Subchondral Bone Microarchitectural and Mineral Properties and Expression of Key Degradative Proteinases by Chondrocytes in Human Hip Osteoarthritis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Macroscopic Evaluation and Osteochondral Plug Extraction
2.3. MicroCT Scanning
2.4. MicroCT Image Analysis
2.5. Histological Processing and Microscopic Grading
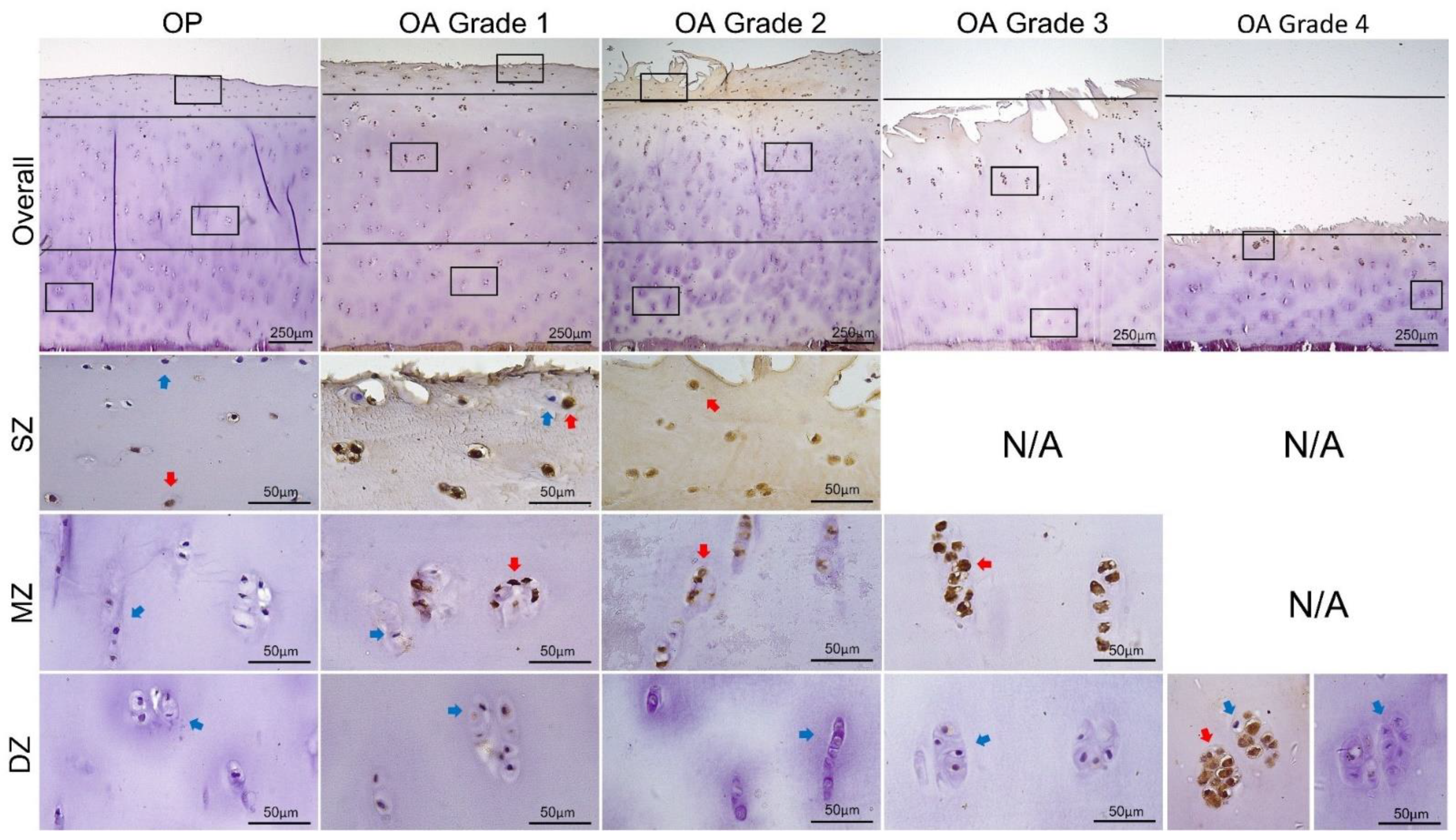
2.6. Immunohistochemical Detection of MMP13 and ADAMTS4
2.7. Measurement of MMP13 and ADAMTS4 Expression
2.8. Statistical Analysis
3. Results
3.1. Microarchitecture and Mineral Properties of Subchondral Bone
3.2. Expression of MMP13 and ADAMTS4 by Chondrocytes
3.3. Correlations between Expression of MMP13 and ADAMTS4 and Subchondral Bone Properties
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Loeser, R.F.; Goldring, S.R.; Scanzello, C.R.; Goldring, M.B. Osteoarthritis: A disease of the joint as an organ. Arthritis Rheum. 2012, 64, 1697–1707. [Google Scholar] [CrossRef] [Green Version]
- Hunter, D.J.; Bierma-Zeinstra, S. Osteoarthritis. Lancet 2019, 393, 1745–1759. [Google Scholar] [CrossRef]
- Madry, H.; van Dijk, C.N.; Mueller-Gerbl, M. The basic science of the subchondral bone. Knee Surg. Sports Traumatol. Arthrosc. 2010, 18, 419–433. [Google Scholar] [CrossRef]
- Sophia Fox, A.J.; Bedi, A.; Rodeo, S.A. The basic science of articular cartilage: Structure, composition, and function. Sports Health 2009, 1, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Chen, Y.; Dou, C.; Dong, S. Microenvironment in subchondral bone: Predominant regulator for the treatment of osteoarthritis. Ann. Rheum. Dis. 2021, 80, 413–422. [Google Scholar] [CrossRef]
- Zhang, L.Z.; Zheng, H.A.; Jiang, Y.; Tu, Y.H.; Jiang, P.H.; Yang, A.L. Mechanical and biologic link between cartilage and subchondral bone in osteoarthritis. Arthritis Care Res. 2012, 64, 960–967. [Google Scholar]
- Lories, R.J.; Luyten, F.P. The bone–cartilage unit in osteoarthritis. Nat. Rev. Rheumatol. 2011, 7, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Yin, J.; Gao, J.; Cheng, T.S.; Pavlos, N.J.; Zhang, C.; Zheng, M.H. Subchondral bone in osteoarthritis: Insight into risk factors and microstructural changes. Arthritis Res. Ther. 2013, 15, 223. [Google Scholar] [CrossRef] [Green Version]
- Karsdal, M.; Bay-Jensen, A.; Lories, R.; Abramson, S.; Spector, T.; Pastoureau, P.; Christiansen, C.; Attur, M.; Henriksen, K.; Goldring, S.R.; et al. The coupling of bone and cartilage turnover in osteoarthritis: Opportunities for bone antiresorptives and anabolics as potential treatments? Ann. Rheum. Dis. 2014, 73, 336–348. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liem, Y.; Dall’Ara, E.; Sullivan, N.; Ahmed, H.; Blom, A.; Sharif, M. Subchondral bone microarchitecture and mineral density in human osteoarthritis and osteoporosis: A regional and compartmental analysis. J. Orthop. Res. 2021, 8, 607764. [Google Scholar]
- Finnilä, M.A.; Thevenot, J.; Aho, O.M.; Tiitu, V.; Rautiainen, J.; Kauppinen, S.; Nieminen, M.T.; Pritzker, K.; Valkealahti, M.; Lehenkari, P.; et al. Association between subchondral bone structure and osteoarthritis histopathological grade. J. Orthop. Res. 2017, 35, 785–792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cox, L.G.E.; van Donkelaar, C.C.; van Rietbergen, B.; Emans, P.J.; Ito, K. Decreased bone tissue mineralization can partly explain subchondral sclerosis observed in osteoarthritis. Bone 2012, 50, 1152–1161. [Google Scholar] [CrossRef] [PubMed]
- Klose-Jensen, R.; Hartlev, L.B.; Boel, L.W.T.; Laursen, M.B.; Stengaard-Pedersen, K.; Keller, K.K.; Hauge, E.-M. Subchondral bone turnover, but not bone volume, is increased in early stage osteoarthritic lesions in the human hip joint. Osteoarthr. Cartil. 2015, 23, 2167–2173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bobinac, D.; Spanjol, J.; Zoricic, S.; Maric, I. Changes in articular cartilage and subchondral bone histomorphometry in osteoarthritic knee joints in humans. Bone 2003, 32, 284–290. [Google Scholar] [CrossRef]
- Chappard, C.; Peyrin, F.; Bonnassie, A.; Lemineur, G.; Brunet-Imbault, B.; Lespessailles, E.; Brunet-Imbault, B.; Lespessailles, E.; Benhamou, C.-L. Subchondral bone micro-architectural alterations in osteoarthritis: A synchrotron micro-computed tomography study. Osteoarthr. Cartil. 2006, 14, 215–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rapagna, S.; Roberts, B.C.; Solomon, L.B.; Reynolds, K.J.; Thewlis, D.; Perilli, E. Tibial cartilage, subchondral bone plate and trabecular bone microarchitecture in varus-and valgus-osteoarthritis versus controls. J. Orthop. Res. 2020, 1–12. [Google Scholar] [CrossRef]
- Troeberg, L.; Nagase, H. Proteases involved in cartilage matrix degradation in osteoarthritis. Biochim. Et Biophys. Acta 2012, 1824, 133–145. [Google Scholar] [CrossRef] [Green Version]
- Kevorkian, L.; Young, D.A.; Darrah, C.; Donell, S.T.; Shepstone, L.; Porter, S.; Brockbank, S.M.V.; Edwards, D.R.; Parker, A.E.; Clark, I.M. Expression profiling of metalloproteinases and their inhibitors in cartilage. Arthritis Rheum. 2004, 50, 131–141. [Google Scholar] [CrossRef]
- Bau, B.; Gebhard, P.M.; Haag, J.; Knorr, T.; Bartnik, E.; Aigner, T. Relative messenger RNA expression profiling of collagenases and aggrecanases in human articular chondrocytes in vivo and in vitro. Arthritis Rheum. 2002, 46, 2648–2657. [Google Scholar] [CrossRef]
- Hayami, T.; Pickarski, M.; Wesolowski, G.A.; Mclane, J.; Bone, A.; Destefano, J.; Rodan, G.A.; Duong, L.T. The role of subchondral bone remodeling in osteoarthritis: Reduction of cartilage degeneration and prevention of osteophyte formation by alendronate in the rat anterior cruciate ligament transection model. Arthritis Rheum. 2004, 50, 1193–1206. [Google Scholar] [CrossRef]
- Zhen, G.; Wen, C.; Jia, X.; Li, Y.; Crane, J.L.; Mears, S.C.; Askin, F.B.; Frassica, F.J.; Chang, W.; Yao, J.; et al. Inhibition of TGF-β signaling in mesenchymal stem cells of subchondral bone attenuates osteoarthritis. Nat. Med. 2013, 19, 704. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Sun, Y.; Pan, X.; Ho, K.; Li, G. Joint distraction attenuates osteoarthritis by reducing secondary inflammation, cartilage degeneration and subchondral bone aberrant change. Osteoarthr. Cartil. 2015, 23, 1728–1735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Li, P.; Zhu, S.; Bi, R. Comparison of early-stage changes of osteoarthritis in cartilage and subchondral bone between two different rat models. PeerJ 2020, 8, e8934. [Google Scholar] [CrossRef] [PubMed]
- Camacho, P.M.; Petak, S.M.; Binkley, N.; Diab, D.L.; Eldeiry, L.S.; Farooki, A.; Harris, S.T.; Hurley, D.L.; Kelly, J.; Lewiecki, M.; et al. American Association of Clinical Endocrinologists/American College of Endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis—2020 update. Endocr. Pract. 2020, 26, 1–46. [Google Scholar] [CrossRef]
- Altman, R.; Alarcón, G.; Appelrouth, D.; Bloch, D.; Borenstein, D.; Brandt, K.; Brown, C.; Cooke, T.D.; Daniel, W.; Feldman, D.; et al. The American College of Rheumatology criteria for the classification and reporting of osteoarthritis of the hip. Arthritis Rheum. 1991, 34, 505–514. [Google Scholar] [CrossRef]
- Bouxsein, M.L.; Boyd, S.K.; Christiansen, B.A.; Guldberg, R.E.; Jepsen, K.J.; Muller, R. Guidelines for Assessment of Bone Microstructure in Rodents Using Micro-Computed Tomography. J. Bone Miner. Res. 2010, 25, 1468–1486. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, N.; Laverty, S.; Kraus, V.; Aigner, T. Basic methods in histopathology of joint tissues. Osteoarthr. Cartil. 2010, 18, S113–S116. [Google Scholar] [CrossRef] [Green Version]
- Pritzker, K.; Gay, S.; Jimenez, S.; Ostergaard, K.; Pelletier, J.-P.; Revell, P.; Salter, D.; Berg, W.V.D. Osteoarthritis cartilage histopathology: Grading and staging. Osteoarthr. Cartil. 2006, 14, 13–29. [Google Scholar] [CrossRef] [Green Version]
- Koo, T.K.; Li, M.Y. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef] [Green Version]
- Pauli, C.; Whiteside, R.; Heras, F.L.; Nesic, D.; Koziol, J.; Grogan, S.P.; Matyas, J.; Pritzker, K.P.H.; D’Lima, D.D.; Lotz, M.K. Comparison of cartilage histopathology assessment systems on human knee joints at all stages of osteoarthritis development. Osteoarthr. Cartil. 2012, 20, 476–485. [Google Scholar] [CrossRef] [Green Version]
- Moldovan, F.; Pelletier, J.P.; Hambor, J.; Cloutier, J.M.; Martel-Pelletier, J. Collagenase-3 (matrix metalloprotease 13) is preferentially localized in the deep layer of human arthritic cartilage in situ. In vitro mimicking effect by transforming growth factor β. Arthritis Rheum. 1997, 40, 1653–1661. [Google Scholar] [CrossRef]
- Tetlow, L.C.; Adlam, D.J.; Woolley, D.E. Matrix metalloproteinase and proinflammatory cytokine production by chondrocytes of human osteoarthritic cartilage: Associations with degenerative changes. Arthritis Rheum. 2001, 44, 585–594. [Google Scholar] [CrossRef]
- Wu, W.; Billinghurst, R.C.; Pidoux, I.; Antoniou, J.; Zukor, D.; Tanzer, M.; Poole, A.R. Sites of collagenase cleavage and denaturation of type II collagen in aging and osteoarthritic articular cartilage and their relationship to the distribution of matrix metalloproteinase 1 and matrix metalloproteinase 13. Arthritis Rheum. 2002, 46, 2087–2094. [Google Scholar] [CrossRef] [PubMed]
- Roach, H.I.; Yamada, N.; Cheung, K.S.; Tilley, S.; Clarke, N.M.; Oreffo, R.O.; Kokubun, S.; Bronner, F. Association between the abnormal expression of matrix-degrading enzymes by human osteoarthritic chondrocytes and demethylation of specific CpG sites in the promoter regions. Arthritis Rheum. 2005, 52, 3110–3124. [Google Scholar] [CrossRef]
- Naito, S.; Shiomi, T.; Okada, A.; Kimura, T.; Chijiiwa, M.; Fujita, Y.; Yatabe, T.; Komiya, K.; Enomoto, H.; Fujikawa, K.; et al. Expression of ADAMTS4 (aggrecanase-1) in human osteoarthritic cartilage. Pathol. Int. 2007, 57, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Cheung, K.S.; Hashimoto, K.; Yamada, N.; Roach, H.I. Expression of ADAMTS-4 by chondrocytes in the surface zone of human osteoarthritic cartilage is regulated by epigenetic DNA de-methylation. Rheumatol. Int. 2009, 29, 525–534. [Google Scholar] [CrossRef]
- Tchetina, E.V.; Squires, G.; Poole, A.R. Increased type II collagen degradation and very early focal cartilage degeneration is associated with upregulation of chondrocyte differentiation related genes in early human articular cartilage lesions. J. Rheumatol. 2005, 32, 876–886. [Google Scholar]
- Van der Kraan, P.; Van den Berg, W. Chondrocyte hypertrophy and osteoarthritis: Role in initiation and progression of cartilage degeneration? Osteoarthr. Cartil. 2012, 20, 223–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldring, M.B.; Otero, M. Inflammation in osteoarthritis. Curr. Opin. Rheumatol. 2011, 23, 471. [Google Scholar] [CrossRef] [PubMed]
- Buckwalter, J.; Mankin, H.J. Articular cartilage: Degeneration and osteoarthritis, repair, regeneration, and transplantation. Instr. Course Lect. 1998, 47, 487–504. [Google Scholar]
- Thijssen, E.; Van Caam, A.; Van Der Kraan, P.M. Obesity and osteoarthritis, more than just wear and tear: Pivotal roles for inflamed adipose tissue and dyslipidaemia in obesity-induced osteoarthritis. Rheumatology 2015, 54, 588–600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berenbaum, F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!). Osteoarthr. Cartil. 2013, 21, 16–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldring, S.R. Alterations in periarticular bone and cross talk between subchondral bone and articular cartilage in osteoarthritis. Ther. Adv. Musculoskelet. Dis. 2012, 4, 249–258. [Google Scholar] [CrossRef] [Green Version]
- Goldring, S.R.; Goldring, M.B. Changes in the osteochondral unit during osteoarthritis: Structure, function and cartilage-bone crosstalk. Nat. Rev. Rheumatol. 2016, 12, 632–644. [Google Scholar] [CrossRef]
- Goldring, S.R. Role of bone in osteoarthritis pathogenesis. Med. Clin. N. Am. 2009, 93, 25–35. [Google Scholar] [CrossRef]
- Allen, M.R.; Burr, D.B. Bone Growth, Modeling, and Remodeling. In Basic and Applied Bone Biology; Allen, M.R., Burr, D.B., Eds.; Academic Press: San Diego, CA, USA, 2019; Chapter 5; pp. 85–100. [Google Scholar]
- Mobasheri, A.; Batt, M. An update on the pathophysiology of osteoarthritis. Ann. Phys. Rehabil. Med. 2016, 59, 333–339. [Google Scholar] [CrossRef] [PubMed]
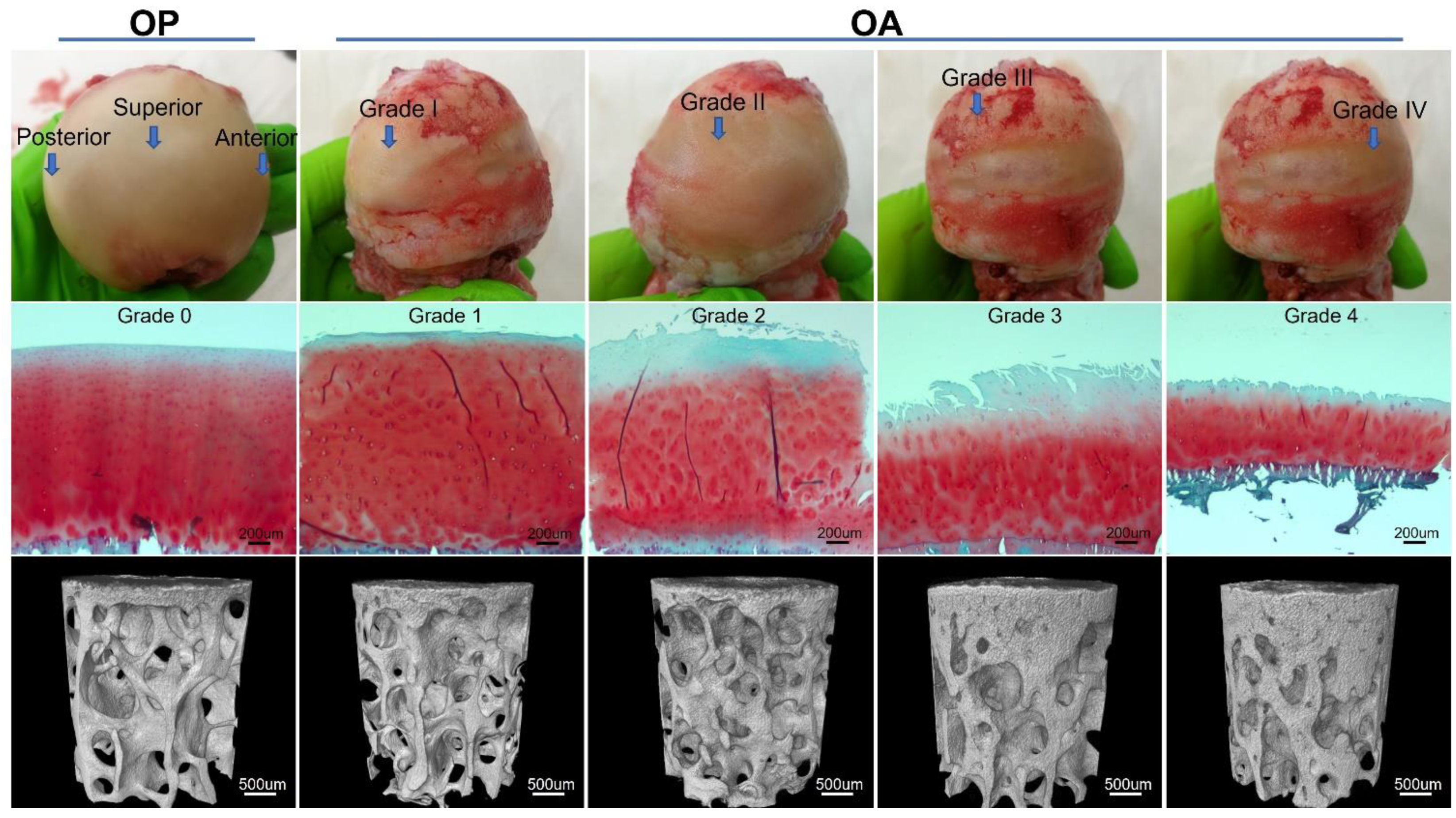
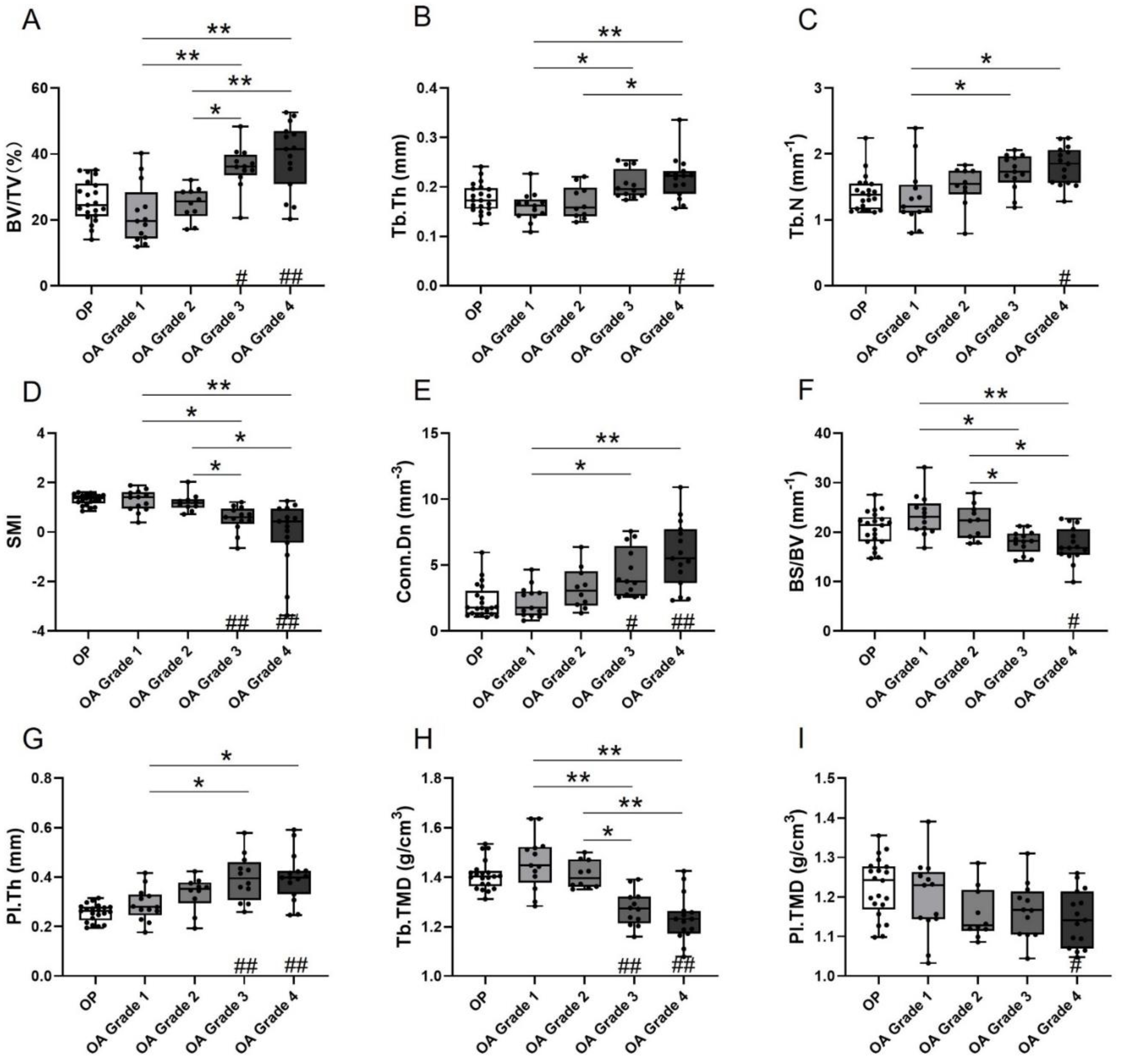
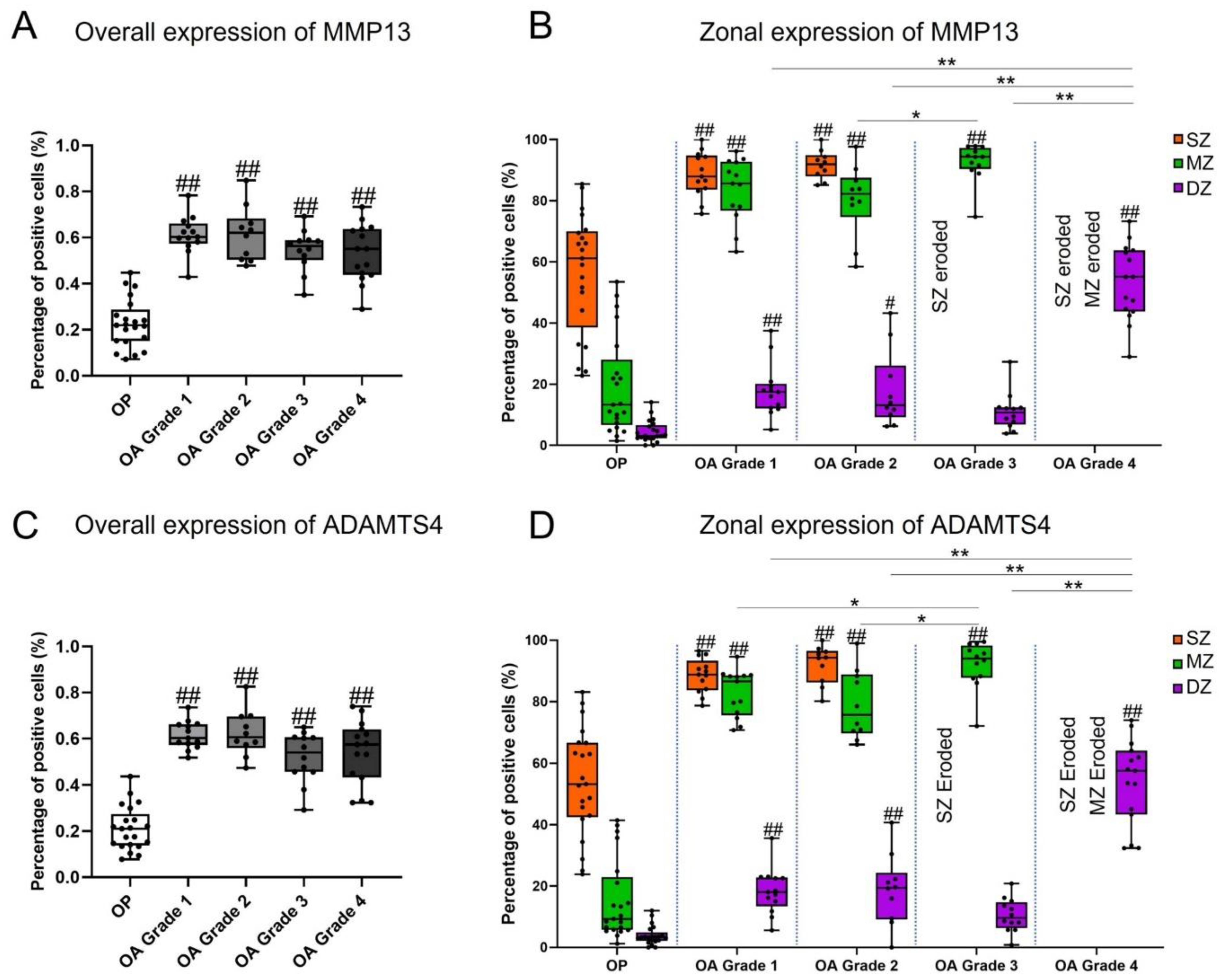
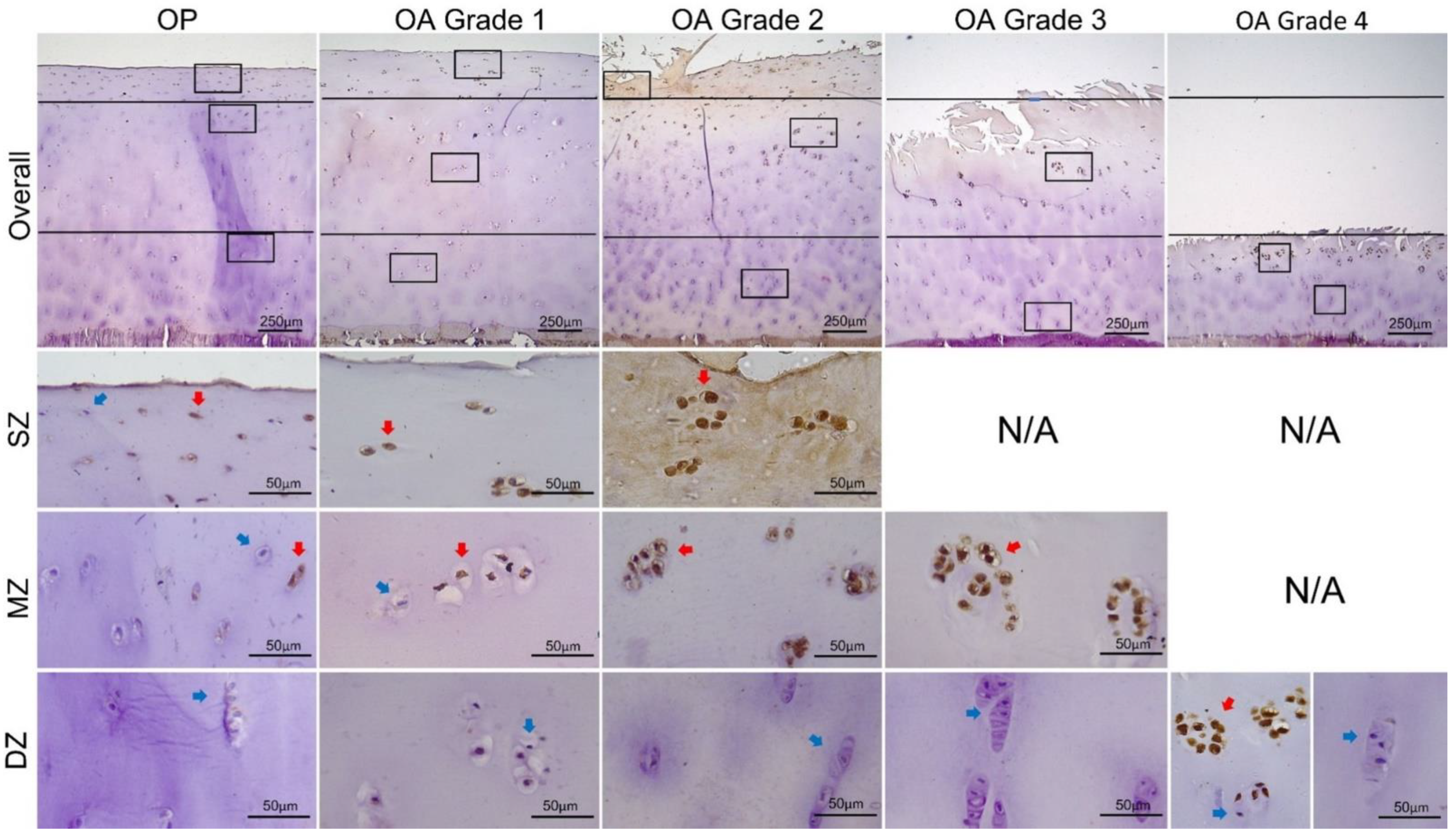
| OP | OA Grade 1 | OA Grade 2 | OA Grade 3 | OA Grade 4 | p-Value (OA Grades Only) | p-Value (OP and OA Grades) | |
|---|---|---|---|---|---|---|---|
| BV/TV (%) | 25.64 ± 6.24 | 21.79 ± 9.12 | 24.95 ± 5.00 | 35.94 ± 6.53 | 39.17 ± 10.34 | <0.001 | <0.001 |
| Tb.Th (mm) | 0.18 ± 0.03 | 0.16 ± 0.03 | 0.17 ± 0.03 | 0.20 ± 0.03 | 0.22 ± 0.04 | <0.001 | <0.001 |
| Tb.N (mm−1) | 1.40 ± 0.27 | 1.35 ± 0.46 | 1.51 ± 0.31 | 1.72 ± 0.27 | 1.82 ± 0.29 | 0.004 | <0.001 |
| SMI (–) | 1.31 ± 0.22 | 1.26 ± 0.43 | 1.20 ± 0.36 | 0.54 ± 0.52 | −0.07 ± 1.34 | <0.001 | <0.001 |
| Conn.Dn (mm−3) | 2.25 ± 1.28 | 2.23 ± 1.18 | 3.26 ± 1.58 | 4.42 ± 1.87 | 5.82 ± 2.54 | <0.001 | <0.001 |
| BS/BV (mm−1) | 20.64 ± 3.46 | 23.35 ± 4.17 | 22.14 ± 3.48 | 18.02 ± 2.34 | 17.42 ± 3.52 | <0.001 | <0.001 |
| Pl.Th (mm) | 0.26 ± 0.04 | 0.29 ± 0.07 | 0.33 ± 0.07 | 0.40 ± 0.09 | 0.40 ± 0.10 | 0.003 | <0.001 |
| Tb.TMD (g/cm3) | 1.41 ± 0.06 | 1.46 ± 0.11 | 1.41 ± 0.06 | 1.27 ± 0.07 | 1.23 ± 0.10 | <0.001 | <0.001 |
| Pl.TMD (g/cm3) | 1.22 ± 0.07 | 1.20 ± 0.10 | 1.16 ± 0.07 | 1.17 ± 0.07 | 1.15 ± 0.07 | 0.35 | 0.03 |
| OP | OA Grade 1 | OA Grade 2 | OA Grade 3 | OA Grade 4 | |
|---|---|---|---|---|---|
| MMP13 | |||||
| Overall | 22.76 ± 10.57 | 61.22 ± 8.29 | 61.53 ± 11.82 | 54.55 ± 8.99 | 53.17 ± 12.37 |
| SZ | 56.39 ± 19.62 | 88.64 ± 7.33 | 91.73 ± 4.64 | / | / |
| MZ | 19.25 ± 16.11 ** | 83.59 ± 10.35 | 80.22 ± 11.82 | 92.52 ± 6.34 | / |
| DZ | 4.45 ± 3.58 * | 17.90 ± 8.67 ** | 17.87 ± 12.55 ** | 11.07 ± 6.26 ** | 53.17 ± 12.37 |
| ADAMTS4 | |||||
| Overall | 21.12 ± 9.48 | 61.59 ± 6.06 | 62.37 ± 10.02 | 51.96 ± 10.97 | 53.86 ± 13.84 |
| SZ | 53.90 ± 17.34 | 88.48 ± 5.59 | 92.07 ± 6.26 | / | / |
| MZ | 15.17 ± 12.96 ** | 82.82 ± 7.68 | 78.97 ± 11.26 * | 92.00 ± 7.76 | / |
| DZ | 3.93 ± 3.11 * | 18.38 ± 7.48 ** | 18.71 ± 11.52 ** | 10.48 ± 5.46 ** | 53.86 ± 13.84 |
| OP | OA | |||||||
|---|---|---|---|---|---|---|---|---|
| SZ | MZ | DZ | Overall | SZ | MZ | DZ | Overall | |
| MMP13 | ||||||||
| BV/TV | 0.53 (−0.14) | 0.19 (−0.30) | 0.88 (0.04) | 0.40 (−0.19) | 0.06 (0.40) | 0.17 (0.24) | 0.049 (0.28) * | 0.12 (−0.22) |
| Tb.Th | 0.86 (−0.04) | 0.56 (−0.13) | 0.76 (0.07) | 0.94 (0.02) | 0.78 (0.06) | 0.55 (0.10) | 0.03 (0.31) * | 0.14 (−0.21) |
| Tb.N | 0.73 (−0.08) | 0.20 (−0.29) | 0.92 (0.02) | 0.69 (−0.09) | 0.10 (0.21) | 0.17 (0.24) | 0.55 (0.09) | 0.14 (−0.21) |
| SMI | 0.14 (0.34) | 0.61 (0.12) | 0.11 (0.36) | 0.28 (0.25) | 0.12 (−0.33) | 0.30 (−0.31) | 0.09 (−0.24) | 0.2 (−0.18) |
| Conn.Dn | 0.84 (0.05) | 0.27 (−0.25) | 0.35 (−0.22) | 0.67 (−0.10) | 0.10 (0.35) | 0.86 (0.03) | 0.11 (0.23) | 0.01 (−0.35) * |
| BS/BV | 0.54 (0.14) | 0.36 (0.21) | 0.99 (−0.00) | 0.71 (0.08) | 0.54 (−0.14) | 0.37 (−0.16) | 0.06 (−0.27) | 0.14 (0.21) |
| Pl.Th | 0.95 (−0.01) | 0.16 (−0.32) | 0.27 (−0.25) | 0.60 (−0.12) | 0.93 (−0.02) | 0.87 (0.03) | 0.37 (0.13) | 0.20 (−0.18) |
| Tb.TMD | 0.59 (0.13) | 0.21 (0.29) | 0.45 (0.17) | 0.34 (0.22) | 0.06 (−0.40) | 0.06 (−0.32) | 0.02 (−0.34) * | 0.12 (0.22) |
| Pl.TMD | 0.40 (−0.19) | 0.78 (−0.07) | 0.52 (0.15) | 0.94 (−0.02) | 0.47 (0.16) | 0.96 (0.01) | 0.51 (−0.10) | 0.67 (0.06) |
| ADAMTS4 | ||||||||
| BV/TV | 0.74 (−0.08) | 0.09 (−0.38) | 0.99 (0.003) | 0.45 (−0.18) | 0.24 (0.25) | 0.20 (0.22) | 0.12 (0.22) | 0.049 (−0.28) * |
| Tb.Th | 0.72 (−0.08) | 0.98 (0.01) | 0.09 (0.38) | 0.64 (0.11) | 0.35 (0.21) | 0.06 (0.32) | 0.10 (0.24) | 0.03 (−0.30) * |
| Tb.N | 0.98 (0.01) | 0.30 (−0.28) | 0.41 (−0.26) | 0.23 (−0.28) | 0.51 (0.14) | 0.57 (0.10) | 0.36 (0.13) | 0.22 (−0.18) |
| SMI | 0.30 (0.24) | 0.22 (0.28) | 0.13 (0.34) | 0.41 (0.19) | 0.54 (0.13) | 0.16 (−0.24) | 0.15 (−0.21) | 0.08 (0.25) |
| Conn.Dn | 0.91 (−0.02) | 0.15 (−0.33) | 0.50 (−0.16) | 0.27 (−0.25) | 0.06 (0.40) | 0.52 (0.11) | 0.19 (0.19) | 0.15 (−0.21) |
| BS/BV | 0.52 (0.15) | 0.65 (0.11) | 0.18 (−0.31) | 0.93 (−0.02) | 0.50 (−0.15) | 0.05 (−0.33) | 0.15 (−0.21) | 0.06 (0.26) |
| Pl.Th | 0.33 (0.22) | 0.58 (−0.13) | 0.73 (0.08) | 0.63 (0.11) | 0.28 (0.24) | 0.17 (0.24) | 0.18 (0.19) | 0.67 (−0.06) |
| Tb.TMD | 0.91 (0.03) | 0.11 (0.36) | 0.96 (0.01) | 0.57 (0.13) | 0.25 (−0.25) | 0.21 (−0.22) | 0.08 (−0.25) | 0.02 (0.33) * |
| Pl.TMD | 0.74 (−0.08) | 0.78 (−0.06) | 0.93 (−0.07) | 0.84 (0.05) | 0.31 (−0.22) | 0.82 (−0.04) | 0.05 (−0.28) | 0.41 (−0.12) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Liem, Y.; Zamli, Z.; Sullivan, N.; Dall’Ara, E.; Ahmed, H.; Sellers, G.M.; Blom, A.; Sharif, M. Subchondral Bone Microarchitectural and Mineral Properties and Expression of Key Degradative Proteinases by Chondrocytes in Human Hip Osteoarthritis. Biomedicines 2021, 9, 1593. https://doi.org/10.3390/biomedicines9111593
Li Y, Liem Y, Zamli Z, Sullivan N, Dall’Ara E, Ahmed H, Sellers GM, Blom A, Sharif M. Subchondral Bone Microarchitectural and Mineral Properties and Expression of Key Degradative Proteinases by Chondrocytes in Human Hip Osteoarthritis. Biomedicines. 2021; 9(11):1593. https://doi.org/10.3390/biomedicines9111593
Chicago/Turabian StyleLi, Yunfei, Yulia Liem, Zaitunnatakhin Zamli, Niall Sullivan, Enrico Dall’Ara, Haroon Ahmed, Grace Matilda Sellers, Ashley Blom, and Mohammed Sharif. 2021. "Subchondral Bone Microarchitectural and Mineral Properties and Expression of Key Degradative Proteinases by Chondrocytes in Human Hip Osteoarthritis" Biomedicines 9, no. 11: 1593. https://doi.org/10.3390/biomedicines9111593
APA StyleLi, Y., Liem, Y., Zamli, Z., Sullivan, N., Dall’Ara, E., Ahmed, H., Sellers, G. M., Blom, A., & Sharif, M. (2021). Subchondral Bone Microarchitectural and Mineral Properties and Expression of Key Degradative Proteinases by Chondrocytes in Human Hip Osteoarthritis. Biomedicines, 9(11), 1593. https://doi.org/10.3390/biomedicines9111593







