.; Aufricht, C.; Gebeshuber, C.A. Podocyte RNA sequencing reveals Wnt- and ECM-associated genes as central in FSGS. PLoS ONE 2020, 15, e0231898. [Google Scholar] [CrossRef]
- Dal Canton, A.; Fuiano, G.; Sepe, V.; Caglioti, A.; Ferrone, S. Mesangial expression of intercellular adhesion molecule-1 in primary glomerulosclerosis. Kidney Int. 1992, 41, 951–955. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Kim, B.K.; Moon, K.C.; Hong, H.K.; Lee, H.S. Activation of the TGF-beta/Smad signaling pathway in focal segmental glomerulosclerosis. Kidney Int. 2003, 64, 1715–1721. [Google Scholar] [CrossRef] [Green Version]
- Reidy, K.; Kaskel, F.J. Pathophysiology of focal segmental glomerulosclerosis. Pediatr. Nephrol. 2007, 22, 350–354. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Zeng, C.; Fu, Y.; Cheng, Z.; Zhang, J.; Liu, Z. Biomarkers of endothelial dysfunction in patients with primary focal segmental glomerulosclerosis. Nephrology 2012, 17, 338–345. [Google Scholar] [CrossRef]
- Zhong, J.; Whitman, J.B.; Yang, H.C.; Fogo, A.B. Mechanisms of scarring in focal segmental glomerulosclerosis. J. Histochem. Cytochem. 2019, 67, 623–632. [Google Scholar] [CrossRef]
- Venner, J.M.; Hidalgo, L.G.; Famulski, K.S.; Chang, J.; Halloran, P.F. The molecular landscape of antibody-mediated kidney transplant rejection: Evidence for NK involvement through CD16a Fc receptors. Am. J. Transpl. 2015, 15, 1336–1348. [Google Scholar] [CrossRef] [Green Version]
- Hidalgo, L.G.; Sis, B.; Sellares, J.; Campbell, P.M.; Mengel, M.; Einecke, G.; Chang, J.; Halloran, P.F. NK cell transcripts and NK cells in kidney biopsies from patients with donor-specific antibodies: Evidence for NK cell involvement in antibody-mediated rejection. Am. J. Transpl. 2010, 10, 1812–1822. [Google Scholar] [CrossRef]
- Hidalgo, L.G.; Sellares, J.; Sis, B.; Mengel, M.; Chang, J.; Halloran, P.F. Interpreting NK cell transcripts versus T cell transcripts in renal transplant biopsies. Am. J. Transpl. 2012, 12, 1180–1191. [Google Scholar] [CrossRef] [PubMed]
- Garcia, E.; Li, M. Caveolin-1 immunohistochemical analysis in differentiating chromophobe renal cell carcinoma from renal oncocytoma. Am. J. Clin. Pathol. 2006, 125, 392–398. [Google Scholar] [CrossRef]
- Ostalska-Nowicka, D.; Nowicki, M.; Zachwieja, J.; Kasper, M.; Witt, M. The significance of caveolin-1 expression in parietal epithelial cells of Bowman’s capsule. Histopathology 2007, 51, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Sorensson, J.; Fierlbeck, W.; Heider, T.; Schwarz, K.; Park, D.S.; Mundel, P.; Lisanti, M.; Ballermann, B.J. Glomerular endothelial fenestrae in vivo are not formed from caveolae. J. Am. Soc. Nephrol. 2002, 13, 2639–2647. [Google Scholar] [CrossRef] [Green Version]
- Kanzaki, G.; Shimizu, A. Currently available useful immunohistochemical markers of renal pathology for the diagnosis of renal allograft rejection. Nephrology 2015, 20 (Suppl. 2), 9–15. [Google Scholar] [CrossRef] [PubMed]
- Van der Hauwaert, C.; Savary, G.; Pincon, C.; Gnemmi, V.; Noel, C.; Broly, F.; Labalette, M.; Perrais, M.; Pottier, N.; Glowacki, F.; et al. Donor caveolin 1 (CAV1) genetic polymorphism influences graft function after renal transplantation. Fibrogenesis Tissue Repair 2015, 8, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chand, S.; Edwards, N.C.; Chue, C.D.; Jesky, M.; Stringer, S.; Simmonds, M.J.; Duff, C.E.; Cockwell, P.; Harper, L.; Steeds, R.P.; et al. Caveolin-1 single-nucleotide polymorphism and arterial stiffness in non-dialysis chronic kidney disease. Nephrol. Dial. Transpl. 2016, 31, 1140–1144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sis, B.; Jhangri, G.S.; Bunnag, S.; Allanach, K.; Kaplan, B.; Halloran, P.F. Endothelial gene expression in kidney transplants with alloantibody indicates antibody-mediated damage despite lack of C4d staining. Am. J. Transpl. 2009, 9, 2312–2323. [Google Scholar] [CrossRef] [PubMed]
- Adam, B.; Afzali, B.; Dominy, K.M.; Chapman, E.; Gill, R.; Hidalgo, L.G.; Roufosse, C.; Sis, B.; Mengel, M. Multiplexed color-coded probe-based gene expression assessment for clinical molecular diagnostics in formalin-fixed paraffin-embedded human renal allograft tissue. Clin. Transpl. 2016, 30, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Dominy, K.M.; Willicombe, M.; Al Johani, T.; Beckwith, H.; Goodall, D.; Brookes, P.; Cook, H.T.; Cairns, T.; McLean, A.; Roufosse, C. Molecular assessment of C4d-positive renal transplant biopsies without evidence of rejection. Kidney Int. Rep. 2019, 4, 148–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gavriatopoulou, M.; Ntanasis-Stathopoulos, I.; Dimopoulos, M.A.; Terpos, E. Anti-BCMA antibodies in the future management of multiple myeloma. Expert Rev. Anticancer Ther. 2019, 19, 319–326. [Google Scholar] [CrossRef]
- Cho, S.F.; Anderson, K.C.; Tai, Y.T. Targeting B Cell Maturation Antigen (BCMA) in multiple myeloma: Potential uses of BCMA-based immunotherapy. Front. Immunol. 2018, 9, 1821. [Google Scholar] [CrossRef] [PubMed]
- Tai, Y.T.; Anderson, K.C. Targeting B-cell maturation antigen in multiple myeloma. Immunotherapy 2015, 7, 1187–1199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgan, G.J.; Williams, L. Antibody-based targeting of BCMA in multiple myeloma. Lancet Oncol. 2020, 21, 186–187. [Google Scholar] [CrossRef]
- Kroczek, R.A.; Henn, V. The role of XCR1 and its ligand XCL1 in antigen cross-presentation by murine and human dendritic cells. Front. Immunol. 2012, 3, 14. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Sharp, J.S.; Handel, T.M.; Prestegard, J.H. Chemokine oligomerization in cell signaling and migration. Prog. Mol. Biol. Transl. Sci. 2013, 117, 531–578. [Google Scholar] [CrossRef] [Green Version]
- Madill-Thomsen, K.; Perkowska-Ptasinska, A.; Bohmig, G.A.; Eskandary, F.; Einecke, G.; Gupta, G.; Halloran, P.F.; Group, M.M.-K.S. Discrepancy analysis comparing molecular and histology diagnoses in kidney transplant biopsies. Am. J. Transpl. 2020, 20, 1341–1350. [Google Scholar] [CrossRef] [PubMed]
- Patey-Mariaud de Serre, N. Méthodes d’études d’une biopsie rénale. In Atlas de Pathologie Rénale; Flammarion: Paris, France, 2008. [Google Scholar]
- Nietner, T.; Jarutat, T.; Mertens, A. Systematic comparison of tissue fixation with alternative fixatives to conventional tissue fixation with buffered formalin in a xenograft-based model. Virchows Arch. 2012, 461, 259–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mella, A.; Gallo, E.; Messina, M.; Caorsi, C.; Amoroso, A.; Gontero, P.; Verri, A.; Maletta, F.; Barreca, A.; Fop, F.; et al. Treatment with plasmapheresis, immunoglobulins and rituximab for chronic-active antibody-mediated rejection in kidney transplantation: Clinical, immunological and pathological results. World J. Transpl. 2018, 8, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Lavacca, A.; Presta, R.; Gai, C.; Mella, A.; Gallo, E.; Camussi, G.; Abbasciano, I.; Barreca, A.; Caorsi, C.; Fop, F.; et al. Early effects of first-line treatment with anti-interleukin-6 receptor antibody tocilizumab for chronic active antibody-mediated rejection in kidney transplantation. Clin. Transpl. 2020, 34, e13908. [Google Scholar] [CrossRef] [PubMed]
- Gallo, E.; Abbasciano, I.; Mingozzi, S.; Lavacca, A.; Presta, R.; Bruno, S.; Deambrosis, I.; Barreca, A.; Romagnoli, R.; Mella, A.; et al. Prevention of acute rejection after rescue with belatacept by association of low-dose tacrolimus maintenance in medically complex kidney transplant recipients with early or late graft dysfunction. PLoS ONE 2020, 15, e0240335. [Google Scholar] [CrossRef]
- Zanotto, E.; Allesina, A.; Barreca, A.; Sidoti, F.; Gallo, E.; Bottino, P.; Iannaccone, M.; Bianco, G.; Biancone, L.; Cavallo, R.; et al. Renal allograft biopsies with polyomavirus BK nephropathy: Turin transplant center, 2015–2019. Viruses 2020, 12, 1047. [Google Scholar] [CrossRef]
- Dotti, I.; Bonin, S.; Basili, G.; Nardon, E.; Balani, A.; Siracusano, S.; Zanconati, F.; Palmisano, S.; De Manzini, N.; Stanta, G. Effects of formalin, methacarn, and fineFIX fixatives on RNA preservation. Diagn. Mol. Pathol. 2010, 19, 112–122. [Google Scholar] [CrossRef]
- Masuda, N.; Ohnishi, T.; Kawamoto, S.; Monden, M.; Okubo, K. Analysis of chemical modification of RNA from formalin-fixed samples and optimization of molecular biology applications for such samples. Nucleic Acids Res. 1999, 27, 4436–4443. [Google Scholar] [CrossRef]
- Perry, C.; Chung, J.Y.; Ylaya, K.; Choi, C.H.; Simpson, A.; Matsumoto, K.T.; Smith, W.A.; Hewitt, S.M. A buffered alcohol-based fixative for histomorphologic and molecular applications. J. Histochem. Cytochem. 2016, 64, 425–440. [Google Scholar] [CrossRef] [Green Version]




| Cav-1 Expression | Percentage of Positive Peritubular Capillaries or Glomeruli | Score |
|---|---|---|
| Negative | 0 | Grade 0 |
| Minimal | 1—<10 | Grade I |
| Focal | 10—50 | Grade II |
| Diffuse | >50 | Grade III |
| Characteristics | c-ABMR (n = 22) | Control Group (n = 11) | p-Value | |
|---|---|---|---|---|
| Gender | Female | 7 | 6 | 0.208 |
| Male | 15 | 5 | ||
| Age at diagnosis | Median (interval) | 54 (17–70) | 49 (31–69) | 0.902 |
| Native disease | Idiopathic CKD | 5 | 3 | 0.964 |
| Immune-mediated glomerulonephritis | 5 | 3 | ||
| APKD | 4 | 1 | ||
| Urinary tract malformation | 4 | 1 | ||
| Idiopathic chronic glomerular disease | 2 | 1 | ||
| Alport syndrome | 1 | 1 | ||
| Other | 1 | 1 | ||
| Donor type | Deceased | 17 | 11 | 0.086 |
| Living | 5 | 0 | ||
| Transplanted kidney | Single | 22 | 10 | 0.151 |
| Double | 0 | 1 | ||
| Re-transplantation | No | 19 | 11 | 0.199 |
| Yes | 3 | 0 | ||
| Comorbidities | No | 8 | 2 | 0.284 |
| Yes | 14 | 9 | ||
| Comorbidities type | None | 8 | 2 | 0.077 |
| Hypertension | 6 | 2 | ||
| Hypertension and metabolic disorders | 1 | 3 | ||
| Hypertension and other causes | 1 | 3 | ||
| Other | 6 | 1 | ||
| Treatment | Single-immunosuppressant therapy | 7 | 3 | 0.106 |
| Double-immunosuppressant therapy | 9 | 8 | ||
| Triple-immunosuppressant therapy | 6 | 0 | ||
| DSA | Not applicable | 0 | 11 | - |
| Class I | 8 | 0 | ||
| Class II | 12 | 0 | ||
| Both | 2 | 0 | ||
| PTO (g/24 h) | Median (interval) | 0.875 (0.2–8) | 0.12 (0–0.46) | 0.026 |
| Crs (mg/dL) | Median (interval) | 2.55 (1.25–4.6) | 1.49 (0.71–3.67) | 0.013 |
| Focal segmental glomerulosclerosis (FSGS) | Not applicable | 0 | 11 | - |
| No | 12 | 0 | ||
| Yes | 10 | 0 | ||
| Active ABMR (g and/or ptc) | Not applicable | 0 | 11 | - |
| No | 8 | 0 | ||
| Yes | 14 | 0 | ||
| Glomerulitis score (g) | Not applicable | 0 | 11 | - |
| 0 | 11 | 0 | ||
| 1 | 6 | 0 | ||
| 2 | 4 | 0 | ||
| 3 | 1 | 0 | ||
| Peritubular capillaritis score (ptc) | Not applicable | 0 | 11 | - |
| 0 | 9 | 0 | ||
| 1 | 2 | 0 | ||
| 2 | 10 | 0 | ||
| 3 | 1 | 0 | ||
| Transplant glomerulopathy score (TG) | Not applicable | 0 | 11 | - |
| 0 | 0 | 0 | ||
| 1 | 3 | 0 | ||
| 2 | 6 | 0 | ||
| 3 | 13 | 0 | ||
| C4d score | Not applicable | 0 | 11 | - |
| 0 | 12 | 0 | ||
| 1 | 2 | 0 | ||
| 2 | 2 | 0 | ||
| 3 | 6 | 0 | ||
| Graft failure | No | 19 | 11 | 0.199 |
| Yes | 3 | 0 | ||
| Median follow-up | (25th–75th) | 9.9 (6.4–13.3) | 4.6 (1.9–5.6) | - |
| Median rejection time | (25th–75th) | 5.6 (3.6–9.8) | Not applicable | - |
| C4d Negative (n = 12) | C4d Positive (n = 10) | p-Value (*: Log Rank Test) | ||
|---|---|---|---|---|
| Gender | Female | 5 | 2 | 0.277 |
| Male | 7 | 8 | ||
| Age at diagnosis | Median (interval) | 53 (18–70) | 55 (17–69) | 0.669 |
| Native disease | Idiopathic CKD | 3 | 2 | 0.247 |
| Immune-mediated glomerulonephritis | 1 | 4 | ||
| APKD | 4 | 0 | ||
| Urinary tract malformation | 2 | 2 | ||
| Idiopathic chronic glomerular disease | 1 | 1 | ||
| Alport syndrome | 0 | 1 | ||
| Other | 1 | 0 | ||
| Donor type | Deceased | 10 | 7 | 0.457 |
| Living | 2 | 3 | ||
| Re-transplantation | No | 9 | 10 | 0.089 |
| Yes | 3 | 0 | ||
| Comorbidities | No | 4 | 4 | 0.746 |
| Yes | 8 | 6 | ||
| Comorbidities type | None | 4 | 4 | 0.529 |
| Hypertension | 4 | 2 | ||
| Hypertension and metabolic disorders | 0 | 1 | ||
| Hypertension and other causes | 0 | 1 | ||
| Other | 4 | 2 | ||
| Treatment | Single-immunosuppressant therapy | 3 | 4 | 0.616 |
| Double-immunosuppressant therapy | 6 | 3 | ||
| Triple-immunosuppressant therapy | 3 | 3 | ||
| DSA | Class I | 6 | 2 | 0.146 |
| Class II | 6 | 6 | ||
| Both | 0 | 2 | ||
| PTO (g/24 h) | Median (interval) | 0.58 (0.2–8) | 1.22 (0.5–5.91) | 0.468 |
| Crs (mg/dL) | Median (interval) | 2.85 (1.25–3.4) | 2.1 (1.6–4.6) | 0.905 |
| Focal segmental glomerulosclerosis (FSGS) | No | 7 | 5 | 0.696 |
| Yes | 5 | 5 | ||
| Active ABMR (g and/or ptc) | No | 4 | 4 | 0.746 |
| Yes | 8 | 6 | ||
| Glomerulitis score (g) | 0 | 5 | 6 | 0.458 |
| 1 | 4 | 2 | ||
| 2 | 3 | 1 | ||
| 3 | 0 | 1 | ||
| Peritubular capillaritis score (ptc) | 0 | 4 | 5 | 0.340 |
| 1 | 2 | 0 | ||
| 2 | 6 | 4 | ||
| 3 | 0 | 1 | ||
| Transplant glomerulopathy score (TG) | 1 | 3 | 0 | 0.122 |
| 2 | 4 | 2 | ||
| 3 | 5 | 8 | ||
| Graft failure | No | 9 | 10 | 0.089 |
| Yes | 3 | 0 | ||
| Median follow-up | (25th–75th) | 7.4 (5.6–12.6) | 10.4 (8.7–21.3) | 0.061 * |
| Median rejection time | (25th–75th) | 4.3 (2.2–6.1) | 8.5 (5.6–20.4) | 0.023 * |
| c-ABMR (n = 22) | Control Group (n = 11) | p-Value | |||
|---|---|---|---|---|---|
| Cav-1 peritubular capillaries expression score | Expression | Negative | 0 | 11 | <0.0001 |
| Positive | 22 | 0 | |||
| Score | Grade 0 | 0 | 11 | - | |
| Grade I | 0 | 0 | |||
| Grade II | 2 | 0 | |||
| Grade III | 20 | 0 | |||
| Cav-1 glomerular capillaries expression score | Expression | Negative | 0 | 11 | <0.0001 |
| Positive | 22 | 0 | |||
| Score | Grade 0 | 0 | 11 | - | |
| Grade I | 4 | 0 | |||
| Grade II | 11 | 0 | |||
| Grade III | 7 | 0 | |||
| C4d Negative (n = 12) | C4d Positive (n = 10) | p-Value | |||
|---|---|---|---|---|---|
| Cav-1 peritubular capillaries expression score | Expression | Negative | 0 | 0 | - |
| Positive | 12 | 10 | |||
| Score | Grade I | 0 | 0 | 0.176 | |
| Grade II | 2 | 0 | |||
| Grade III | 10 | 10 | |||
| Cav-1 glomerular capillaries expression score | Expression | Negative | 0 | 0 | - |
| Positive | 12 | 10 | |||
| Score | Grade I | 3 | 1 | 0.035 | |
| Grade II | 8 | 3 | |||
| Grade III | 1 | 6 |
| Annotated Pathways | Total Number of Genes Analyzed | Total Number of Overexpressed Genes (Percentage) | # OGs (p < 0.01) | # OGs (p < 0.05) |
|---|---|---|---|---|
| Adaptive Immune System | 127 | 52 (40.9) | 48 | 4 |
| Angiogenesis | 22 | 12 (54.5) | 10 | 2 |
| Apoptosis and Cell Cycle Regulation | 52 | 23 (44.2) | 17 | 6 |
| Autophagy | 17 | 3 (17.6) | 1 | 2 |
| B-cell Receptor Signaling | 45 | 24 (53.3) | 21 | 3 |
| Cell–ECM Interaction | 101 | 39 (38.6) | 34 | 5 |
| Chemokine Signaling | 57 | 19 (33.3) | 16 | 3 |
| Complement System | 31 | 12 (38.7) | 8 | 4 |
| Cytokine Signaling | 98 | 15 (15.3) | 13 | 2 |
| Cytosolic DNA Sensing | 19 | 5 (26.3) | 3 | 2 |
| Cytotoxicity | 54 | 21 (38.9) | 19 | 2 |
| Epigenetics and Transcription | 16 | 1 (6.2) | 0 | 1 |
| Hematopoiesis | 204 | 62 (30.4) | 43 | 19 |
| Inflammasomes | 11 | 4 (36.4) | 2 | 2 |
| Innate Immune System | 165 | 59 (35.7) | 46 | 13 |
| Lymphocyte Trafficking | 21 | 11 (52.4) | 10 | 1 |
| MAPK | 63 | 15 (23.8) | 7 | 8 |
| Metabolism | 68 | 22 (32.3) | 18 | 4 |
| MHC Class I Antigen Presentation | 33 | 19 (57.5) | 18 | 1 |
| MHC Class II Antigen Presentation | 14 | 11 (78.6) | 11 | 0 |
| mTOR | 14 | 3 (21.4) | 1 | 2 |
| NF-kappa B Signaling | 56 | 20 (35.7) | 17 | 3 |
| NLR Signaling | 54 | 18 (33.3) | 14 | 4 |
| Oxidative Stress | 62 | 15 (24.2) | 10 | 5 |
| T-cell Checkpoint Signaling | 28 | 4 (14.3) | 4 | 0 |
| T-cell Receptor Signaling | 66 | 28 (42.4) | 25 | 3 |
| TGF-beta Signaling | 30 | 6 (20.0) | 4 | 2 |
| Th1 Differentiation | 16 | 6 (37.5) | 4 | 2 |
| Th17 Differentiation | 39 | 8 (20.5) | 5 | 3 |
| Th17 Mediated Biology | 39 | 9 (23.1) | 7 | 2 |
| Th2 Differentiation | 17 | 5 (29.4) | 4 | 1 |
| Tissue Homeostasis | 39 | 8 (20.5) | 3 | 5 |
| TNF Family Signaling | 61 | 19 (31.1) | 13 | 6 |
| Toll-like Receptor Signaling | 70 | 21 (30.0) | 17 | 4 |
| Treg Differentiation | 13 | 5 (38.5) | 3 | 2 |
| Type I Interferon Signaling | 39 | 19 (48.7) | 16 | 3 |
| Type II Interferon Signaling | 44 | 28 (63.6) | 26 | 2 |
| Viral Detection | 4 | 0 (0) | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gambella, A.; Barreca, A.; Osella-Abate, S.; Bottasso, E.; Giarin, M.M.; Papotti, M.; Biancone, L.; Metovic, J.; Collemi, G.; Cassoni, P.; et al. Caveolin-1 in Kidney Chronic Antibody-Mediated Rejection: An Integrated Immunohistochemical and Transcriptomic Analysis Based on the Banff Human Organ Transplant (B-HOT) Gene Panel. Biomedicines 2021, 9, 1318. https://doi.org/10.3390/biomedicines9101318
Gambella A, Barreca A, Osella-Abate S, Bottasso E, Giarin MM, Papotti M, Biancone L, Metovic J, Collemi G, Cassoni P, et al. Caveolin-1 in Kidney Chronic Antibody-Mediated Rejection: An Integrated Immunohistochemical and Transcriptomic Analysis Based on the Banff Human Organ Transplant (B-HOT) Gene Panel. Biomedicines. 2021; 9(10):1318. https://doi.org/10.3390/biomedicines9101318
Chicago/Turabian StyleGambella, Alessandro, Antonella Barreca, Simona Osella-Abate, Emanuel Bottasso, Manuela Maria Giarin, Mauro Papotti, Luigi Biancone, Jasna Metovic, Giammarco Collemi, Paola Cassoni, and et al. 2021. "Caveolin-1 in Kidney Chronic Antibody-Mediated Rejection: An Integrated Immunohistochemical and Transcriptomic Analysis Based on the Banff Human Organ Transplant (B-HOT) Gene Panel" Biomedicines 9, no. 10: 1318. https://doi.org/10.3390/biomedicines9101318
APA StyleGambella, A., Barreca, A., Osella-Abate, S., Bottasso, E., Giarin, M. M., Papotti, M., Biancone, L., Metovic, J., Collemi, G., Cassoni, P., & Bertero, L. (2021). Caveolin-1 in Kidney Chronic Antibody-Mediated Rejection: An Integrated Immunohistochemical and Transcriptomic Analysis Based on the Banff Human Organ Transplant (B-HOT) Gene Panel. Biomedicines, 9(10), 1318. https://doi.org/10.3390/biomedicines9101318