Sex Differences in the Effect of Diabetes on Cerebral Glucose Metabolism
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Preparation
2.2. Positron Emission Tomography Experiments and Data Analysis
2.3. Histological Assessments
2.4. Statistical Analysis
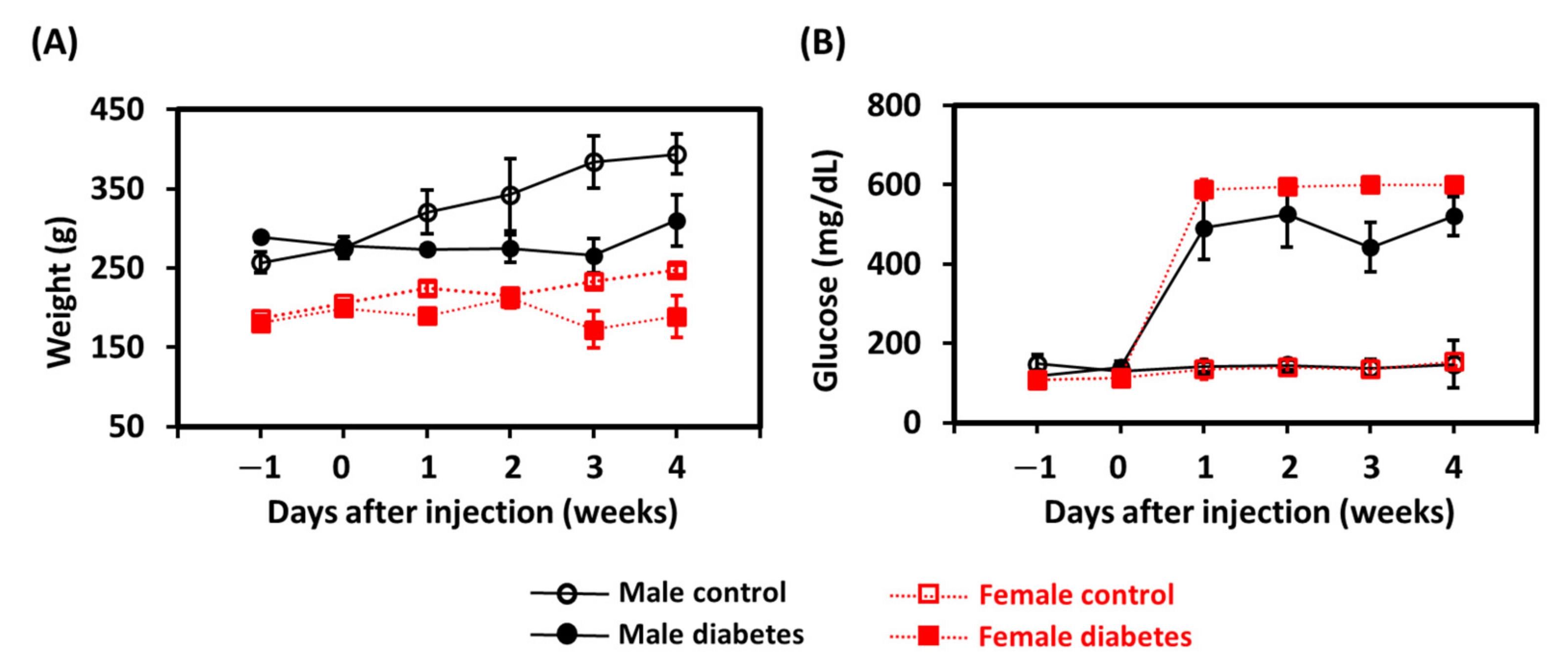
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Halter, J.B. Diabetes mellitus in an aging population: The challenge ahead. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2012, 67, 1297–1299. [Google Scholar] [CrossRef] [PubMed]
- Cooper, I.D.; Brookler, K.H.; Crofts, C.A.P. Rethinking Fragility Fractures in Type 2 Diabetes: The Link between Hyperinsulinaemia and Osteofragilitas. Biomedicines 2021, 9, 1165. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.M.; Huang, Y.C.; Shih, C.T.; Chen, Y.F.; Peng, S.L. MRI-based measurements of whole-brain global cerebral blood flow: Comparison and validation at 1.5T and 3T. J. Magn. Reson. Imaging 2018, 48, 1273–1280. [Google Scholar] [CrossRef]
- Chen, R.; Ovbiagele, B.; Feng, W. Diabetes and Stroke: Epidemiology, Pathophysiology, Pharmaceuticals and Outcomes. Am. J. Med. Sci. 2016, 351, 380–386. [Google Scholar] [CrossRef] [Green Version]
- Arvanitakis, Z.; Wilson, R.S.; Bienias, J.L.; Evans, D.A.; Bennett, D.A. Diabetes mellitus and risk of Alzheimer disease and decline in cognitive function. Arch. Neurol. 2004, 61, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Cukierman, T.; Gerstein, H.C.; Williamson, J.D. Cognitive decline and dementia in diabetes--systematic overview of prospective observational studies. Diabetologia 2005, 48, 2460–2469. [Google Scholar] [CrossRef] [PubMed]
- Roberts, R.O.; Knopman, D.S.; Przybelski, S.A.; Mielke, M.M.; Kantarci, K.; Preboske, G.M.; Senjem, M.L.; Pankratz, V.S.; Geda, Y.E.; Boeve, B.F.; et al. Association of type 2 diabetes with brain atrophy and cognitive impairment. Neurology 2014, 82, 1132–1141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruehl, H.; Wolf, O.T.; Sweat, V.; Tirsi, A.; Richardson, S.; Convit, A. Modifiers of cognitive function and brain structure in middle-aged and elderly individuals with type 2 diabetes mellitus. Brain Res. 2009, 1280, 186–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, W.; Wang, S.; Sun, Z.; Bai, F.; Zhou, Y.; Yang, Y.; Wang, P.; Huang, Y.; Yuan, Y. Altered baseline brain activity in type 2 diabetes: A resting-state fMRI study. Psychoneuroendocrinology 2013, 38, 2493–2501. [Google Scholar] [CrossRef]
- Cui, Y.; Jiao, Y.; Chen, Y.C.; Wang, K.; Gao, B.; Wen, S.; Ju, S.; Teng, G.J. Altered spontaneous brain activity in type 2 diabetes: A resting-state functional MRI study. Diabetes 2014, 63, 749–760. [Google Scholar] [CrossRef] [Green Version]
- Yagihashi, S.; Mizukami, H.; Sugimoto, K. Mechanism of diabetic neuropathy: Where are we now and where to go? J. Diabetes Investig. 2011, 2, 18–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hempel, R.; Onopa, R.; Convit, A. Type 2 diabetes affects hippocampus volume differentially in men and women. Diabetes Metab. Res. Rev. 2012, 28, 76–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrett-Connor, E.; Giardina, E.G.; Gitt, A.K.; Gudat, U.; Steinberg, H.O.; Tschoepe, D. Women and heart disease: The role of diabetes and hyperglycemia. Arch. Intern. Med. 2004, 164, 934–942. [Google Scholar] [CrossRef]
- Crowley, A.; Menon, V.; Lessard, D.; Yarzebski, J.; Jackson, E.; Gore, J.M.; Goldberg, R.J. Sex differences in survival after acute myocardial infarction in patients with diabetes mellitus (Worcester Heart Attack Study). Am. Heart J. 2003, 146, 824–831. [Google Scholar] [CrossRef]
- Garcia-Casares, N.; Berthier, M.L.; Jorge, R.E.; Gonzalez-Alegre, P.; Gutierrez Cardo, A.; Rioja Villodres, J.; Acion, L.; Ariza Corbo, M.J.; Nabrozidis, A.; Garcia-Arnes, J.A.; et al. Structural and functional brain changes in middle-aged type 2 diabetic patients: A cross-sectional study. J. Alzheimers Dis. 2014, 40, 375–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Risacher, S.L.; Huang, E.; Saykin, A.J.; Alzheimer’s Disease Neuroimaging, I. Type 2 diabetes mellitus is associated with brain atrophy and hypometabolism in the ADNI cohort. Neurology 2016, 87, 595–600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, H.; Lin, Q.; Wang, D.; Xu, P.; Zhao, L.; Hu, W.; Bai, G.; Yan, Z.; Gao, H. NMR-based metabolomics reveals brain region-specific metabolic alterations in streptozotocin-induced diabetic rats with cognitive dysfunction. Metab. Brain Dis. 2017, 32, 585–593. [Google Scholar] [CrossRef]
- Huang, M.; Gao, L.; Yang, L.; Lin, F.; Lei, H. Abnormalities in the brain of streptozotocin-induced type 1 diabetic rats revealed by diffusion tensor imaging. Neuroimage Clin. 2012, 1, 57–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furman, B.L. Streptozotocin-Induced Diabetic Models in Mice and Rats. Curr. Protoc. Pharmacol. 2015, 70, 5–47. [Google Scholar] [CrossRef]
- Chen, L.Y.; Cheng, H.L.; Kuan, Y.H.; Liang, T.J.; Chao, Y.Y.; Lin, H.C. Therapeutic Potential of Luteolin on Impaired Wound Healing in Streptozotocin-Induced Rats. Biomedicines 2021, 9, 761. [Google Scholar] [CrossRef]
- Vital, P.; Larrieta, E.; Hiriart, M. Sexual dimorphism in insulin sensitivity and susceptibility to develop diabetes in rats. J. Endocrinol. 2006, 190, 425–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daulatzai, M.A. Cerebral hypoperfusion and glucose hypometabolism: Key pathophysiological modulators promote neurodegeneration, cognitive impairment, and Alzheimer’s disease. J. Neurosci. Res. 2017, 95, 943–972. [Google Scholar] [CrossRef] [PubMed]
- Eagan, D.E.; Gonzales, M.M.; Tarumi, T.; Tanaka, H.; Stautberg, S.; Haley, A.P. Elevated serum C-reactive protein relates to increased cerebral myoinositol levels in middle-aged adults. Cardiovasc. Psychiatry Neurol. 2012, 2012, 120540. [Google Scholar] [CrossRef] [PubMed]
- De Rekeneire, N.; Peila, R.; Ding, J.; Colbert, L.H.; Visser, M.; Shorr, R.I.; Kritchevsky, S.B.; Kuller, L.H.; Strotmeyer, E.S.; Schwartz, A.V.; et al. Diabetes, hyperglycemia, and inflammation in older individuals: The health, aging and body composition study. Diabetes Care 2006, 29, 1902–1908. [Google Scholar] [CrossRef] [Green Version]
- Espeland, M.A.; Brinton, R.D.; Hugenschmidt, C.; Manson, J.E.; Craft, S.; Yaffe, K.; Weitlauf, J.; Vaughan, L.; Johnson, K.C.; Padula, C.B.; et al. Impact of Type 2 Diabetes and Postmenopausal Hormone Therapy on Incidence of Cognitive Impairment in Older Women. Diabetes Care 2015, 38, 2316–2324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brinton, R.D. The healthy cell bias of estrogen action: Mitochondrial bioenergetics and neurological implications. Trends Neurosci. 2008, 31, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Brinton, R.D. Estrogen-induced plasticity from cells to circuits: Predictions for cognitive function. Trends Neurosci. 2009, 30, 212–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LaManna, J.C.; Salem, N.; Puchowicz, M.; Erokwu, B.; Koppaka, S.; Flask, C.; Lee, Z. Ketones suppress brain glucose consumption. Adv. Exp. Med. Biol. 2009, 645, 301–306. [Google Scholar] [PubMed] [Green Version]
- Stork, C.; Renshaw, P.F. Mitochondrial dysfunction in bipolar disorder: Evidence from magnetic resonance spectroscopy research. Mol. Psychiatry 2005, 10, 900–919. [Google Scholar] [CrossRef] [Green Version]
- Lying-Tunell, U.; Lindblad, B.S.; Malmlund, H.O.; Persson, B. Cerebral blood flow and metabolic rate of oxygen, glucose, lactate, pyruvate, ketone bodies and amino acids. Acta Neurol. Scand. 1980, 62, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Peters, S.A.; Woodward, M.; Mejia Arango, S.; Batty, G.D.; Beckett, N.; Beiser, A.; Borenstein, A.R.; Crane, P.K.; Haan, M.; et al. Type 2 Diabetes as a Risk Factor for Dementia in Women Compared With Men: A Pooled Analysis of 2.3 Million People Comprising More Than 100,000 Cases of Dementia. Diabetes Care 2016, 39, 300–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kautzky-Willer, A.; Harreiter, J.; Pacini, G. Sex and Gender Differences in Risk, Pathophysiology and Complications of Type 2 Diabetes Mellitus. Endocr. Rev. 2016, 37, 278–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fatih, N.; Chaturvedi, N.; Lane, C.; Parker, T.; Lu, K.; Cash, D.; Malone, I.; Silverwood, R.; Wong, A.; Barnes, J.; et al. Sex-related differences in whole brain volumes at age 70 in association with hyperglycemia during adult life. Neurobiol. Aging 2021. [Google Scholar] [CrossRef]
- Last, D.; Alsop, D.C.; Abduljalil, A.M.; Marquis, R.P.; de Bazelaire, C.; Hu, K.; Cavallerano, J.; Novak, V. Global and regional effects of type 2 diabetes on brain tissue volumes and cerebral vasoreactivity. Diabetes Care 2007, 30, 1193–1199. [Google Scholar] [CrossRef] [Green Version]
- Simpkins, J.W.; Yang, S.H.; Wen, Y.; Singh, M. Estrogens, progestins, menopause and neurodegeneration: Basic and clinical studies. Cell. Mol. Life Sci. 2005, 62, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, P. The Laboratory Rat: Relating Its Age with Human’s. Int. J. Prev. Med. 2013, 4, 624–630. [Google Scholar]
- Huxley, R.; Barzi, F.; Woodward, M. Excess risk of fatal coronary heart disease associated with diabetes in men and women: Meta-analysis of 37 prospective cohort studies. BMJ 2006, 332, 73–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pilote, L.; Dasgupta, K.; Guru, V.; Humphries, K.H.; McGrath, J.; Norris, C.; Rabi, D.; Tremblay, J.; Alamian, A.; Barnett, T.; et al. A comprehensive view of sex-specific issues related to cardiovascular disease. Can. Med. Assoc. J. 2007, 176, S1–S44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuanetti, G.; Latini, R.; Maggioni, A.P.; Santoro, L.; Franzosi, M.G. Influence of diabetes on mortality in acute myocardial infarction: Data from the GISSI-2 study. J. Am. Coll. Cardiol. 1993, 22, 1788–1794. [Google Scholar] [CrossRef] [Green Version]
- Shahrani, M.; Asgharzadeh, N.; Kheiri, S.; Karimi, R.; Sadeghimanesh, A.; Asgharian, S.; Lorigooini, Z. Astragalus fascicolifolius manna abortifacient risk and effects on sex hormones in BALB/c mice. BioMedicine 2020, 10, 11–17. [Google Scholar] [CrossRef]
- Peng, S.L.; Dumas, J.A.; Park, D.C.; Liu, P.; Filbey, F.M.; McAdams, C.J.; Pinkham, A.E.; Adinoff, B.; Zhang, R.; Lu, H. Age-related increase of resting metabolic rate in the human brain. NeuroImage 2014, 98, 176–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, H.; Xu, F.; Rodrigue, K.M.; Kennedy, K.M.; Cheng, Y.; Flicker, B.; Hebrank, A.C.; Uh, J.; Park, D.C. Alterations in cerebral metabolic rate and blood supply across the adult lifespan. Cereb. Cortex 2011, 21, 1426–1434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baxter, L.R., Jr.; Mazziotta, J.C.; Phelps, M.E.; Selin, C.E.; Guze, B.H.; Fairbanks, L. Cerebral glucose metabolic rates in normal human females versus normal males. Psychiatry Res. 1987, 21, 237–245. [Google Scholar] [CrossRef]
- Miura, S.A.; Schapiro, M.B.; Grady, C.L.; Kumar, A.; Salerno, J.A.; Kozachuk, W.E.; Wagner, E.; Rapoport, S.I.; Horwitz, B. Effect of gender on glucose utilization rates in healthy humans: A positron emission tomography study. J. Neurosci. Res. 1990, 27, 500–504. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Kanno, I.; Uemura, K.; Shishido, F.; Inugami, A.; Ogawa, T.; Murakami, M.; Suzuki, K. Reduction in regional cerebral metabolic rate of oxygen during human aging. Stroke 1986, 17, 1220–1228. [Google Scholar] [CrossRef] [Green Version]
- Peng, S.L.; Chiu, H.; Wu, C.Y.; Huang, C.W.; Chung, Y.H.; Shih, C.T.; Shen, W.C. The effect of caffeine on cerebral metabolism during alpha-chloralose anesthesia differs from isoflurane anesthesia in the rat brain. Psychopharmacology 2019, 236, 1749–1757. [Google Scholar] [CrossRef] [PubMed]
- Luft, C.; Greggio, S.; Venturin, G.T.; da Costa, M.S.; da Costa, J.C.; Donadio, M.V.F. Sex differences in the effects of acute stress on cerebral glucose metabolism: A microPET study. Brain Res. 2019, 1722, 146355. [Google Scholar] [CrossRef] [PubMed]
- Brands, A.M.; Biessels, G.J.; de Haan, E.H.; Kappelle, L.J.; Kessels, R.P. The effects of type 1 diabetes on cognitive performance: A meta-analysis. Diabetes Care 2005, 28, 726–735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manschot, S.M.; Brands, A.M.; van der Grond, J.; Kessels, R.P.; Algra, A.; Kappelle, L.J.; Biessels, G.J.; Utrecht Diabetic Encephalopathy Study Group. Brain magnetic resonance imaging correlates of impaired cognition in patients with type 2 diabetes. Diabetes 2006, 55, 1106–1113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shih, Y.Y.; Chiang, Y.C.; Chen, J.C.; Huang, C.H.; Chen, Y.Y.; Liu, R.S.; Chang, C.; Jaw, F.S. Brain nociceptive imaging in rats using (18)f-fluorodeoxyglucose small-animal positron emission tomography. Neuroscience 2008, 155, 1221–1226. [Google Scholar] [CrossRef] [PubMed]
- Patterson, E.; Ryan, P.M.; Cryan, J.F.; Dinan, T.G.; Ross, R.P.; Fitzgerald, G.F.; Stanton, C. Gut microbiota, obesity and diabetes. Postgrad. Med. J. 2016, 92, 286–300. [Google Scholar] [CrossRef] [PubMed]
- Corb Aron, R.A.; Abid, A.; Vesa, C.M.; Nechifor, A.C.; Behl, T.; Ghitea, T.C.; Munteanu, M.A.; Fratila, O.; Andronie-Cioara, F.L.; Toma, M.M.; et al. Recognizing the Benefits of Pre-/Probiotics in Metabolic Syndrome and Type 2 Diabetes Mellitus Considering the Influence of Akkermansia muciniphila as a Key Gut Bacterium. Microorganisms 2021, 9, 618. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Kibe, R.; Ooga, T.; Aiba, Y.; Sawaki, E.; Koga, Y.; Benno, Y. Cerebral low-molecular metabolites influenced by intestinal microbiota: A pilot study. Front. Syst. Neurosci. 2013, 7, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, C.-Y.; Lin, Y.-H.; Hsieh, H.-H.; Lin, J.-J.; Peng, S.-L. Sex Differences in the Effect of Diabetes on Cerebral Glucose Metabolism. Biomedicines 2021, 9, 1661. https://doi.org/10.3390/biomedicines9111661
Wu C-Y, Lin Y-H, Hsieh H-H, Lin J-J, Peng S-L. Sex Differences in the Effect of Diabetes on Cerebral Glucose Metabolism. Biomedicines. 2021; 9(11):1661. https://doi.org/10.3390/biomedicines9111661
Chicago/Turabian StyleWu, Chun-Yi, Yu-Hsin Lin, Hsin-Hua Hsieh, Jia-Jia Lin, and Shin-Lei Peng. 2021. "Sex Differences in the Effect of Diabetes on Cerebral Glucose Metabolism" Biomedicines 9, no. 11: 1661. https://doi.org/10.3390/biomedicines9111661





