Sex-Specific Metabolic Pathways Were Associated with Alzheimer’s Disease (AD) Endophenotypes in the European Medical Information Framework for AD Multimodal Biomarker Discovery Cohort
Abstract
:1. Introduction
2. Methods
2.1. Participants
2.2. Clinical and Cognitive Data
2.3. Metabolomics Data
2.4. Statistical Analyses
3. Results
3.1. Demographics
3.2. Sex-Specific Association of Blood Metabolites with AD Endophenotypes
3.3. Interactive Effect of APOE ε4 and Sex
3.4. Sex-Specific Metabolites as Diagnostic Biomarkers
4. Discussion
4.1. Metabolites Associated with AD and Other Endophenotypes
4.2. Sex-Specific Biomarkers
4.3. Limitations and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scheltens, P.; de Strooper, B.; Kivipelto, M.; Holstege, H.; Chételat, G.; Teunissen, C.E.; Cummings, J.; van der Flier, W.M. Alzheimer’s disease. Lancet 2021, 397, 1577–1590. [Google Scholar] [CrossRef]
- Jack, C.R., Jr.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018, 14, 535–562. [Google Scholar] [CrossRef] [PubMed]
- Clifford, R.J., Jr.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Feldman, H.H.; Frisoni, G.B.; Hampel, H.; Jagust, W.J.; Johnson, K.A.; Knopman, D.S.; et al. A/T/N: An unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology 2016, 87, 539–547. [Google Scholar] [CrossRef]
- Vinters, H.V. Emerging Concepts in Alzheimer’s Disease. Annu. Rev. Pathol. Mech. Dis. 2015, 10, 291–319. [Google Scholar] [CrossRef]
- Pike, C.J. Sex and the development of Alzheimer’s disease. J. Neurosci. Res. 2017, 95, 671–680. [Google Scholar] [CrossRef] [Green Version]
- Karikari, T.K.; Pascoal, T.A.; Ashton, N.J.; Janelidze, S.; Benedet, A.L.; Rodriguez, J.L.; Chamoun, M.; Savard, M.; Kang, M.S.; Therriault, J.; et al. Blood phosphorylated tau 181 as a biomarker for Alzheimer’s disease: A diagnostic performance and prediction modelling study using data from four prospective cohorts. Lancet Neurol. 2020, 19, 422–433. [Google Scholar] [CrossRef]
- Palmqvist, S.; Janelidze, S.; Quiroz, Y.T.; Zetterberg, H.; Lopera, F.; Stomrud, E.; Su, Y.; Chen, Y.; Serrano, G.E.; Leuzy, A.; et al. Discriminative Accuracy of Plasma Phospho-tau217 for Alzheimer Disease vs Other Neurodegenerative Disorders. JAMA 2020, 324, 772–781. [Google Scholar] [CrossRef]
- Verberk, I.M.; Laarhuis, M.B.; van den Bosch, K.A.; Ebenau, J.L.; van Leeuwenstijn, M.; Prins, N.D.; Scheltens, P.; Teunissen, C.E.; van der Flier, W.M. Serum Markers Glial Fibrillary Acidic Protein and Neurofilament Light for Prognosis and Monitoring in Cognitively Normal Older People: A Prospective Memory Clinic-Based Cohort Study. Lancet Healthy Longev. 2021, 2, E87–E95. [Google Scholar] [CrossRef]
- Xu, W.; Tan, L.; Wang, H.-F.; Jiang, T.; Tan, M.-S.; Tan, L.; Zhao, Q.-F.; Li, J.-Q.; Wang, J.; Yu, J.-T. Meta-analysis of modifiable risk factors for Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 2015, 86, 1299–1306. [Google Scholar] [CrossRef]
- Ruigrok, A.N.; Salimi-Khorshidi, G.; Lai, M.-C.; Baron-Cohen, S.; Lombardo, M.; Tait, R.J.; Suckling, J. A meta-analysis of sex differences in human brain structure. Neurosci. Biobehav. Rev. 2014, 39, 34–50. [Google Scholar] [CrossRef] [Green Version]
- Mielke, M.M. Sex and Gender Differences in Alzheimer’s Disease Dementia. Psychiatr. Times 2018, 35, 14–17. [Google Scholar]
- Ferretti, M.T.; Women’s Brain Project and the Alzheimer Precision Medicine Initiative; Iulita, M.F.; Cavedo, E.; Chiesa, P.A.; Dimech, A.S.; Chadha, A.S.; Baracchi, F.; Girouard, H.; Misoch, S.; et al. Sex differences in Alzheimer disease—The gateway to precision medicine. Nat. Rev. Neurol. 2018, 14, 457–469. [Google Scholar] [CrossRef]
- Dementia UK: Update [Internet]. Available online: https://www.alzheimers.org.uk/sites/default/files/migrate/downloads/dementia_uk_update.pdf (accessed on 28 August 2020).
- Shaw, C.; Hayes-Larson, E.; Glymour, M.M.; Dufouil, C.; Hohman, T.J.; Whitmer, R.A.; Kobayashi, L.C.; Brookmeyer, R.; Mayeda, E.R. Evaluation of Selective Survival and Sex/Gender Differences in Dementia Incidence Using a Simulation Model. JAMA Netw. Open 2021, 4, e211001. [Google Scholar] [CrossRef]
- Stamate, D.; Kim, M.; Proitsi, P.; Westwood, S.; Baird, A.; Nevado-Holgado, A.; Hye, A.; Bos, I.; Vos, S.J.; Vandenberghe, R.; et al. A metabolite-based machine learning approach to diagnose Alzheimer-type dementia in blood: Results from the European Medical Information Framework for Alzheimer disease biomarker discovery cohort. Alzheimers Dement. Transl. Res. Clin. Interv. 2019, 5, 933–938. [Google Scholar] [CrossRef]
- Kim, M.; Snowden, S.; Suvitaival, T.; Ali, A.; Merkler, D.J.; Ahmad, T.; Westwood, S.; Baird, A.; Proitsi, P.; Nevado-Holgado, A.; et al. Primary fatty amides in plasma associated with brain amyloid burden, hippocampal volume, and memory in the European Medical Information Framework for Alzheimer’s Disease biomarker discovery cohort. Alzheimers Dement. 2019, 15, 817–827. [Google Scholar] [CrossRef]
- Bos, I.; Vos, S.; Vandenberghe, R.; Scheltens, P.; Engelborghs, S.; Frisoni, G.; Molinuevo, J.L.; Wallin, A.; Lleó, A.; Popp, J.; et al. The EMIF-AD Multimodal Biomarker Discovery study: Design, methods and cohort characteristics. Alzheimers Res. Ther. 2018, 10, 64. [Google Scholar] [CrossRef] [Green Version]
- Reijs, B.L.; Ramakers, I.H.; Elias-Sonnenschein, L.; Teunissen, C.E.; Koel-Simmelink, M.; Tsolaki, M.; Wahlund, L.-O.; Waldemar, G.; Hausner, L.; Johannsen, P.; et al. Relation of Odor Identification with Alzheimer’s Disease Markers in Cerebrospinal Fluid and Cognition. J. Alzheimers Dis. 2017, 60, 1025–1034. [Google Scholar] [CrossRef]
- Galluzzi, S.; Marizzoni, M.; Babiloni, C.; Albani, D.; Antelmi, L.; Bagnoli, C.; Bartrés-Faz, D.; Cordone, S.; Didic, M.; Farotti, L.; et al. Clinical and biomarker profiling of prodromal Alzheimer’s disease in workpackage 5 of the Innovative Medicines Initiative PharmaCog project: A ‘European ADNI study’. J. Intern. Med. 2016, 279, 576–591. [Google Scholar] [CrossRef]
- Visser, P.; Verhey, F.; Boada, M.; Bullock, R.; De Deyn, P.; Frisoni, G.B.; Frölich, L.; Hampel, H.; Jolles, J.; Jones, R.; et al. Development of Screening Guidelines and Clinical Criteria for Predementia Alzheimer’s Disease. Neuroepidemiology 2008, 30, 254–265. [Google Scholar] [CrossRef] [Green Version]
- Van der Flier, W.M.; Pijnenburg, Y.A.L.; Prins, N.; Lemstra, A.W.; Bouwman, F.H.; Teunissen, C.E.; van Berckel, B.N.M.; Stam, C.J.; Barkhof, F.; Visser, P.J.; et al. Optimizing patient care and research: The Amsterdam Dementia Cohort. J. Alzheimers Dis. 2014, 41, 313–327. [Google Scholar] [CrossRef] [Green Version]
- Somers, C.; Struyfs, H.; Goossens, J.; Niemantsverdriet, E.; Luyckx, J.; De Roeck, N.; De Roeck, E.; De Vil, B.; Cras, P.; Martin, J.-J.; et al. A Decade of Cerebrospinal Fluid Biomarkers for Alzheimer’s Disease in Belgium. J. Alzheimers Dis. 2016, 54, 383–395. [Google Scholar] [CrossRef]
- Fortea, J.; Sala-Llonch, R.; Bartrés-Faz, D.; Bosch, B.; Lladó, A.; Bargalló, N.; Molinuevo, J.L.; Sánchez-Valle, R. Increased cortical thickness and caudate volume precede atrophy in PSEN1 mutation carriers. J. Alzheimers Dis. 2010, 22, 909–922. [Google Scholar] [CrossRef]
- Adamczuk, K.; Schaeverbeke, J.; Nelissen, N.; Neyens, V.; Vandenbulcke, M.; Goffin, K.; Lilja, J.; Hilven, K.; Dupont, P.; Van Laere, K.; et al. Amyloid imaging in cognitively normal older adults: Comparison between 18F-flutemetamol and 11C-Pittsburgh compound B. Eur. J. Nucl. Med. Mol. Imaging 2015, 43, 142–151. [Google Scholar] [CrossRef] [Green Version]
- Estanga, A.; Ecay-Torres, M.; Ibañez, A.; Izagirre, A.; Villanua, J.; Garcia-Sebastian, M.; Gaspar, M.T.I.; Otaegui-Arrazola, A.; Iriondo, A.; Clerigue, M.; et al. Beneficial effect of bilingualism on Alzheimer’s disease CSF biomarkers and cognition. Neurobiol. Aging 2017, 50, 144–151. [Google Scholar] [CrossRef]
- Alcolea, D.; Clarimón, J.; Carmona-Iragui, M.; Illán-Gala, I.; Morenas-Rodríguez, E.; Barroeta, I.; Ribosa-Nogué, R.; Sala, I.; Sánchez-Saudinós, M.B.; Videla, L.; et al. The Sant Pau Initiative on Neurodegeneration (SPIN) cohort: A data set for biomarker discovery and validation in neurodegenerative disorders. Alzheimers Dement. Transl. Res. Clin. Interv. 2019, 5, 597–609. [Google Scholar] [CrossRef]
- Wallin, A.; Nordlund, A.; Jonsson, M.; Blennow, K.; Zetterberg, H.; Öhrfelt, A.; Stalhammar, J.; Eckerström, M.; Carlsson, M.; Olsson, E.; et al. Alzheimer’s disease—Subcortical vascular disease spectrum in a hospital-based setting: Overview of results from the Gothenburg MCI and dementia studies. Br. J. Pharmacol. 2015, 36, 95–113. [Google Scholar] [CrossRef]
- Tautvydaitė, D.; Kukreja, D.; Antonietti, J.-P.; Henry, H.; Von Gunten, A.; Popp, J. Interaction between personality traits and cerebrospinal fluid biomarkers of Alzheimer’s disease pathology modulates cognitive performance. Alzheimers Res. Ther. 2017, 9, 6. [Google Scholar] [CrossRef] [Green Version]
- Petersen, R.C. Mild cognitive impairment as a diagnostic entity. J. Intern. Med. 2004, 256, 183–194. [Google Scholar] [CrossRef]
- McKhann, G.; Drachman, D.; Folstein, M.; Katzman, R.; Price, D.; Stadlan, E.M. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984, 34, 939–944. [Google Scholar] [CrossRef] [Green Version]
- Thomas, L.E.; O’Brien, E.C.; Piccini, J.P.; D’Agostino, R.B.; Pencina, M.J. Application of net reclassification index to non-nested and point-based risk prediction models: A review. Eur. Heart J. 2018, 40, 1880–1887. [Google Scholar] [CrossRef]
- Pencina, M.J.; D’Agostino, R.B.; D’Agostino, R.B.; Vasan, R.S. Evaluating the added predictive ability of a new marker: From area under the ROC curve to reclassification and beyond. Stat. Med. 2008, 27, 157–172. [Google Scholar] [CrossRef]
- Pencina, M.J.; Steyerberg, E.W.; D’Agostino Sr, R.B. Net reclassification index at event rate: Properties and relationships. Stat. Med. 2017, 36, 4455–4467. [Google Scholar] [CrossRef]
- Cristofano, A.; Sapere, N.; La Marca, G.; Angiolillo, A.; Vitale, M.; Corbi, G.; Scapagnini, G.; Intrieri, M.; Russo, C.; Corso, G.; et al. Serum Levels of Acyl-Carnitines along the Continuum from Normal to Alzheimer’s Dementia. PLoS ONE 2016, 11, e0155694. [Google Scholar] [CrossRef] [Green Version]
- Cao, B.; Wang, D.; Pan, Z.; Brietzke, E.; McIntyre, R.S.; Musial, N.; Mansur, R.B.; Subramanieapillai, M.; Zeng, J.; Huang, N.; et al. Characterizing Acyl-Carnitine Biosignatures for Schizophrenia: A Longitudinal Pre- and Post-Treatment Study. Transl. Psychiatry 2019, 9, 19. [Google Scholar] [CrossRef] [Green Version]
- Van der Velpen, V.; Teav, T.; Gallart-Ayala, H.; Mehl, F.; Konz, I.; Clark, C.; Oikonomidi, A.; Peyratout, G.; Henry, H.; Delorenzi, M.; et al. Systemic and central nervous system metabolic alterations in Alzheimer’s disease. Alzheimers Res. Ther. 2019, 11, 93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Von Arnim, C.A.F.; Herbolsheimer, F.; Nikolaus, T.; Peter, R.; Biesalski, H.K.; Ludolph, A.C.; Riepe, M.; Nagel, G.; ActiFE Ulm Study Group. Dietary antioxidants and dementia in a population-based case-control study among older people in South Germany. J. Alzheimers Dis. 2012, 31, 717–724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giil, L.M.; Midttun, Ø.; Refsum, H.; Ulvik, A.; Advani, R.; Smith, A.D.; Ueland, P.M. Kynurenine Pathway Metabolites in Alzheimer’s Disease. J. Alzheimers Dis. 2017, 60, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Wood, P.L. Lipidomics of Alzheimer’s disease: Current status. Alzheimers Res. Ther. 2012, 4, 5. [Google Scholar] [CrossRef] [Green Version]
- Li, N.-J.; Liu, W.-T.; Li, W.; Li, S.-Q.; Chen, X.-H.; Bi, K.-S.; He, P. Plasma metabolic profiling of Alzheimer’s disease by liquid chromatography/mass spectrometry. Clin. Biochem. 2010, 43, 992–997. [Google Scholar] [CrossRef]
- Kaddurah-Daouk, R.; Zhu, H.; Sharma, S.; Bogdanov, M.; Rozen, S.G.; Matson, W.; Oki, N.O.; Motsinger-Reif, A.A.; Churchill, E.; Lei, Z.; et al. Alterations in metabolic pathways and networks in Alzheimer’s disease. Transl. Psychiatry 2013, 3, e244. [Google Scholar] [CrossRef] [Green Version]
- Martínez, M.F.; Martín, X.E.; Alcelay, L.G.; Flores, J.C.; Valiente, J.M.U.; Juanbeltz, B.I.; Beldarraín, M.; Ángeles, G.; López, J.M.; Gonzalez-Fernández, M.C.; et al. The COMT Val158 Met polymorphism as an associated risk factor for Alzheimer disease and mild cognitive impairment in APOE 4 carriers. BMC Neurosci. 2009, 10, 125. [Google Scholar] [CrossRef] [Green Version]
- Gulyás, B.; Pavlova, E.; Kasa, P.; Gulya, K.; Bakota, L.; Várszegi, S.; Keller, É.; Horváth, M.C.; Nag, S.; Hermecz, I.; et al. Activated MAO-B in the brain of Alzheimer patients, demonstrated by [11C]-l-deprenyl using whole hemisphere autoradiography. Neurochem. Int. 2011, 58, 60–68. [Google Scholar] [CrossRef]
- Koistinen, V.M.; Kärkkäinen, O.; Borewicz, K.; Zarei, I.; Jokkala, J.; Micard, V.; Rosa-Sibakov, N.; Auriola, S.; Aura, A.-M.; Smidt, H.; et al. Contribution of gut microbiota to metabolism of dietary glycine betaine in mice and in vitro colonic fermentation. Microbiome 2019, 7, 103. [Google Scholar] [CrossRef]
- Aswad, M.; Rayan, M.; Abu-Lafi, S.; Falah, M.; Raiyn, J.; Abdallah, Z.; Rayan, A. Nature is the best source of anti-inflammatory drugs: Indexing natural products for their anti-inflammatory bioactivity. Inflamm. Res. 2018, 67, 67–75. [Google Scholar] [CrossRef]
- Sun, H.; Cai, W.; Wang, X.; Liu, Y.; Hou, B.; Zhu, X.; Qiu, L. Vaccaria hypaphorine alleviates lipopolysaccharide-induced inflammation via inactivation of NFκB and ERK pathways in Raw 264.7 cells. BMC Complement. Altern. Med. 2017, 17, 120. [Google Scholar] [CrossRef] [Green Version]
- Hartai, Z.; Juhász, A.; Rimanóczy, Á.; Janáky, T.; Donkó, T.; Dux, L.; Penke, B.; Tóth, G.K.; Janka, Z.; Kálmán, J. Decreased serum and red blood cell kynurenic acid levels in Alzheimer’s disease. Neurochem. Int. 2007, 50, 308–313. [Google Scholar] [CrossRef]
- Gulaj, E.; Pawlak, K.; Bien, B.; Pawlak, D. Kynurenine and its metabolites in Alzheimer’s disease patients. Adv. Med. Sci. 2010, 55, 204–211. [Google Scholar] [CrossRef]
- Poeggeler, B.; Rassoulpour, A.; Wu, H.-Q.; Guidetti, P.; Roberts, R.; Schwarcz, R. Dopamine receptor activation reveals a novel, kynurenate-sensitive component of striatal N-methyl-d-aspartate neurotoxicity. Neuroscience 2007, 148, 188–197. [Google Scholar] [CrossRef] [Green Version]
- Raison, C.L.; Rook, G.W.; Miller, A.H.; Begay, T.K. Chapter 26—Role of Inflammation in Psychiatric Disease. In Neurobiology of Brain Disorders; Zigmond, M.J., Rowland, L.P., Coyle, J.T., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 396–421. Available online: http://www.sciencedirect.com/science/article/pii/B9780123982704000264 (accessed on 12 January 2021).

| Sample Size (N Max) | CTL | MCI | AD | p-Value * | |
|---|---|---|---|---|---|
| Age | All (695) | 65 (7.9) | 70 (8.1) | 70 (8.49) | 6.52 × 10−14 |
| Sex (f/m) | All (377/318) | 155/128 | 141/134 | 81/56 | 3.13 × 10−1 |
| APOE ε4 (+/−) | All (348/347) | 111/172 | 153/122 | 84/53 | 7.30 × 10−6 |
| Aβ z-score | 693 | −0.22 (1.11) | −0.82 (0.99) | −1.22 (0.64) | <2.00 × 10−16 |
| p-tau z-score | 640 | −0.00087 (0.99) | −0.94 (1.38) | −1.31 (1.66) | <2.00 × 10−16 |
| t-tau z-score | 637 | 0.032 (0.84) | −0.98 (1.25) | −1.65 (1.60) | <2.00 × 10−16 |
| Attention z-score | 644 | 0.21 (1.13) | −0.91 (1.63) | −1.92 (1.99) | <2.00 × 10−16 |
| Executive z-score | 526 | 0.16 (1.16) | −0.79 (2.04) | −2.26 (2.55) | <2.00 × 10−16 |
| Language z-score | 674 | −0.18 (0.99) | −1.0 (1.25) | −2.08 (1.27) | <2.00 × 10−16 |
| Memory delayed z-score | 551 | −0.037 (1.15) | −1.52 (1.40) | −2.40 (1.070 | <2.00 × 10−16 |
| Memory immediate z-score | 637 | −0.50 (1.77) | −1.57 (1.39) | −2.34 (1.24) | <2.00 × 10−16 |
| Visuo-construction z-score | 436 | −0.20 (1.34) | −0.14 (1.47) | −1.36 (1.98) | 1.93 × 10−8 |
| Hippocampal left | 455 | 3761.21 (453.57) | 3272.52 (634.570 | 3017.90 (487.63) | <2.00 × 10−16 |
| Hippocampal right | 455 | 3878.12 (436.76) | 3388.14 (628.15) | 3146.17 (500.52) | <2.00 × 10−16 |
| Hippocampal sum | 455 | 7639.32 (857.95) | 6660.69 (1210.53) | 6182.10 (913.81) | <2.00 × 10−16 |
| Cortical thickness in whole brain | 420 | 2.30 (0.12) | 2.30 (0.11) | 2.28 (0.11) | 5.47 × 10−1 |
| Cortical thickness in AD regions | 420 | 2.66 (0.16) | 2.63 (0.15) | 2.58 (0.17) | 1.95 × 10−4 |
| Taking AChEI, yes/no | 76/149 | 1/40 | 50/78 | 25/31 | 1.3 × 10−5 |
| Taking other AD medications, yes/no | 23/201 | 0/41 | 16/112 | 7/48 | 1.55 × 10−1 |
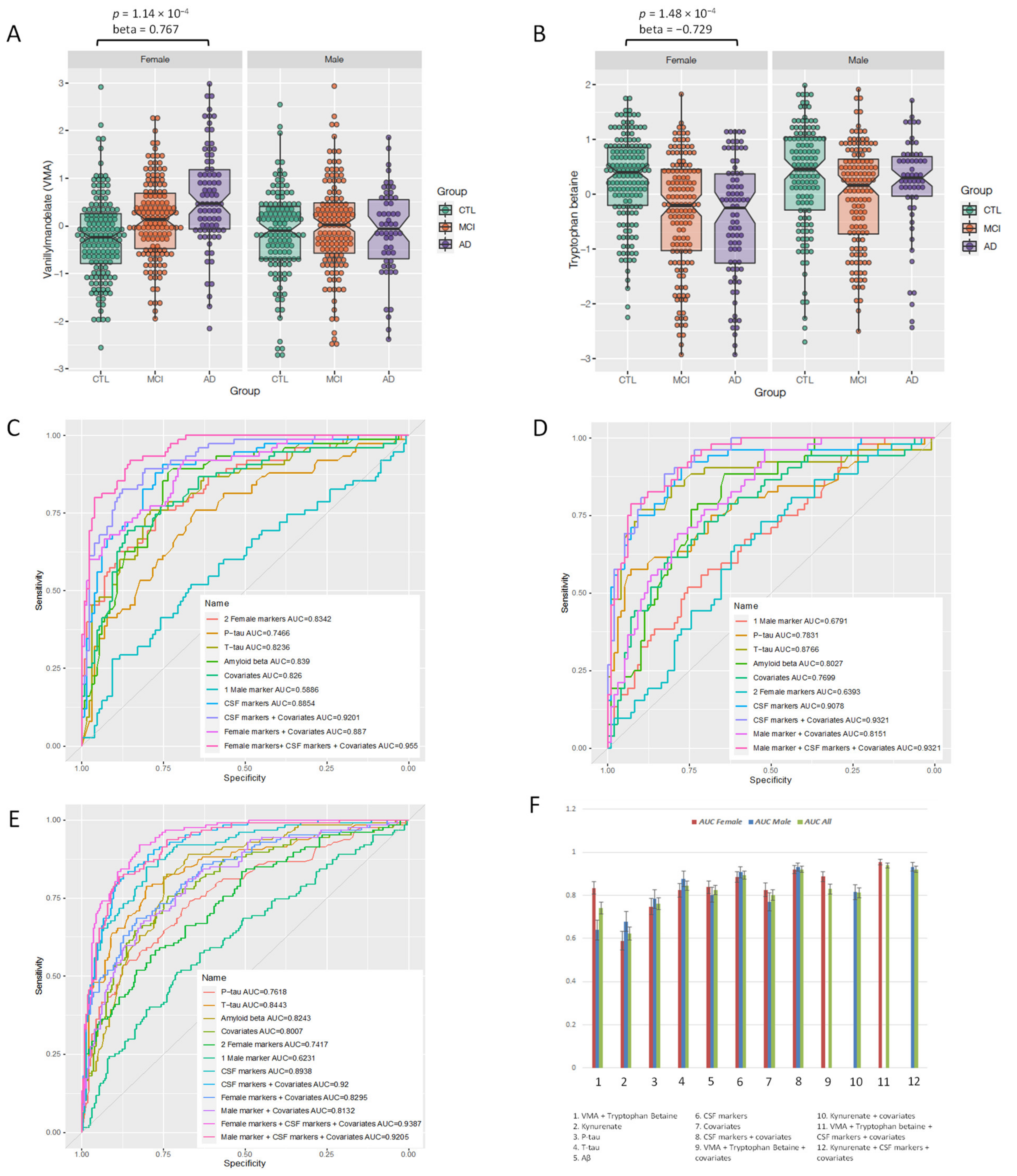
| Metabolite | Interaction Effect | Interaction p-Value | Female Effect | Female p-Value | 95% CI | Male Effect | Male p-Value | 95% CI |
|---|---|---|---|---|---|---|---|---|
| VMA | 0.84 | 1.66 × 10−3 | 0.77 | 1.14 × 10−4 | 0.04 to 1.18 | −0.16 | 0.438 | |
| Tryptophan betaine | −0.58 | 1.92 × 10−2 | −0.73 | 1.48 × 10−4 | −1.12 to 0.36 | −0.015 | 0.934 | |
| Kynurenate | 0.69 | 2.35 × 10−2 | 0.37 | 0.043 | −1.04 | 7.63 × 10−5 | −1.58 to 0.54 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Green, R.; Kim, M.; Lord, J.; Ebshiana, A.; Westwood, S.; Baird, A.L.; Nevado-Holgado, A.J.; Shi, L.; Hye, A.; et al. Sex-Specific Metabolic Pathways Were Associated with Alzheimer’s Disease (AD) Endophenotypes in the European Medical Information Framework for AD Multimodal Biomarker Discovery Cohort. Biomedicines 2021, 9, 1610. https://doi.org/10.3390/biomedicines9111610
Xu J, Green R, Kim M, Lord J, Ebshiana A, Westwood S, Baird AL, Nevado-Holgado AJ, Shi L, Hye A, et al. Sex-Specific Metabolic Pathways Were Associated with Alzheimer’s Disease (AD) Endophenotypes in the European Medical Information Framework for AD Multimodal Biomarker Discovery Cohort. Biomedicines. 2021; 9(11):1610. https://doi.org/10.3390/biomedicines9111610
Chicago/Turabian StyleXu, Jin, Rebecca Green, Min Kim, Jodie Lord, Amera Ebshiana, Sarah Westwood, Alison L. Baird, Alejo J. Nevado-Holgado, Liu Shi, Abdul Hye, and et al. 2021. "Sex-Specific Metabolic Pathways Were Associated with Alzheimer’s Disease (AD) Endophenotypes in the European Medical Information Framework for AD Multimodal Biomarker Discovery Cohort" Biomedicines 9, no. 11: 1610. https://doi.org/10.3390/biomedicines9111610
APA StyleXu, J., Green, R., Kim, M., Lord, J., Ebshiana, A., Westwood, S., Baird, A. L., Nevado-Holgado, A. J., Shi, L., Hye, A., Snowden, S. G., Bos, I., Vos, S. J. B., Vandenberghe, R., Teunissen, C. E., Kate, M. T., Scheltens, P., Gabel, S., Meersmans, K., ... on behalf of the European Medical Information Framework Consortium. (2021). Sex-Specific Metabolic Pathways Were Associated with Alzheimer’s Disease (AD) Endophenotypes in the European Medical Information Framework for AD Multimodal Biomarker Discovery Cohort. Biomedicines, 9(11), 1610. https://doi.org/10.3390/biomedicines9111610









