Microbiome First Approaches to Rescue Public Health and Reduce Human Suffering
Abstract
:1. Introduction
2. Recent Failures of the Public Health Promise
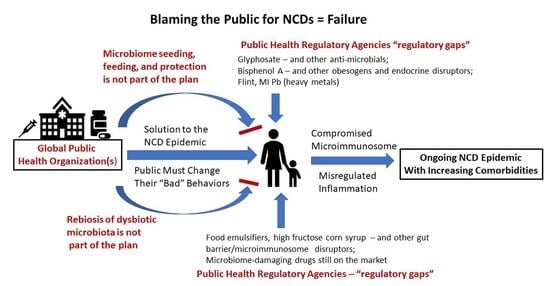
3. The Blame Game
4. Change Is Overdue
5. The Updated Science–Application Gap: Ancient Personal Responsibility Solutions to Stop NCDs
6. The Global Burden of Disease Study
7. NHANES Results Illustrate That Multimorbid NCDs with Polypharmacy Are the New Norms
8. Failure to Protect Multimorbid NCD-Bearing, Pro-Inflammatory Seniors against the SARS-CoV-2-Induced Cytokine Storm
9. The National Children’s Study
10. Public Health Failures among Regulatory Agencies
11. The Final Group of Problematic Public Health-Related Activities
12. Transforming Public Health for Impactful Successes against the NCD Epidemic
13. The WHO and Its Four Modifiable Behaviors to Defeat NCDs
14. WHO Behavioral Modification #1: Eat a Healthy Diet (in Spite of the Microbiota-Driven Sense Control)
14.1. Taste
14.2. Smell
14.3. Satiety
15. WHO Behavior Modifications #2 and #3: Consume Less Alcohol and Stop Using Tobacco (in Spite of the Microbiome’s Role in Addiction and Withdrawal)
16. WHO’s Behavioral Modification #4: Stop the Inactive Lifestyle and Exercise More (in Spite the Inherent Nature of NCDs)
16.1. Early-Life Programming of NCDs vs. Exercise
16.2. Priority of the Microbiome and the First 1000 Days Concept
17. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Duffy, J. The Sanitarians: A History of American Public Health, 1st ed.; University of Illinois Press: Champaign, IL, USA, 1992; 344p, ISBN 9780252062766. [Google Scholar]
- Cranor, C.F. Tragic Failures: How and Why We are Harmed by Toxic Chemicals, 1st ed.; Oxford University Press: Oxford, UK, 2017; 264p, ISBN 978-0190635756. [Google Scholar]
- Editorial. The catastrophic failures of public health. Lancet 2004, 363, 745. [Google Scholar] [CrossRef]
- Bellinger, D.C. Lead Contamination in Flint—An Abject Failure to Protect Public Health. N. Engl. J. Med. 2016, 374, 1101–1103. [Google Scholar] [CrossRef] [PubMed]
- Maffini, M.V.; Neltner, T.G.; Vogel, S. We are what we eat: Regulatory gaps in the United States that put our health at risk. PLoS Biol. 2017, 15, e2003578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Myers, J.P.; Antoniou, M.N.; Blumberg, B.; Carroll, L.; Colborn, T.; Everett, L.G.; Hansen, M.; Landrigan, P.J.; Lanphear, B.P.; Mesnage, R.; et al. Concerns over use of glyphosate-based herbicides and risks associated with exposures: A consensus statement. Environ. Health 2016, 15, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lobstein, T.; Brownell, K.D. Endocrine-disrupting chemicals and obesity risk: A review of recommendations for obesity prevention policies. Obes. Rev. 2021, 22, e13332. [Google Scholar] [CrossRef]
- Wilkinson, J.E.; Franzosa, E.A.; Everett, C.; Li, C.; HCMPH Researchers and Trainees; HCMPH Investigators; Hu, F.B.; Wirth, D.F.; Song, M.; Chan, A.T.; et al. A framework for microbiome science in public health. Nat. Med. 2021, 27, 766–774. [Google Scholar] [CrossRef]
- Dietert, R.R. Microbiome First Medicine in Health and Safety. Biomedicines 2021, 9, 1099. [Google Scholar] [CrossRef]
- King, D.E.; Xiang, J.; Pilkerton, C.S. Multimorbidity Trends in United States Adults, 1988–2014. J. Am. Board Fam. Med. 2018, 31, 503–513. [Google Scholar] [CrossRef] [Green Version]
- Bloom, D.E.; Cafiero, E.T.; Jané-Llopis, E.; Abrahams-Gessel, S.; Bloom, L.R.; Fathima, S.; Feigl, B.; Gaziano, T.; Mowafi, M.; Pandya, A.; et al. The Global Economic Burden of Noncommunicable Diseases; World Economic Forum: Geneva, Switzerland, 2011; 48p. [Google Scholar]
- World Health Organization. Noncommunicable Diseases Fact Sheet; World Health Organization: Geneva, Switzerland, 2021; Available online: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases (accessed on 27 August 2021).
- World Health Organization. Preventing Noncommunicable Diseases. Available online: https://www.who.int/activities/preventing-noncommunicable-diseases (accessed on 27 August 2021).
- Newsholme, A. The possible association of the consumption of alcohol with excessive mortality from cancer. Br. Med. J. 1903, 2, 1529–1531. [Google Scholar] [CrossRef] [Green Version]
- The Tobacco Heart. The Hospital 1901, 30, 6. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5186887/?page=1 (accessed on 9 October 2021).
- Foxcroft, L. Calories and Corsets: A History of Dieting over Two Thousand Years; Profile Books: London, UK, 2012; 320p, ISBN 978-1846684258. [Google Scholar]
- Tipton, C.M. The history of “Exercise Is Medicine” in ancient civilizations. Adv. Physiol. Educ. 2014, 38, 109–117. [Google Scholar] [CrossRef]
- Franklin, B., III. Dialog Between Franklin and the Gout. In The Oxford Book of American Essays; Matthews, B., Ed.; Oxford University Press: New York, NY, USA, 1914; 508p, Available online: https://www.bartleby.com/109/3.html (accessed on 9 October 2021).
- GBD 2019 Risk Factors Collaborators 2020. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1223–1249. [Google Scholar] [CrossRef]
- Dean, E.; Skinner, M.; Yu, H.P.; Jones, A.Y.; Gosselink, R.; Söderlund, A. Why COVID-19 strengthens the case to scale up assault on non-communicable diseases: Role of health professionals including physical therapists in mitigating pandemic waves. AIMS Public Health 2021, 8, 369–375. [Google Scholar] [CrossRef]
- Dietert, R.R. Lessons for human holobiont medicine in the era of SARS-Cov-2. Am. J. Biomed. Sci. Res. 2021, 13, 152–156. [Google Scholar] [CrossRef]
- Chan, E.Y.Y.; Kim, J.H.; Lo, E.S.K.; Huang, Z.; Hung, H.; Hung, K.K.C.; Wong, E.; Lee, E.K.P.; Wong, M.C.S.; Wong, S.Y.S. What Happened to People with Non-Communicable Diseases during COVID-19: Implications of H-EDRM Policies. Int. J. Environ. Res. Public Health 2020, 17, 5588. [Google Scholar] [CrossRef]
- Finlay, B.B.; Amato, K.R.; Azad, M.; Blaser, M.J.; Bosch, T.C.G.; Chu, H.; Dominguez-Bello, M.G.; Ehrlich, S.D.; Elinav, E.; Geva-Zatorsky, N.; et al. The hygiene hypothesis, the COVID pandemic, and consequences for the human microbiome. Proc. Natl. Acad. Sci. USA 2021, 118, e2010217118. [Google Scholar] [CrossRef]
- Dietert, R.R. The microbiological basis of human superorganism freedom. Am. J. Biomed. Sci. Res. 2021, 13, 653–662. [Google Scholar] [CrossRef]
- Office of the Director—Eunice Kennedy Shriver National Institute of Child Health and Human Development. National Children’s Study Archive (version 5/12/20). Available online: https://www.nichd.nih.gov/research/supported/NCS (accessed on 26 August 2021).
- Hudak, M.L.; Park, C.H.; Annett, R.D.; Hale, D.E.; McGovern, P.M.; McLaughlin, T.J.; Dole, N.; Kaar, J.L.; Balsam, M.J. The National Children’s Study: An Introduction and Historical Overview. Pediatrics 2016, 137, S213–S218. [Google Scholar] [CrossRef] [Green Version]
- Kaiser, J. NIH Cancels Massive U.S. Children’s Study. Science. 2014. Available online: https://www.science.org/news/2014/12/nih-cancels-massive-us-children-s-study (accessed on 27 August 2021).
- Schmidt, C. The Death of the National Children’s Study: What Went Wrong? Undark. 2016. Available online: https://undark.org/2016/05/25/the-death-of-a-study-national-childrens-study/ (accessed on 30 August 2021).
- Greenstreet, R. Adjustment of Rates of Guillain-Barré Syndrome among Recipients of Swine Flu Vaccine, 1976–1977. J. R. Soc. Med. 1983, 76, 620–621. [Google Scholar] [CrossRef] [Green Version]
- Sencer, D.J.; Millar, J.D. Reflections on the 1976 swine flu vaccination program. Emerg. Infect. Dis. 2006, 12, 29–33. [Google Scholar] [CrossRef] [Green Version]
- Arachi, D.; Furuya, S.; David, A.; Mangwiro, A.; Chimed-Ochir, O.; Lee, K.; Tighe, P.; Takala, J.; Driscoll, T.; Takahashi, K. Development of the “National Asbestos Profile” to Eliminate Asbestos-Related Diseases in 195 Countries. Int. J. Environ. Res. Public Health 2021, 18, 1804. [Google Scholar] [CrossRef]
- Banerjee, N.; Wang, H.; Wang, G.; Boor, P.J.; Khan, M.F. Redox-sensitive Nrf2 and MAPK signaling pathways contribute to trichloroethene-mediated autoimmune disease progression. Toxicology 2021, 457, 152804. [Google Scholar] [CrossRef]
- Ben Maamar, M.; Nilssonm, E.; Thorson, J.L.M.; Beck, D.; Skinner, M.K. Transgenerational disease specific epigenetic sperm biomarkers after ancestral exposure to dioxin. Environ. Res. 2021, 192, 110279. [Google Scholar] [CrossRef]
- McCann, M.S.; Fernandez, H.R.; Flowers, S.A.; Maguire-Zeiss, K.A. Polychlorinated biphenyls induce oxidative stress and metabolic responses in astrocytes. Neurotoxicology 2021, 86, 59–68. [Google Scholar] [CrossRef]
- Pirozzi, C.; Lama, A.; Annunziata, C.; Cavaliere, G.; Ruiz-Fernandez, C.; Monnolo, A.; Comella, F.; Gualillo, O.; Stornaiuolo, M.; Mollica, M.P.; et al. Oral Bisphenol A Worsens Liver Immune-Metabolic and Mitochondrial Dysfunction Induced by High-Fat Diet in Adult Mice: Cross-Talk between Oxidative Stress and Inflammasome Pathway. Antioxidants 2020, 9, 1201. [Google Scholar] [CrossRef]
- Pandey, N.; Maske, P.; Mote, C.; Dighe, V. Exposure to Atrazine through gestation and lactation period led to impaired sexual maturation and subfertility in F1 male rats with congenital deformities in F2 progeny. Food Chem. Toxicol. 2021, 29, 112586. [Google Scholar] [CrossRef]
- Wang, D.; Liu, J.; Jiang, H. Triclosan regulates the Nrf2/HO-1 pathway through the PI3K/Akt/JNK signaling cascade to induce oxidative damage in neurons. Environ. Toxicol. 2021, 36, 1953–1964. [Google Scholar] [CrossRef]
- Qiu, L.; Wang, H.; Dong, T.; Huang, J.; Li, T.; Ren, H.; Wang, X.; Qu, J.; Wang, S. Perfluorooctane sulfonate (PFOS) disrupts testosterone biosynthesis via CREB/CRTC2/StAR signaling pathway in Leydig cells. Toxicology 2021, 449, 152663. [Google Scholar] [CrossRef]
- Kannan, K.; Vimalkuma, K. A Review of Human Exposure to Microplastics and Insights Into Microplastics as Obesogens. Front. Endocrinol. 2021, 12, 724989. [Google Scholar] [CrossRef]
- Chen, L.; Nie, P.; Yao, L.; Tang, Y.; Hong, W.; Liu, W.; Fu, F.; Xu, H. TiO2 NPs induce the reproductive toxicity in mice with gestational diabetes mellitus through the effects on the endoplasmic reticulum stress signaling pathway. Ecotoxicol. Environ. Saf. 2021, 226, 112814. [Google Scholar] [CrossRef]
- Hu, J.; Lesseur, C.; Miao, Y.; Manservisi, F.; Panzacchi, S.; Mandrioli, D.; Belpoggi, F.; Chen, J.; Petrick, L. Low-dose exposure of glyphosate-based herbicides disrupt the urine metabolome and its interaction with gut microbiota. Sci. Rep. 2021, 11, 3265. [Google Scholar] [CrossRef]
- Le Bastard, Q.; Berthelot, L.; Soulillou, J.P.; Montassier, E. Impact of non-antibiotic drugs on the human intestinal microbiome. Expert Rev. Mol. Diagn. 2021, 15, 1–14. [Google Scholar] [CrossRef]
- Wang, X.; Tang, Q.; Hou, H.; Zhang, W.; Li, M.; Chen, D.; Gu, Y.; Wang, B.; Hou, J.; Liu, Y.; et al. Gut Microbiota in NSAID Enteropathy: New Insights From Inside. Front. Cell Infect. Microbiol. 2021, 11, 679396. [Google Scholar] [CrossRef] [PubMed]
- Inghammar, M.; Svanström, H.; Voldstedlund, M.; Melbye, M.; Hviid, A.; Mølbak, K.; Pasterna, B. Proton-Pump Inhibitor Use and the Risk of Community-Associated Clostridium difficile Infection. Clin. Infect. Dis. 2021, 72, e1084–e1089. [Google Scholar] [CrossRef] [PubMed]
- Bancil, A.S.; Sandall, A.M.; Rossi, M.; Chassaing, B.; Lindsay, J.O.; Whelan, K. Food Additive Emulsifiers and Their Impact on Gut Microbiome, Permeability, and Inflammation: Mechanistic Insights in Inflammatory Bowel Disease. J. Crohns Colitis 2021, 15, 1068–1079. [Google Scholar] [CrossRef]
- Naimi, S.; Viennois, E.; Gewirtz, A.T.; Chassaing, B. Direct impact of commonly used dietary emulsifiers on human gut microbiota. Microbiome 2021, 9, 66. [Google Scholar] [CrossRef]
- Jin, G.; Tang, Q.; Ma, J.; Liu, X.; Zhou, B.; Sun, Y.; Pang, X.; Guo, Z.; Xie, R.; Liu, T.; et al. Maternal Emulsifier P80 Intake Induces Gut Dysbiosis in Offspring and Increases Their Susceptibility to Colitis in Adulthood. mSystems 2021, 6, e01337-20. [Google Scholar] [CrossRef]
- Latham, J. The Failure of the Genome. The Guardian (UK). 2011. Available online: https://www.theguardian.com/commentisfree/2011/apr/17/human-genome-genetics-twin-studies (accessed on 9 October 2021).
- DeWitt, R.D. Pediatric lead exposure and the water crisis in Flint, Michigan. J. Am. Acad. PAs 2017, 30, 43–46. [Google Scholar] [CrossRef]
- Hanna-Attisha, M.; Lanphear, B.; Landrigan, P. Lead Poisoning in the 21st Century: The Silent Epidemic Continues. Am. J. Public Health 2018, 108, 1430. [Google Scholar] [CrossRef]
- Block, J. Vaccinating people who have had covid-19: Why doesn’t natural immunity count in the US? BMJ 2021, 374, n2101. [Google Scholar] [CrossRef]
- Bejarano, D.A.; Schlitzer, A. Breathing more breadth into COVID-19 T cell responses. Med 2021, 2, 999–1001. [Google Scholar] [CrossRef]
- CDC Statement. Natural Immunity and Vaccinations. CDC Media Relations. 2021. Available online: https://www.cdc.gov/media/releases/2021/s0806-vaccination-protection.html (accessed on 1 October 2021).
- Zabetakis, I.; Lordan, R.; Norton, C.; Tsoupras, A. COVID-19: The Inflammation Link and the Role of Nutrition in Potential Mitigation. Nutrients 2020, 12, 1466. [Google Scholar] [CrossRef]
- Nikoloski, Z.; Alqunaibet, A.M.; Alfawaz, R.A.; Almudarra, S.S.; Herbst, C.H.; El-Saharty, S.; Alsukait, R.; Algwizani, A. Covid-19 and non-communicable diseases: Evidence from a systematic literature review. BMC Public Health 2021, 21, 1068. [Google Scholar] [CrossRef]
- Dietert, R.R.; Etzel, R.A.; Chen, D.; Halonen, M.; Holladay, S.D.; Jarabek, A.M.; Landreth, K.; Peden, D.B.; Pinkerton, K.; Smialowicz, R.J.; et al. Workshop to identify critical windows of exposure for children’s health: Immune and respiratory systems work group summary. Environ. Health Perspect. 2000, 108, 483–490. [Google Scholar] [CrossRef] [Green Version]
- Barouki, R.; Gluckman, P.D.; Grandjean, P.; Hanson, M.; Heindel, J.J. Developmental origins of non-communicable disease: Implications for research and public health. Environ. Health 2012, 11, 42. [Google Scholar] [CrossRef] [Green Version]
- Anway, M.D.; Skinner, M.K. Epigenetic transgenerational actions of endocrine disruptors. Endocrinology 2006, 147, S43–S49. [Google Scholar] [CrossRef]
- George, F.; Mahieux, S.; Daniel, C.; Titécat, M.; Beauval, N.; Houcke, I.; Neut, C.; Allorge, D.; Borges, F.; Jan, G.; et al. Assessment of Pb(II), Cd(II), and Al(III) Removal Capacity of Bacteria from Food and Gut Ecological Niches: Insights into Biodiversity to Limit Intestinal Biodisponibility of Toxic Metals. Microorganisms 2021, 9, 456. [Google Scholar] [CrossRef]
- Duan, H.; Yu, L.; Tian, F.; Zhai, Q.; Fan, L.; Chen, W. Gut microbiota: A target for heavy metal toxicity and a probiotic protective strategy. Sci. Total Environ. 2020, 742, 140429. [Google Scholar] [CrossRef]
- Su, H.; Liu, J.; Wu, G.; Long, Z.; Fan, J.; Xu, Z.; Liu, J.; Yu, Z.; Cao, M.; Liao, N.; et al. Homeostasis of gut microbiota protects against polychlorinated biphenyl 126-induced metabolic dysfunction in liver of mice. Sci. Total Environ. 2020, 720, 137597. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, L.; Duan, H.; Zhao, R.; Xiao, Y.; Guo, M.; Zhao, J.; Zhang, H.; Chen, W.; Tian, F. The Protection of Lactiplantibacillus plantarum CCFM8661 Against Benzopyrene-Induced Toxicity via Regulation of the Gut Microbiota. Front. Immunol. 2021, 12, 736129. [Google Scholar] [CrossRef]
- Dietert, R.R.; Dietert, J.M. Twentieth Century Dogmas Prevent Sustainable Healthcare. Am. J. Biomed. Sci. Res. 2021, 13, 409–417. [Google Scholar] [CrossRef]
- Cani, P.D.; Everard, A. Keeping gut lining at bay: Impact of emulsifiers. Trends Endocrinol. Metab. 2015, 26, 273–274. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhao, M.; Zhang, H.; Li, Y.; Liu, M.; Feng, F. Antimicrobial Emulsifier-Glycerol Monolaurate Induces Metabolic Syndrome, Gut Microbiota Dysbiosis, and Systemic Low-Grade Inflammation in Low-Fat Diet Fed Mice. Mol. Nutr. Food Res. 2018, 62, 1700547. [Google Scholar] [CrossRef]
- Godinez Puig, L.; Lusk, K.; Glick, D.; Einstein, K.L.; Palmer, M.; Fox, S.; Wang, M.L. Perceptions of Public Health Priorities and Accountability Among US Mayors. Public Health Rep. 2021, 136, 161–171. [Google Scholar] [CrossRef]
- WHO Obesity and Overweight. Fact Sheet. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 9 October 2021).
- Vogel, S.A. The politics of plastics: The making and unmaking of bisphenol a “safety”. Am. J. Public Health 2009, 99, S559–S566. [Google Scholar] [CrossRef]
- Andújar, N.; Gálvez-Ontiveros, Y.; Zafra-Gómez, A.; Rodrigo, L.; Álvarez-Cubero, M.J.; Aguilera, M.; Monteagudo, C.; Rivas, A.A. Bisphenol A Analogues in Food and Their Hormonal and Obesogenic Effects: A Review. Nutrients 2019, 11, 2136. [Google Scholar] [CrossRef] [Green Version]
- Desai, M.; Ferrini, M.G.; Jellyman, J.K.; Han, G.; Ross, M.G. In vivo and in vitro bisphenol A exposure effects on adiposity. J. Dev. Orig. Health Dis. 2018, 9, 678–687. [Google Scholar] [CrossRef]
- Pérez-Bermejo, M.; Mas-Pérez, I.; Murillo-Llorente, M.T. The Role of the Bisphenol A in Diabetes and Obesity. Biomedicines 2021, 9, 666. [Google Scholar] [CrossRef]
- Linares, R.; Fernández, M.F.; Gutiérrez, A.; García-Villalba, R.; Suárez, B.; Zapater, P.; Martínez-Blázquez, J.A.; Caparrós, E.; Tomás-Barberán, F.A.; Francés, R. Endocrine disruption in Crohn’s disease: Bisphenol A enhances systemic inflammatory response in patients with gut barrier translocation of dysbiotic microbiota products. FASEB J. 2021, 35, e21697. [Google Scholar] [CrossRef]
- Roberts, L. Genome Mapping Goal Now in Reach: James Watson has promised to complete a map of the human genome within 5 years; now it looks like it might be doable. Science 1989, 244, 424–425. [Google Scholar] [CrossRef]
- Anderson, G.C. Human genome project. The honeymoon is over. Nature 1990, 346, 309. [Google Scholar] [CrossRef]
- Saey, T.H. More than a Chicken, Fewer than a Grape. Science News. 2010. Available online: https://www.sciencenews.org/article/more-chicken-fewer-grape (accessed on 11 October 2021).
- Craft-Blacksheare, M.G. Lessons Learned From the Crisis in Flint, Michigan Regarding the Effects of Contaminated Water on Maternal and Child Health. J. Obstet. Gynecol. Neonatal Nurs. 2017, 46, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Akdis, C.A. Does the epithelial barrier hypothesis explain the increase in allergy, autoimmunity and other chronic conditions? Nat. Rev. Immunol. 2021, 21, 739–751. [Google Scholar] [CrossRef] [PubMed]
- Swaney, M.H.; Kalan, L.R. Living in Your Skin: Microbes, Molecules, and Mechanisms. Infect. Immun. 2021, 89, e00695-20. [Google Scholar] [CrossRef] [PubMed]
- Nakatsuji, T.; Chen, T.H.; Narala, S.; Chun, K.A.; Two, A.M.; Yun, T.; Shafiq, F.; Kotol, P.F.; Bouslimani, A.; Melnik, A.V.; et al. Antimicrobials from human skin commensal bacteria protect against Staphylococcus aureus and are deficient in atopic dermatitis. Sci. Transl. Med. 2017, 9, eaah4680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan-Lim, C.S.C.; Esteban-Ipac, N.A.R.; Recto, M.S.T.; Castor, M.A.R.; Casis-Hao, R.J.; Nano, A.L.M. Comparative effectiveness of probiotic strains on the prevention of pediatric atopic dermatitis: A systematic review and network meta-analysis. Pediatr. Allergy Immunol. 2021, 32, 1255–1270. [Google Scholar] [CrossRef] [PubMed]
- Cukrowska, B.; Ceregra, A.; Maciorkowska, E.; Surowska, B.; Zegadło-Mylik, M.A.; Konopka, E.; Trojanowska, I.; Zakrzewska, M.; Bierła, J.B.; Zakrzewski, M.; et al. The Effectiveness of Probiotic Lactobacillus rhamnosus and Lactobacillus casei Strains in Children with Atopic Dermatitis and Cow’s Milk Protein Allergy: A Multicenter, Randomized, Double Blind, Placebo Controlled Study. Nutrients 2021, 13, 1169. [Google Scholar] [CrossRef]
- Callewaert, C.; Knödlseder, N.; Karoglan, A.; Güel, M.; Paetzold, B. Skin microbiome transplantation and manipulation: Current state of the art. Comput. Struct. Biotechnol. J. 2021, 19, 624–631. [Google Scholar] [CrossRef]
- Abuqwider, J.N.; Mauriello, G.; Altamimi, M. Akkermansia muciniphila, a New Generation of Beneficial Microbiota in Modulating Obesity: A Systematic Review. Microorganisms 2021, 9, 1098. [Google Scholar] [CrossRef]
- Ouyang, J.; Lin, J.; Isnard, S.; Fombuena, B.; Peng, X.; Marette, A.; Routy, B.; Messaoudene, M.; Chen, Y.; Routy, J.P. The Bacterium Akkermansia muciniphila: A Sentinel for Gut Permeability and Its Relevance to HIV-Related Inflammation. Front. Immunol. 2020, 11, 645. [Google Scholar] [CrossRef]
- Ottman, N.; Reunanen, J.; Meijerink, M.; Pietilä, T.E.; Kainulainen, V.; Klievink, J.; Huuskonen, L.; Aalvink, S.; Skurnik, M.; Boeren, S.; et al. Pili-like proteins of Akkermansia muciniphila modulate host immune responses and gut barrier function. PLoS ONE 2017, 12, e0173004. [Google Scholar] [CrossRef]
- Anhê, F.F.; Pilon, G.; Roy, D.; Desjardins, Y.; Levy, E.; Marette, A. Triggering Akkermansia with dietary polyphenols: A new weapon to combat the metabolic syndrome? Gut Microbes 2016, 7, 146–153. [Google Scholar] [CrossRef] [Green Version]
- Huang, D.; Gao, J.; Li, C.; Nong, C.; Huang, W.; Zheng, X.; Li, S.; Peng, Y. A potential probiotic bacterium for antipsychotic-induced metabolic syndrome: Mechanisms underpinning how Akkermansia muciniphila subtype improves olanzapine-induced glucose homeostasis in mice. Psychopharmacology 2021, 238, 2543–2553. [Google Scholar] [CrossRef]
- Carlier, F.M.; de Fays, C.; Pilette, C. Epithelial Barrier Dysfunction in Chronic Respiratory Diseases. Front. Physiol. 2021, 12, 691227. [Google Scholar] [CrossRef]
- Nino, G.; Rodriguez-Martinez, C.E.; Gutierrez, M.J. Early Microbial-Immune Interactions and Innate Immune Training of the Respiratory System during Health and Disease. Children 2021, 8, 413. [Google Scholar] [CrossRef]
- Coleman, M.; Dietert, R.R.; North, D.W.; Stephenson, M.M. Enhancing Human Superorganism Ecosystem Resilience by Holistically ‘Managing our Microbes’. J. Appl. Microbiol. 2021, in press. [Google Scholar] [CrossRef]
- Nordengrün, M.; Abdurrahman, G.; Treffon, J.; Wächter, H.; Kahl, B.C.; Bröker, B.M. Allergic Reactions to Serine Protease-Like Proteins of Staphylococcus aureus. Front. Immunol. 2021, 12, 651060. [Google Scholar] [CrossRef]
- Lokken-Toyli, K.L.; de Steenhuijsen Piters, W.A.A.; Zangari, T.; Martel, R.; Kuipers, K.; Shopsin, B.; Loomis, C.; Bogaert, D.; Weiser, J.N. Decreased production of epithelial-derived antimicrobial molecules at mucosal barriers during early life. Mucosal Immunol. 2021, 14, 1358–1368. [Google Scholar] [CrossRef]
- Cole, A.L.; Sundar, M.; Lopez, A.; Forsman, A.; Yooseph, S.; Cole, A.M. Identification of Nasal Gammaproteobacteria with Potent Activity against Staphylococcus aureus: Novel Insights into the “Noncarrier” State. mSphere 2021, 6, e01015-20. [Google Scholar] [CrossRef]
- Accorsi, E.K.; Franzosa, E.A.; Hsu, T.; Joice Cordy, R.; Maayan-Metzger, A.; Jaber, H.; Reiss-Mandel, A.; Kline, M.; DuLong, C.; Lipsitch, M.; et al. Determinants of Staphylococcus aureus carriage in the developing infant nasal microbiome. Genome Biol. 2020, 21, 301. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, Q.; Meng, H.; Lv, H.; Liu, Y.; Liu, J.; Wang, H.; He, L.; Qin, J.; Wang, Y.; et al. Staphylococcus epidermidis Contributes to Healthy Maturation of the Nasal Microbiome by Stimulating Antimicrobial Peptide Production. Cell Host Microbe 2020, 27, 68–78. [Google Scholar] [CrossRef]
- Leung, R.; Covasa, M. Do Gut Microbes Taste? Nutrients 2021, 13, 2581. [Google Scholar] [CrossRef]
- Ellender, G.; Moynihan, P. Oral Health Impacts on Flavor and Significance in Dental Treatment. JDR Clin. Trans. Res. 2021, 6, 460–462. [Google Scholar] [CrossRef]
- Jurczak, A.; Jamka-Kasprzyk, M.; Bębenek, Z.; Staszczyk, M.; Jagielski, P.; Kościelniak, D.; Gregorczyk-Maga, I.; Kołodziej, I.; Kępisty, M.; Kukurba-Setkowicz, M.; et al. Differences in Sweet Taste Perception and Its Association with the Streptococcus mutans Cariogenic Profile in Preschool Children with Caries. Nutrients 2020, 12, 2592. [Google Scholar] [CrossRef]
- Burcham, Z.M.; Garneau, N.L.; Comstock, S.S.; Tucker, R.M.; Knight, R.; Metcalf, J.L.; Genetics of Taste Lab Citizen Scientists. Patterns of Oral Microbiota Diversity in Adults and Children: A Crowdsourced Population Study. Sci. Rep. 2020, 10, 2133. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Cui, J.; Liu, Y.; Chen, K.; Huang, L.; Liu, Y. Oral, Tongue-Coating Microbiota, and Metabolic Disorders: A Novel Area of Interactive Research. Front. Cardiovasc. Med. 2021, 8, 730203. [Google Scholar] [CrossRef]
- Esberg, A.; Haworth, S.; Hasslöf, P.; Lif Holgerson, P.; Johansson, I. Oral Microbiota Profile Associates with Sugar Intake and Taste Preference Genes. Nutrients 2020, 12, 681. [Google Scholar] [CrossRef] [Green Version]
- Besnard, P.; Christensen, J.E.; Bernard, A.; Collet, X.; Verges, B.; Burcelin, R. Fatty taste variability in obese subjects: The oral microbiota hypothesis. Oilseeds Fats Crop. Lipids 2020, 27, 38. [Google Scholar] [CrossRef]
- Besnard, P.; Christensen, J.E.; Bernard, A.; Simoneau-Robin, I.; Collet, X.; Verges, B.; Burcelin, R. Identification of an oral microbiota signature associated with an impaired orosensory perception of lipids in insulin-resistant patients. Acta Diabetol. 2020, 57, 1445–1451. [Google Scholar] [CrossRef]
- Bernard, A.; Le Beyec-Le Bihan, J.; Radoi, L.; Coupaye, M.; Sami, O.; Casanova, N.; Le May, C.; Collet, X.; Delaby, P.; Le Bourgot, C.; et al. Orosensory Perception of Fat/Sweet Stimuli and Appetite-Regulating Peptides before and after Sleeve Gastrectomy or Gastric Bypass in Adult Women with Obesity. Nutrients 2021, 13, 878. [Google Scholar] [CrossRef]
- Khan, A.S.; Keast, R.; Khan, N.A. Preference for dietary fat: From detection to disease. Prog. Lipid Res. 2020, 78, 101032. [Google Scholar] [CrossRef]
- Mameli, C.; Cattaneo, C.; Panelli, S.; Comandatore, F.; Sangiorgio, A.; Bedogni, G.; Bandi, C.; Zuccotti, G.; Pagliarini, E. Taste perception and oral microbiota are associated with obesity in children and adolescents. PLoS ONE 2019, 14, e0221656. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, C.; Riso, P.; Laureati, M.; Gargari, G.; Pagliarini, E. Exploring Associations between Interindividual Differences in Taste Perception, Oral Microbiota Composition, and Reported Food Intake. Nutrients 2019, 11, 1167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pocheron, A.L.; Le Dréan, G.; Billard, H.; Moyon, T.; Pagniez, A.; Heberden, C.; Le Chatelier, E.; Darmaun, D.; Michel, C.; Parnet, P. Maternal Microbiota Transfer Programs Offspring Eating Behavior. Front. Microbiol. 2021, 12, 672224. [Google Scholar] [CrossRef] [PubMed]
- Bernard, A.; Ancel, D.; Neyrinck, A.M.; Dastugue, A.; Bindels, L.B.; Delzenne, N.M.; Besnard, P. A Preventive Prebiotic Supplementation Improves the Sweet Taste Perception in Diet-Induced Obese Mice. Nutrients 2019, 11, 549. [Google Scholar] [CrossRef] [Green Version]
- Heys, C.; Fisher, A.M.; Dewhurst, A.D.; Lewis, Z.; Lizé, A. Exposure to foreign gut microbiota can facilitate rapid dietary shifts. Sci. Rep. 2021, 11, 16791. [Google Scholar] [CrossRef]
- Leitão-Gonçalves, R.; Carvalho-Santos, Z.; Francisco, A.P.; Fioreze, G.T.; Anjos, M.; Baltazar, C.; Elias, A.P.; Itskov, P.M.; Piper, M.D.W.; Ribeiro, C. Commensal bacteria and essential amino acids control food choice behavior and reproduction. PLoS Biol. 2017, 15, e2000862. [Google Scholar] [CrossRef]
- Schwartz, M.; Canon, F.; Feron, G.; Neiers, F.; Gamero, A. Impact of Oral Microbiota on Flavor Perception: From Food Processing to In-Mouth Metabolization. Foods 2021, 10, 2006. [Google Scholar] [CrossRef]
- Thompson, M.D.; DeBosch, B.J. Maternal Fructose Diet-Induced Developmental Programming. Nutrients 2021, 13, 3278. [Google Scholar] [CrossRef]
- Beisner, J.; Gonzalez-Granda, A.; Basrai, M.; Damms-Machado, A.; Bischoff, S.C. Fructose-Induced Intestinal Microbiota Shift Following Two Types of Short-Term High-Fructose Dietary Phases. Nutrients 2020, 12, 3444. [Google Scholar] [CrossRef]
- Naudon, L.; François, A.; Mariadassou, M.; Monnoye, M.; Philippe, C.; Bruneau, A.; Dussauze, M.; Rué, O.; Rabot, S.; Meunier, N. First step of odorant detection in the olfactory epithelium and olfactory preferences differ according to the microbiota profile in mice. Behav. Brain Res. 2020, 384, 112549. [Google Scholar] [CrossRef] [Green Version]
- Bienenstock, J.; Kunze, W.A.; Forsythe, P. Disruptive physiology: Olfaction and the microbiome-gut-brain axis. Biol. Rev. Camb. Philos Soc. 2018, 93, 390–403. [Google Scholar] [CrossRef]
- Koskinen, K.; Reichert, J.L.; Hoier, S.; Schachenreiter, J.; Duller, S.; Moissl-Eichinger, C.; Schöpf, V. The nasal microbiome mirrors and potentially shapes olfactory function. Sci. Rep. 2018, 8, 1296. [Google Scholar] [CrossRef] [Green Version]
- Casadei, E.; Tacchi, L.; Lickwar, C.R.; Espenschied, S.T.; Davison, J.M.; Muñoz, P.; Rawls, J.F.; Salinas, I. Commensal Bacteria Regulate Gene Expression and Differentiation in Vertebrate Olfactory Systems Through Transcription Factor REST. Chem. Senses 2019, 44, 615–630. [Google Scholar] [CrossRef]
- Morquecho-Campos, P.; de Graaf, K.; Boesveldt, S. Smelling our appetite? The influence of food odors on congruent appetite, food preferences and intake. Food Qual. Prefer. 2020, 85, 103959. [Google Scholar] [CrossRef]
- Uauy, R.; Díaz, E. Consequences of food energy excess and positive energy balance. Public Health Nutr. 2005, 8, 1077–1099. [Google Scholar] [CrossRef] [Green Version]
- Rautmann, A.W.; de La Serre, C.B. Microbiota’s Role in Diet-Driven Alterations in Food Intake: Satiety, Energy Balance, and Reward. Nutrients 2021, 13, 3067. [Google Scholar] [CrossRef]
- Han, H.; Yi, B.; Zhong, R.; Wang, M.; Zhang, S.; Ma, J.; Yin, Y.; Yin, J.; Chen, L.; Zhang, H. From gut microbiota to host appetite: Gut microbiota-derived metabolites as key regulators. Microbiome 2021, 9, 162. [Google Scholar] [CrossRef]
- Pizarroso, N.A.; Fuciños, P.; Gonçalves, C.; Pastrana, L.; Amado, I.R. A Review on the Role of Food-Derived Bioactive Molecules and the Microbiota-Gut-Brain Axis in Satiety Regulation. Nutrients 2021, 13, 632. [Google Scholar] [CrossRef]
- Leeuwendaal, N.K.; Cryan, J.F.; Schellekens, H. Gut peptides and the microbiome: Focus on ghrelin. Curr. Opin. Endocrinol. Diabetes Obes. 2021, 28, 243–252. [Google Scholar] [CrossRef]
- Sanchez, M.; Darimont, C.; Panahi, S.; Drapeau, V.; Marette, A.; Taylor, V.H.; Doré, J.; Tremblay, A. Effects of a Diet-Based Weight-Reducing Program with Probiotic Supplementation on Satiety Efficiency, Eating Behaviour Traits, and Psychosocial Behaviours in Obese Individuals. Nutrients 2017, 9, 284. [Google Scholar] [CrossRef]
- Narmaki, E.; Borazjani, M.; Ataie-Jafari, A.; Hariri, N.; Doost, A.H.; Qorbani, M.; Saidpour, A. The combined effects of probiotics and restricted calorie diet on the anthropometric indices, eating behavior, and hormone levels of obese women with food addiction: A randomized clinical trial. Nutr. Neurosci. 2020, 15, 1–13. [Google Scholar] [CrossRef]
- Cheng, Y.C.; Liu, J.R. Effect of Lactobacillus rhamnosus GG on Energy Metabolism, Leptin Resistance, and Gut Microbiota in Mice with Diet-Induced Obesity. Nutrients 2020, 12, 2557. [Google Scholar] [CrossRef]
- Wang, B.; Yu, H.; He, Y.; Wen, L.; Gu, J.; Wang, X.; Miao, X.; Qiu, G.; Wang, H. Effect of soybean insoluble dietary fiber on prevention of obesity in high-fat diet fed mice via regulation of the gut microbiota. Food Funct. 2021, 12, 7923–7937. [Google Scholar] [CrossRef]
- Russell, J.T.; Zhou, Y.; Weinstock, G.M.; Bubier, J.A. The Gut Microbiome and Substance Use Disorder. Front Neurosci. 2021, 15, 725500. [Google Scholar] [CrossRef]
- Dietert, R.R.; Dietert, J.M. Microbiome First Approaches in Pain Prevention and Management. Am. J. Biomed. Sci. Res. 2021, 14, 184–192. [Google Scholar] [CrossRef]
- Forouzan, S.; McGrew, K.; Kosten, T.A. Drugs and bugs: Negative affect, psychostimulant use and withdrawal, and the microbiome. Am. J. Addict. 2021, 30, 525–538. [Google Scholar] [CrossRef]
- O’Sullivan, S.J.; Schwaber, J.S. Similarities in alcohol and opioid withdrawal syndromes suggest common negative reinforcement mechanisms involving the interoceptive antireward pathway. Neurosci. Biobehav. Rev. 2021, 125, 355–364. [Google Scholar] [CrossRef]
- Xiao, H.W.; Ge, C.; Feng, G.X.; Li, Y.; Luo, D.; Dong, J.L.; Li, H.; Wang, H.; Cui, M.; Fan, S.J. Gut microbiota modulates alcohol withdrawal-induced anxiety in mice. Toxicol. Lett. 2018, 287, 23–30. [Google Scholar] [CrossRef]
- Zhao, W.; Hu, Y.; Li, C.; Li, N.; Zhu, S.; Tan, X.; Li, M.; Zhang, Y.; Xu, Z.; Ding, Z.; et al. Transplantation of fecal microbiota from patients with alcoholism induces anxiety/depression behaviors and decreases brain mGluR1/PKC ε levels in mouse. Biofactors 2020, 46, 38–54. [Google Scholar] [CrossRef]
- Rodriguez-Gonzalez, A.; Orio, L. Microbiota and Alcohol Use Disorder: Are Psychobiotics a Novel Therapeutic Strategy? Curr. Pharm. Des. 2020, 26, 2426–2437. [Google Scholar] [CrossRef]
- Lucerne, K.E.; Osman, A.; Meckel, K.R.; Kiraly, D.D. Contributions of neuroimmune and gut-brain signaling to vulnerability of developing substance use disorders. Neuropharmacology 2021, 192, 108598. [Google Scholar] [CrossRef] [PubMed]
- Freedman, Z.G.; Kane, J.A.; King, T.S.; Graziane, N.M. The effect of prescribing antibiotics with opioids on the development of opioid use disorder: A national database study. J. Addict. Dis. 2021, 25, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ezquer, F.; Quintanilla, M.E.; Moya-Flores, F.; Morales, P.; Munita, J.M.; Olivares, B.; Landskron, G.; Hermoso, M.A.; Ezquer, M.; Herrera-Marschitz, M.; et al. Innate gut microbiota predisposes to high alcohol consumption. Addict. Biol. 2021, 26, e13018. [Google Scholar] [CrossRef] [PubMed]
- Carbia, C.; Lannoy, S.; Maurage, P.; López-Caneda, E.; O’Riordan, K.J.; Dinan, T.G.; Cryan, J.F. A biological framework for emotional dysregulation in alcohol misuse: From gut to brain. Mol. Psychiatry 2021, 26, 1098–1118. [Google Scholar] [CrossRef]
- García-Cabrerizo, R.; Carbia, C.; O’ Riordan, K.J.; Schellekens, H.; Cryan, J.F. Microbiota-gut-brain axis as a regulator of reward processes. J. Neurochem. 2021, 157, 1495–1524. [Google Scholar] [CrossRef]
- Novelle, M.G. Decoding the Role of Gut-Microbiome in the Food Addiction Paradigm. Int. J. Environ. Res. Public Health 2021, 18, 6825. [Google Scholar] [CrossRef]
- Agustí, A.; Campillo, I.; Balzano, T.; Benítez-Páez, A.; López-Almela, I.; Romaní-Pérez, M.; Forteza, J.; Felipo, V.; Avena, N.M.; Sanz, Y. Bacteroides uniformis CECT 7771 Modulates the Brain Reward Response to Reduce Binge Eating and Anxiety-Like Behavior in Rat. Mol. Neurobiol. 2021, 58, 4959–4979. [Google Scholar] [CrossRef]
- Thomaz, A.C.; Iyer, V.; Woodward, T.J.; Hohmann, A.G. Fecal microbiota transplantation and antibiotic treatment attenuate naloxone-precipitated opioid withdrawal in morphine-dependent mice. Exp. Neurol. 2021, 343, 113787. [Google Scholar] [CrossRef]
- Zawertailo, L.; Attwells, S.; deRuiter, W.K.; Le, T.L.; Dawson, D.; Selby, P. Food Addiction and Tobacco Use Disorder: Common Liability and Shared Mechanisms. Nutrients 2020, 12, 3834. [Google Scholar] [CrossRef]
- Morrow, J.D.; Castaldi, P.J.; Chase, R.P.; Yun, J.H.; Lee, S.; Liu, Y.Y.; Hersh, C.P. Peripheral blood microbial signatures in current and former smokers. Sci. Rep. 2021, 11, 19875. [Google Scholar] [CrossRef]
- Prakash, A.; Peters, B.A.; Cobbs, E.; Beggs, D.; Choi, H.; Li, H.; Hayes, R.B.; Ahn, J. Tobacco Smoking and the Fecal Microbiome in a Large, Multi-ethnic Cohort. Cancer Epidemiol. Biomark. Prev. 2021, 30, 1328–1335. [Google Scholar] [CrossRef]
- Kosciolek, T.; Victor, T.A.; Kuplicki, R.; Rossi, M.; Estaki, M.; Ackermann, G.; Tulsa 1000 Investigators; Knight, R.; Paulus, M.P. Individuals with substance use disorders have a distinct oral microbiome pattern. Brain Behav. Immun. Health 2021, 15, 100271. [Google Scholar] [CrossRef]
- Gopinath, D.; Wie, C.C.; Banerjee, M.; Thangavelu, L.; Kumar, R.P.; Nallaswamy, D.; Botelho, M.G.; Johnson, N.W. Compositional profile of mucosal bacteriome of smokers and smokeless tobacco users. Clin. Oral Investig. 2021, 1–10. [Google Scholar] [CrossRef]
- He, L.; Zhou, Y.X.; Zhang, Y.; Hang, B.; Chang, H.; Schick, S.F.; Celniker, S.E.; Xia, Y.; Snijders, A.M.; Mao, J.H. Thirdhand cigarette smoke leads to age-dependent and persistent alterations in the cecal microbiome of mice. MicrobiologyOpen 2021, 10, e1198. [Google Scholar] [CrossRef]
- Kelley, S.T.; Liu, W.; Quintana, P.J.E.; Hoh, E.; Dodder, N.G.; Mahabee-Gittens, E.M.; Padilla, S.; Ogden, S.; Frenzel, S.; Sisk-Hackworth, L.; et al. Altered microbiomes in thirdhand smoke-exposed children and their home environments. Pediatr. Res. 2021, 1–8. [Google Scholar] [CrossRef]
- Biedermann, L.; Zeitz, J.; Mwinyi, J.; Sutter-Minder, E.; Rehman, A.; Ott, S.J.; Steurer-Stey, C.; Frei, A.; Frei, P.; Scharl, M.; et al. Smoking cessation induces profound changes in the composition of the intestinal microbiota in humans. PLoS ONE 2013, 8, e59260. [Google Scholar] [CrossRef]
- Alberg, A.J.; Carter, C.L.; Carpenter, M.J. Weight gain as an impediment to cigarette smoking cessation: A lingering problem in need of solutions. Prev. Med. 2007, 44, 296–297. [Google Scholar] [CrossRef]
- Simpson, S.; Kimbrough, A.; Boomhower, B.; McLellan, R.; Hughes, M.; Shankar, K.; de Guglielmo, G.; George, O. Depletion of the Microbiome Alters the Recruitment of Neuronal Ensembles of Oxycodone Intoxication and Withdrawal. eNeuro 2020, 7, ENEURO.0312-19.2020. [Google Scholar] [CrossRef]
- Lewis, C.B.; Laflin, M.; Gray, D.L. Exercise as Medicine for Older Women. Clin. Geriatr. Med. 2021, 37, 639–650. [Google Scholar] [CrossRef]
- Nascimento, M.M. Dance, aging, and neuroplasticity: An integrative review. Neurocase 2021, 13, 1–10. [Google Scholar] [CrossRef]
- Carapellotti, A.M.; Stevenson, R.; Doumas, M. The efficacy of dance for improving motor impairments, non-motor symptoms, and quality of life in Parkinson’s disease: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0236820. [Google Scholar] [CrossRef]
- Ruiz-Muelle, A.; López-Rodríguez, M.M. Dance for People with Alzheimer’s Disease: A Systematic Review. Curr. Alzheimer Res. 2019, 16, 919–933. [Google Scholar] [CrossRef]
- Sakellariou, X.M.; Papafaklis, M.I.; Domouzoglou, E.M.; Katsouras, C.S.; Michalis, L.K.; Naka, K.K. Exercise-mediated adaptations in vascular function and structure: Beneficial effects in coronary artery disease. World J. Cardiol. 2021, 13, 399–415. [Google Scholar] [CrossRef]
- Cominato, L.; Franco, R.; Damiani, D. Adolescent obesity treatments: News, views, and evidence. Arch. Endocrinol. Metab. 2021. [Google Scholar] [CrossRef]
- Richards, N.C.; Gouda, H.N.; Durham, J.; Rampatige, R.; Rodney, A.; Whittaker, M. Disability, noncommunicable disease and health information. Bull. World Health Organ. 2016, 94, 230–232. [Google Scholar] [CrossRef]
- Lee, M.C.; Hsu, Y.J.; Ho, H.H.; Kuo, Y.W.; Lin, W.Y.; Tsai, S.Y.; Chen, W.L.; Lin, C.L.; Huang, C.C. Effectiveness of human-origin Lactobacillus plantarum PL-02 in improving muscle mass, exercise performance and anti-fatigue. Sci. Rep. 2021, 11, 19469. [Google Scholar] [CrossRef]
- Agosti, M.; Tandoi, F.; Morlacchi, L.; Bossi, A. Nutritional and metabolic programming during the first thousand days of life. Pediatr. Med. Chir. 2017, 39, 157. [Google Scholar] [CrossRef] [Green Version]
- Reddy, S.P.; Mbewu, A.D. The Implications of the Developmental Origins of Health and Disease on Public Health Policy and Health Promotion in South Africa. Healthcare 2016, 4, 83. [Google Scholar] [CrossRef] [Green Version]
- Barker, D.J. The fetal and infant origins of adult disease. BMJ 1990, 301, 1111. [Google Scholar] [CrossRef] [Green Version]
- Hanson, M.; Gluckman, P. Developmental origins of noncommunicable disease: Population and public health implications. Am. J. Clin. Nutr. 2011, 94, 1754S–1758S. [Google Scholar] [CrossRef] [Green Version]
- Adair, L.S. Long-term consequences of nutrition and growth in early childhood and possible preventive interventions. Nestlé Nutr. Inst. Workshop Ser. 2014, 78, 111–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raspini, B.; Porri, D.; De Giuseppe, R.; Chieppa, M.; Liso, M.; Cerbo, R.M.; Civardi, E.; Garofoli, F.; Monti, M.C.; Vacca, M.; et al. Prenatal and postnatal determinants in shaping offspring’s microbiome in the first 1000 days: Study protocol and preliminary results at one month of life. Ital. J. Pediatr. 2020, 46, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scher, M.S. “The First Thousand Days” Define a Fetal/Neonatal Neurology Program. Front. Pediatr. 2021, 9, 683138. [Google Scholar] [CrossRef] [PubMed]
- Subcomisión DOHaD—SAP “Origen de la Salud y Enfermedad en el Curso de la Vida”—Sociedad Argentina de Pediatría. Concepto de Developmental Origins of Health and Disease: El ambiente en los primeros mil días de vida y su asociación con las enfermedades no transmisibles [Developmental Origins of Health and Disease Concept: The environment in the first 1000 days of life and its association with noncommunicable diseases]. Arch. Argent. Pediatr. 2020, 118, S118–S129. (In Spanish) [Google Scholar] [CrossRef]
- Moreno Villares, J.M.; Collado, M.C.; Larqué, E.; Leis Trabazo, R.; Saenz De Pipaón, M.; Moreno Aznar, L.A. Los primeros 1000 días: Una oportunidad para reducir la carga de las enfermedades no transmisibles [The first 1000 days: An opportunity to reduce the burden of noncommunicable diseases]. Nutr. Hosp. 2019, 36, 218–232. (In Spanish) [Google Scholar] [CrossRef] [PubMed]
- Chilvers, A. The first 1000 days. The impact of microbes on health outcomes. J. Fam. Health 2016, 26, 25–26. [Google Scholar]
- Schwartzenberger, S.J. Your Baby’s First 1000 Days: AAP Policy Explained; American Academy of Pediatrics: Itasca, IL, USA, 2018; Available online: https://www.healthychildren.org/English/ages-stages/baby/Pages/Babys-First-1000-Days-AAP-Policy-Explained.aspx (accessed on 6 October 2021).
| Public Health Initiatives, Challenges, and Responses | Reference(s) |
|---|---|
| The World Health Organization recently tabulated that the vast majority of global deaths (71%) are caused by NCDs. However, they offer no plans seemingly capable of eliminating NCDs as the major cause of death. | [13] |
| The Global Burden of Disease Study illustrates the ongoing NCD epidemic but fails to even mention the microbiome among 87 risk factors and 369 diseases considered across hundreds of countries. It concluded that people are living more years in poor health despite medical advancements. | [19] |
| The extent of the failure of public health initiatives to address the decades-long NCD epidemic was revealed via a recent NHANES study survey. The study found that 91.8% of senior adults in the United States carry two or more NCDs. | [9,10] |
| The public health failure regarding the epidemic of multimorbid NCDs associated with aging was compounded when public health institutions failed to adequately protect the NCD-riddled, pro-inflammatory, and hyper-vulnerable geriatric population against the SARS-CoV-2-induced lethal cytokine storm. | [20,21,22] |
| Public health mandates during the SARS-CoV-2 pandemic that further eroded the human microbiome instead of protecting the microbiome and the microimmunosome. | [23,24] |
| The National Children’s Study was a grand 2000 Congressionally mandated, NIH-led inter-federal agency plan to prioritize early life health risk identification and prevention as the keys to better health both for children and across the lifespan. It was closed in 2014 with little to show for the very expensive initiative. | [25,26,27,28] |
| The Swine Flu incident beginning at Ft. Dix, NJ in 1976 and the rushed national vaccination program for a pandemic that never showed up proved to cause more health damage than good. | [29,30] |
| Public Health protection programs have repeatedly experienced “regulatory gaps” that permitted millions of people across multiple generations to be exposed to NCD-promoting toxicants before the hazard was eventually recognized. These safety testing gaps are not tied to a lack of microbiome safety evaluation. Examples of such global exposures to “safe” chemicals include: asbestos, trichloroethelene, dioxin, polychlorinated biphenyls, plasticizers including bisphenol A, atrazine, triclosan, perfluorinated compounds, microplastics, certain nanoparticles, and other endocrine disruptors and obesogens. | [31,32,33,34,35,36,37,38,39,40] |
| A plethora of food, food additives, drugs, and environmental chemicals previously approved by the FDA, the USDA, and the EPA have been shown to significantly damage the microimmunosome posing a significant risk to human health. Screening for microimmunsome safety would have been useful as would regulatory action based on identified toxicity for the microbiome. | [41,42,43,44,45,46,47] |
| Medical Journal calls to reverse the Public Health failure of the NCD epidemic has produced little effect. | [3] |
| The Human Genome Project was touted as the keystone through which most human diseases would be cured. Instead, it resulted in an underwhelming number of chromosomal genes identified and few diseases cured to date. | [48] |
| Risk–benefit decisions in Flint, MI resulted in years of exposure of children and adults to the neurotoxic, microimmunosome-damaging, heavy metal Pb (lead). | [4,49,50] |
| Disowning fundamental immunology and the role of natural immunity during the SARS-CoV-2 pandemic and the importance of T cell responses to viruses in heterologous adaptive immunity | [51,52,53] |
| Sense | Test Species/Group | Microbe(s) Involved/Subjects Discussed | Effects | Reference(s) |
|---|---|---|---|---|
| Taste | Human (with some mouse research brought in) | Staphylococci, Streptococci Actinomyces, Lactobacillus Prevotella, Porphyromonas Actinobacteria and Bacteroidetes Actinomyces, Oribacterium, Solobacterium, Catonella, Campylobacter Clostridia Proteobacteria, Prevotella Streptococci mutans | In this review article, these bacteria have been associated with changes in specific aspects of taste. | [96] |
| Taste | Human (emphasis on dental patients) | General review of broad scope on taste and including smell. The impact of biofilms is considered. | This review emphasizes the life course ramification of flavor biases and the potential risk to the aging population. | [97] |
| Taste thresholds | Human (preschool children) | Oral microbiota affecting sweet taste thresholds in children | This is an important study showing that preschool with a lower threshold for perceiving sugar consumed less sugar, had fewer dental caries, and a general lack of oral Streptococcus mutans. The reverse was true for children with high thresholds for perceiving sugar. | [98] |
| Taste | Human (adults and youth) | This was a crowdsourced population study of adults and youth. Treponema was found in the oral microbiome of adults with dental problems and of obese youth. | The observation of Treponema in youth suggests it might be a biomarker for later oral health problems and connected in some way to the childhood obesity. This study did not find a microbial sweetness taste difference among the crowdsourced sampling. | [99] |
| Taste | Human | Oral-tongue review of how the mouth microbiome affects the gut microbiome, barrier integrity, inflammation, indirectly the gut–brain axis, the liver and all through taste regulation | The tongue microbiome and its dysbiosis can be a large contributor to metabolic disorders that facilitate obesity, diabetes, and cardiovascular disease. | [100] |
| Taste | Human (dental patients, teenagers and young adults) | Oral microbiota were characterized among dental patients with differing sugar intake and caries vs. fewer caries | Specific oral microbiota were associated with sugar intake. However, there were several distinct ecological combinations of microbiota that were associated with high sugar intake. | [101] |
| Taste | Human (men) | Examined obese men for oral microbiota signatures within the circumvallate papillae (CVP) that relate to fatty taste perception. | Decreased fatty taste perception was associated with elevated Bacteroides genus and Clostridium_XIV and decreased Lactobacillus compared against the high fatty taste perception group. | [102] |
| Taste | Human (diabetic patients) | Examined type 2 diabetic patients for oral microbiota signatures within the circumvallate papillae (CVP) that relate to fatty taste perception. | Impaired fatty acid perception is not driven by insulin resistance but rather is affected by microbiota dysbiosis. Additionally, some drugs (e.g., metformin, statins) may affect lipid sensitivity perception | [103] |
| Taste thresholds and intensity | Human | Analysis of orosensory perception of lipids and sweets in adult females following different types of gastric surgery | Low numbers of patients and high individual variability produced few statistically significant differences beyond a microbiome signature. | [104] |
| Taste sensitivity | Human | This is a review article examining fat taste sensitivity and microbiota. | The article focuses on insensitivity to long-chain dietary fatty acids, the microbiota that are associated with reduced dietary fat detection, and this physiological–microbiological change as a path to obesity. | [105] |
| Taste distinctions | Human | Taste perception, oral microbiota, and childhood obesity were compared in the cross-sectional study | In this cross-sectional study, obese children vs. controls had difficulty identifying taste quality. A lower number of Fungiform Papillae, a lower oral microbiome alpha diversity, and some subtle differences in microbiota representation were reported. | [106] |
| Taste perceptions and food preferences | Human | Oral microbiota, perceptions, and dietary preferences | In a study of 59 volunteers, the results indicated a correlation between tongue dorsum microbiota, gustatory function, and specific food intake. The Clostridia class was associated with high energy, protein, and fat intake while Prevotalla genus bacteria were associated with high fiber intake. | [107] |
| Taste/Eating Behaviors | Rat | Maternal microbiota program the offspring’s eating behavior. | Maternal microbiota transfer from obese prone or obese resistance dams into F344 strain neonates helped to establish that neonatal microbiota can program juveniles and adult eating behaviors. This programming did not require the transferred microbiota to persist into adulthood. It is a microbiome-based example of DOHaD. | [108] |
| Taste Perception | Mouse | Prebiotic modulates sweet taste perception in obese mice | An inulin-type fructan prebiotic was administered to diet-induced obese mice for 12 weeks. The supplementation produced an elevation of cecal Bifidobacteria and Akkermansia and improved the orosensory perception of sweet compounds. | [109] |
| Taste/Food choice behavior | Drosophila research model study | Ingestion of foreign microbiota produced a strong shift in dietary preferences. | A strong food aversion was evolved into a strong food preference by repeated ingestion of microbiota derived from a different Drosophila species. | [110] |
| Taste/Food choice behavior | Drosophila research model study | Strong dietary preferences controlled by the metabolism of commensal bacteria. | Commensal bacteria were shown to direct food preferences via metabolic activity and could overcome some direct effects of the food itself. | [111] |
| Taste and Smell | Review | Oral microbiota metabolism affects flavor perception thresholds via multiple routes. Taste and smell perceptions are both affected. | Comprehensive coverage of the multiple pathways through which both taste and smell are affected by microbiota. | [112] |
| Sense | Test Species/Group | Microbe(s) Involved/Subjects Discussed | Effects | Reference(s) |
|---|---|---|---|---|
| Smell | Mouse (three groups) | Characterization of microbiota among three distinct groups of mice: C3H/HeN, Swiss, and BALB/cByJ. Germ free mice (C3H/HeN) were installed with microbiota from each of the three groups permitting olfactory, electro-olfactogram recordings (EOG) of the epithelium and microbiota comparisons on the same mouse genetic background. | Among 11 odorants examined, several differentially activated the olfactory epithelium linked to the microbiota profile. The findings suggest the importance of the microbiota in the olfactory epithelium physiology (e.g., EOG). | [115] |
| Smell | Mouse and Human (Review) | Chemosensory links between microbiota, olfaction and emotion are described and the “odorome” concept is introduced. An example of bacterial products discussed is β-phenylethylamine. | This is an important review article covering the capacity of bacterial products to affect olfactory receptors and, in turn, to elicit specific emotions. | [116] |
| Smell | Human | Bacterial signatures from among Actinobacteria, Bacteroidia, Bacilli, Clostridia and Proteobacteria were associated with hyposmic (i.e., odor detection) threshold, low discrimination and low identification performance. Corynebacterium and Faecalibacterium were often biomarkers for reduced odor discrimination and threshold. Comamonadaceae and Enterobacteriaceae were linked with reduce thresholds and identification. Porphyromonas and unclassified Lachnospiraceae were associated with poor performance across all three olfactory performance categories. | Odor thresholds, identification and discrimination were all evaluated and found to be linked by microbiota composition. Specific groups of microbiota affected combinations of the three categories. | [117] |
| Smell | Mice and Zebrafish | Mechanistic study in two animal models | Found evidence for nasal microbiota regulation of olfactory transcriptional factors | [118] |
| Sense | Test Species/Group | Microbe(s) Involved/Subjects Discussed | Effects | Reference(s) |
|---|---|---|---|---|
| Satiety | Review of multi-species studies (primarily rodent with some human) | This recent review article details the variety of mechanisms through which gut microbiota control satiety | Microbiota were demonstrated to control both central and peripheral food intake mechanisms. | [121] |
| Satiety | Review of human and mouse studies | Review article covering probiotic and prebiotic studies on eating and satiety. It also discusses the categories of microbial peptides, hormones, and products as well as metabolites that affect hunger, eating, and satiety. Most of the probiotic studies cited used Lactobacillus and/or Bifidobacterium species. | This review describes the control of multiple regulatory factors affecting satiety that are embedded within the gut microbiome. It also summarizes numerous clinical and research studies on microbiota and appetite control. | [122] |
| Satiety | Review | Review article covering microbiota–gut–brain axis in satiety regulation | The article is focused on how we move toward microbiota–gut–brain axis on a chip in vitro assessment. | [123] |
| Satiety | Review | Review article detailing the regulation of gut peptides and particularly ghrelin via gut microbiota | This review article provides satiety-related evidence that gut microbiota regulates ghrelin levels via short chain fatty acids, specific amino acids, formyl peptides, LPS, and H2S, and affects ghrelin receptor signaling. | [124] |
| Satiety | Obese adults | Lactobacillus rhamnosus CGMCC1.3724 (LPR) | This was a 24-week duration (two 12-week phases) double-blind, randomized, placebo-controlled trial examining control of appetite, weight loss, and mood. Positive significant effects on satiety were seen in both men and woman with the latter experiencing the greater benefit. | [125] |
| Satiety | Obese women | A multi-species probiotic mix or placebo was used in combination with a caloric restricted diet. The probiotic mix contained: Lactobacillus acidophilus, Bifidobacterium bifidum, Bifidobacterium lactis, Bifidobacterium longum, Lactobacillus rhamnosus, Lactobacillus reuteri, magnesium stearate, and maltodextrin | This was a 12-week duration, randomized, double-blind, placebo-controlled clinical trial of obese women. Positive effects were seen in the probiotic supplemented group for both eating behavior as well as anthropometric indices. | [126] |
| Satiety | Mouse | Lactobacillus rhamnosus GG | Aged Balb/c mice were fed a regular diet or a high fat diet and two different doses of probiotic supplementation were examined for effects on obesity-related biomarkers including leptin resistance. High dose probiotic reversed the leptin-resistance associated with diet-induced obesity. | [127] |
| Satiety | Mouse | A prebiotic soybean insoluble dietary fiber was administered during a 24-week intervention in high fat diet mice. The specialized fiber induced increases in Lactobacillus and Lachnospirace_Nk4A136_group with decreases in Lachnospiraceae and Bacteroidesacidifaciens | The outcomes of the 24-week prebiotics intervention and microbiome shift were changes in short chain fatty acid production and an elevation in satiety hormones. | [128] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dietert, R.R. Microbiome First Approaches to Rescue Public Health and Reduce Human Suffering. Biomedicines 2021, 9, 1581. https://doi.org/10.3390/biomedicines9111581
Dietert RR. Microbiome First Approaches to Rescue Public Health and Reduce Human Suffering. Biomedicines. 2021; 9(11):1581. https://doi.org/10.3390/biomedicines9111581
Chicago/Turabian StyleDietert, Rodney R. 2021. "Microbiome First Approaches to Rescue Public Health and Reduce Human Suffering" Biomedicines 9, no. 11: 1581. https://doi.org/10.3390/biomedicines9111581