Over-Dose Lithium Toxicity as an Occlusive-like Syndrome in Rats and Gastric Pentadecapeptide BPC 157
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Drugs
2.3. Experimental Protocol
2.4. Statistical Analysis
3. Results
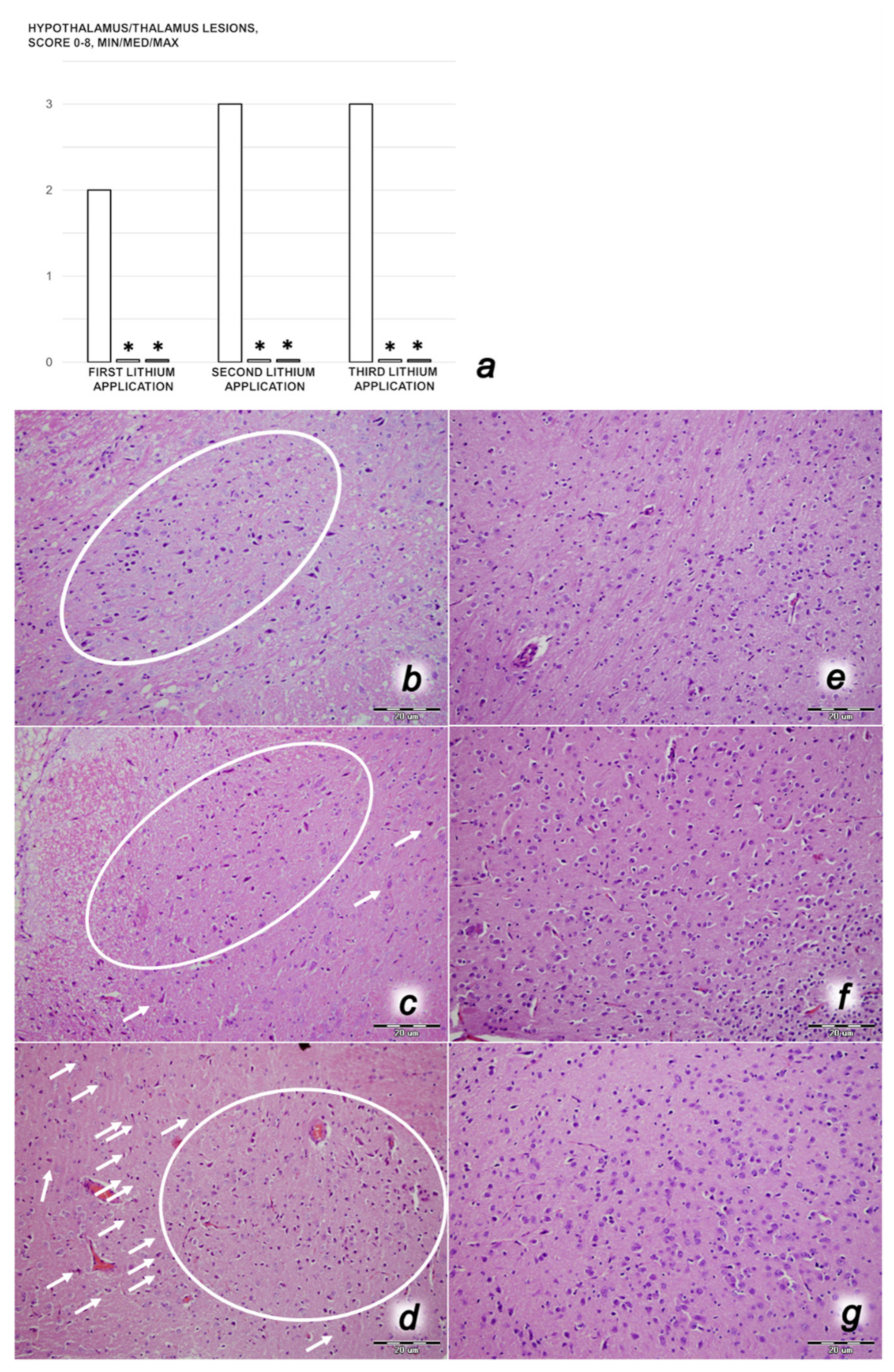
3.1. A Perilous Syndrome Occurred Centrally and Peripherally
3.1.1. Portal and Caval Veins, Abdominal Aorta, and Superior Sagittal Sinus Pressure Recordings
3.1.2. Thrombosis
3.1.3. ECG Recordings
3.2. Perilous Syndrome Occurred Peripherally
3.2.1. Vein Congestion
3.2.2. Heart, Lung, Liver, Kidney, and Gastrointestinal Lesions
Heart Damage
Lung Damage
Liver Damage
Kidney Damage
Gastrointestinal Lesions
3.3. Perilous Syndrome Occurred Centrally
3.3.1. Brain Swelling and Counteraction
3.3.2. Brain Damage
3.4. Oxidative Stress
3.5. Muscular Weakness
3.6. Lithium Serum Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Bosche, B.; Molcanyi, M.; Noll, T.; Rej, S.; Zatschler, B.; Doeppner, T.R.; Hescheler, J.; Müller, D.J.; Macdonald, R.L.; Härtel, F.V. A differential impact of lithium on endothelium-dependent but not on endothelium-independent vessel relaxation. Prog. Neuropsychopharmacol. Biol. Psychiatry 2016, 67, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Vukojevic, J.; Siroglavic, M.; Kasnik, K.; Kralj, T.; Stancic, D.; Kokot, A.; Kolaric, D.; Drmic, D.; Sever, A.Z.; Barisic, I.; et al. Rat inferior caval vein (ICV) ligature and particular new insights with the stable gastric pentadecapeptide BPC 157. Vasc. Pharmacol. 2018, 106, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Kolovrat, M.; Gojkovic, S.; Krezic, I.; Malekinusic, D.; Vrdoljak, B.; Kasnik Kovac, K.; Kralj, T.; Drmic, D.; Barisic, I.; Horvat Pavlov, K.; et al. Pentadecapeptide BPC 157 resolves Pringle maneuver in rats, both ischemia and reperfusion. World J. Hepatol. 2020, 12, 184–206. [Google Scholar] [CrossRef] [PubMed]
- Knezevic, M.; Gojkovic, S.; Krezic, I.; Zizek, H.; Malekinusic, D.; Vrdoljak, B.; Vranes, H.; Knezevic, T.; Barisic, I.; Horvat Pavlov, K.; et al. Occlusion of the superior mesenteric artery in rats reversed by collateral pathways activation: Gastric pentadecapeptide BPC 157 therapy counteracts multiple organ dysfunction syndrome; intracranial, portal and caval hypertension; and aortal hypotension. Biomedicines 2021, 9, 609. [Google Scholar] [CrossRef] [PubMed]
- Gojkovic, S.; Krezic, I.; Vrdoljak, B.; Malekinusic, D.; Barisic, I.; Petrovic, A.; Horvat Pavlov, K.; Kolovrat, M.; Duzel, A.; Knezevic, M.; et al. Pentadecapeptide BPC 157 resolves suprahepatic occlusion of the inferior caval vein, Budd-Chiari syndrome model in rats. World J. Gastrointest. Pathophysiol. 2020, 11, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Knezevic, M.; Gojkovic, S.; Krezic, I.; Zizek, H.; Malekinusic, D.; Vrdoljak, B.; Knezevic, T.; Vranes, H.; Drmic, D.; Staroveski, M.; et al. Occluded superior mesenteric artery and vein. Therapy with the stable gastric pentadecapeptide BPC 157. Biomedicines 2021, 9, 792. [Google Scholar] [CrossRef]
- Knezevic, M.; Gojkovic, S.; Krezic, I.; Zizek, H.; Vranes, H.; Malekinusic, D.; Vrdoljak, B.; Knezevic, T.; Pavlov, K.H.; Drmic, D.; et al. Complex syndrome of the complete occlusion of the end of the superior mesenteric vein, opposed with the stable gastric pentadecapeptide BPC 157 in rats. Biomedicines 2021, 9, 1029. [Google Scholar] [CrossRef]
- Gojkovic, S.; Krezic, I.; Vranes, H.; Zizek, H.; Drmic, D.; Pavlov, K.H.; Petrovic, A.; Batelja, L.; Milavic, M.; Sikiric, S.; et al. BPC 157 therapy and the permanent occlusion of the superior sagittal sinus in rat. Vascular recruitment. Biomedicines 2021, 9, 744. [Google Scholar] [CrossRef]
- Gojkovic, S.; Krezic, I.; Vranes, H.; Zizek, H.; Drmic, D.; Batelja Vuletic, L.; Milavic, M.; Sikiric, S.; Stilinovic, I.; Simeon, P.; et al. Robert’s intragastric alcohol-induced gastric lesion model as an escalated general peripheral and central syndrome, counteracted by the stable gastric pentadecapeptide BPC 157. Biomedicines 2021, 9, 1300. [Google Scholar] [CrossRef]
- Sikiric, P.; Hahm, K.B.; Blagaic, A.B.; Tvrdeic, A.; Pavlov, K.H.; Petrovic, A.; Kokot, A.; Gojkovic, S.; Krezic, I.; Drmic, D.; et al. Stable gastric pentadecapeptide BPC 157, Robert’s stomach cytoprotection/adaptive cytoprotection/organoprotection, and Selye’s stress coping response: Progress, achievements, and the future. Gut Liver 2020, 14, 153–167. [Google Scholar] [CrossRef]
- Sikiric, P.; Rucman, R.; Turkovic, B.; Sever, M.; Klicek, R.; Radic, B.; Drmic, D.; Stupnisek, M.; Misic, M.; Vuletic, L.B.; et al. Novel cytoprotective mediator, stable gastric pentadecapeptide BPC 157. Vascular recruitment and gastrointestinal tract healing. Curr. Pharm. Des. 2018, 24, 1990–2001. [Google Scholar] [CrossRef]
- Sikiric, P.; Seiwerth, S.; Rucman, R.; Kolenc, D.; Vuletic, L.B.; Drmic, D.; Grgic, T.; Strbe, S.; Zukanovic, G.; Crvenkovic, D.; et al. Brain-gut axis and pentadecapeptide BPC 157: Theoretical and practical implications. Curr. Neuropharmacol. 2016, 14, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Seiwerth, S.; Milavic, M.; Vukojevic, J.; Gojkovic, S.; Krezic, I.; Vuletic, L.B.; Pavlov, K.H.; Petrovic, A.; Sikiric, S.; Vranes, H.; et al. Stable gastric pentadecapeptide BPC 157 and wound healing. Front. Pharmacol. 2021, 12, 627533. [Google Scholar] [CrossRef]
- Vukojevic, J.; Milavic, M.; Perovic, D.; Ilic, S.; Zemba Cilic, A.; Duran, N.; Strbe, S.; Zoricic, Z.; Filipcic, I.; Brecic, P.; et al. Pentadecapeptide BPC 157 and the central nervous system. Neural. Regen. Res. 2022, 17, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Strbe, S.; Filipcic, I.; Seiwerth, S.; Sikiric, P. Pentadecapeptide BPC 157 counteracts the adverse effect of lithium overdose in rats. FASEB J. 2019, 33, 822–824. [Google Scholar] [CrossRef]
- Medvidovic-Grubisic, M.; Stambolija, V.; Kolenc, D.; Katancic, J.; Murselovic, T.; Plestina-Borjan, I.; Strbe, S.; Drmic, D.; Barisic, I.; Sindic, A.; et al. Hypermagnesemia disturbances in rats, NO-related: Pentadecapeptide BPC 157 abrogates, L-NAME and L-arginine worsen. Inflammopharmacology 2017, 25, 439–449. [Google Scholar] [CrossRef]
- O’Donnell, K.C.; Gould, T.D. The behavioral actions of lithium in rodent models. Leads to develop novel therapeutics. Neurosci. Biobehav. Rev. 2007, 31, 932–962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamijo, Y.; Soma, K.; Hamanaka, S.; Nagai, T.; Kurihara, K. Dural sinus thrombosis with severe hypernatremia developing in a patient on long-term lithium therapy. J. Toxicol. Clin. Toxicol. 2003, 41, 359–362. [Google Scholar] [CrossRef]
- Lyles, M.R. Deep venous thrombophlebitis associated with lithium toxicity. J. Natl. Med. Assoc. 1984, 76, 633–634. [Google Scholar]
- Cannon, S.R. Intestinal vasculitis and lithium carbonate-associated diarrhoea. Postgrad. Med. J. 1982, 58, 445–447. [Google Scholar] [CrossRef] [Green Version]
- Duzel, A.; Vlainic, J.; Antunovic, M.; Malekinusic, D.; Vrdoljak, B.; Samara, M.; Gojkovic, S.; Krezic, I.; Vidovic, T.; Bilic, Z.; et al. Stable gastric pentadecapeptide BPC 157 in the treatment of colitis and ischemia and reperfusion in rats: New insights. World J. Gastroenterol. 2017, 23, 8465–8488. [Google Scholar] [CrossRef]
- Amic, F.; Drmic, D.; Bilic, Z.; Krezic, I.; Zizek, H.; Peklic, M.; Klicek, R.; Pajtak, A.; Amic, E.; Vidovic, T.; et al. Bypassing major venous occlusion and duodenal lesions in rats, and therapy with the stable gastric pentadecapeptide BPC 157, L-NAME and L-arginine. World J. Gastroenterol. 2018, 24, 5366–5378. [Google Scholar] [CrossRef]
- Drmic, D.; Samara, M.; Vidovic, T.; Malekinusic, D.; Antunovic, M.; Vrdoljak, B.; Ruzman, J.; Milkovic, P.M.; Horvat, K.P.; Jeyakumar, J.; et al. Counteraction of perforated cecum lesions in rats: Effects of pentadecapeptide BPC 157, L-NAME and L-arginine. World J. Gastroenterol. 2018, 24, 5462–5476. [Google Scholar] [CrossRef]
- Sever, A.Z.; Sever, M.; Vidovic, T.; Lojo, N.; Kolenc, D.; Vuletic, L.B.; Drmic, D.; Kokot, A.; Zoricic, I.; Coric, M.; et al. Stable gastric pentadecapeptide BPC 157 in the therapy of the rats with bile duct ligation. Eur. J. Pharmacol. 2019, 847, 130–142. [Google Scholar] [CrossRef]
- Vukojevic, J.; Vrdoljak, B.; Malekinusic, D.; Siroglavic, M.; Milavic, M.; Kolenc, D.; Boban Blagaic, A.; Bateljam, L.; Drmic, D.; Seiwerth, S.; et al. The effect of pentadecapeptide BPC 157 on hippocampal ischemia/reperfusion injuries in rats. Brain Behav. 2020, 10, e01726. [Google Scholar] [CrossRef]
- Zlatar, M.; Kokot, A.; Vuletic, L.B.; Masnec, S.; Kralj, T.; Perisa, M.M.; Barisic, I.; Radic, B.; Milanovic, K.; Drmic, D.; et al. BPC 157 as a therapy for retinal ischemia induced by retrobulbar application of L-NAME in rats. Front. Pharmacol 2021, 12, 632295. [Google Scholar] [CrossRef] [PubMed]
- Park, J.M.; Lee, H.J.; Sikiric, P.; Hahm, K.B. BPC 157 rescued NSAID-cytotoxicity via stabilizing intestinal permeability and enhancing cytoprotection. Curr. Pharm. Des. 2020, 26, 2971–2981. [Google Scholar] [CrossRef] [PubMed]
- Kang, E.A.; Han, Y.M.; An, J.M.; Park, Y.J.; Sikiric, P.; Kim, D.H.; Kwon, K.A.; Kim, Y.J.; Yang, D.; Tchah, H.; et al. BPC157 as potential agent rescuing from cancer cachexia. Curr. Pharm. Des. 2018, 24, 1947–1956. [Google Scholar] [CrossRef]
- Hsieh, M.J.; Lee, C.H.; Chueh, H.Y.; Chang, G.J.; Huang, H.Y.; Lin, Y.; Pang, J.S. Modulatory effects of BPC 157 on vasomotor tone and the activation of Src-Caveolin-1-endothelial nitric oxide synthase pathway. Sci. Rep. 2020, 10, 17078. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.H.; Tsai, W.C.; Lin, M.S.; Hsu, Y.H.; Pang, J.H.S. The promoting effect of pentadecapeptide BPC 157 on tendon healing involves tendon outgrowth, cell survival, and cell migration. J. Appl. Physiol. 2011, 110, 774–780. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.H.; Tsai, W.C.; Hsu, Y.H.; Pang, J.H.S. Pentadecapeptide BPC 157 enhances the growth hormone receptor expression in tendon fibroblasts. Molecules 2014, 19, 19066–19077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, T.; Zhang, K.; Sun, L.; Xue, X.; Zhang, C.; Shu, Z.; Mu, N.; Gu, J.; Zhang, W.; Wang, Y.; et al. Body protective compound-157 enhances alkali-burn wound healing in vivo and promotes proliferation, migration, and angiogenesis in vitro. Drug Des. Devel. Ther. 2015, 9, 2485–2499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsieh, M.J.; Liu, H.T.; Wang, C.N.; Huang, H.Y.; Lin, Y.; Ko, Y.S.; Wang, J.S.; Chang, V.H.; Pang, J.S. Therapeutic potential of pro-angiogenic BPC157 is associated with VEGFR2 activation and up-regulation. J. Mol. Med. 2017, 95, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Tkalcevic, V.I.; Cuzic, S.; Brajsa, K.; Mildner, B.; Bokulic, A.; Situm, K.; Perovic, D.; Glojnaric, I.; Parnham, M.J. Enhancement by PL 14736 of granulation and collagen organization in healing wounds and the potential role of egr-1 expression. Eur. J. Pharmacol. 2007, 570, 212–221. [Google Scholar] [CrossRef]
- Wang, X.Y.; Qu, M.; Duan, R.; Shi, D.; Jin, L.; Gao, J.; Wood, J.D.; Li, J.; Wang, G.D. Cytoprotective mechanism of the novel gastric peptide BPC157 in gastrointestinal tract and cultured enteric neurons and glial cells. Neurosci. Bull. 2019, 35, 167–170. [Google Scholar] [CrossRef]
- Sikiric, P.; Seiwerth, S.; Rucman, R.; Turkovic, B.; Rokotov, D.S.; Brcic, L.; Sever, M.; Klicek, R.; Radic, B.; Drmic, D.; et al. Stable gastric pentadecapeptide BPC 157-NO-system relation. Curr. Pharm. Des. 2014, 20, 1126–1135. [Google Scholar] [CrossRef]
- Sikiric, P.; Seiwerth, S.; Rucman, R.; Turkovic, B.; Rokotov, D.S.; Brcic, L.; Sever, M.; Klicek, R.; Radic, B.; Drmic, D.; et al. Toxicity by NSAIDs. Counteraction by stable gastric pentadecapeptide BPC 157. Curr. Pharm. Des. 2013, 19, 76–83. [Google Scholar]
- Konosic, S.; Petricevic, M.; Ivancan, V.; Konosic, L.; Goluza, E.; Krtalic, B.; Drmic, D.; Stupnisek, M.; Seiwerth, S.; Sikiric, P. Intragastric application of aspirin, clopidogrel, cilostazol, and BPC 157 in rats: Platelet aggregation and blood clot. Oxid. Med. Cell Longev. 2019, 2019, 9084643. [Google Scholar] [CrossRef]
- Luetic, K.; Sucic, M.; Vlainic, J.; Halle, Z.B.; Strinic, D.; Vidovic, T.; Luetic, F.; Marusic, M.; Gulic, S.; Pavelic, T.T.; et al. Cyclophosphamide induced stomach and duodenal lesions as a NO-system disturbance in rats: L-NAME, L-arginine, stable gastric pentadecapeptide BPC 157. Inflammopharmacology 2017, 25, 255–264. [Google Scholar] [CrossRef]
- Sucic, M.; Luetic, K.; Jandric, I.; Drmic, D.; Sever, A.Z.; Vuletic, L.B.; Halle, Z.B.; Strinic, D.; Kokot, A.; Seiwerth, R.S.; et al. Therapy of the rat hemorrhagic cystitis induced by cyclophosphamide. Stable gastric pentadecapeptide BPC 157, l-arginine, l-NAME. Eur. J. Pharmacol. 2019, 861, 172593. [Google Scholar] [CrossRef]
- Belosic Halle, Z.; Vlainic, J.; Drmic, D.; Strinic, D.; Luetic, K.; Sucic, M.; Medvidovic-Grubisic, M.; Pavelic Turudic, T.; Petrovic, I.; Seiwerth, S.; et al. Class side effects: Decreased pressure in the lower oesophageal and the pyloric sphincters after the administration of dopamine antagonists, neuroleptics, anti-emetics, L-NAME, pentadecapeptide BPC 157 and l-arginine. Inflammopharmacology 2017, 25, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Jelovac, N.; Sikirić, P.; Rucman, R.; Petek, M.; Perović, D.; Konjevoda, P.; Marović, A.; Seiwerth, S.; Grabarević, Z.; Sumajstorcić, J.; et al. A novel pentadecapeptide, BPC 157, blocks the stereotypy produced acutely by amphetamine and the development of haloperidol-induced supersensitivity to amphetamine. Biol. Psychiatry 1998, 43, 511–519. [Google Scholar] [CrossRef]
- Sikiric, P.; Jelovac, N.; Jelovac-Gjeldum, A.; Dodig, G.; Staresinic, M.; Anic, T.; Zoricic, I.; Rak, D.; Perovic, D.; Aralica, G.; et al. Pentadecapeptide BPC 157 attenuates chronic amphetamine-induced behavior disturbances. Acta Pharmacol. Sin. 2002, 23, 412–422. [Google Scholar]
- Zemba Cilic, A.; Zemba, M.; Cilic, M.; Balenovic, I.; Strbe, S.; Ilic, S.; Vukojevic, J.; Zoricic, Z.; Filipcic, I.; Kokot, A.; et al. Pentadecapeptide BPC 157 counteracts L-NAME-induced catalepsy. BPC 157, L-NAME, L-arginine, NO-relation, in the suited rat acute and chronic models resembling ‘positive-like’ symptoms of schizophrenia. Behav. Brain Res. 2021, 396, 112919. [Google Scholar] [CrossRef] [PubMed]
- Jelovac, N.; Sikiric, P.; Rucman, R.; Petek, M.; Marovic, A.; Perovic, D.; Seiwerth, S.; Mise, S.; Turkovic, B.; Dodig, G.; et al. Pentadecapeptide BPC 157 attenuates disturbances induced by neuroleptics: The effect on catalepsy and gastric ulcers in mice and rats. Eur. J. Pharmacol. 1999, 379, 19–31. [Google Scholar] [CrossRef]
- Sikiric, P.; Separovic, J.; Buljat, G.; Anic, T.; Stancic-Rokotov, D.; Mikus, D.; Duplancic, B.; Marovic, A.; Zoricic, I.; Prkacin, I.; et al. Gastric mucosal lesions induced by complete dopamine system failure in rats. The effects of dopamine agents, ranitidine, atropine, omeprazole and pentadecapeptide BPC 157. J. Physiol. Paris 2000, 94, 105–110. [Google Scholar] [CrossRef]
- Bilic, I.; Zoricic, I.; Anic, T.; Separovic, J.; Stancic-Rokotov, D.; Mikus, D.; Buljat, G.; Ivankovic, D.; Aralica, G.; Prkacin, I.; et al. Haloperidol-stomach lesions attenuation by pentadecapeptide BPC 157, omeprazole, bromocriptine, but not atropine, lansoprazole, pantoprazole, ranitidine, cimetidine and misoprostol in mice. Life Sci. 2001, 68, 1905–1912. [Google Scholar] [CrossRef]
- Strinic, D.; Belosic Halle, Z.; Luetic, K.; Nedic, A.; Petrovic, I.; Sucic, M.; Zivanovic Posilovic, G.; Balenovic, D.; Strbe, S.; Udovicic, M.; et al. BPC 157 counteracts QTc prolongation induced by haloperidol, fluphenazine, clozapine, olanzapine, quetiapine, sulpiride, and metoclopramide in rats. Life Sci. 2017, 186, 66–79. [Google Scholar] [CrossRef]
- Sikiric, P.; Separovic, J.; Buljat, G.; Anic, T.; Stancic-Rokotov, D.; Mikus, D.; Marovic, A.; Prkacin, I.; Duplancic, B.; Zoricic, I.; et al. The antidepressant effect of an antiulcer pentadecapeptide BPC 157 in Porsolt’s test and chronic unpredictable stress in rats. A comparison with antidepressants. J. Physiol. Paris 2000, 94, 99–104. [Google Scholar] [CrossRef]
- Tohyama, Y.; Sikirić, P.; Diksic, M. Effects of pentadecapeptide BPC157 on regional serotonin synthesis in the rat brain: Alpha-methyl-L-tryptophan autoradiographic measurements. Life Sci. 2004, 76, 345–357. [Google Scholar] [CrossRef]
- Verimer, T.; Goodale, D.B.; Long, J.P.; Flynn, J.R. Lithium effects on haloperidol-induced pre- and postsynaptic dopamine receptor supersensitivity. J. Pharm. Pharmacol. 1980, 32, 665–666. [Google Scholar] [CrossRef]
- Barisic, I.; Balenovic, D.; Klicek, R.; Radic, B.; Nikitovic, B.; Drmic, D.; Udovicic, M.; Strinic, D.; Bardak, D.; Berkopic, L.; et al. Mortal hyperkalemia disturbances in rats are NO-system related. The life saving effect of pentadecapeptide BPC 157. Regul. Pept. 2013, 181, 50–66. [Google Scholar] [CrossRef] [PubMed]
- Stambolija, V.; Stambolija, T.P.; Holjevac, J.K.; Murselovic, T.; Radonic, J.; Duzel, V.; Duplancic, B.; Uzun, S.; Zivanovic-Posilovic, G.; Kolenc, D.; et al. BPC 157: The counteraction of succinylcholine, hyperkalemia, and arrhythmias. Eur J. Pharmacol 2016, 781, 83–91. [Google Scholar] [CrossRef]
- Meinen, S.; Lin, S.; Rüegg, M.A.; Punga, A.R. Fatigue and muscle atrophy in a mouse model of myasthenia gravis is paralleled by loss of sarcolemmal nNOS. PLoS ONE 2012, 7, e44148. [Google Scholar] [CrossRef] [Green Version]
- Eustis, S.; Elwell, M.; MacKenzie, W. (Eds.) Boorman’s Pathology of the Rat 2nd Edition Reference and Atlas; Academic Press: Cambridge, MA, USA, 2017. [Google Scholar]
- Bona, E.; Hagberg, H.; Løberg, E.M.; Bågenholm, R.; Thoresen, M. Protective effects of moderate hypothermia after neonatal hypoxia-ischemia: Short- and long-term outcome. Pediatr. Res. 1998, 43, 738–745. [Google Scholar] [CrossRef] [Green Version]
- Murao, Y.; Loomis, W.; Wolf, P.; Hoyt, D.B.; Junger, W.G. Effect of dose of hypertonic saline on its potential to prevent lung tissue damage in a mouse model of hemorrhagic shock. Shock 2003, 20, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.Y.; Abdul, A.B.H.; Ibrahim, T.A.T.; Abdelwahab, S.I.; Elhassan, M.M.; Syam, M.M. Evaluation of acute toxicity and the effect of single injected doses of zerumbone on the kidney and liver functions in Sprague Dawley rats. Afr. J. Biotechnol. 2010, 9, 442–445. [Google Scholar]
- Chui, C.J.; McArdle, A.H.; Brown, R.; Scott, H.; Gurd, F. Intestinal mucosal lesion in low-flow states. Arch. Surg. 1970, 101, 478–483. [Google Scholar] [CrossRef]
- Lane, J.S.; Todd, K.E.; Lewis, M.P.; Gloor, B.; Ashley, S.W.; Reber, H.A.; McFadden, D.W.; Chandler, C.F. Interleukin-10 reduces the systemic inflammatory response in a murine model of intestinal ischemia/reperfusion. Surgery 1997, 122, 288–294. [Google Scholar] [CrossRef]
- Novinscak, T.; Brcic, L.; Staresinic, M.; Jukic, I.; Radic, B.; Pevec, D.; Mise, S.; Tomasovic, S.; Brcic, I.; Banic, T.; et al. Gastric pentadecapeptide BPC 157 as an effective therapy for muscle crush injury in the rat. Surg Today 2008, 38, 716–725. [Google Scholar] [CrossRef] [PubMed]
- Pevec, D.; Novinscak, T.; Brcic, L.; Sipos, K.; Jukic, I.; Staresinic, M.; Mise, S.; Brcic, I.; Kolenc, D.; Klicek, R.; et al. Impact of pentadecapeptide BPC 157 on muscle healing impaired by systemic corticosteroid application. Med. Sci. Monit. 2010, 16, BR81–BR88. [Google Scholar] [PubMed]
- Staresinic, M.; Petrovic, I.; Novinscak, T.; Jukic, I.; Pevec, D.; Suknaic, S.; Kokic, N.; Batelja, L.; Brcic, L.; Boban-Blagaic, A.; et al. Effective therapy of transected quadriceps muscle in rat: Gastric pentadecapeptide BPC 157. J. Orthop Res. 2006, 24, 1109–1117. [Google Scholar] [CrossRef]
- Orct, T.; Jurasović, J.; Micek, V.; Karaica, D.; Sabolić, I. Macro- and microelements in the rat liver, kidneys, and brain tissues; sex differences and effect of blood removal by perfusion in vivo. J. Trace Elem. Med. Biol. 2017, 40, 104–111. [Google Scholar] [CrossRef]
- Chen, P.; Tang, H.; Zhang, Q.; Xu, L.; Zhou, W.; Hu, X.; Deng, Y.; Zhang, L. Basic fibroblast growth factor (bFGF) protects the blood-brain barrier by binding of FGFR1 and Activating the ERK signaling pathway after intra-abdominal hypertension and traumatic brain injury. Med. Sci. Monit. 2020, 26, e922009. [Google Scholar]
- Malbrain, M.L.; Wilmer, A. The polycompartment syndrome: Towards an understanding of the interactions between different compartments! Intensive Care Med. 2007, 33, 1869–1872. [Google Scholar] [CrossRef]
- Langham, R.J.; Syme, G.J.; Syme, L.A. Voluntary lithium intake, antidotal thirst’ and concurrent behavior of rats. Br. J. Pharmacol. 1975, 55, 409–413. [Google Scholar] [CrossRef]
- Röther, J.; Waggie, K.; van Bruggen, N.; de Crespigny, A.J.; Moseley, M.E. Experimental cerebral venous thrombosis: Evaluation using magnetic resonance imaging. J. Cereb. Blood Flow Metab. 1996, 16, 1353–1361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Röttger, C.; Madlener, K.; Heil, M.; Gerriets, T.; Walberer, M.; Wessels, T.; Bachmann, G.; Kaps, M.; Stolz, E. Is heparin treatment the optimal management for cerebral venous thrombosis? Effect of abciximab, recombinant tissue plasminogen activator, and enoxaparin in experimentally induced superior sagittal sinus thrombosis. Stroke 2005, 36, 841–846. [Google Scholar] [CrossRef] [Green Version]
- Ben Saad, A.; Dalel, B.; Rjeibi, I.; Smida, A.; Ncib, S.; Zouari, N.; Zourgui, L. Phytochemical, antioxidant and protective effect of cactus cladodes extract against lithium-induced liver injury in rats. Pharm. Biol. 2017, 55, 516–525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toplan, S.; Ozdemir, S.; Tanriverdi, G.; Akyolcu, M.C.; Ozcelik, D.; Darıyerli, N. The effects of lithium administration on oxidant/antioxidant status in rats: Biochemical and histomorphological evaluations. Biol. Trace Elem. Res. 2016, 169, 279–284. [Google Scholar] [CrossRef]
- Eskandari, M.R.; Fard, J.K.; Hosseini, M.J.; Pourahmad, J. Glutathione mediated reductive activation and mitochondrial dysfunction play key roles in lithium induced oxidative stress and cytotoxicity in liver. Biometals 2012, 25, 863–873. [Google Scholar] [CrossRef]
- Nciri, R.; Allagui, M.S.; Bourogaa, E.; Saoudi, M.; Murat, J.C.; Croute, F.; Elfeki, A. Lipid peroxidation, antioxidant activities and stress protein (HSP72/73, GRP94) expression in kidney and liver of rats under lithium treatment. J. Physiol. Biochem. 2012, 68, 11–18. [Google Scholar] [CrossRef]
- Sung, C.C.; Chen, L.; Limbutara, K.; Jung, H.J.; Gilmer, G.G.; Yang, C.R.; Lin, S.H.; Khositseth, S.; Chou, C.L.; Knepper, M.A. RNA-Seq and protein mass spectrometry in microdissected kidney tubules reveal signaling processes initiating lithium-induced nephrogenic diabetes insipidus. Kidney Int. 2019, 96, 363–377. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Sharma, M.; Rana, A.K.; Joshi, R.; Swarnkar, M.K.; Acharya, V.; Singh, D. Deciphering key regulators involved in epilepsy-induced cardiac damage through whole transcriptome and proteome analysis in a rat model. Epilepsia 2021, 62, 504–516. [Google Scholar] [CrossRef]
- Abdel Hamid, O.I.; Ibrahim, E.M.; Hussien, M.H.; ElKhateeb, S.A. The molecular mechanisms of lithium-induced cardiotoxicity in male rats and its amelioration by N-acetyl cysteine. Hum. Exp. Toxicol 2020, 39, 696–711. [Google Scholar] [CrossRef]
- Sahin, O.; Sulak, O.; Yavuz, Y.; Uz, E.; Eren, I.; Ramazan Yilmaz, H.; Malas, M.A.; Altuntas, I.; Songur, A. Lithium-induced lung toxicity in rats: The effect of caffeic acid phenethyl ester (CAPE). Pathology 2006, 38, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Sironval, V.; Scagliarini, V.; Murugadoss, S.; Tomatis, M.; Yakoub, Y.; Turci, F.; Hoet, P.; Lison, D.; van den Brule, S. LiCoO(2) particles used in Li-ion batteries induce primary mutagenicity in lung cells via their capacity to generate hydroxyl radicals. Part. Fibre Toxicol. 2020, 17, 6. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, Y.; Guo, L.; Zhou, J.; Niu, J.; Wang, P.; Qiang, Y.; Liu, K.; Wen, Y.; Zhang, L.; et al. GPER1 modulates synaptic plasticity during the development of temporal lobe epilepsy in rats. Neurochem. Res. 2021, 46, 2019–2032. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Chen, S.; Qin, J.; Chen, B.; Ni, G.; Chen, Z.; Zhou, J.; Li, Z.; Ning, Y.; Wu, C.; et al. Pluronic P85-coated poly(butylcyanoacrylate) nanoparticles overcome phenytoin resistance in P-glycoprotein overexpressing rats with lithium-pilocarpine-induced chronic temporal lobe epilepsy. Biomaterials 2016, 97, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Sikiric, P.; Seiwerth, S.; Grabarevic, Z.; Rucman, R.; Petek, M.; Jagic, V.; Turkovic, B.; Rotkvic, I.; Mise, S.; Zoricic, I.; et al. The influence of a novel pentadecapeptide, BPC 157, on N(G)-nitro-L-arginine methylester and L-arginine effects on stomach mucosa integrity and blood pressure. Eur. J. Pharmacol. 1997, 332, 23–33. [Google Scholar] [CrossRef]
- Turkovic, B.; Sikiric, P.; Seiwerth, S.; Mise, S.; Anic, T.; Petek, M. Stable gastric pentadecapeptide BPC 157 studied for inflammatory bowel disease (PLD-116, PL14736, Pliva) induces nitric oxide synthesis. Gastroenterology 2004, 126, 287. [Google Scholar]
- Stupnisek, M.; Kokot, A.; Drmic, D.; Hrelec Patrlj, M.; Zenko Sever, A.; Kolenc, D.; Radic, B.; Suran, J.; Bojic, D.; Vcev, A.; et al. Pentadecapeptide BPC 157 reduces bleeding and thrombocytopenia after amputation in rats treated with heparin, warfarin, l-NAME and l-arginine. PLoS ONE 2015, 10, e0123454. [Google Scholar] [CrossRef] [PubMed]
- Stupnisek, M.; Franjic, S.; Drmic, D.; Hrelec, M.; Kolenc, D.; Radic, B.; Bojic, D.; Vcev, A.; Seiwerth, S.; Sikiric, P. Pentadecapeptide BPC 157 reduces bleeding time and thrombocytopenia after amputation in rats treated with heparin, warfarin or aspirin. Thromb. Res. 2012, 129, 652–659. [Google Scholar] [CrossRef]
- Drmic, D.; Kolenc, D.; Ilic, S.; Bauk, L.; Sever, M.; Zenko Sever, A.; Luetic, K.; Suran, J.; Seiwerth, S.; Sikiric, P. Celecoxib-induced gastrointestinal, liver and brain lesions in rats, counteraction by BPC 157 or l-arginine, aggravation by l-NAME. World J. Gastroenterol. 2017, 23, 5304–5312. [Google Scholar] [CrossRef] [PubMed]
- Ilic, S.; Brcic, I.; Mester, M.; Filipovicm, M.; Sever, M.; Klicek, R.; Barisic, I.; Radic, B.; Zoricic, Z.; Bilic, V.; et al. Over-dose insulin and stable gastric pentadecapeptide BPC 157. Attenuated gastric ulcers, seizures, brain lesions, hepatomegaly, fatty liver, breakdown of liver glycogen, profound hypoglycemia and calcification in rats. J. Physiol. Pharmacol. 2009, 60, 107–114. [Google Scholar] [PubMed]
- Ilic, S.; Drmic, D.; Zarkovic, K.; Kolenc, D.; Coric, M.; Brcic, L.; Klicek, R.; Radic, B.; Sever, M.; Djuzel, V.; et al. High hepatotoxic dose of paracetamol produces generalized convulsions and brain damage in rats. A counteraction with the stable gastric pentadecapeptide BPC 157 (PL 14736). J. Physiol. Pharmacol. 2010, 61, 241–250. [Google Scholar] [PubMed]
- Ilic, S.; Drmic, D.; Franjic, S.; Kolenc, D.; Coric, M.; Brcic, L.; Klicek, R.; Radic, B.; Sever, M.; Djuzel, V.; et al. Pentadecapeptide BPC 157 and its effects on a NSAID toxicity model: Diclofenac-induced gastrointestinal, liver, and encephalopathy lesions. Life Sci. 2011, 88, 535–542. [Google Scholar] [CrossRef]
- Ilic, S.; Drmic, D.; Zarkovic, K.; Kolenc, D.; Brcic, L.; Radic, B.; Djuzel, V.; Blagaic, A.B.; Romic, Z.; Dzidic, S.; et al. Ibuprofen hepatic encephalopathy, hepatomegaly, gastric lesion and gastric pentadecapeptide BPC 157 in rats. Eur. J. Pharmacol. 2011, 667, 322–329. [Google Scholar] [CrossRef]
- Lojo, N.; Rasic, Z.; Sever, A.Z.; Kolenc, D.; Vukusic, D.; Drmic, D.; Zoricic, I.; Sever, M.; Seiwerth, S.; Sikiric, P. Effects of diclofenac, L-NAME, L-arginine, and pentadecapeptide BPC157 on gastrointestinal, liver, and brain lesions, failed anastomosis, and intestinal adaptation deterioration in 24 h-short-bowel rats. PLoS ONE 2016, 11, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Sikiric, P.; Seiwerth, S.; Grabarevic, Z.; Rucman, R.; Petek, M.; Jagic, V.; Turkovic, B.; Rotkvic, I.; Mise, S.; Zoricic, I.; et al. Pentadecapeptide BPC 157 positively affects both non-steroidal anti-inflammatory agent-induced gastrointestinal lesions and adjuvant arthritis in rats. J. Physiol. Paris 1997, 91, 113–122. [Google Scholar] [CrossRef]
- Balenovic, D.; Bencic, M.L.; Udovicic, M.; Simonji, K.; Hanzevacki, J.S.; Barisic, I.; Kranjcevic, S.; Prkacin, I.; Coric, V.; Brcic, L.; et al. Inhibition of methyldigoxin-induced arrhythmias by pentadecapeptide BPC 157: A relation with NO-system. Regul. Pept. 2009, 156, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Lovric-Bencic, M.; Sikiric, P.; Hanzevacki, J.S.; Seiwerth, S.; Rogic, D.; Kusec, V.; Aralica, G.; Konjevoda, P.; Batelja, L.; Blagaic, A.B. Doxorubicine-congestive heart failure-increased big endothelin-1 plasma concentration: Reversal by amlodipine, losartan, and gastric pentadecapeptide BPC157 in rat and mouse. J. Pharmacol. Sci. 2004, 95, 19–26. [Google Scholar] [CrossRef] [Green Version]
- Zivanovic-Posilovic, G.; Balenovic, D.; Barisic, I.; Strinic, D.; Stambolija, V.; Udovicic, M.; Uzun, S.; Drmic, D.; Vlainic, J.; Bencic, M.L.; et al. Stable gastric pentadecapeptide BPC 157 and bupivacaine. Eur. J. Pharmacol. 2016, 793, 56–65. [Google Scholar] [CrossRef]
- Grabarevic, Z.; Tisljar, M.; Artukovic, B.; Bratulic, M.; Dzaja, P.; Seiwerth, S.; Sikiric, P.; Peric, J.; Geres, D.; Kos, J. The influence of BPC 157 on nitric oxide agonist and antagonist induced lesions in broiler chicken. J. Physiol. Paris 1997, 91, 139–149. [Google Scholar] [CrossRef]
- Udovicic, M.; Sever, M.; Kavur, L.; Loncaric, K.; Barisic, I.; Balenovic, D.; Zivanovic Posilovic, G.; Strinic, D.; Uzun, S.; Batelja Vuletic, L.; et al. Stable gastric pentadecapeptide BPC 157 therapy for monocrotaline-induced pulmonary hypertension in rats leads to prevention and reversal. Biomedicines 2021, 9, 822. [Google Scholar] [CrossRef]
- Stancic-Rokotov, D.; Slobodnjak, Z.; Aralica, J.; Aralica, G.; Perovic, D.; Staresinic, M.; Gjurasin, M.; Anic, T.; Zoricic, I.; Buljat, G.; et al. Lung lesions and anti-ulcer agents beneficial effect: Anti-ulcer agents pentadecapeptide BPC 157, ranitidine, omeprazole and atropine ameliorate lung lesion in rats. J. Physiol. Paris 2001, 95, 303–308. [Google Scholar] [CrossRef]
- Fernandes, S.C.; Braga Ade, F.; Braga, F.S.; Loyola, Y.C.; Souza, S.R.; Barcelos, C.C. Influence of lithium on the neuromuscular blockade produced by atracurium and cisatracurium: Study on rat phrenic nerve-diaphragm preparations. Rev. Bras. Anestesiol 2007, 57, 289–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dehpour, A.R.; Samadian, T.; Rassaee, N. Diabetic rats show more resistance to neuromuscular blockade induced by aminoglycoside antibiotics. Gen. Pharmacol. 1993, 24, 1415–1418. [Google Scholar] [CrossRef]
- Dehpour, A.R.; Samadian, T.; Roushanzamir, F. Interaction of aminoglycoside antibiotics and lithium at the neuromuscular junctions. Drugs Exp. Clin. Res. 1992, 18, 383–387. [Google Scholar] [PubMed]
- Klicek, R.; Kolenc, D.; Suran, J.; Drmic, D.; Brcic, L.; Aralica, G.; Sever, M.; Holjevac, J.; Radic, B.; Turudic, T.; et al. Stable gastric pentadecapeptide BPC 157 heals cysteamine-colitis and colon-colon-anastomosis and counteracts cuprizone brain injuries and motor disability. J. Physiol. Pharmacol. 2013, 64, 597–612. [Google Scholar] [PubMed]
- Girardi, P.; Brugnoli, R.; Manfredi, G.; Sani, G. Lithium in bipolar disorder: Optimizing therapy using prolonged-release formulations. Drugs R D 2016, 16, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Malhi, G.S.; Tanious, M.; Das, P.; Coulston, C.M.; Berk, M. Potential mechanisms of action of lithium in bipolar disorder. Current understanding. CNS Drugs 2013, 27, 135–153. [Google Scholar] [CrossRef]
- Smith, D.F. Lithium and motor activity of animals: Effects and possible mechanism of action. Int. Pharm. 1980, 15, 197–217. [Google Scholar] [CrossRef] [PubMed]
- Matussek, N.; Linsmayer, M. The effect of lithium and amphetamine on desmethylimipramine-Ro 4-1284 induced motor hyperactivity. Life Sci. 1968, 7, 371–375. [Google Scholar] [CrossRef]
- Berggren, U.; Tallstedt, L.; Ahlenius, S.; Engel, J. The effect of lithium on amphetamine-induced locomotor stimulation. Psychopharmacology 1978, 59, 41–45. [Google Scholar] [CrossRef]
- Petersen, K.P. Effect of age and route of administration on LD50 of lithium chloride in the rat. Acta Pharmacol. Toxicol. 1980, 47, 351–354. [Google Scholar] [CrossRef]
- Guidance for Industry, Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers, Pharmacology and Toxicology, U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER), July 2005. Available online: http://www.fda.gov/cder/guidance/index.htm (accessed on 19 October 2021).
- Cade, J.F.J. Lithium salts in the treatment of psychotic excitement. Med. J. Aust. 1949, 2, 349–352. [Google Scholar] [CrossRef] [Green Version]
- Veljaca, M.; Pavić-Sladoljev, D.; Mildner, B.; Brajsa, K.; Krnic, Z.; Bubenik, M.; Stipanicic, S.; Tabak-Slosic, M.; Brnic, L.; Khan, Z.; et al. Safety, tolerability and pharmacokinetics of PL 14736, a novel agent for treatment of ulcerative colitis, in healthy male volunteers. Gut 2003, 51 (Suppl. III), A309. [Google Scholar]
- Ruenzi, M.; Stolte, M.; Veljaca, M.; Oreskovic, K.; Peterson, J.; Ulcerative Colitis Study Group. A multicenter, randomized, double blind, placebo controlled phase II study of PL 14736 enema in the treatment of mild-to-moderate ulcerative colitis. Gastroenterology 2005, 128, 584. [Google Scholar]
- Veljaca, M.; Lesch, C.A.; Pllana, R.; Sanchez, B.; Chan, K.; Guglietta, A. BPC-15 reduces trinitrobenzene sulfonic acid-induced colonic damage in rats. J. Pharmacol. Exp. Ther. 1995, 272, 417–422. [Google Scholar]
- Tlak Gajger, I.; Ribarić, J.; Smodiš Škerl, M.; Vlainić, J.; Sikirić, P. Stable gastric pentadecapeptide BPC 157 in honeybee (Apis mellifera) therapy, to control Nosema ceranae invasions in apiary conditions. J. Vet. Pharmacol. Ther. 2018, 41, 614–621. [Google Scholar] [CrossRef]
- Sikiric, P.; Drmic, D.; Sever, M.; Klicek, R.; Blagaic, A.B.; Tvrdeic, A.; Kralj, T.; Kovac, K.K.; Vukojevic, J.; Siroglavic, M.; et al. Fistulas healing. Stable gastric pentadecapeptide BPC 157 therapy. Curr. Pharm. Des. 2020, 26, 2991–3000. [Google Scholar] [CrossRef] [PubMed]
- Seiwerth, S.; Rucman, R.; Turkovic, B.; Sever, M.; Klicek, R.; Radic, B.; Drmic, D.; Stupnisek, M.; Misic, M.; Vuletic, L.B.; et al. BPC 157 and standard angiogenic growth factors. Gastrointestinal tract healing, lessons from tendon, ligament, muscle and bone healing. Curr. Pharm. Des. 2018, 24, 1972–1989. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Sun, L.; Ren, F.; Huang, P.; Tian, Z.; Cui, J.; Zhang, W.; Wang, S.; Zhang, K.; He, L.; et al. Preclinical safety evaluation of body protective compound-157, a potential drug for treating various wounds. Regul. Toxicol. Pharmacol. 2020, 114, 104665. [Google Scholar] [CrossRef] [PubMed]
- Bosche, B.; Molcanyi, M.; Rej, S.; Doeppner, T.R.; Obermann, M.; Müller, D.J.; Das, A.; Hescheler, J.; Macdonald, R.L.; Noll, T.; et al. Low-dose lithium stabilizes human endothelial barrier by decreasing MLC phosphorylation and universally augments cholinergic vasorelaxation capacity in a direct manner. Front. Physiol. 2016, 7, 593. [Google Scholar] [CrossRef] [Green Version]
- Kan, C.; Liu, A.; Fang, H.; Dirsch, O.; Dahmen, U.; Boettcher, M. Induction of autophagy reduces ischemia/reperfusion injury in steatotic rat livers. J. Surg. Res. 2017, 216, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Fang, H.; Dahmen, U.; Dirsch, O. Chronic lithium treatment protects against liver ischemia/reperfusion injury in rats. Liver Transpl. 2013, 19, 762–772. [Google Scholar] [CrossRef]
- Yildirim, S.; Celikezen, F.C.; Oto, G.; Sengul, E.; Bulduk, M.; Tasdemir, M.; Ali Cinar, D. An investigation of protective effects of lithium borate on blood and histopathological parameters in acute cadmium-inudced rats. Biol. Trace Elem. Res. 2018, 182, 287–294. [Google Scholar] [CrossRef]
- Vošahlíková, M.; Roubalová, L.; Brejchová, J.; Alda, M.; Svoboda, P. Therapeutic lithium alters polar head-group region of lipid bilayer and prevents lipid peroxidation in forebrain cortex of sleep-deprived rats. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2021, 1866, 158962. [Google Scholar] [CrossRef]
- Kanazawa, L.K.S.; Radulski, D.R.; Pereira, G.S.; Prickaerts, J.; Schwarting, R.K.W.; Acco, A.; Andreatini, R. Andrographolide blocks 50-kHz ultrasonic vocalizations, hyperlocomotion and oxidative stress in an animal model of mania. J. Psychiatr. Res. 2021, 139, 91–98. [Google Scholar] [CrossRef]
- Albayrak, A.; Halici, Z.; Polat, B.; Karakus, E.; Cadirci, E.; Bayir, Y.; Kunak, S.; Karcioglu, S.S.; Yigit, S.; Unal, D.; et al. Protective effects of lithium: A new look at an old drug with potential antioxidative and anti-inflammatory effects in an animal model of sepsis. Int. Immunopharmacol. 2013, 16, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Khairova, R.; Pawar, R.; Salvadore, G.; Juruena, M.F.; de Sousa, R.T.; Soeiro-de-Souza, M.G.; Salvador, M.; Zarate, C.A.; Gattaz, W.F.; Machado-Vieira, R. Effects of lithium on oxidative stress parameters in healthy subjects. Mol. Med. Rep. 2012, 5, 680–682. [Google Scholar] [PubMed]
- Mezni, A.; Aoua, H.; Khazri, O.; Limam, F.; Aouani, E. Lithium induced oxidative damage and inflammation in the rat’s heart: Protective effect of grape seed and skin extract. Biomed. Pharmacother. 2017, 95, 1103–1111. [Google Scholar] [CrossRef] [PubMed]



















| Brain Area | Grading | Percent Area Affected | Morphological Changes |
|---|---|---|---|
| Cerebral and cerebellar cortex, hypothalamus, thalamus, hippocampus | 1 | ≤10 | Small, patchy, complete, or incomplete infarcts |
| 2 | 20–30 | Partly confluent complete or incomplete infarcts | |
| 3 | 40–60 | Large confluent compete infarcts | |
| 4 | >75 | In cortex; total disintegration of the tissue, in hypothalamus, thalamus, hippocampus; large complete infarcts | |
| Cerebral and cerebellar cortex, hypothalamus, thalamus, hippocampus | 1 | ≤20 | A few karyopyknotic of neuronal cells |
| 2 | 50 | Patchy areas of karyopyknotic areas | |
| 3 | 75 | More extensive of karyopyknotic areas | |
| 4 | 100 | Complete infarction |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strbe, S.; Gojkovic, S.; Krezic, I.; Zizek, H.; Vranes, H.; Barisic, I.; Strinic, D.; Orct, T.; Vukojevic, J.; Ilic, S.; et al. Over-Dose Lithium Toxicity as an Occlusive-like Syndrome in Rats and Gastric Pentadecapeptide BPC 157. Biomedicines 2021, 9, 1506. https://doi.org/10.3390/biomedicines9111506
Strbe S, Gojkovic S, Krezic I, Zizek H, Vranes H, Barisic I, Strinic D, Orct T, Vukojevic J, Ilic S, et al. Over-Dose Lithium Toxicity as an Occlusive-like Syndrome in Rats and Gastric Pentadecapeptide BPC 157. Biomedicines. 2021; 9(11):1506. https://doi.org/10.3390/biomedicines9111506
Chicago/Turabian StyleStrbe, Sanja, Slaven Gojkovic, Ivan Krezic, Helena Zizek, Hrvoje Vranes, Ivan Barisic, Dean Strinic, Tatjana Orct, Jaksa Vukojevic, Spomenko Ilic, and et al. 2021. "Over-Dose Lithium Toxicity as an Occlusive-like Syndrome in Rats and Gastric Pentadecapeptide BPC 157" Biomedicines 9, no. 11: 1506. https://doi.org/10.3390/biomedicines9111506







