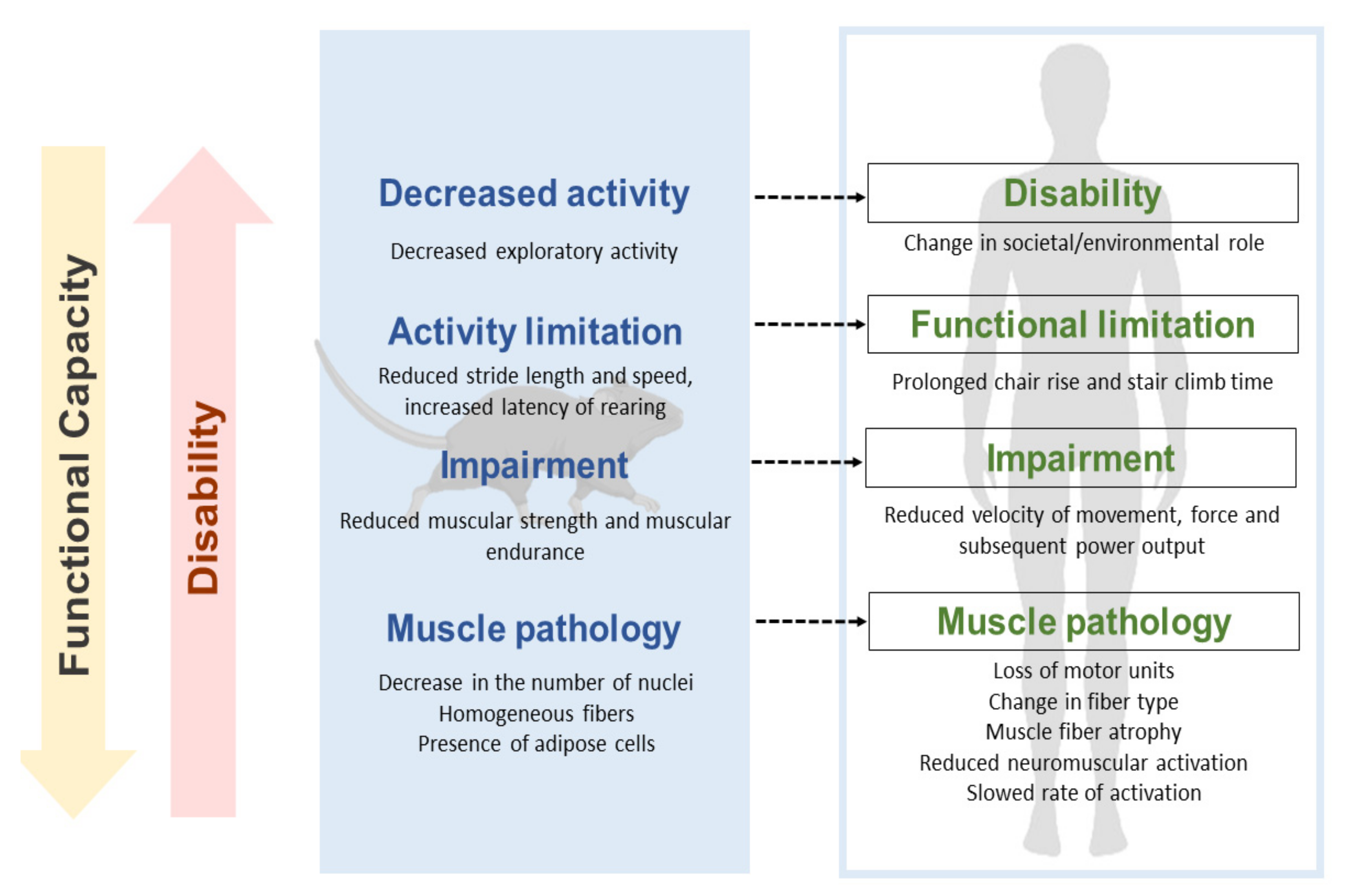
Modeling Functional Limitations, Gait Impairments, and Muscle Pathology in Alzheimer’s Disease: Studies in the 3xTg-AD Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Design
2.3. Behavioral Assessments
2.3.1. Activity—Spontaneous Gait and Exploration
2.3.2. Body Function—Mobility and Muscular Strength
2.3.3. Body Structure—Joints and Muscles
2.3.4. Motor Performance, Geotaxis, and Hindlimb Clasping
2.4. Statistics
3. Results
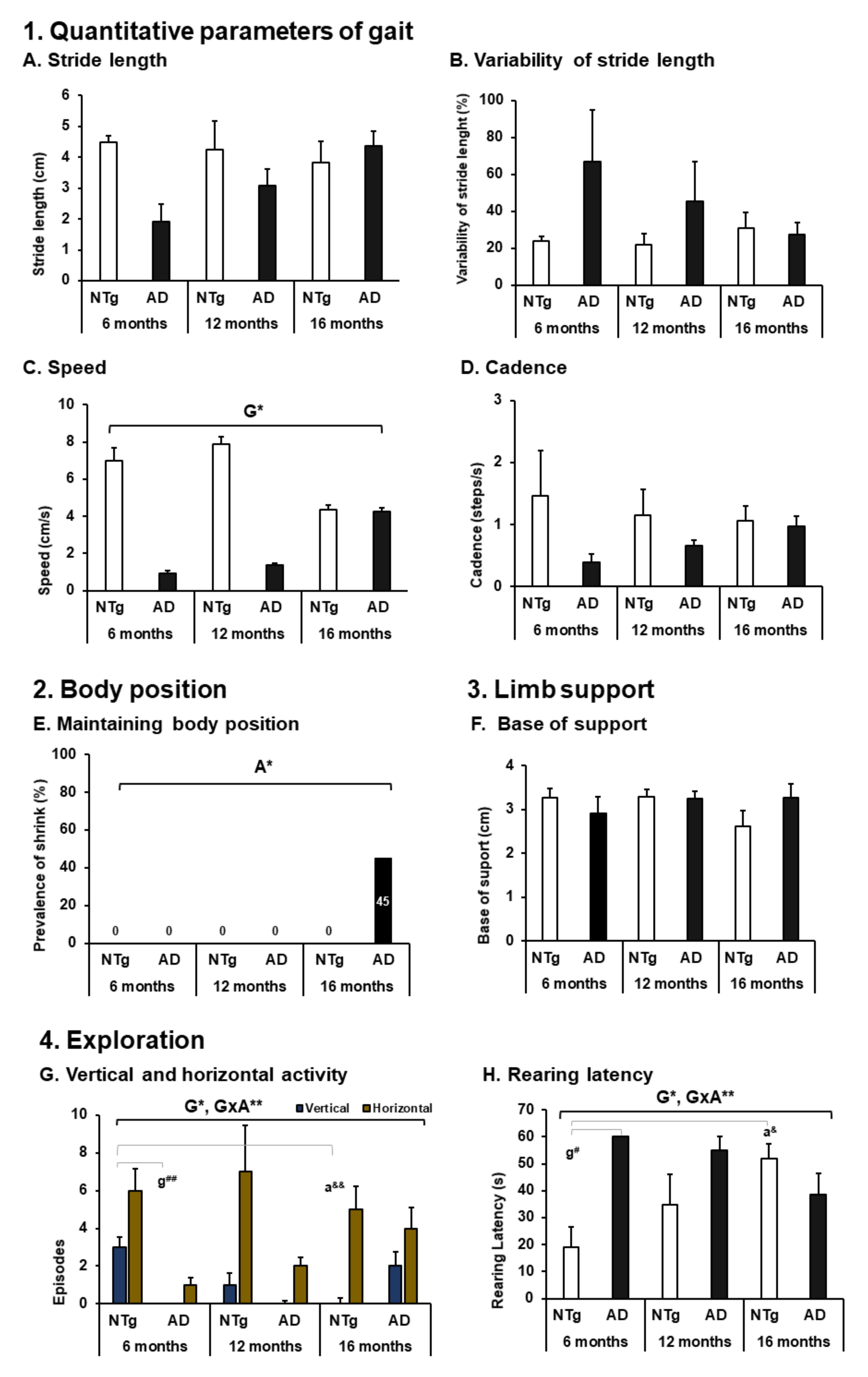
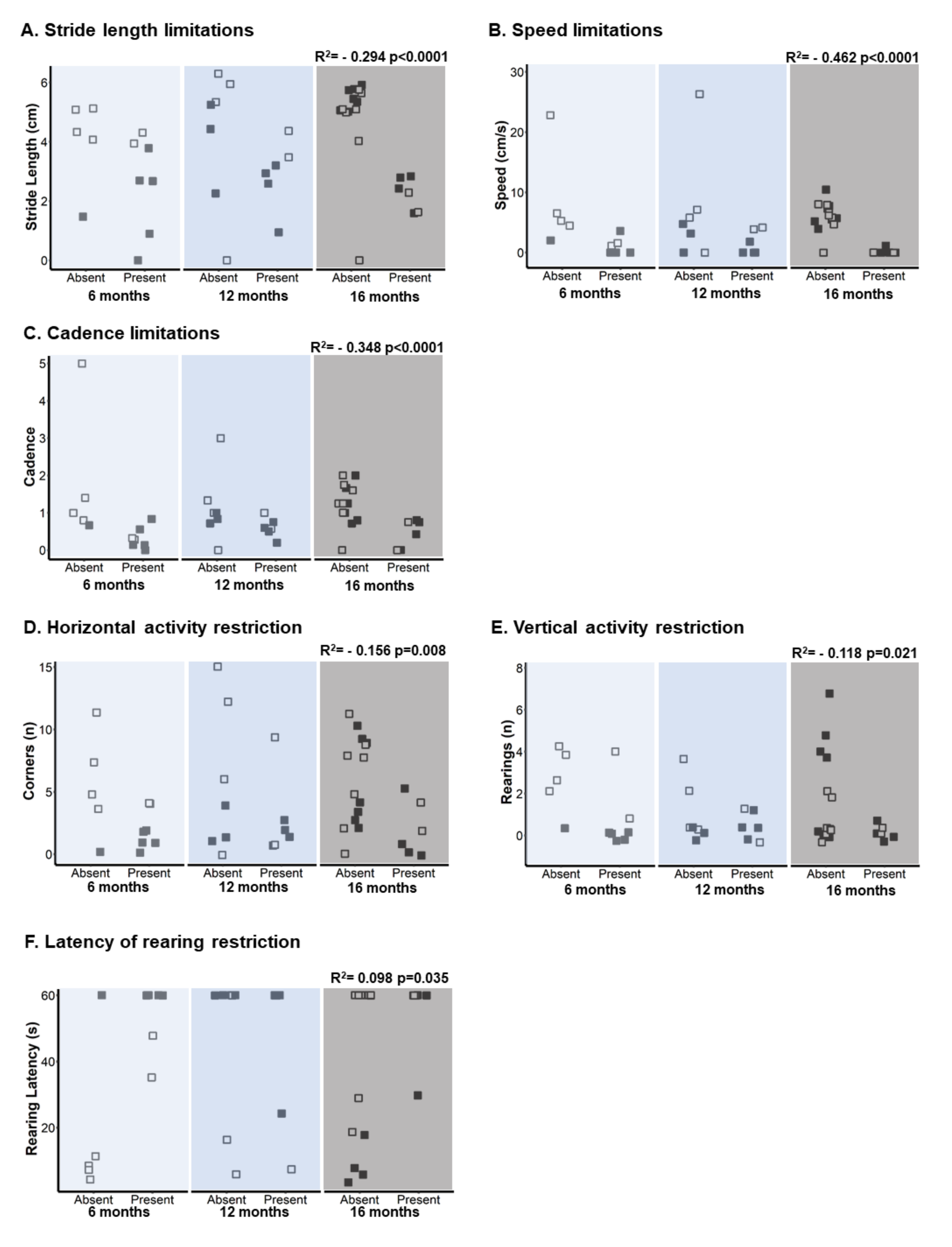
3.1. Activity—Spontaneous Gait and Exploration
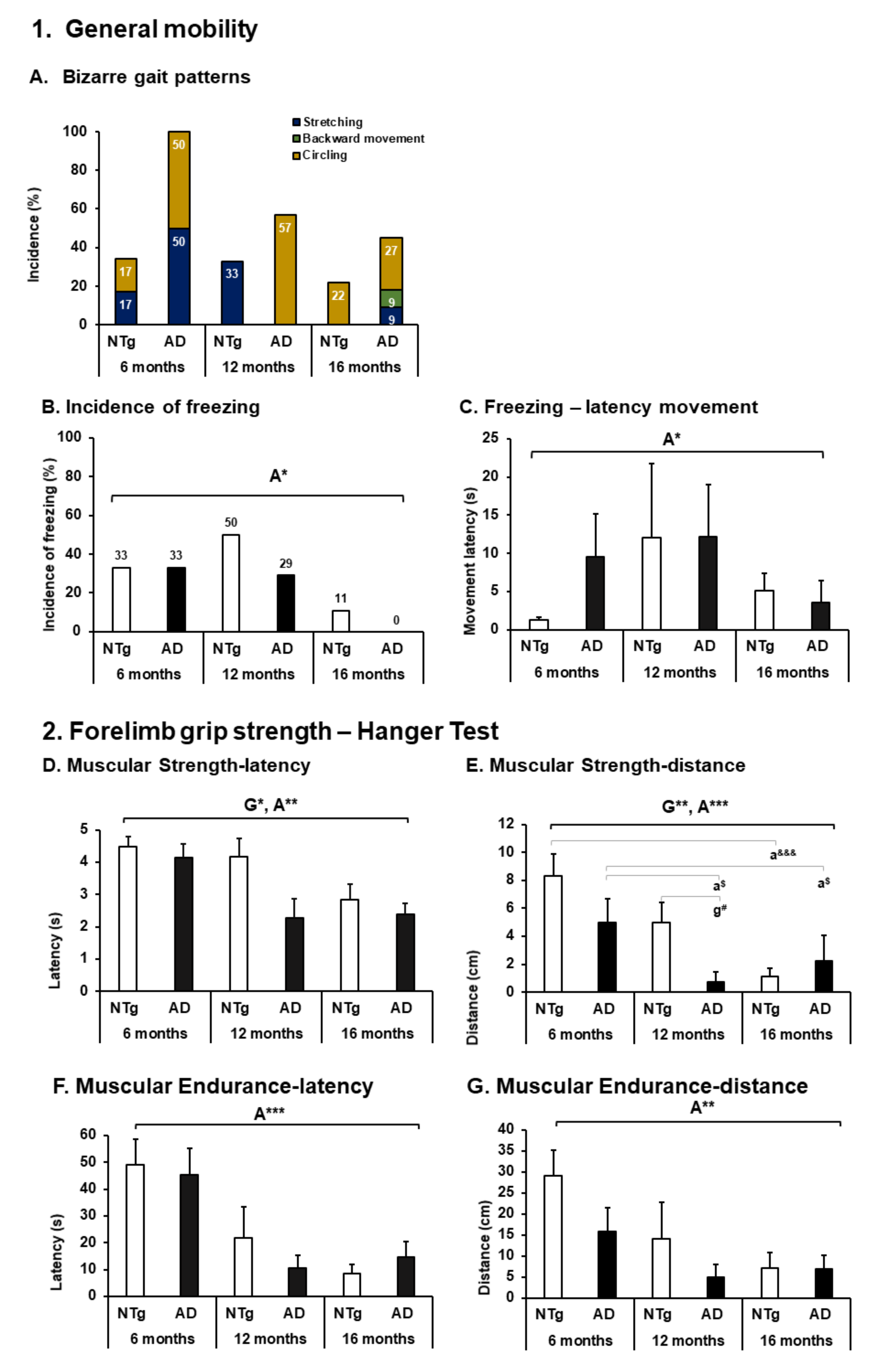
3.2. Body Function—Mobility and Muscular Strength
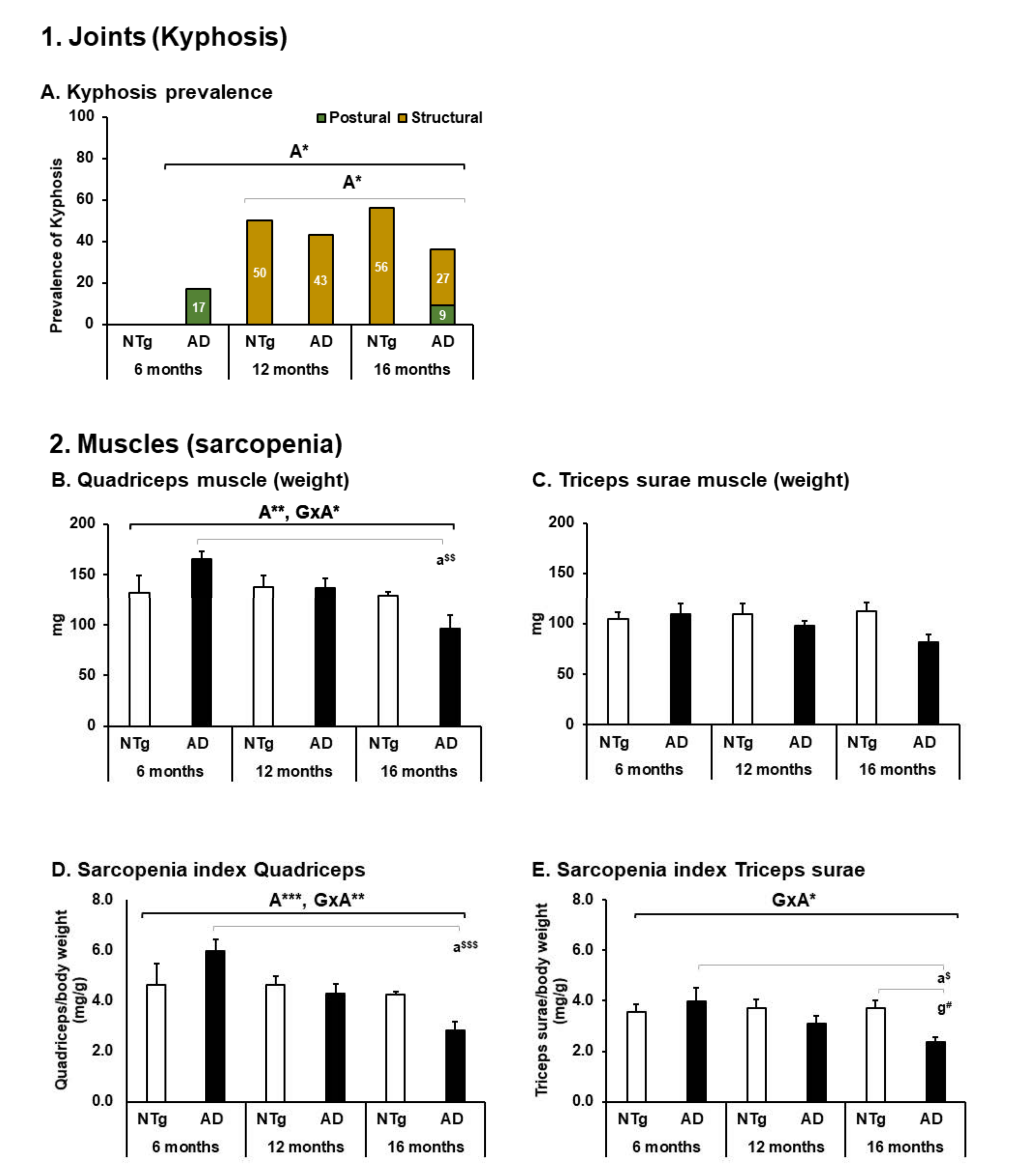
3.3. Body Structure—Joints and Muscles
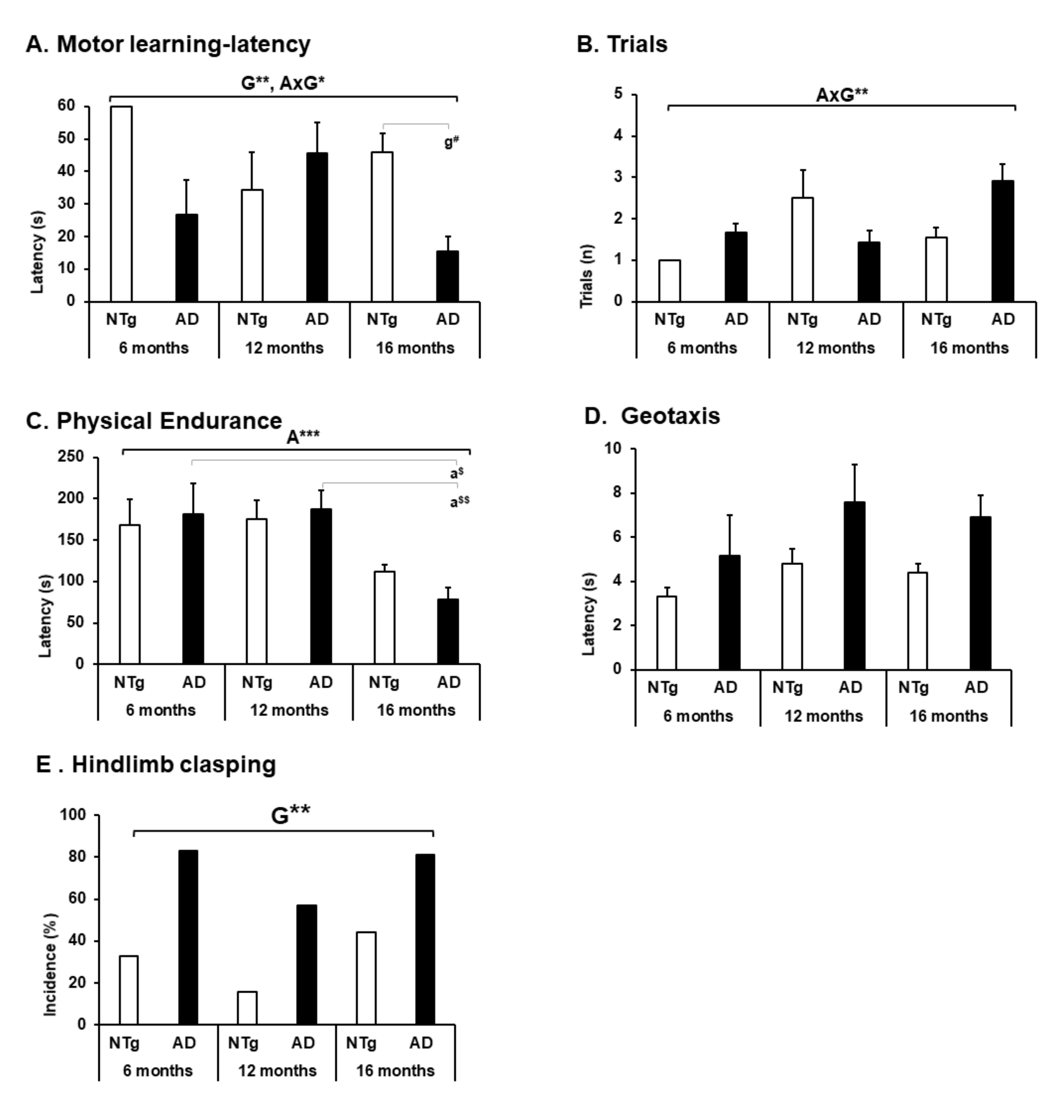
3.4. Motor Performance, Geotaxis, and Hindlimb Clasping
3.5. Survival, Kyphosis, and Frailty Phenotype
4. Discussion
4.1. Activity—Spontaneous Gait and Exploration
4.2. Body Function—Mobility and Muscular Strength
4.3. Body Structure—Joints and Muscles
4.4. Motor Performance, Geotaxis, and Hindlimb Clasping
4.5. Survival, Kyphosis, and Frailty Phenotype
5. Conclusions
- (1)
- The 3xTg-AD mice show more significant functional impairment in gait and exploratory activity quantitative variables.
- (2)
- The presence of movement limitations and muscle weakness mark the functional decline related to the disease severity stages that intensify with increasing age.
- (3)
- Motor performance in 3xTg-AD is accompanied by a series of bizarre behaviors that interfere with the trajectory, which allows us to infer poor neurological control.
- (4)
- Signs of physical frailty accompany the functional deterioration of these animals.
- (5)
- Signs of sarcopenia are present in an advanced stage of AD, with differences in fibre distribution, number of cell nuclei, and presence of adipose tissue.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goyal, D.; Tjandra, D.; Migrino, R.Q.; Giordani, B.; Syed, Z.; Wiens, J. Characterizing heterogeneity in the progression of Alzheimer’s disease using longitudinal clinical and neuroimaging biomarkers. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2018, 10, 629–637. [Google Scholar] [CrossRef]
- Ryan, J.; Fransquet, P.; Wrigglesworth, J.; Lacaze, P. Phenotypic Heterogeneity in Dementia: A Challenge for Epidemiology and Biomarker Studies. Front. Public Health 2018, 6, 181. [Google Scholar] [CrossRef]
- Zidan, M.; ArCoverde, C.; BoM de araújo, N.; Vasques, P.; Rios, A.; Laks, J.; Deslandes, A. Motor and functional changes in different stages of Alzheimer’s disease. Rev. Psiq. Clín. 2012, 39, 161–165. [Google Scholar] [CrossRef]
- Albers, M.W.; Gilmore, G.C.; Kaye, J.; Murphy, C.; Wingfield, A.; Bennett, D.A.; Boxer, A.L.; Buchman, A.S.; Cruickshanks, K.J.; Devanand, D.P.; et al. At the interface of sensory and motor dysfunctions and Alzheimer’s Disease. Alzheimers Dement. 2015, 11, 70. [Google Scholar] [CrossRef] [PubMed]
- Montero-Odasso, M.; Perry, G. Gait Disorders in Alzheimer’s Disease and Other Dementias: There is Something in the Way You Walk. J. Alzheimer’s Dis. 2019, 71, S1–S4. [Google Scholar] [CrossRef] [PubMed]
- Beauchet, O.; Allali, G.; Montero-Odasso, M.; Sejdić, E.; Fantino, B.; Annweiler, C. Motor phenotype of decline in cognitive performance among community-dwellers without dementia: Population-based study and meta-analysis. PLoS ONE 2014, 9, e99318. [Google Scholar] [CrossRef]
- Montero-Odasso, M.; Speechley, M. Falls in Cognitively Impaired Older Adults: Implications for Risk Assessment And Prevention. J. Am. Geriatr Soc. 2018, 66, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Muir, S.W.; Speechley, M.; Wells, J.; Borrie, M.; Gopaul, K.; Montero-Odasso, M. Gait assessment in mild cognitive impairment and Alzheimer’s disease: The effect of dual-task challenges across the cognitive spectrum. Gait Posture. 2012, 35, 96–100. [Google Scholar] [CrossRef]
- Pirker, W.; Katzenschlager, R. Gait disorders in adults and the elderly: A clinical guide. Wien. Klin. Wochenschr. 2017, 129, 81. [Google Scholar] [CrossRef]
- Baker, J.M. Gait Disorders. Am. J. Med. 2018, 131, 602–607. [Google Scholar] [CrossRef]
- Scherder, E.; Eggermont, L.; Swaab, D.; van Heuvelen, M.; Kamsma, Y.; de Greef, M.; van Wijck, R.; Mulder, T. Gait in ageing and associated dementias; its relationship with cognition. Neurosci. Biobehav. Rev. 2007, 31, 485–497. [Google Scholar] [CrossRef] [PubMed]
- Valenti, G.; Bonomi, A.G.; Westerterp, K.R. Walking as a Contributor to Physical Activity in Healthy Older Adults: 2 Week Longitudinal Study Using Accelerometry and the Doubly Labeled Water Method. JMIR Mhealth Uhealth 2016, 4, e5445. [Google Scholar] [CrossRef]
- Leisman, G.; Moustafa, A.A.; Shafir, T. Thinking, Walking, Talking: Integratory Motor and Cognitive Brain Function. Front. Public Health 2016, 4, 4. [Google Scholar] [CrossRef]
- Taborri, J.; Palermo, E.; Rossi, S.; Cappa, P. Gait Partitioning Methods: A Systematic Review. Sensor 2016, 16, 66. [Google Scholar] [CrossRef] [PubMed]
- Umberger, B.R. Stance and swing phase costs in human walking. J. R. Soc. Interface 2010, 7, 1329. [Google Scholar] [CrossRef]
- Wert, D.M.; Brach, J.; Perera, S.; VanSwearingen, J.M. Gait Biomechanics, Spatial and Temporal Characteristics, and the Energy Cost of Walking in Older Adults With Impaired Mobility. Phys. Ther. 2010, 90, 977. [Google Scholar] [CrossRef]
- Ganglius, Y. Evaluación de la marcha en el adulto mayor. Cart. Geriátrico Gerontológica 2011, 4, 1–36. [Google Scholar]
- Salazar Pachón, J.D.; Ramírez Villada, J.F.; Chaparro, D.; León, H.H. Revisión Sistemática Sobre el Impacto de la Actividad Física en los Trastornos de la Marcha en el Adulto Mayor. Apunt. Educ. Física Y Deport. 2014, 118, 30–39. [Google Scholar] [CrossRef][Green Version]
- Menz, H.B.; Lord, S.R.; Fitzpatrick, R.C. Age-related differences in walking stability. Age Ageing 2003, 32, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Boyer, K.A.; Johnson, R.T.; Banks, J.J.; Jewell, C.; Hafer, J.F. Systematic review and meta-analysis of gait mechanics in young and older adults. Exp. Gerontol. 2017, 95, 63–70. [Google Scholar] [CrossRef]
- Ostrosky, K.M.; VanSwearingen, J.M.; Burdett, R.G.; Gee, Z. A comparison of gait characteristics in young and old subjects. Phys. Ther. 1994, 74, 637–646. [Google Scholar] [CrossRef]
- Montgomery, G.; McPhee, J.; Pääsuke, M.; Sipilä, S.; Maier, A.B.; Hogrel, J.-Y.; Degens, H. Determinants of Performance in the Timed up-and-go and Six-Minute Walk Tests in Young and Old Healthy Adults. J. Clin. Med. 2020, 9, 1561. [Google Scholar] [CrossRef] [PubMed]
- Guralnik, J.M.; Ferrucci, L.; Simonsick, E.M.; Salive, M.E.; Wallace, R.B. Lower-Extremity Function in Persons over the Age of 70 Years as a Predictor of Subsequent Disability. N. Engl. J. Med. 2009, 332, 556–561. [Google Scholar] [CrossRef] [PubMed]
- Bohannon, R.W. Relevance of Muscle Strength to Gait Performance in Patients with Neurologic Disability. J. Neurol. Rehab. 1989, 3, 97–100. [Google Scholar] [CrossRef]
- Boyle, P.A.; Buchman, A.S.; Wilson, R.S.; Leurgans, S.E.; Bennett, D.A. Association of muscle strength with the risk of Alzheimer disease and the rate of cognitive decline in community-dwelling older persons. Arch. Neurol. 2009, 66, 1339–1344. [Google Scholar] [CrossRef]
- Sui, S.X.; Holloway-Kew, K.L.; Hyde, N.K.; Williams, L.J.; Leach, S.; Pasco, J.A. Muscle strength and gait speed rather than lean mass are better indicators for poor cognitive function in older men. Sci. Rep. 2020, 10, 10367. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, Y.; Kaneko, Y.; Sato, T.; Shimizu, S.; Kanetaka, H.; Hanyu, H. Sarcopenia and Muscle Functions at Various Stages of Alzheimer Disease. Front. Neurol. 2018, 9, 170. [Google Scholar] [CrossRef]
- Arrieta, H.; Rezola-Pardo, C.; Echeverria, I.; Iturburu, M.; Gil, S.M.; Yanguas, J.J.; Irazusta, J.; Rodriguez-Larrad, A. Physical activity and fitness are associated with verbal memory, quality of life and depression among nursing home residents: Preliminary data of a randomized controlled trial. BMC Geriatr. 2018, 18, 80. [Google Scholar] [CrossRef]
- Beeri, M.S.; Leugrans, S.E.; Delbono, O.; Bennett, D.A.; Buchman, A.S. Sarcopenia is associated with incident Alzheimer’s dementia, mild cognitive impairment, and cognitive decline. J. Am. Geriatr. Soc. 2021, 69, 1826–1835. [Google Scholar] [CrossRef] [PubMed]
- Baeta-Corral, R.; Giménez-Llort, L. Bizarre behaviors and risk assessment in 3xTg-AD mice at early stages of the disease. Behav. Brain Res. 2014, 258, 97–105. [Google Scholar] [CrossRef]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving Bioscience Research Reporting: The ARRIVE Guidelines for Reporting Animal Research. PLoS Biol. 2010, 8, e1000412. [Google Scholar] [CrossRef]
- Castillo-Mariqueo, L.; Giménez-Llort, L. Translational Modeling of Psychomotor Function in Normal and AD-Pathological Aging With Special Concerns on the Effects of Social Isolation. Front. Aging 2021, 2, 648567. [Google Scholar] [CrossRef]
- Verbrugge, L.M.; Jette, A.M. The disablement process. Soc. Sci. Med. 1994, 38, 1–14. [Google Scholar] [CrossRef]
- Reid, K.F.; Fielding, R.A. Skeletal Muscle Power: A Critical Determinant of Physical Functioning In Older Adults. Exerc. Sport Sci. Rev. 2012, 40, 4. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Q.M.; Meng, Z.; Yin, Z.; Luo, X.; Yu, D. Gait disorder as a predictor of spatial learning and memory impairment in aged mice. PeerJ 2017, 5, e2854. [Google Scholar] [CrossRef] [PubMed]
- Giménez-Llort, L.; Fernández-Teruel, A.; Escorihuela, R.M.; Fredholm, B.B.; Tobeña, A.; Pekny, M.; Johansson, B. Mice lacking the adenosine A1 receptor are anxious and aggressive, but are normal learners with reduced muscle strength and survival rate. Eur. J. Neurosci. 2002, 16, 547–550. [Google Scholar] [CrossRef] [PubMed]
- Castillo Mariqueo, L.; Alveal-Mellado, D.; Gimenez-LLort, L. Hindlimb clasping, kyphosis and piloerection: Frailty markers from middle to very old ages in mice. Eur. J. Neurol. 2021, 28, 447. [Google Scholar]
- Edström, E.; Ulfhake, B. Sarcopenia is not due to lack of regenerative drive in senescent skeletal muscle. Aging Cell 2005, 4, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Allan, L.M.; Ballard, C.G.; Burn, D.J.; Kenny, R.A. Prevalence and Severity of Gait Disorders in Alzheimer’s and Non-Alzheimer’s Dementias. J. Am. Geriatr. Soc. 2005, 53, 1681–1687. [Google Scholar] [CrossRef]
- Broom, L.; Ellison, B.A.; Worley, A.; Wagenaar, L.; Sörberg, E.; Ashton, C.; Bennett, D.A.; Buchman, A.S.; Saper, C.B.; Shih, L.C.; et al. A translational approach to capture gait signatures of neurological disorders in mice and humans. Sci. Rep. 2017, 7, 3225. [Google Scholar] [CrossRef] [PubMed]
- Buchman, A.S.; Bennett, D.A. Loss of motor function in preclinical Alzheimer’s disease. Expert Rev. Neurother. 2011, 11, 665–676. [Google Scholar] [CrossRef] [PubMed]
- Chiaramonte, R.; Cioni, M. Critical spatiotemporal gait parameters for individuals with dementia: A systematic review and meta-analysis. Hong Kong Physiother. J. 2021, 41, 1–14. [Google Scholar] [CrossRef]
- Aggarwal, N.T.; Wilson, R.S.; Beck, T.L.; Bienias, J.L.; Bennett, D.A. Motor Dysfunction in Mild Cognitive Impairment and the Risk of Incident Alzheimer Disease. Arch. Neurol. 2006, 63, 1763–1769. [Google Scholar] [CrossRef]
- Kurlan, R.; Richard, I.H.; Papka, M.; Marshall, F. Movement disorders in Alzheimer’s disease: More rigidity of definitions is needed. Mov. Disord. 2000, 15, 24–29. [Google Scholar] [CrossRef]
- Wirths, O.; Bayer, T.A. Motor impairment in Alzheimer’s disease and transgenic Alzheimer’s disease mouse models. Genes Brain Behav. 2008, 7 (Suppl. 1), 1–5. [Google Scholar] [CrossRef]
- Castillo Mariqueo, L.; Gimenez-LLort, L. Gait impairments and functional limitations in the exploratory activity in an animal model of Alzheimer’s disease. Eur. J. Neurol. 2021, 28, 446. [Google Scholar]
- Castillo Mariqueo, L.; Giménez Llort, L. Bizarre behaviors limit exploratory activity and impair spontaneous gait performance in aged mice with AD pathology. In Behavioral Neuroscience, Proceedings of the 2nd International Electronic Conference on Brain Sciences, online, 15–20 July 2021; MDPI: Basel, Switzerland, 2021. [Google Scholar] [CrossRef]
- Coelho, F.G.; Stella, F.; de Andrade, L.P.; Barbieri, F.A.; Santos-Galduróz, R.F.; Gobbi, S.; Costa, J.L.; Gobbi, L.T. Gait and risk of falls associated with frontal cognitive functions at different stages of Alzheimer’s disease. Aging. Neuropsychol. Cogn. 2012, 19, 644–656. [Google Scholar] [CrossRef] [PubMed]
- Setogawa, S.; Yamaura, H.; Arasaki, T.; Endo, S.; Yanagihara, D. Deficits in memory-guided limb movements impair obstacle avoidance locomotion in Alzheimer’s disease mouse model. Sci. Rep. 2014, 41, 7220. [Google Scholar] [CrossRef] [PubMed]
- Aoki, S.; Sato, Y.; Yanagihara, D. Lesion in the lateral cerebellum specifically produces overshooting of the toe trajectory in leading forelimb during obstacle avoidance in the rat. J. Neurophysiol. 2013, 110, 1511–1524. [Google Scholar] [CrossRef][Green Version]
- Tarantini, S.; Yabluchanskiy, A.; Fülöp, G.A.; Kiss, T.; Perz, A.; O’Connor, D.; Johnson, E.; Sorond, F.; Ungvari, Z.I.; Csiszar, A. Age-Related Alterations in Gait Function in Freely Moving Male C57BL/6 Mice: Translational Relevance of Decreased Cadence and Increased Gait Variability. J. Gerontol. Ser. A 2019, 74, 1417. [Google Scholar] [CrossRef]
- Akula, S.K.; McCullough, K.B.; Weichselbaum, C.; Dougherty, J.D.; Maloney, S.E. The trajectory of gait development in mice. Brain Behav. 2020, 10, e01636. [Google Scholar] [CrossRef]
- Broom, L.; Worley, A.; Gao, F.; Hernandez, L.D.; Ashton, C.E.; Shih, L.C.; VanderHorst, V.G. Translational methods to detect asymmetries in temporal and spatial walking metrics in parkinsonian mouse models and human subjects with Parkinson’s disease. Sci. Rep. 2019, 9, 2437. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, K.; Gardin, J.; Sabnis, G.; Peer, A.; Darrell, M.; Deats, S.; Geuther, B.; Lutz, C.M.; Kumar, V. Gait-level analysis of mouse open field behavior using deep learning-based pose estimation. bioRxiv 2020. [Google Scholar] [CrossRef]
- J España, J.; Giménez-Llort, L.; Valero, J.; Miñano, A.; Rábano, A.; Rodriguez-Alvarez, J.; LaFerla, F.M.; Saura, C.A. Intraneuronal β-Amyloid Accumulation in the Amygdala Enhances Fear and Anxiety in Alzheimer’s Disease Transgenic Mice. Biol. Psychiatry 2010, 67, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Giménez-Llort, L.; Blázquez, G.; Cañete, T.; Johansson, B.; Oddo, S.; Tobeña, A.; LaFerla, F.M.; Fernández-Teruel, A.; Gimeez-Llort, L.; Blaquez, G.; et al. Modeling behavioral and neuronal symptoms of Alzheimer’s disease in mice: A role for intraneuronal amyloid. Neurosci. Biobehav. Rev. 2007, 31, 125–147. [Google Scholar] [CrossRef] [PubMed]
- Roda, A.R.; Esquerda-Canals, G.; Martí-Clúa, J.; Villegas, S. Cognitive Impairment in the 3xTg-AD Mouse Model of Alzheimer’s Disease is Affected by Aβ-ImmunoTherapy and Cognitive Stimulation. Pharmaceutics 2020, 12, 944. [Google Scholar] [CrossRef]
- Muntsant-Soria, A.; Gimenez-Llort, L. Impact of social isolation on the behavioral and functional profiles and hippocampal atrophy asymmetry in dementia in times of coronavirus pandemic (COVID-19): A translational neuroscience approach. Front. Psychiatry 2020, 11, 1126. [Google Scholar] [CrossRef]
- Cordón-Barris, L.; Pascual-Guiral, S.; Yang, S.; Giménez-Llort, L.; Lope-Piedrafita, S.; Niemeyer, C.; Claro, E.; Lizcano, J.M.; Bayascas, J.R. Mutation of the 3-Phosphoinositide-Dependent Protein Kinase 1 (PDK1) Substrate-Docking Site in the Developing Brain Causes Microcephaly with Abnormal Brain Morphogenesis Independently of Akt, Leading to Impaired Cognition and Disruptive Behaviors. Mol. Cell. Biol. 2016, 36, 2967–2982. [Google Scholar] [CrossRef]
- Castillo-Mariqueo, L.; Giménez-Llort, L. Indexes for flotation and circling, two non-search behaviors in the water maze, sensitive to D-galactose–induced accelerated aging and Alzheimer’s disease. Behav. Brain Res. 2019, 377, 112229. [Google Scholar] [CrossRef]
- Baeta-Corral, R.; Giménez-Llort, L. Persistent hyperactivity and distinctive strategy features in the Morris water maze in 3xTg-AD mice at advanced stages of disease. Behav. Neurosci. 2015, 129, 129–137. [Google Scholar] [CrossRef]
- Miljkovic, N.; Lim, J.Y.; Miljkovic, I.; Frontera, W.R. Aging of skeletal muscle fibers. Ann. Rehabil. Med. 2015, 39, 155–162. [Google Scholar] [CrossRef]
- Larsson, L.; Degens, H.; Li, M.; Salviati, L.; Lee, Y.I.; Thompson, W.; Kirkland, J.L.; Sandri, M. Sarcopenia: Aging-Related Loss of Muscle Mass and Function. Physiol. Rev. 2019, 99, 427–511. [Google Scholar] [CrossRef] [PubMed]
- Goodpaster, B.H.; Park, S.W.; Harris, T.B.; Kritchevsky, S.B.; Nevitt, M.; Schwartz, A.V.; Simonsick, E.M.; Tylavsky, F.A.; Visser, M.; Newman, A.B. The loss of skeletal muscle strength, mass, and quality in older adults: The health, aging and body composition study. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2006, 61, 1059–1064. [Google Scholar] [CrossRef]
- Stover, K.R.; Campbell, M.A.; Van Winssen, C.M.; Brown, R.E. Analysis of motor function in 6-month-old male and female 3xTg-AD mice. Behav. Brain Res. 2015, 281, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Garvock-de Montbrun, T.; Fertan, E.; Stover, K.; Brown, R.E. Motor deficits in 16-month-old male and female 3xTg-AD mice. Behav. Brain Res. 2019, 356, 305–313. [Google Scholar] [CrossRef]
- Shavlakadze, T.; McGeachie, J.; Grounds, M.D. Delayed but excellent myogenic stem cell response of regenerating geriatric skeletal muscles in mice. Biogerontology 2010, 11, 363–376. [Google Scholar] [CrossRef] [PubMed]
- Van Dijk, M.; Nagel, J.; Dijk, F.J.; Salles, J.; Verlaan, S.; Walrand, S.; van Norren, K.; Luiking, Y. Sarcopenia in older mice is characterized by a decreased anabolic response to a protein meal. Arch. Gerontol. Geriatr. 2017, 69, 134–143. [Google Scholar] [CrossRef]
- Sayer, A.A.; Robinson, S.M.; Patel, H.P.; Shavlakadze, T.; Cooper, C.; Grounds, M.D. New horizons in the pathogenesis, diagnosis and management of sarcopenia. Age Ageing 2013, 42, 1145–1150. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Listrat, A.; Meunier, B.; Gueugneau, M.; Coudy-Gandilhon, C.; Combaret, L.; Taillandier, D.; Polge, C.; Attaix, D.; Lethias, C.; et al. Apoptosis in capillary endothelial cells in ageing skeletal muscle. Aging Cell 2014, 13, 254–262. [Google Scholar] [CrossRef]
- Mankhong, S.; Kim, S.; Moon, S.; Kwak, H.-B.; Park, D.-H.; Kang, J.-H. Experimental Models of Sarcopenia: Bridging Molecular Mechanism and Therapeutic Strategy. Cells 2020, 9, 1385. [Google Scholar] [CrossRef] [PubMed]
- Kadoguchi, T.; Shimada, K.; Miyazaki, T.; Kitamura, K.; Kunimoto, M.; Aikawa, T.; Sugita, Y.; Ouchi, S.; Shiozawa, T.; Yokoyama-Nishitani, M.; et al. Promotion of oxidative stress is associated with mitochondrial dysfunction and muscle atrophy in aging mice. Geriatr. Gerontol. Int. 2020, 20, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; He, M.; Yu, D.; Wu, Y.; Wang, X.; Lv, S.; Xiao, W.; Li, Y. Mouse models of sarcopenia: Classification and evaluation. J. Cachexia. Sarcopenia Muscle 2021, 12, 538–554. [Google Scholar] [CrossRef]
- Lalonde, R.; Strazielle, C. Brain regions and genes affecting limb-clasping responses. Brain Res. Rev. 2011, 67, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Lieu, C.A.; Chinta, S.J.; Rane, A.; Andersen, J.K. Age-related behavioral phenotype of an astrocytic monoamine oxidase-B transgenic mouse model of Parkinson’s disease. PLoS ONE 2013, 8, e54200. [Google Scholar] [CrossRef] [PubMed]
- Muntsant, A.; Jiménez-Altayó, F.; Puertas-Umbert, L.; Jiménez-Xarrie, E.; Vila, E.; Giménez-Llort, L. Sex-dependent end-of-life mental and vascular scenarios for compensatory mechanisms in mice with normal and ad-neurodegenerative aging. Biomedicines 2021, 9, 111. [Google Scholar] [CrossRef]

| Translational Functioning and Disability (ICF) | ||||||
|---|---|---|---|---|---|---|
| NTg Mice | 3xTg-AD Mice | ||||
| 1. Quantitative parameters of gait (see Figure 2) | 6 Months | 12 Months | 16 Months | 6 Months | 12 Months | 16 Months |
| A. Stride length (cm) | ABSENT | MILD | MILD | MODERATE | MODERATE | MILD |
| B. Variability of stride length (%) | ABSENT | ABSENT | MILD | SEVERE | MODERATE | MODERATE |
| C. Speed (cm/s) | ABSENT | ABSENT | MILD | SEVERE | SEVERE | MODERATE |
| D. Cadence (steps/s) | ABSENT | ABSENT | ABSENT | SEVERE | MODERATE | MODERATE |
| 2. Body position (see Figure 2) | ||||||
| E. Maintaining body position (%) | ABSENT | ABSENT | ABSENT | ABSENT | ABSENT | MILD |
| 3. Limb support | ||||||
| F. Base of support (cm) | ABSENT | ABSENT | MILD | ABSENT | ABSENT | ABSENT |
| 4. Exploration (see Figure 2) | ||||||
| G. Vertical and horizontal activity (n episodes) | MILD | MILD | MILD | MODERATE | SEVERE | MILD |
| H. Rearing latency (s) | MILD | MODERATE | SEVERE | COMPLETE | SEVERE | MODERATE |
| ||||||
| 1. General mobility (see Figure 3) | ||||||
| A. Bizarre gait patterns (incidence %) | MILD | MODERATE | MILD | MODERATE | MODERATE | MILD |
| B. Freezing (movement latency) | ABSENT | MODERATE | MILD | MODERATE | MODERATE | MILD |
| C. Freezing—latency movement | ABSENT | MODERATE | MILD | MODERATE | MODERATE | MILD |
| 2. Forelimb grip strength—Hanger Test (see Figure 3) | ||||||
| D. Muscular Strength (latency) | ABSENT | MILD | MODERATE | MILD | MODERATE | MODERATE |
| E. Muscular Strength (distance) | ABSENT | MILD | SEVERE | MILD | SEVERE | MODERATE |
| F. Muscular Endurance (latency) | ABSENT | MODERATE | SEVERE | MILD | SEVERE | SEVERE |
| G. Muscular Endurance (distance) | ABSENT | MODERATE | SEVERE | MODERATE | SEVERE | SEVERE |
| ||||||
| 1. Joints (see Figure 4) | ||||||
| A. Kyphosis prevalence | ABSENT | MODERATE | MODERATE | MILD | MILD | MILD |
| 2. Muscles (see Figure 4) | ||||||
| B. Quadriceps muscle (weight) | MILD | MILD | MILD | ABSENT | MILD | MODERATE |
| C. Triceps surae muscle (weight) | MILD | MILD | MILD | MILD | MILD | MODERATE |
| D. Sarcopenia index—Quadriceps | MILD | MILD | MILD | ABSENT | MILD | MODERATE |
| E. Sarcopenia index—Triceps | MILD | MILD | MILD | MILD | MILD | MODERATE |
| Qualifiers: Generic qualifier with the negative scale used to indicate the extent or magnitude of an impairment: NO impairment, (absent) 0–4%; MILD impairment, (slight, low) 5–24%; MODERATE impairment, (medium, fair) 25–49%; SEVERE impairment, (high, extreme), 50–95%; COMPLETE impairment, (total) 96–100%—not specified in the ICF for humans. Activity limitations are difficulties an individual may have in executing activities: NO difficulty, (absent) 0–4%; MILD difficulty, (slight, low) 5–24%; MODERATE difficulty, (medium, fair) 25–49%; SEVERE difficulty, (high, extreme) 50–95%; COMPLETE difficulty, (total) 96–100%—not specified in the ICF for humans. | ||||||
| Morphological Features | NTg Mice | 3xTg-AD Mice | ||||
|---|---|---|---|---|---|---|
| 6 Months | 12 Months | 16 Months | 6 Months | 12 Months | 16 Months | |
| Quadriceps | ||||||
| 1. Nuclei | ||||||
| Localization | Peripheral nuclei | Peripheral nuclei | Peripheral nuclei | Peripheral nuclei | Peripheral nuclei | Peripheral nuclei |
| Number | ++++ | +++ | +++ | +++ | ++ | +++ |
| 2. Fiber | ||||||
| Distribution | Homogeneous | Homogeneous | Homogeneous | Homogeneous | Homogeneous | Homogeneous |
| 3. Adipose tissue | ||||||
| Localization | Intramuscular | Intramuscular | Intramuscular | Intramuscular | Intramuscular | Peripheral |
| Number | + | + | ++ | ++ | + | + |
| Triceps surae | ||||||
| 1. Nuclei | ||||||
| Localization | Peripheral nuclei | Peripheral nuclei | Peripheral nuclei | Peripheral nuclei | Peripheral nuclei | Peripheral nuclei |
| Number | ++++ | +++ | +++ | +++ | ++ | +++ |
| 2. Fiber | ||||||
| Distribution | Homogeneous | Homogeneous | Heterogeneous | Homogeneous | Homogeneous | Heterogeneous |
| 3. Adipose tissue | - | - | ||||
| Localization | Intramuscular | Peripheral | Intramuscular | Intramuscular | ||
| Number | + | ++++ | + | ++++ | ||
| Qualifier: 75–100% = ++++. 50–75% = +++. 25–50% = ++. 0–25% = +. 0% = -. | ||||||
| Conditions | NTg Mice | 3xTg-AD Mice | Statistics | ||||
|---|---|---|---|---|---|---|---|
| 6 Months | 12 Months | 16 Months | 6 Months | 12 Months | 16 Months | ||
| 1. Survival (mean + SEM days) (Mortality ratio) | 329 + 25.26 3/15 (20%) | 337 + 29.09 3/9 (33.3%) | 350 + 15.60 20/40 (50%) | 208 + 1.26 0/15 (0%) | 395 + 9.63 1/16 (6.2%) | 481 + 25.31 5/24 (20.8%) | S && |
| 2. Kyphosis (animals, %) | - | 3/6 (50%) | 5/9 (56%) | 1/6 (17%) | 3/7 (43%) | 4/11 (36%) | A ** |
| - | - | - | 1/6 (17%) | - | 1/11 (9%) | n.s. |
| - | 3/6 (50%) | 5/9 (56%) | - | 3/7 (43%) | 3/11 (27%) | A* |
| 3. Physical conditions (animals, %) | |||||||
| 30 g. | 30 g. | 30 g. | 28 g. | 33 g. | 34g. | A *, a # |
| 2/6 (33%) | 4/6 (67%) | 5/9 (56%) | 1/6 (17%) | 4/7 (57%) | 4/11 (36%) | n.s. |
| - | - | - | - | - | 5/11 (45%) | a # |
| - | - | - | - | - | 4/11 (36%) | a # |
| - | 1/6 (17%) | 2/9 (22%) | - | - | 6/11 (55%) | A * |
| - | - | - | - | - | 4/11 (36%) | a # |
| - | 1/6 (17%) | - | - | - | 9/11 (82%) | A **, G * |
| Kaplan–Meier, Log Rank: S && p < 0.01. X2, A: age, ** p < 0.01 * p < 0.05, G: genotype, * p < 0.05. n.s. p > 0.05. # p < 0.05. | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castillo-Mariqueo, L.; Pérez-García, M.J.; Giménez-Llort, L. Modeling Functional Limitations, Gait Impairments, and Muscle Pathology in Alzheimer’s Disease: Studies in the 3xTg-AD Mice. Biomedicines 2021, 9, 1365. https://doi.org/10.3390/biomedicines9101365
Castillo-Mariqueo L, Pérez-García MJ, Giménez-Llort L. Modeling Functional Limitations, Gait Impairments, and Muscle Pathology in Alzheimer’s Disease: Studies in the 3xTg-AD Mice. Biomedicines. 2021; 9(10):1365. https://doi.org/10.3390/biomedicines9101365
Chicago/Turabian StyleCastillo-Mariqueo, Lidia, M. José Pérez-García, and Lydia Giménez-Llort. 2021. "Modeling Functional Limitations, Gait Impairments, and Muscle Pathology in Alzheimer’s Disease: Studies in the 3xTg-AD Mice" Biomedicines 9, no. 10: 1365. https://doi.org/10.3390/biomedicines9101365
APA StyleCastillo-Mariqueo, L., Pérez-García, M. J., & Giménez-Llort, L. (2021). Modeling Functional Limitations, Gait Impairments, and Muscle Pathology in Alzheimer’s Disease: Studies in the 3xTg-AD Mice. Biomedicines, 9(10), 1365. https://doi.org/10.3390/biomedicines9101365







