Ovotransferrin Supplementation Improves the Iron Absorption: An In Vitro Gastro-Intestinal Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagent Preparation
2.2. Cell Culture
2.3. In Vitro Gastric Barrier Model
2.4. In Vitro Intestinal Barrier Model
2.5. D Gastro-Intestinal Tract In Vitro Model
2.6. MTT Assay
2.7. Iron Quantification Assay
2.8. Transferrin Quantification Assay
2.9. SOD Assay
2.10. Western Blot
2.11. Occludin Quantification Assay
2.12. Claudin 1 Detection Assay
2.13. Human Tight Junction Protein 1 (ZO-1) Detection
2.14. Statistical Analysis
3. Results
3.1. Dose-Response and Time-Dependent Study on GTL-16 and Caco-2 Cells
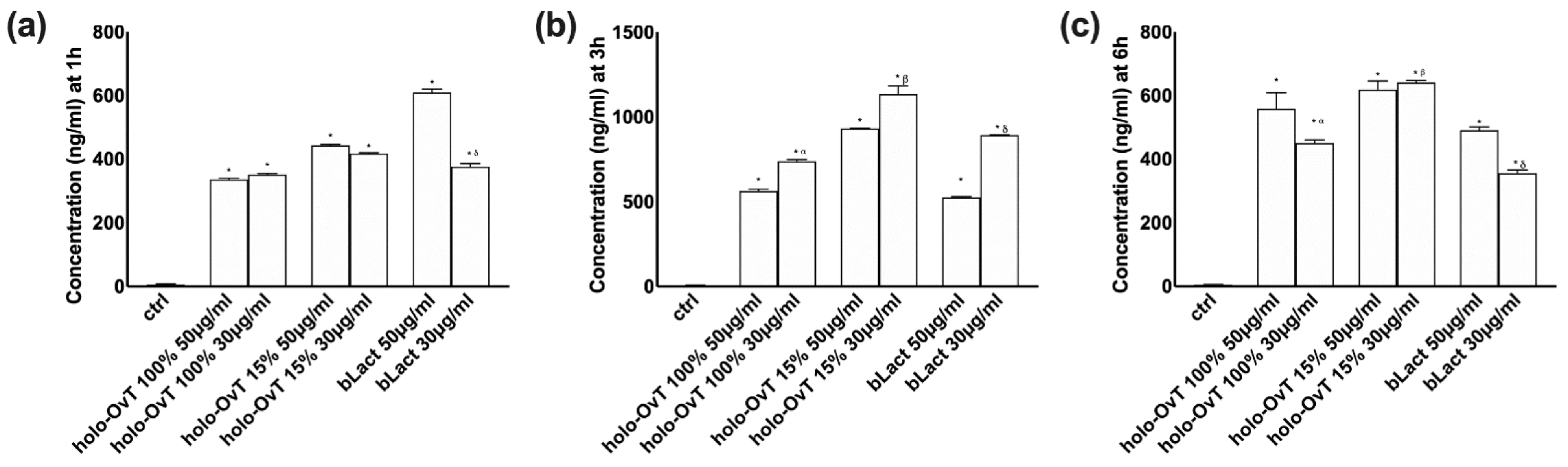
3.2. Analysis of Transferrin after Gastric and Intestinal Barrier In Vitro
3.3. Three-Dimensional (3D) In Vitro Model Mimicking In Vivo Complexity of the Gastro-Intestinal Barrier
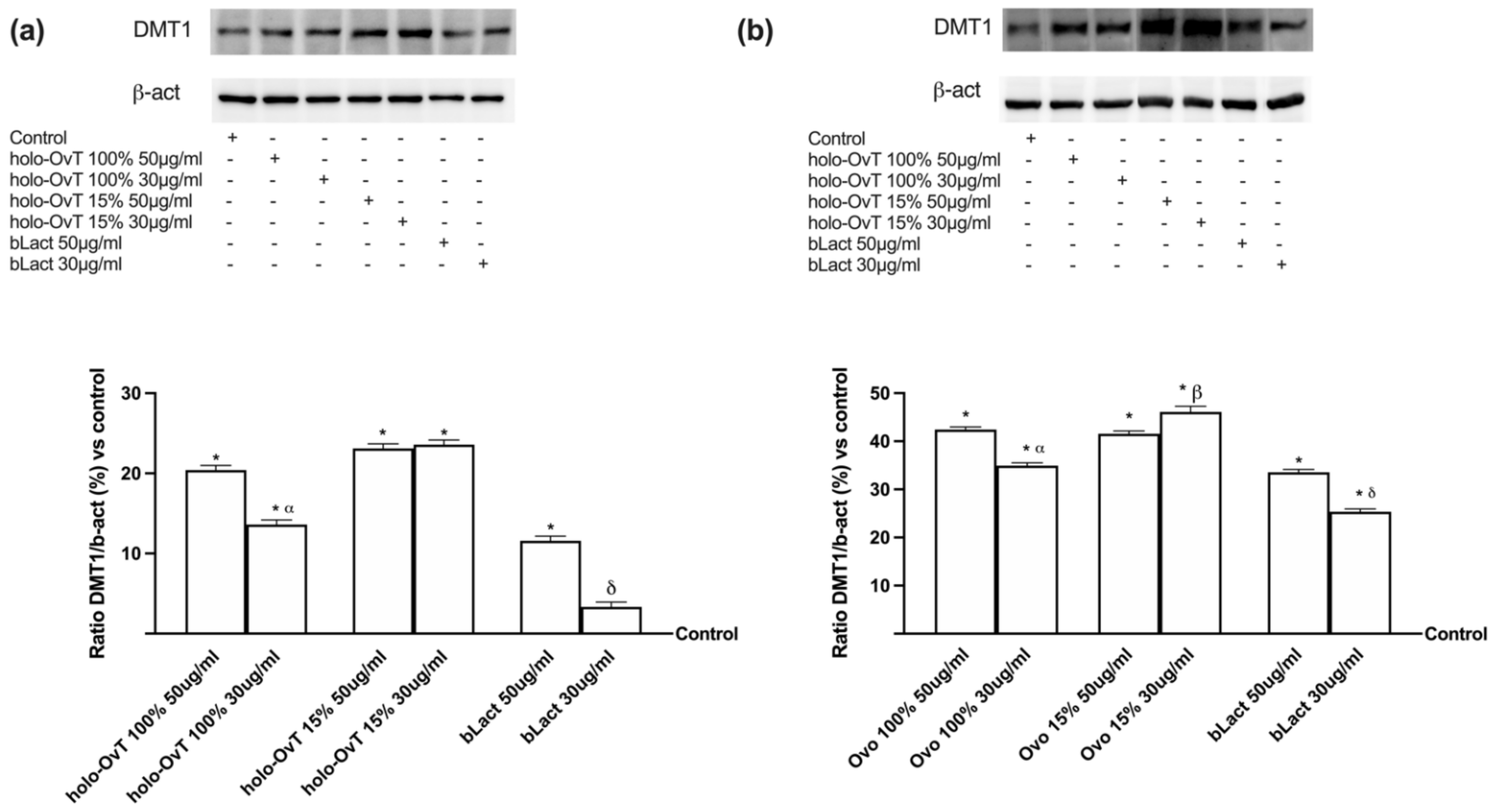
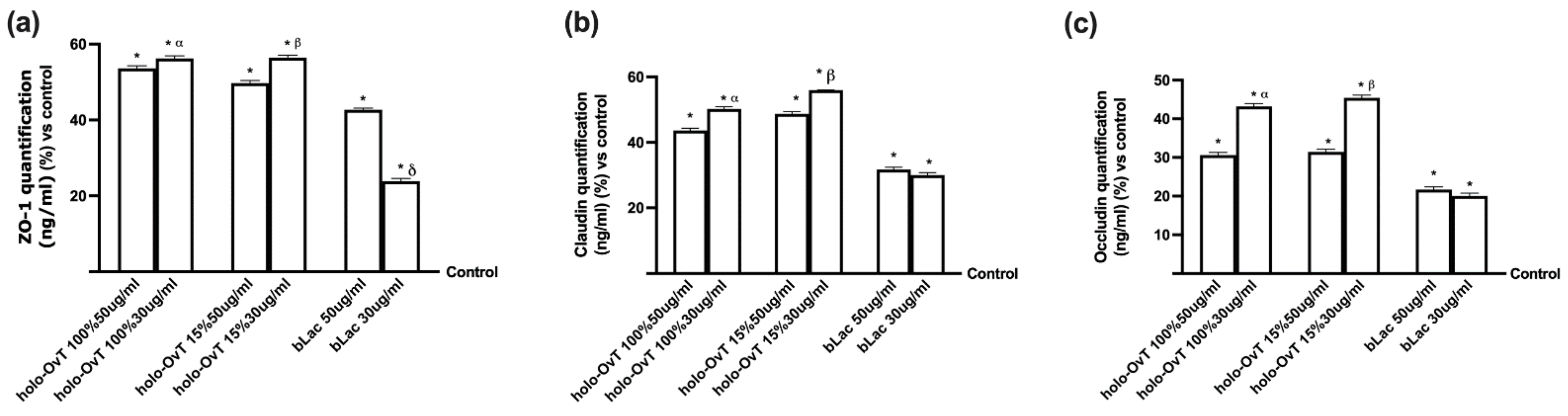
3.4. Analysis of the Mechanism of Action by Intracellular Mechanism Activated on 3D Model
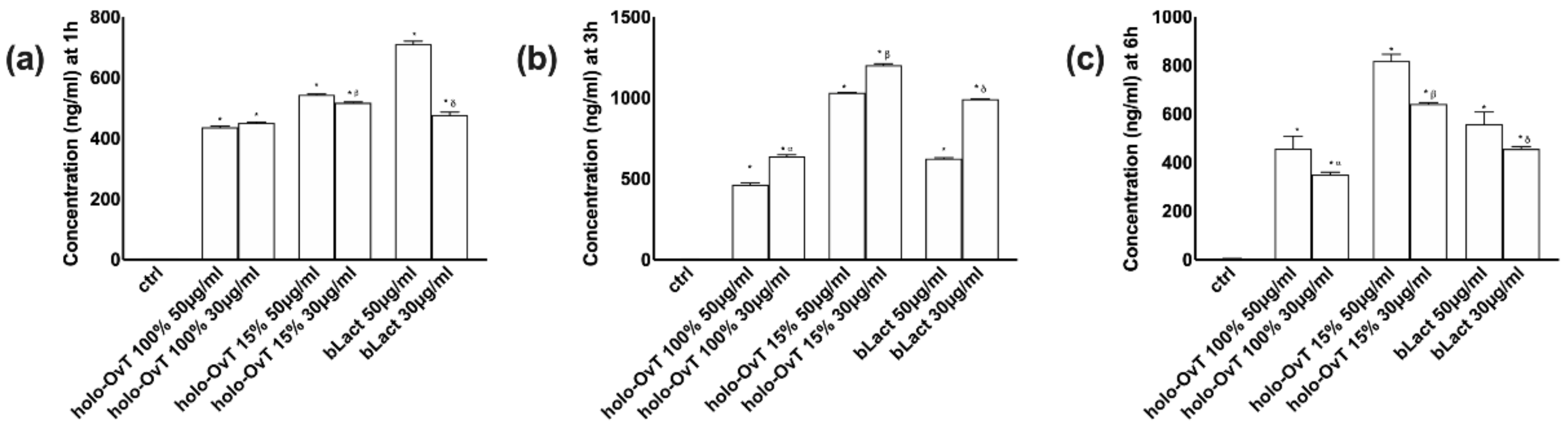
3.5. Effects of Holo-OvT and bLact on 3D Model in Presence of Iron
3.6. Intracellular Mechanisms Activated by OvT and bLact on 3D Model in Presence of Iron
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bresson, J.L.; Burlingame, B.; Dean, T.; Fairweather-Tait, S.; Heinonen, M.; Hirsch-Ernst, K.I.; Mangelsdorf, I.; McArdle, H.; Naska, A.; Neuhauser-Berthold, M.; et al. Scientific Opinion on Dietary Reference Values for iron. EFSA J. 2015, 13. [Google Scholar] [CrossRef]
- Goroll, A.H.; Mulley, A.G. Office evaluation and management of the adult patient. In Primary Care Medicine; Ergodebooks: Houston, TX, USA, 2009; Volume 82, pp. 607–608. [Google Scholar]
- Theil, E.C. Ferritin: Structure, gene regulation, and cellular function in animals, plants, and microorganism. Annu. Rev. Biochem. 1987, 56, 289–315. [Google Scholar] [CrossRef] [PubMed]
- Qamar, K.; Saboor, M.; Qudsia, F.; Khosa, S.M.; Moinuddin Usman, M. Malabsorption of iron as a cause of iron deficiency anemia in postmenopausal women. Pak. J. Med. Sci 2015, 31, 304–308. [Google Scholar]
- WHO. INT WHO. Report: Priorities in the Assessment of Vitamin A and Iron Status in Populations, Panama City, Panama, 15–17 September 2010; World Health Organization: Geneva, Switzerland, 2012. [Google Scholar]
- WHO. INT WHO/NHD/01.3-Iron Deficiency Anaemia Assessment, Prevention, and Control a Guide for Programme Man-Agers. Available online: https://www.who.int/nutrition/publications/en/ida_assessment_prevention_control.pdf (accessed on 1 December 2012).
- Abu Hashim, H.; Foda, O.; Ghayaty, E. Lactoferrin or ferrous salts for iron deficiency anemia in pregnancy: A meta-analysis of randomized trials. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 219, 45–52. [Google Scholar] [CrossRef]
- Spik, G.; Coddeville, B.; Montreuil, J. Comparative study of the primary structures of sero-, lacto- and ovotransferrin glycans from different species. Biochimie 1988, 70, 1459–1469. [Google Scholar] [CrossRef]
- Deeming, D.C. Behavior patterns during incubation. In Avian Incubation: Behaviour, Environment, and Evolution; Deeming, D.C., Ed.; Oxford University Press: Oxford, UK, 2002; pp. 63–87. [Google Scholar]
- Saxena, I.; Tayyab, S. Protein proteinase inhibitors from avian egg whites. Cell. Mol. Life Sci. 1997, 53, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Miyagawa, S.; Matsumoto, K.; Kamata, R.; Okamura, R.; Maeda, H. Spreading of Serratia marcescens in experimental keratitis and growth suppression by chicken egg white ovomacroglobulin. Jpn. J. Ophthalmol. 1991, 35. [Google Scholar]
- Board, R.G.; Fuller, R. Non-specific antimicrobial defences of the avian egg, ambryo and neonate. Biol. Rev. 1974, 49, 15–49. [Google Scholar] [CrossRef]
- Mason, A.B.; Woodworth, R.C.; Oliver, R.W.A.; Green, B.N.; Lin, L.-N.; Brandts, J.F.; Savage, K.J.; Tam, B.M.; MacGillivray, R.T.A. Association of the two lobes of ovotransferrin is a prerequisite for receptor recognition. Studies with recombinant ovotransferrins. Biochem. J. 1996, 319, 361–368. [Google Scholar] [CrossRef] [Green Version]
- Giansanti, F.; Leboffe, L.; Angelucci, F.; Antonini, G. The Nutraceutical Properties of Ovotransferrin and Its Potential Utilization as a Functional Food. Nutrients 2015, 7, 9105–9115. [Google Scholar] [CrossRef] [Green Version]
- Rathnapala, E.C.N.; Ahn, D.U.; Abeyrathne, S. Functional properties of ovotransferrin from chicken egg white and its derived peptides: A review. Food Sci. Biotechnol. 2021, 30, 619–630. [Google Scholar] [CrossRef] [PubMed]
- Egbuna, C.; Dable-Tupas, G. Functional Foods and Nutraceuticals, 1st ed.; Springer Nature: Cham, Switzerland, 2020; pp. 1–632. [Google Scholar]
- Williams, J.; Elleman, T.C.; Kingston, I.B.; Wilkins, A.G.; Kuhn, K.A. The Primary Structure of Hen Ovotransferrin. JBIC J. Biol. Inorg. Chem. 1982, 122, 297–303. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov—ClinicalTrials.gov Identifier: NCT04643054. Available online: https://clinicaltrials.gov/ct2/show/NCT04643054 (accessed on 15 July 2021.).
- Abeyrathne, E.D.N.S.; Lee, H.Y.; Ham, J.S.; Ahn, D.U. Separation of ovotransferrin from chicken egg white without using organic solvents. Poult. Sci. 2013, 92, 1091–1097. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, I.; Mizutani, K.; Hirose, M. Iron-Binding Process in the Amino- and Carboxyl-Terminal Lobes of Ovotransferrin: Quantitative Studies Utilizing Single Fe3+-Binding Mutants. Biochemistry 2004, 43, 11118–11125. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Xu, X.-X.; Liu, Y.; Xi, E.-Z.; An, J.-J.; Tabys, D.; Liu, N. The In Vitro Protective Role of Bovine Lactoferrin on Intestinal Epithelial Barrier. Molecules 2019, 24, 148. [Google Scholar] [CrossRef] [PubMed]
- Uberti, F.; Morsanuto, V.; Ruga, S.; Galla, R.; Farghali, M.; Notte, F.; Bozzo, C.; Magnani, C.; Nardone, A.; Molinari, C. Study of Magnesium Formulations on Intestinal Cells to Influence Myometrium Cell Relaxation. Nutrients 2020, 12, 573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fossati, L.; Dechaume, R.; Hardillier, E.; Chevillon, D.; Prevost, C.; Bolze, S.; Maubon, N. Use of simulated intestinal fluid for Caco-2 permeability assay of lipophilic drugs. Int. J. Pharm. 2008, 360, 148–155. [Google Scholar] [CrossRef]
- DiMarco, R.L.; Hunt, D.R.; Dewi, R.E.; Heilshorn, S.C. Improvement of paracellular transport in the Caco-2 drug screening model using protein-engineered substrates. Biomaterials 2017, 129, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Christides, T.; Ganis, J.C.; Sharp, P.A. In vitro assessment of iron availability from commercial Young Child Formulae sup-plemented with prebiotics. Eur. J. Nutr. 2018, 57, 669–678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- FDA.GOV. Available online: https://www.fda.gov/media/117974/download (accessed on 12 May 2021).
- EMA.EU. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-m9-biopharmaceutics-classification-system-based-biowaivers-step-2b-first-version_en.pdf (accessed on 6 August 2018).
- Uberti, F.; Morsanuto, V.; Ghirlanda, S.; Molinari, C. Iron Absorption from Three Commercially Available Supplements in Gastrointestinal Cell Lines. Nutrients 2017, 9, 1008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christides, T.; Wray, D.; McBride, R.; Fairweather, R.; Sharp, P. Iron bioavailability from commercially available iron supplements. Eur. J. Nutr. 2015, 54, 1345–1352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giordano, S.; Di Renzo, M.F.; Ferracini, R.; Chiadò-Piat, L.; Comoglio, P.M. p145, a protein with associated tyrosine kinase activity in a human gastric carcinoma cell line. Mol. Cell Biol. 1988, 8, 3510–3517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uberti, F.; Bardelli, C.; Morsanuto, V.; Ghirlanda, S.; Molinari, C. Role of vitamin D3 combined to alginates in preventing acid and oxidative injury in cultured gastric epithelial cells. BMC Gastroenterol. 2016, 16, 127. [Google Scholar] [CrossRef] [Green Version]
- Lourenço, B.N.; Santos, T.; Oliveira, C.; Barrias, C.C.; Granja, P.L. Bioengineering a novel 3D in vitro model of gastric mucosa for stomach permeability studies. Acta Biomater. 2018, 82, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Lemieux, M.; Bouchard, F.; Gosselin, P.; Paquin, J.; Mateescu, M.A. The NCI-N87 cell line as a gastric epithelial barrier model for drug permeability assay. Biochem. Biophys. Res. Commun. 2011, 412, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Guha, S.; Alvarez, S.; Majumder, K. Transport of Dietary Anti-Inflammatory Peptide, γ-Glutamyl Valine (γ-EV), across the Intestinal Caco-2 Monolayer. Nutrients 2021, 13, 1448. [Google Scholar] [CrossRef]
- Hubatsch, I.; Ragnarsson, E.G.; Artursson, P. Determination of drug permeability and prediction of drug absorption in Caco-2 monolayers. Nat. Protoc. 2007, 2, 2111–2119. [Google Scholar] [CrossRef] [PubMed]
- Candini, O.; Grisendi, G.; Foppiani, E.M.; Brogli, M.; Aramini, B.; Masciale, V.; Spano, C.; Petrachi, T.; Veronesi, E.; Conte, P.; et al. A Novel 3D In Vitro Platform for Pre-Clinical Investigations in Drug Testing, Gene Therapy, and Immuno-oncology. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Molinari, C.; Ruga, S.; Farghali, M.; Galla, R.; Fernandez-Godino, R.; Clemente, N.; Uberti, F. Effects of a New Combination of Natural Extracts on Glaucoma-Related Retinal Degeneration. Foods 2021, 10, 1885. [Google Scholar] [CrossRef]
- Majka, G.; Więcek, G.; Śróttek, M.; Śpiewak, K.; Brindell, M.; Koziel, J.; Marcinkiewicz, J.; Strus, M. The impact of lactoferrin with different levels of metal saturation on the intestinal epithelial barrier function and mucosal inflammation. BioMetals 2016, 29, 1019–1033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manzoni, P.; Rinaldi, M.; Cattani, S.; Pugni, L.; Romeo, M.G.; Messner, H.; Stolfi, I.; Decembrino, L.; Laforgia, N.; Vagnarelli, F.; et al. Bovine Lactoferrin Supplementation for Prevention of Late-Onset Sepsis in Very Low-Birth-Weight Neonates a Randomized Trial. JAMA 2009, 302, 1421–1428. [Google Scholar] [CrossRef] [PubMed]
- García-Montoya, I.A.; Cendón, T.S.; Arévalo-Gallegos, S.; Rascón-Cruz, Q. Lactoferrin a multiple bioactive protein: An over-view. Biochim. Biophys. Acta 2012, 1820, 226–236. [Google Scholar] [CrossRef]
- Hering, N.A.; Luettig, J.; Krug, S.M.; Wiegand, S.; Gross, G.; van Tol, E.A.; Schulzke, J.D.; Rosenthal, R. Lactoferrin protects against intestinal inflammation and bacteria-induced barrier dysfunction in vitro. Ann. N. Y. Acad. Sci. 2017, 1405, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Mann, J.K.; Ndung’U, T. The potential of lactoferrin, ovotransferrin and lysozyme as antiviral and immune-modulating agents in COVID-19. Futur. Virol. 2020, 15, 609–624. [Google Scholar] [CrossRef]
- Giansanti, F.; Rossi, P.; Massucci, M.T.; Botti, D.; Antonini, G.; Valenti, P.; Seganti, L. Antiviral activity of ovotransferrin dis-closes an evolutionary strategy for the defensive activities of lactoferrin. Biochem. Cell. Biol. 2002, 80, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Abeyrathne, E.D.N.S.; Lee, H.Y.; Ahn, D.U. Egg white proteins and their potential use in food processing or as nutraceutical and pharmaceutical agents—A review. Poult. Sci. 2013, 92, 3292–3299. [Google Scholar] [CrossRef]
- Blais, A.; Fan, C.; Voisin, T.; Aattouri, N.; Dubarry, M.; Blachier, F.; Tomé, D. Effects of lactoferrin on intestinal epithelial cell growth and differentiation: An in vivo and in vitro study. BioMetals 2014, 27, 857–874. [Google Scholar] [CrossRef]
- Koch, S.; Nusrat, A. Dynamic Regulation of Epithelial Cell Fate and Barrier Function by Intercellular Junctions. Ann. N. Y. Acad. Sci. 2009, 1165, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Weber, C.R. Dynamic properties of the tight junction barrier. Ann. N. Y. Acad. Sci. 2012, 1257, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Reinhold, A.K.; Rittner, H. Barrier function in the peripheral and central nervous system—A review. Pflug. Arch. 2016, 469, 123–134. [Google Scholar] [CrossRef]
- Barmeyer, C.; Schulzke, J.D.; Fromm, M. Claudin-related intestinal diseases. Semin. Cell. Dev. Biol. 2015, 42, 30–38. [Google Scholar] [CrossRef] [PubMed]













Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galla, R.; Grisenti, P.; Farghali, M.; Saccuman, L.; Ferraboschi, P.; Uberti, F. Ovotransferrin Supplementation Improves the Iron Absorption: An In Vitro Gastro-Intestinal Model. Biomedicines 2021, 9, 1543. https://doi.org/10.3390/biomedicines9111543
Galla R, Grisenti P, Farghali M, Saccuman L, Ferraboschi P, Uberti F. Ovotransferrin Supplementation Improves the Iron Absorption: An In Vitro Gastro-Intestinal Model. Biomedicines. 2021; 9(11):1543. https://doi.org/10.3390/biomedicines9111543
Chicago/Turabian StyleGalla, Rebecca, Paride Grisenti, Mahitab Farghali, Laura Saccuman, Patrizia Ferraboschi, and Francesca Uberti. 2021. "Ovotransferrin Supplementation Improves the Iron Absorption: An In Vitro Gastro-Intestinal Model" Biomedicines 9, no. 11: 1543. https://doi.org/10.3390/biomedicines9111543
APA StyleGalla, R., Grisenti, P., Farghali, M., Saccuman, L., Ferraboschi, P., & Uberti, F. (2021). Ovotransferrin Supplementation Improves the Iron Absorption: An In Vitro Gastro-Intestinal Model. Biomedicines, 9(11), 1543. https://doi.org/10.3390/biomedicines9111543






