Genetically Encoded Fluorescent Biosensors for Biomedical Applications
Abstract
:1. Introduction
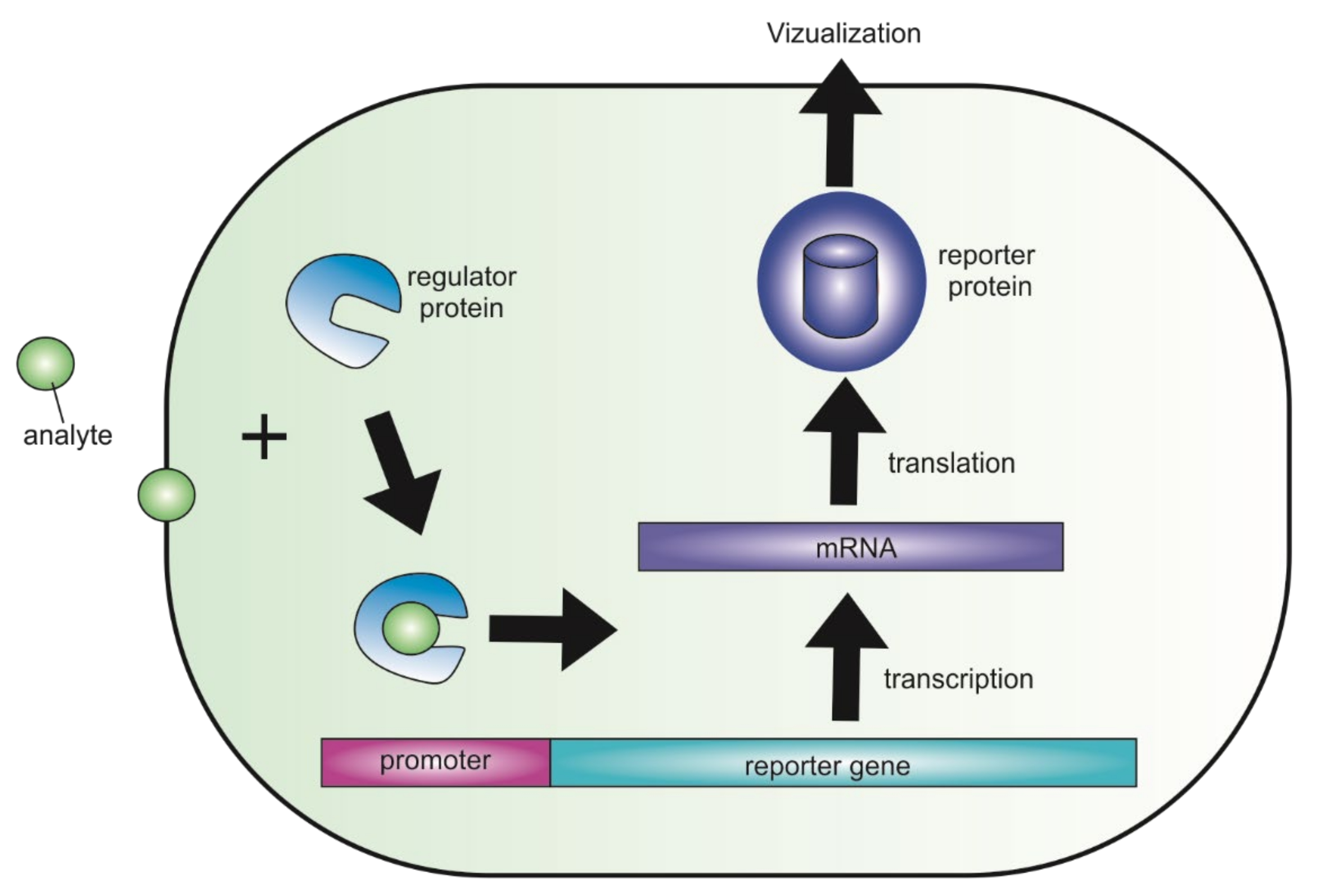
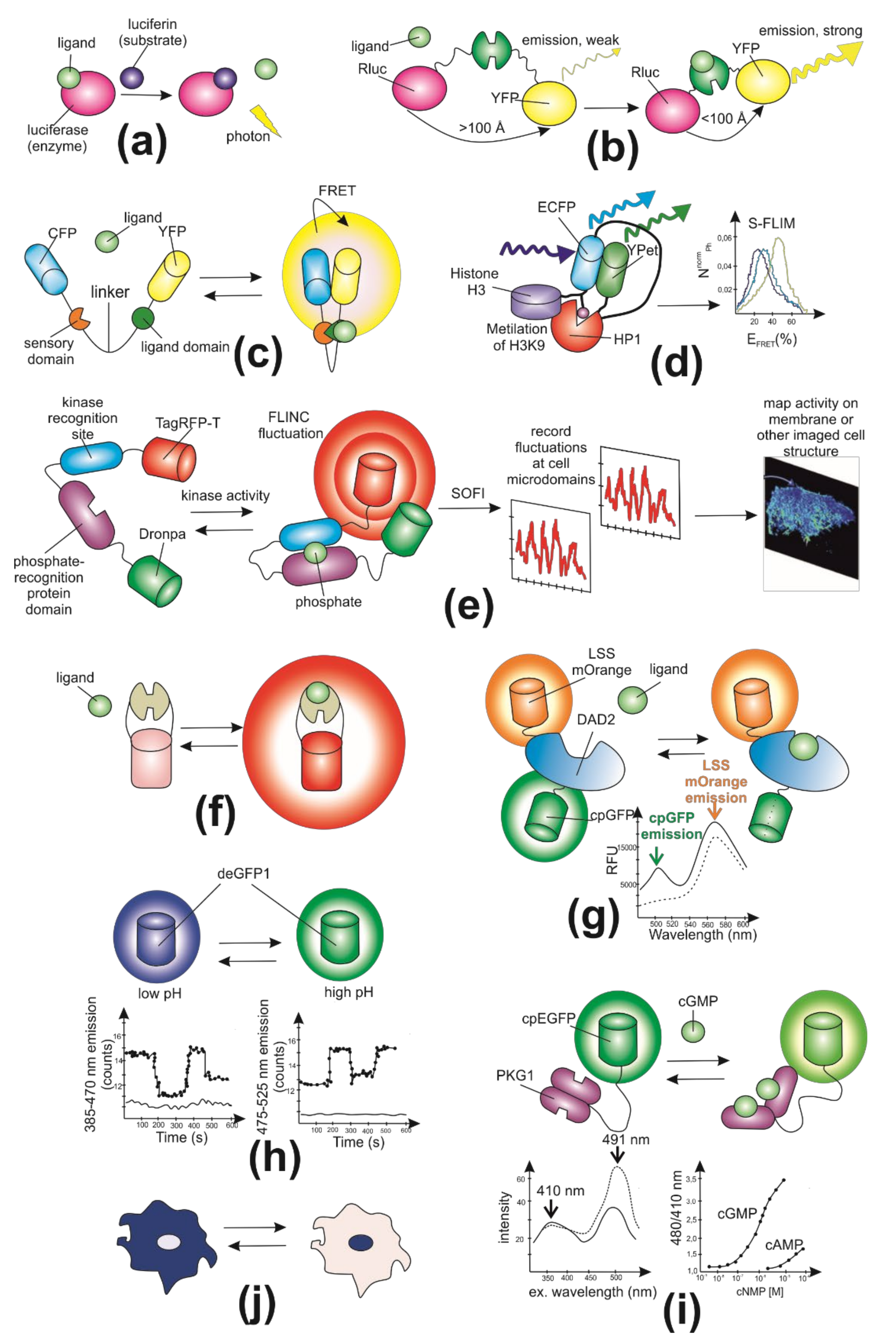
2. General Characteristics of Genetically Encoded Fluorescent Biosensors
3. Key Parameters in Biosensor Design
- (1)
- Ease of use. One of the critical factors when choosing a biosensor is the availa-bility of the equipment needed for the measurements. For example, ratiometric biosensors based on FRET require sophisticated microscopic equipment to quickly (or simultaneously) collect data from two or more fluorescence channels. At the same time, for biosensors that measure fluorescence intensity, uninvolved instruments are needed to collect data. It is also necessary to consider the availability of the materials used. Finally, many luminescent biosensors require an additional step of adding a substrate such as coelenterazine.
- (2)
- Sensitivity of a biosensor is the dynamic range, which is the ratio between the minimum and maximum values that the biosensor can detect. The sensitivity of the biosensor used is application-specific. For example, to measure constant concentrations of a molecule or ion, it is recommended to choose a sensor with a dissociation constant equal to or close to the expected concentration. An even more rigorous approach is to measure the concentration at rest using several biosensors, each of which has a slightly different dissociation constant value. If the goal is to measure a change in concentration or activity when the signal is expected to be weak, then the biosensor with the highest dynamic range and dissociation constant value, at which the concentration change will be within 5 dissociation constant, will be most sensitive.
- (3)
- The detection limit of a sensor is defined often by the range in which the binding isotherm has near-linear characteristics, that is, between 10% and 90% saturation; often two orders of magnitude for a FRET sensor with a Hill coefficient of 1. Due to the dramatic intensity changes caused by ligand binding in fluorescent sensors, the dynamic range can be extended beyond the linear range. Signal-to-background ratio depends not only on sensor properties, but importantly on the background fluorescence in the specimen under investigation. Autofluorescence can differ substantially. Handling, stress conditions can trigger production of fluorigenic compounds.
- (4)
- Selectivity and specificity are two important features to consider when developing or using existing biosensors. Selectivity and specificity are determined by the structure and conformational flexibility of a protein. The terms are often used interchangeably, but are best used for different aspects: specificity is defined as how restrictive a protein is in its choice of substrate (fewer vs. more substrates). Selectivity is defined by substrate properties and is a quantitative measure of the rate constants for interaction of the protein with the substrate [15]. As Peracchi put it elegantly [16]: “Substrate specificity cannot be absolute and is inherently limited. … discrimination between alternative substrates can be relatively low, … Substrate promiscuity helps to fuel an ‘underground’ network of reactions which may represent a basis for further evolution and diversification of metabolism”. Notably, binding protein selectivity is tested with only few analytes, while the in vivo environment presents a highly complex set of molecules. Rarely, binding protein affinity is suitable for in vivo analyses. Affinity has to be adjusted by mutagenesis and affinity series of the sensors might be required. Mutations in the binding pocket may impact ligand selectivity.
- (5)
- Cytotoxicity and biostability. From first principles, one could argue that the higher the sensor level is, the brighter the signal and the better the ability to discern changes in analyte levels or activity. Strong promoters provide high levels, yet besides likely triggering gene silencing, high sensor levels impact physiology. While sensors are minimally invasive, they can affect cellular functions, either by acting as scavengers or by interacting with other cellular components; essentially posing an “Observer Effect” problem. In the absence of novel, even less invasive technologies, it will be important perform proper controls. Biostability of the biosensor is a very important characteristic especially for biosensors used for continuous monitoring. This feature determines the ability of the biosensor device to resist change in its performance over a period of time in response to interruptions arising from external factors.
4. Biomedical Applications
4.1. Cancer
4.1.1. Protein Kinase Activity
4.1.2. pH Level
4.1.3. Other
4.2. Neurological Disorders
4.2.1. Measurements of Lactate Level
4.2.2. pH Level
4.2.3. Other
4.3. Inflammation
4.4. Other Diseases
5. Advantages, Challenges, and Prospects for the Use of Genetically Encoded Fluorescent Biosensors in Biomedical Research
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gupta, N.; Renugopalakrishnan, V.; Liepmann, D.; Paulmurugan, R.; Malhotra, B.D. Cell-based biosensors: Recent trends, challenges and future perspectives. Biosens. Bioelectron. 2019, 141, 111435. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wu, C.; Cai, H.; Hu, N.; Zhou, J.; Wang, P. Cell-based biosensors and their application in biomedicine. Chem. Rev. 2014, 114, 6423–6461. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, P.; Franz, B.; Bhunia, A.K. Mammalian cell-based sensor system. Adv. Biochem. Eng. Biotechnol. 2010, 117, 21–55. [Google Scholar]
- Greenwald, E.C.; Mehta, S.; Zhang, J. Genetically Encoded Fluorescent Biosensors Illuminate the Spatiotemporal Regulation of Signaling Networks. Chem. Rev. 2018, 118, 11707–11794. [Google Scholar] [CrossRef]
- Yeh, H.W.; Ai, H.W. Development and Applications of Bioluminescent and Chemiluminescent Reporters and Biosensors. Annu. Rev. Anal. Chem. 2019, 12, 129–150. [Google Scholar] [CrossRef] [Green Version]
- Sekar, R.B.; Periasamy, A. Fluorescence resonance energy transfer (FRET) microscopy imaging of live cell protein localizations. J. Cell. Biol. 2003, 160, 629–633. [Google Scholar] [CrossRef] [Green Version]
- San Martin, A.; Ceballo, S.; Ruminot, I.; Lerchundi, R.; Frommer, W.B.; Barros, L.F. A genetically encoded FRET lactate sensor and its use to detect the Warburg effect in single cancer cells. PLoS ONE 2013, 8, e57712. [Google Scholar] [CrossRef] [PubMed]
- Murakoshi, H.; Shibata, A.C.E. ShadowY: A dark yellow fluorescent protein for FLIM-based FRET measurement. Sci. Rep. 2017, 7, 6791. [Google Scholar] [CrossRef] [PubMed]
- Mo, G.C.; Ross, B.; Hertel, F.; Manna, P.; Yang, X.; Greenwald, E.; Booth, C.; Plummer, A.M.; Tenner, B.; Chen, Z.; et al. Genetically encoded biosensors for visualizing live-cell biochemical activity at super-resolution. Nat. Methods 2017, 14, 427–434. [Google Scholar] [CrossRef] [Green Version]
- Zagaynova, E.V.; Druzhkova, I.N.; Mishina, N.M.; Ignatova, N.I.; Dudenkova, V.V.; Shirmanova, M.V. Imaging of Intracellular pH in Tumor Spheroids Using Genetically Encoded Sensor SypHer2. Adv. Exp. Med. Biol. 2017, 1035, 105–119. [Google Scholar] [PubMed]
- Scipioni, L.; Rossetta, A.; Tedeschi, G.; Gratton, E. Phasor S-FLIM: A new paradigm for fast and robust spectral fluorescence lifetime imaging. Nat. Methods 2021, 18, 542–550. [Google Scholar] [CrossRef] [PubMed]
- Chesterfield, R.J.; Whitfield, J.H.; Pouvreau, B.; Cao, D.; Alexandrov, K.; Beveridge, C.A.; Vickers, C.E. Rational Design of Novel Fluorescent Enzyme Biosensors for Direct Detection of Strigolactones. ACS Synth. Biol. 2020, 9, 2107–2118. [Google Scholar] [CrossRef]
- Hanson, G.T.; McAnaney, T.B.; Park, E.S.; Rendell, M.E.; Yarbrough, D.K.; Chu, S.; Xi, L.; Boxer, S.G.; Montrose, M.H.; Remington, S.J. Green fluorescent protein variants as ratiometric dual emission pH sensors. 1. Structural characterization and preliminary application. Biochemistry 2002, 41, 15477–15488. [Google Scholar] [CrossRef] [PubMed]
- Nausch, L.W.; Ledoux, J.; Bonev, A.D.; Nelson, M.T.; Dostmann, W.R. Differential patterning of cGMP in vascular smooth muscle cells revealed by single GFP-linked biosensors. Proc. Natl. Acad. Sci. USA 2008, 105, 365–370. [Google Scholar] [CrossRef] [Green Version]
- Butre, C.I.; Sforza, S.; Gruppen, H.; Wierenga, P.A. Introducing enzyme selectivity: A quantitative parameter to describe enzymatic protein hydrolysis. Anal. Bioanal. Chem. 2014, 406, 5827–5841. [Google Scholar] [CrossRef]
- Peracchi, A. The Limits of Enzyme Specificity and the Evolution of Metabolism. Trends Biochem. Sci. 2018, 43, 984–996. [Google Scholar] [CrossRef] [PubMed]
- Dagogo-Jack, I.; Shaw, A.T. Tumour heterogeneity and resistance to cancer therapies. Nat. Rev. Clin. Oncol. 2018, 15, 81–94. [Google Scholar] [CrossRef]
- Prasetyanti, P.R.; Medema, J.P. Intra-tumor heterogeneity from a cancer stem cell perspective. Mol. Cancer 2017, 16, 41. [Google Scholar] [CrossRef] [Green Version]
- Meacham, C.E.; Morrison, S.J. Tumour heterogeneity and cancer cell plasticity. Nature 2013, 501, 328–337. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Ma, Y.; Taylor, S.S.; Tsien, R.Y. Genetically encoded reporters of protein kinase A activity reveal impact of substrate tethering. Proc. Natl. Acad. Sci. USA 2001, 98, 14997–15002. [Google Scholar] [CrossRef] [Green Version]
- Herbst, K.J.; Allen, M.D.; Zhang, J. Luminescent kinase activity biosensors based on a versatile bimolecular switch. J. Am. Chem. Soc. 2011, 133, 5676–5679. [Google Scholar] [CrossRef] [Green Version]
- Kurokawa, K.; Mochizuki, N.; Ohba, Y.; Mizuno, H.; Miyawaki, A.; Matsuda, M. A pair of fluorescent resonance energy transfer-based probes for tyrosine phosphorylation of the CrkII adaptor protein in vivo. J. Biol. Chem. 2001, 276, 31305–31310. [Google Scholar] [CrossRef] [Green Version]
- Weitsman, G.; Mitchell, N.J.; Evans, R.; Cheung, A.; Kalber, T.L.; Bofinger, R.; Fruhwirth, G.O.; Keppler, M.; Wright, Z.V.F.; Barber, P.R.; et al. Detecting intratumoral heterogeneity of EGFR activity by liposome-based in vivo transfection of a fluorescent biosensor. Oncogene 2017, 36, 3618–3628. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Hupfeld, C.J.; Taylor, S.S.; Olefsky, J.M.; Tsien, R.Y. Insulin disrupts beta-adrenergic signalling to protein kinase A in adipocytes. Nature 2005, 437, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.D.; Zhang, J. Subcellular dynamics of protein kinase A activity visualized by FRET-based reporters. Biochem. Biophys. Res. Commun. 2006, 348, 716–721. [Google Scholar] [CrossRef]
- Stefan, E.; Aquin, S.; Berger, N.; Landry, C.R.; Nyfeler, B.; Bouvier, M.; Michnick, S.W. Quantification of dynamic protein complexes using Renilla luciferase fragment complementation applied to protein kinase A activities in vivo. Proc. Natl. Acad. Sci. USA 2007, 104, 16916–16921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Saulnier, J.L.; Yellen, G.; Sabatini, B.L. A PKA activity sensor for quantitative analysis of endogenous GPCR signaling via 2-photon FRET-FLIM imaging. Front. Pharmacol. 2014, 5, 56. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.F.; Liu, B.; Hong, I.; Mo, A.; Roth, R.H.; Tenner, B.; Lin, W.; Zhang, J.Z.; Molina, R.S.; Drobizhev, M.; et al. An ultrasensitive biosensor for high-resolution kinase activity imaging in awake mice. Nat. Chem. Biol. 2021, 17, 39–46. [Google Scholar] [CrossRef]
- Tang, S.; Yasuda, R. Imaging ERK and PKA Activation in Single Dendritic Spines during Structural Plasticity. Neuron 2017, 93, 1315–1324.e3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, X.; Zhang, J. Spatiotemporal analysis of differential Akt regulation in plasma membrane microdomains. Mol. Biol. Cell 2008, 19, 4366–4373. [Google Scholar] [CrossRef] [Green Version]
- Violin, J.D.; Zhang, J.; Tsien, R.Y.; Newton, A.C. A genetically encoded fluorescent reporter reveals oscillatory phosphorylation by protein kinase C. J. Cell. Biol. 2003, 161, 899–909. [Google Scholar] [CrossRef] [PubMed]
- Ting, A.Y.; Kain, K.H.; Klemke, R.L.; Tsien, R.Y. Genetically encoded fluorescent reporters of protein tyrosine kinase activities in living cells. Proc. Natl. Acad. Sci. USA 2001, 98, 15003–15008. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Botvinick, E.L.; Zhao, Y.; Berns, M.W.; Usami, S.; Tsien, R.Y.; Chien, S. Visualizing the mechanical activation of Src. Nature 2005, 434, 1040–1045. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, T.; Kondo, T.; Darmanin, S.; Tsuda, M.; Tanaka, S.; Tobiume, M.; Asaka, M.; Ohba, Y. A novel FRET-based biosensor for the measurement of BCR-ABL activity and its response to drugs in living cells. Clin. Cancer Res. 2010, 16, 3964–3975. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Clister, T.L.; Lowry, P.R.; Seldin, M.M.; Wong, G.W.; Zhang, J. Dynamic Visualization of mTORC1 Activity in Living Cells. Cell Rep. 2015, 10, 1767–1777. [Google Scholar] [CrossRef] [Green Version]
- Azad, T.; Janse van Rensburg, H.J.; Lightbody, E.D.; Neveu, B.; Champagne, A.; Ghaffari, A.; Kay, V.R.; Hao, Y.; Shen, H.; Yeung, B.; et al. A LATS biosensor screen identifies VEGFR as a regulator of the Hippo pathway in angiogenesis. Nat. Commun. 2018, 9, 1061. [Google Scholar] [CrossRef] [PubMed]
- Tomida, T.; Takekawa, M.; Saito, H. Oscillation of p38 activity controls efficient pro-inflammatory gene expression. Nat. Commun. 2015, 6, 8350. [Google Scholar] [CrossRef] [Green Version]
- Lapenna, S.; Giordano, A. Cell cycle kinases as therapeutic targets for cancer. Nat. Rev. Drug Discov. 2009, 8, 547–566. [Google Scholar] [CrossRef]
- Tunceroglu, A.; Matsuda, M.; Birge, R.B. Real-time fluorescent resonance energy transfer analysis to monitor drug resistance in chronic myelogenous leukemia. Mol. Cancer Ther. 2010, 9, 3065–3073. [Google Scholar] [CrossRef] [Green Version]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Compan, V.; Pierredon, S.; Vanderperre, B.; Krznar, P.; Marchiq, I.; Zamboni, N.; Pouyssegur, J.; Martinou, J.C. Monitoring Mitochondrial Pyruvate Carrier Activity in Real Time Using a BRET-Based Biosensor: Investigation of the Warburg Effect. Mol. Cell 2015, 59, 491–501. [Google Scholar] [CrossRef] [Green Version]
- Moll, U.M.; Petrenko, O. The MDM2-p53 interaction. Mol. Cancer Res. 2003, 1, 1001–1008. [Google Scholar]
- Dudgeon, D.D.; Shinde, S.; Hua, Y.; Shun, T.Y.; Lazo, J.S.; Strock, C.J.; Giuliano, K.A.; Taylor, D.L.; Johnston, P.A.; Johnston, P.A. Implementation of a 220,000-compound HCS campaign to identify disruptors of the interaction between p53 and hDM2 and characterization of the confirmed hits. J. Biomol. Screen. 2010, 15, 766–782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porter, A.G.; Janicke, R.U. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999, 6, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Ip, L.; Luo, H.; Chang, D.C.; Luo, K.Q. A high throughput drug screen based on fluorescence resonance energy transfer (FRET) for anticancer activity of compounds from herbal medicine. Br. J. Pharmacol. 2007, 150, 321–334. [Google Scholar] [CrossRef]
- Kroemer, G.; Galluzzi, L.; Kepp, O.; Zitvogel, L. Immunogenic cell death in cancer therapy. Annu. Rev. Immunol. 2013, 31, 51–72. [Google Scholar] [CrossRef]
- Tanida, I.; Ueno, T.; Kominami, E. LC3 and Autophagy. Methods Mol. Biol. 2008, 445, 77–88. [Google Scholar] [PubMed]
- Liu, P.; Zhao, L.; Pol, J.; Levesque, S.; Petrazzuolo, A.; Pfirschke, C.; Engblom, C.; Rickelt, S.; Yamazaki, T.; Iribarren, K.; et al. Crizotinib-induced immunogenic cell death in non-small cell lung cancer. Nat. Commun. 2019, 10, 1486. [Google Scholar] [CrossRef] [PubMed]
- Lehn, A.; Gelauff, J.; Hoeritzauer, I.; Ludwig, L.; McWhirter, L.; Williams, S.; Gardiner, P.; Carson, A.; Stone, J. Functional neurological disorders: Mechanisms and treatment. J. Neurol. 2016, 263, 611–620. [Google Scholar] [CrossRef]
- Dugger, B.N.; Dickson, D.W. Pathology of Neurodegenerative Diseases. Cold Spring Harb. Perspect. Biol. 2017, 9, a028035. [Google Scholar] [CrossRef]
- Gladden, L.B. Lactate metabolism: A new paradigm for the third millennium. J. Physiol. 2004, 558, 5–30. [Google Scholar] [CrossRef]
- Shimizu, H.; Watanabe, E.; Hiyama, T.Y.; Nagakura, A.; Fujikawa, A.; Okado, H.; Yanagawa, Y.; Obata, K.; Noda, M. Glial Nax channels control lactate signaling to neurons for brain [Na+] sensing. Neuron 2007, 54, 59–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Machler, P.; Wyss, M.T.; Elsayed, M.; Stobart, J.; Gutierrez, R.; von Faber-Castell, A.; Kaelin, V.; Zuend, M.; San Martin, A.; Romero-Gomez, I.; et al. In Vivo Evidence for a Lactate Gradient from Astrocytes to Neurons. Cell Metab. 2016, 23, 94–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pierre, K.; Pellerin, L. Monocarboxylate transporters in the central nervous system: Distribution, regulation and function. J. Neurochem. 2005, 94, 1–14. [Google Scholar] [CrossRef]
- Sada, N.; Lee, S.; Katsu, T.; Otsuki, T.; Inoue, T. Epilepsy treatment. Targeting LDH enzymes with a stiripentol analog to treat epilepsy. Science 2015, 347, 1362–1367. [Google Scholar] [CrossRef]
- Solis-Maldonado, M.; Miro, M.P.; Acuna, A.I.; Covarrubias-Pinto, A.; Loaiza, A.; Mayorga, G.; Beltran, F.A.; Cepeda, C.; Levine, M.S.; Concha, I.I.; et al. Altered lactate metabolism in Huntington’s disease is dependent on GLUT3 expression. CNS Neurosci. Ther. 2018, 24, 343–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Contreras-Baeza, Y.; Ceballo, S.; Arce-Molina, R.; Sandoval, P.Y.; Alegria, K.; Barros, L.F.; San Martin, A. MitoToxy assay: A novel cell-based method for the assessment of metabolic toxicity in a multiwell plate format using a lactate FRET nanosensor, Laconic. PLoS ONE 2019, 14, e0224527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Putney, L.K.; Barber, D.L. Na-H exchange-dependent increase in intracellular pH times G2/M entry and transition. J. Biol. Chem. 2003, 278, 44645–44649. [Google Scholar] [CrossRef] [Green Version]
- Adams, D.S.; Masi, A.; Levin, M. H+ pump-dependent changes in membrane voltage are an early mechanism necessary and sufficient to induce Xenopus tail regeneration. Development 2007, 134, 1323–1335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Markvicheva, K.N.; Bilan, D.S.; Mishina, N.M.; Gorokhovatsky, A.Y.; Vinokurov, L.M.; Lukyanov, S.; Belousov, V.V. A genetically encoded sensor for H2O2 with expanded dynamic range. Bioorg. Med. Chem. 2011, 19, 1079–1084. [Google Scholar] [CrossRef] [PubMed]
- Matlashov, M.E.; Bogdanova, Y.A.; Ermakova, G.V.; Mishina, N.M.; Ermakova, Y.G.; Nikitin, E.S.; Balaban, P.M.; Okabe, S.; Lukyanov, S.; Enikolopov, G.; et al. Fluorescent ratiometric pH indicator SypHer2: Applications in neuroscience and regenerative biology. Biochim. Biophys. Acta 2015, 1850, 2318–2328. [Google Scholar] [CrossRef] [Green Version]
- Poburko, D.; Santo-Domingo, J.; Demaurex, N. Dynamic regulation of the mitochondrial proton gradient during cytosolic calcium elevations. J. Biol. Chem. 2011, 286, 11672–11684. [Google Scholar] [CrossRef] [Green Version]
- Ermakova, Y.G.; Pak, V.V.; Bogdanova, Y.A.; Kotlobay, A.A.; Yampolsky, I.V.; Shokhina, A.G.; Panova, A.S.; Marygin, R.A.; Staroverov, D.B.; Bilan, D.S.; et al. SypHer3s: A genetically encoded fluorescent ratiometric probe with enhanced brightness and an improved dynamic range. Chem. Commun. 2018, 54, 2898–2901. [Google Scholar] [CrossRef]
- Chebotarev, A.S.; Pochechuev, M.S.; Lanin, A.A.; Kelmanson, I.V.; Kotova, D.A.; Fetisova, E.S.; Panova, A.S.; Bilan, D.S.; Fedotov, A.B.; Belousov, V.V.; et al. Enhanced-contrast two-photon optogenetic pH sensing and pH-resolved brain imaging. J. Biophoton. 2021, 14, e202000301. [Google Scholar] [CrossRef]
- Raimondo, J.V.; Tomes, H.; Irkle, A.; Kay, L.; Kellaway, L.; Markram, H.; Millar, R.P.; Akerman, C.J. Tight Coupling of Astrocyte pH Dynamics to Epileptiform Activity Revealed by Genetically Encoded pH Sensors. J. Neurosci. 2016, 36, 7002–7013. [Google Scholar] [CrossRef]
- Ballabio, A.; Bonifacino, J.S. Lysosomes as dynamic regulators of cell and organismal homeostasis. Nat. Rev. Mol. Cell Biol. 2020, 21, 101–118. [Google Scholar] [CrossRef]
- Beyenbach, K.W.; Wieczorek, H. The V-type H+ ATPase: Molecular structure and function, physiological roles and regulation. J. Exp. Biol. 2006, 209, 577–589. [Google Scholar] [CrossRef] [Green Version]
- Clayton, E.L.; Mizielinska, S.; Edgar, J.R.; Nielsen, T.T.; Marshall, S.; Norona, F.E.; Robbins, M.; Damirji, H.; Holm, I.E.; Johannsen, P.; et al. Frontotemporal dementia caused by CHMP2B mutation is characterised by neuronal lysosomal storage pathology. Acta Neuropathol. 2015, 130, 511–523. [Google Scholar] [CrossRef] [Green Version]
- Bourdenx, M.; Daniel, J.; Genin, E.; Soria, F.N.; Blanchard-Desce, M.; Bezard, E.; Dehay, B. Nanoparticles restore lysosomal acidification defects: Implications for Parkinson and other lysosomal-related diseases. Autophagy 2016, 12, 472–483. [Google Scholar] [CrossRef] [Green Version]
- Ponsford, A.H.; Ryan, T.A.; Raimondi, A.; Cocucci, E.; Wycislo, S.A.; Frohlich, F.; Swan, L.E.; Stagi, M. Live imaging of intra-lysosome pH in cell lines and primary neuronal culture using a novel genetically encoded biosensor. Autophagy 2021, 17, 1500–1518. [Google Scholar] [CrossRef]
- Chin, M.Y.; Patwardhan, A.R.; Ang, K.H.; Wang, A.L.; Alquezar, C.; Welch, M.; Nguyen, P.T.; Grabe, M.; Molofsky, A.V.; Arkin, M.R.; et al. Genetically Encoded, pH-Sensitive mTFP1 Biosensor for Probing Lysosomal pH. ACS Sens. 2021, 6, 2168–2180. [Google Scholar] [CrossRef]
- Patriarchi, T.; Cho, J.R.; Merten, K.; Howe, M.W.; Marley, A.; Xiong, W.H.; Folk, R.W.; Broussard, G.J.; Liang, R.; Jang, M.J.; et al. Ultrafast neuronal imaging of dopamine dynamics with designed genetically encoded sensors. Science 2018, 360. [Google Scholar] [CrossRef] [Green Version]
- Sun, F.; Zeng, J.; Jing, M.; Zhou, J.; Feng, J.; Owen, S.F.; Luo, Y.; Li, F.; Wang, H.; Yamaguchi, T.; et al. A Genetically Encoded Fluorescent Sensor Enables Rapid and Specific Detection of Dopamine in Flies, Fish, and Mice. Cell 2018, 174, 481–496.e19. [Google Scholar] [CrossRef] [PubMed]
- Labouesse, M.A.; Cola, R.B.; Patriarchi, T. GPCR-Based Dopamine Sensors-A Detailed Guide to Inform Sensor Choice for In vivo Imaging. Int. J. Mol. Sci. 2020, 21, 8048. [Google Scholar] [CrossRef]
- Sabatini, B.L.; Tian, L. Imaging Neurotransmitter and Neuromodulator Dynamics In Vivo with Genetically Encoded Indicators. Neuron 2020, 108, 17–32. [Google Scholar] [CrossRef]
- Bittner, C.X.; Valdebenito, R.; Ruminot, I.; Loaiza, A.; Larenas, V.; Sotelo-Hitschfeld, T.; Moldenhauer, H.; San Martin, A.; Gutierrez, R.; Zambrano, M.; et al. Fast and reversible stimulation of astrocytic glycolysis by K+ and a delayed and persistent effect of glutamate. J. Neurosci. 2011, 31, 4709–4713. [Google Scholar] [CrossRef]
- Bittner, C.X.; Loaiza, A.; Ruminot, I.; Larenas, V.; Sotelo-Hitschfeld, T.; Gutierrez, R.; Cordova, A.; Valdebenito, R.; Frommer, W.B.; Barros, L.F. High resolution measurement of the glycolytic rate. Front. Neuroenerg. 2010, 2, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruminot, I.; Schmalzle, J.; Leyton, B.; Barros, L.F.; Deitmer, J.W. Tight coupling of astrocyte energy metabolism to synaptic activity revealed by genetically encoded FRET nanosensors in hippocampal tissue. J. Cereb. Blood Flow Metab. 2019, 39, 513–523. [Google Scholar] [CrossRef]
- Diaz-Garcia, C.M.; Lahmann, C.; Martinez-Francois, J.R.; Li, B.; Koveal, D.; Nathwani, N.; Rahman, M.; Keller, J.P.; Marvin, J.S.; Looger, L.L.; et al. Quantitative in vivo imaging of neuronal glucose concentrations with a genetically encoded fluorescence lifetime sensor. J. Neurosci. Res. 2019, 97, 946–960. [Google Scholar] [CrossRef] [Green Version]
- Diaz-Garcia, C.M.; Mongeon, R.; Lahmann, C.; Koveal, D.; Zucker, H.; Yellen, G. Neuronal Stimulation Triggers Neuronal Glycolysis and Not Lactate Uptake. Cell Metab. 2017, 26, 361–374.e4. [Google Scholar] [CrossRef]
- Youssef, S.; Zhang, S.; Ai, H.W. A Genetically Encoded, Ratiometric Fluorescent Biosensor for Hydrogen Sulfide. ACS Sens. 2019, 4, 1626–1632. [Google Scholar] [CrossRef]
- Abe, K.; Kimura, H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J. Neurosci. 1996, 16, 1066–1071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamoun, P.; Belardinelli, M.C.; Chabli, A.; Lallouchi, K.; Chadefaux-Vekemans, B. Endogenous hydrogen sulfide overproduction in Down syndrome. Am. J. Med. Genet. A 2003, 116A, 310–311. [Google Scholar] [CrossRef]
- Kim, G.A.; Ginga, N.J.; Takayama, S. Integration of Sensors in Gastrointestinal Organoid Culture for Biological Analysis. Cell. Mol. Gastroenterol. Hepatol. 2018, 6, 123–131.e1. [Google Scholar] [CrossRef] [Green Version]
- Inda, M.E.; Mimee, M.; Lu, T.K. Cell-based biosensors for immunology, inflammation, and allergy. J. Allergy Clin. Immunol. 2019, 144, 645–647. [Google Scholar] [CrossRef] [Green Version]
- Daeffler, K.N.; Galley, J.D.; Sheth, R.U.; Ortiz-Velez, L.C.; Bibb, C.O.; Shroyer, N.F.; Britton, R.A.; Tabor, J.J. Engineering bacterial thiosulfate and tetrathionate sensors for detecting gut inflammation. Mol. Syst. Biol. 2017, 13, 923. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.G.; Moon, S.J.; Kim, S.K.; Kim, T.H.; Lim, H.S.; Yeon, G.H.; Sung, B.H.; Lee, C.H.; Lee, S.G.; Hwang, J.H.; et al. A designed whole-cell biosensor for live diagnosis of gut inflammation through nitrate sensing. Biosens. Bioelectron. 2020, 168, 112523. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, J.O.; Hellstrom, P.M.; Lundberg, J.M.; Alving, K. Greatly increased luminal nitric oxide in ulcerative colitis. Lancet 1994, 344, 1673–1674. [Google Scholar] [CrossRef]
- Kotula, J.W.; Kerns, S.J.; Shaket, L.A.; Siraj, L.; Collins, J.J.; Way, J.C.; Silver, P.A. Programmable bacteria detect and record an environmental signal in the mammalian gut. Proc. Natl. Acad. Sci. USA 2014, 111, 4838–4843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naydich, A.D.; Nangle, S.N.; Bues, J.J.; Trivedi, D.; Nissar, N.; Inniss, M.C.; Niederhuber, M.J.; Way, J.C.; Silver, P.A.; Riglar, D.T. Synthetic Gene Circuits Enable Systems-Level Biosensor Trigger Discovery at the Host-Microbe Interface. mSystems 2019, 4, e00125-19. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Fu, L.; Xu, M.; Zheng, J.; Yuan, Z. Dual-Modal In Vivo Fluorescence/Photoacoustic Microscopy Imaging of Inflammation Induced by GFP-Expressing Bacteria. Sensors 2019, 19, 238. [Google Scholar] [CrossRef] [Green Version]
- Braubach, O.; Cohen, L.B.; Choi, Y. Historical Overview and General Methods of Membrane Potential Imaging. Adv. Exp. Med. Biol. 2015, 859, 3–26. [Google Scholar] [PubMed]
- Joshi, J.; Rubart, M.; Zhu, W. Optogenetics: Background, Methodological Advances and Potential Applications for Cardiovascular Research and Medicine. Front. Bioeng. Biotechnol. 2019, 7, 466. [Google Scholar] [CrossRef] [Green Version]
- Dempsey, G.T.; Chaudhary, K.W.; Atwater, N.; Nguyen, C.; Brown, B.S.; McNeish, J.D.; Cohen, A.E.; Kralj, J.M. Cardiotoxicity screening with simultaneous optogenetic pacing, voltage imaging and calcium imaging. J. Pharmacol. Toxicol. Methods 2016, 81, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Hochbaum, D.R.; Zhao, Y.; Farhi, S.L.; Klapoetke, N.; Werley, C.A.; Kapoor, V.; Zou, P.; Kralj, J.M.; Maclaurin, D.; Smedemark-Margulies, N.; et al. All-optical electrophysiology in mammalian neurons using engineered microbial rhodopsins. Nat. Methods 2014, 11, 825–833. [Google Scholar] [CrossRef] [Green Version]
- Hou, J.H.; Kralj, J.M.; Douglass, A.D.; Engert, F.; Cohen, A.E. Simultaneous mapping of membrane voltage and calcium in zebrafish heart in vivo reveals chamber-specific developmental transitions in ionic currents. Front. Physiol. 2014, 5, 344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakai, R.; Repunte-Canonigo, V.; Raj, C.D.; Knopfel, T. Design and characterization of a DNA-encoded, voltage-sensitive fluorescent protein. Eur. J. Neurosci. 2001, 13, 2314–2318. [Google Scholar] [CrossRef] [PubMed]
- Lam, A.J.; St-Pierre, F.; Gong, Y.; Marshall, J.D.; Cranfill, P.J.; Baird, M.A.; McKeown, M.R.; Wiedenmann, J.; Davidson, M.W.; Schnitzer, M.J.; et al. Improving FRET dynamic range with bright green and red fluorescent proteins. Nat. Methods 2012, 9, 1005–1012. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Xian, W.; Bellin, M.; Dorn, T.; Tian, Q.; Goedel, A.; Dreizehnter, L.; Schneider, C.M.; Ward-van Oostwaard, D.; Ng, J.K.; et al. Subtype-specific promoter-driven action potential imaging for precise disease modelling and drug testing in hiPSC-derived cardiomyocytes. Eur. Heart J. 2017, 38, 292–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, L.; Han, Z.; Platisa, J.; Wooltorton, J.R.; Cohen, L.B.; Pieribone, V.A. Single action potentials and subthreshold electrical events imaged in neurons with a fluorescent protein voltage probe. Neuron 2012, 75, 779–785. [Google Scholar] [CrossRef] [Green Version]
- Shinnawi, R.; Huber, I.; Maizels, L.; Shaheen, N.; Gepstein, A.; Arbel, G.; Tijsen, A.J.; Gepstein, L. Monitoring Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes with Genetically Encoded Calcium and Voltage Fluorescent Reporters. Stem Cell Rep. 2015, 5, 582–596. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.H.; Kao, H.K.J.; Chang, C.W.; Merleev, A.; Overton, J.L.; Pretto, D.; Yechikov, S.; Maverakis, E.; Chiamvimonvat, N.; Chan, J.W.; et al. Human induced pluripotent stem cell line with genetically encoded fluorescent voltage indicator generated via CRISPR for action potential assessment post-cardiogenesis. Stem Cells 2020, 38, 90–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaheen, N.; Shiti, A.; Huber, I.; Shinnawi, R.; Arbel, G.; Gepstein, A.; Setter, N.; Goldfracht, I.; Gruber, A.; Chorna, S.V.; et al. Human Induced Pluripotent Stem Cell-Derived Cardiac Cell Sheets Expressing Genetically Encoded Voltage Indicator for Pharmacological and Arrhythmia Studies. Stem Cell Rep. 2018, 10, 1879–1894. [Google Scholar] [CrossRef] [PubMed]
- Volkow, N.D.; McLellan, A.T. Opioid Abuse in Chronic Pain—Misconceptions and Mitigation Strategies. N. Engl. J. Med. 2016, 374, 1253–1263. [Google Scholar] [CrossRef] [PubMed]
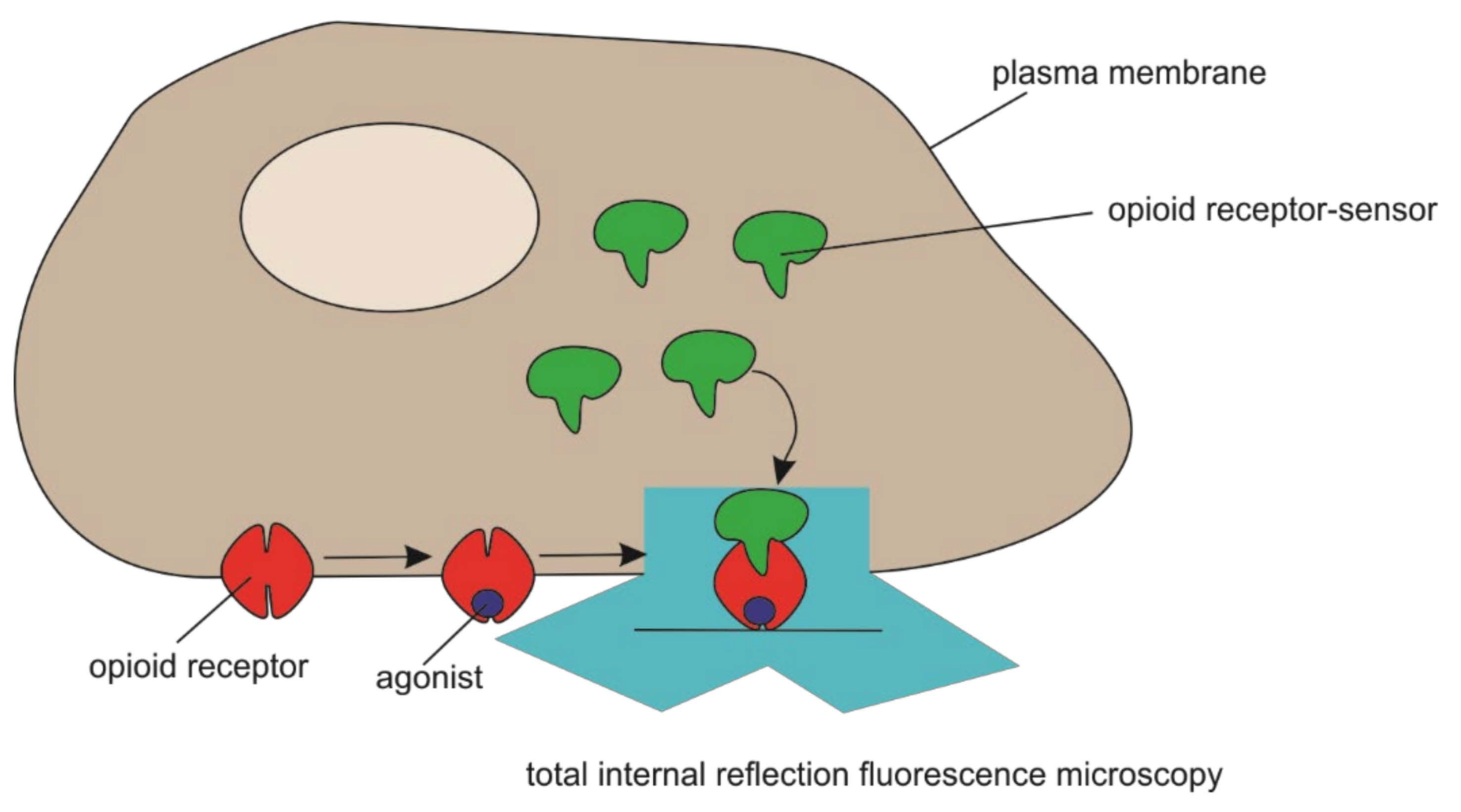
- Stoeber, M.; Jullie, D.; Lobingier, B.T.; Laeremans, T.; Steyaert, J.; Schiller, P.W.; Manglik, A.; von Zastrow, M. A Genetically Encoded Biosensor Reveals Location Bias of Opioid Drug Action. Neuron 2018, 98, 963–976.e5. [Google Scholar] [CrossRef] [Green Version]
- Shivange, A.V.; Borden, P.M.; Muthusamy, A.K.; Nichols, A.L.; Bera, K.; Bao, H.; Bishara, I.; Jeon, J.; Mulcahy, M.J.; Cohen, B.; et al. Determining the pharmacokinetics of nicotinic drugs in the endoplasmic reticulum using biosensors. J. Gen. Physiol. 2019, 151, 738–757. [Google Scholar] [CrossRef]
- Baens, M.; Noels, H.; Broeckx, V.; Hagens, S.; Fevery, S.; Billiau, A.D.; Vankelecom, H.; Marynen, P. The dark side of EGFP: Defective polyubiquitination. PLoS ONE 2006, 1, e54. [Google Scholar] [CrossRef] [Green Version]
- Breckwoldt, M.O.; Pfister, F.M.; Bradley, P.M.; Marinkovic, P.; Williams, P.R.; Brill, M.S.; Plomer, B.; Schmalz, A.; St. Clair, D.K.; Naumann, R.; et al. Multiparametric optical analysis of mitochondrial redox signals during neuronal physiology and pathology in vivo. Nat. Med. 2014, 20, 555–560. [Google Scholar] [CrossRef]
- Stapper, Z.A.; Jahn, T.R. Changes in Glutathione Redox Potential Are Linked to Abeta42-Induced Neurotoxicity. Cell Rep. 2018, 24, 1696–1703. [Google Scholar] [CrossRef] [Green Version]
- Dong, C.; Ly, C.; Dunlap, L.E.; Vargas, M.V.; Sun, J.; Hwang, I.W.; Azinfar, A.; Oh, W.C.; Wetsel, W.C.; Olson, D.E.; et al. Psychedelic-inspired drug discovery using an engineered biosensor. Cell 2021, 184, 2779–2792.e18. [Google Scholar] [CrossRef]
- Misra, T.; Baccino-Calace, M.; Meyenhofer, F.; Rodriguez-Crespo, D.; Akarsu, H.; Armenta-Calderon, R.; Gorr, T.A.; Frei, C.; Cantera, R.; Egger, B.; et al. A genetically encoded biosensor for visualising hypoxia responses in vivo. Biol. Open 2017, 6, 296–304. [Google Scholar] [PubMed] [Green Version]
- Komatsu, N.; Terai, K.; Imanishi, A.; Kamioka, Y.; Sumiyama, K.; Jin, T.; Okada, Y.; Nagai, T.; Matsuda, M. A platform of BRET-FRET hybrid biosensors for optogenetics, chemical screening, and in vivo imaging. Sci. Rep. 2018, 8, 8984. [Google Scholar] [CrossRef] [PubMed]
- Berezin, M.Y.; Achilefu, S. Fluorescence lifetime measurements and biological imaging. Chem. Rev. 2010, 110, 2641–2684. [Google Scholar] [CrossRef] [Green Version]
- Auslander, D.; Eggerschwiler, B.; Kemmer, C.; Geering, B.; Auslander, S.; Fussenegger, M. A designer cell-based histamine-specific human allergy profiler. Nat. Commun. 2014, 5, 4408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chassin, H.; Geering, B.; Schukur, L.; Auslander, D.; Lang, B.; Fussenegger, M. Sensing and responding to allergic response cytokines through a genetically encoded circuit. Nat. Commun. 2017, 8, 1101. [Google Scholar] [CrossRef] [Green Version]
- Schukur, L.; Geering, B.; Charpin-El Hamri, G.; Fussenegger, M. Implantable synthetic cytokine converter cells with AND-gate logic treat experimental psoriasis. Sci. Transl. Med. 2015, 7, 318ra201. [Google Scholar] [CrossRef] [PubMed]



| Kinase | Name | Type of Sensors | Proteins | Reference |
|---|---|---|---|---|
| Protein kinase A | AKAR | FRET | ECFP/YFP | [20] |
| AKAR2 | FRET | ECFP/Citrine | [24] | |
| AKAR3 | FRET | YPet | [25] | |
| Rluc-PCA | Bioluminescence | Renilla luciferase | [26] | |
| FLIM-AKAR | FLIM–FRET | meGFPΔ/cpsREACH | [27] | |
| ExRai-AKAR | Ratiometric | EGFP | [28] | |
| BimAKAR | FRET | YPet | [21] | |
| Protein kinase A/Extracellular signal-regulated kinase | ERK/PKA biosensors | FLIM–FRET | sREAChet/EGFP | [29] |
| Protein kinase B | AktAR | FRET | CFP/Venus | [30] |
| Protein kinase C | BimCKAR | Bioluminescence | Renilla luciferase | [21] |
| CKAR | FRET | CFP/YFP | [31] | |
| Protein tyrosine kinases Src, Abl | Src/Abl indicator | FRET | CFP/YFP | [32] |
| Protein tyrosine kinase Src | Src reporterr | FRET | CFP/YFP | [33] |
| BCR-ABL kinase | Pickles | FRET | CFP/Venus | [34] |
| Serine/threonine protein kinase | TORCAR | FRET | Cerulean/YPet | [35] |
| LATS kinase | LATS-BS | Bioluminescence | Photinus Pyralis luciferase | [36] |
| P38 MAP kinase | PerKy-38 | FRET | YPet/CFP | [37] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ovechkina, V.S.; Zakian, S.M.; Medvedev, S.P.; Valetdinova, K.R. Genetically Encoded Fluorescent Biosensors for Biomedical Applications. Biomedicines 2021, 9, 1528. https://doi.org/10.3390/biomedicines9111528
Ovechkina VS, Zakian SM, Medvedev SP, Valetdinova KR. Genetically Encoded Fluorescent Biosensors for Biomedical Applications. Biomedicines. 2021; 9(11):1528. https://doi.org/10.3390/biomedicines9111528
Chicago/Turabian StyleOvechkina, Vera S., Suren M. Zakian, Sergey P. Medvedev, and Kamila R. Valetdinova. 2021. "Genetically Encoded Fluorescent Biosensors for Biomedical Applications" Biomedicines 9, no. 11: 1528. https://doi.org/10.3390/biomedicines9111528
APA StyleOvechkina, V. S., Zakian, S. M., Medvedev, S. P., & Valetdinova, K. R. (2021). Genetically Encoded Fluorescent Biosensors for Biomedical Applications. Biomedicines, 9(11), 1528. https://doi.org/10.3390/biomedicines9111528






