Effectiveness of Biofunctionalization of Titanium Surfaces with Phosphonic Acid
Abstract
:1. Introduction
2. Materials and Methods
- Untreated Ti screws;
- Ti screws treated with carboxylphosphonic acid using the immersion method;
- Ti screws treated with acid + BMP-2- (additional group during cellular behavior assay).
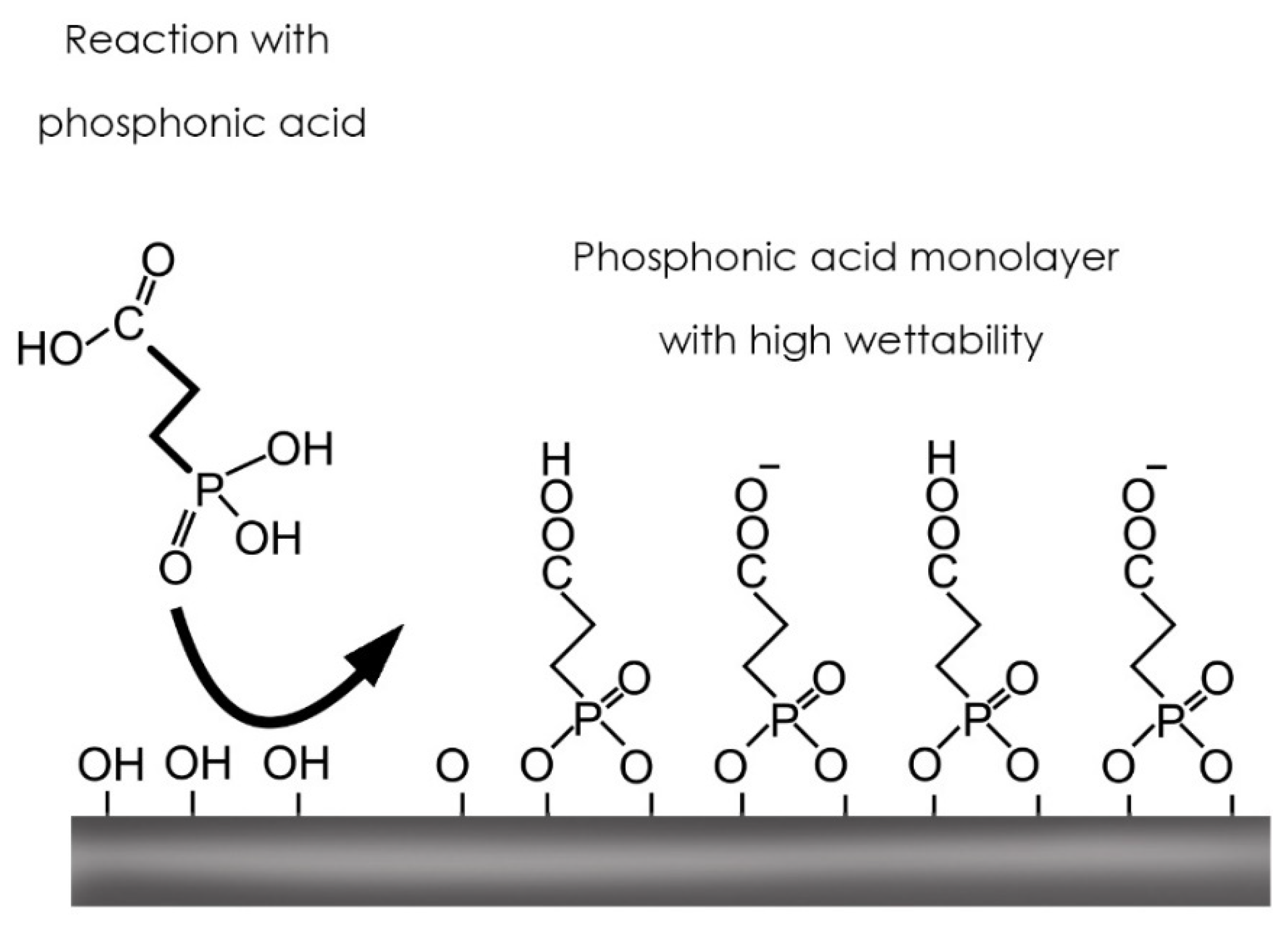
2.1. Surface Modification: Carboxyethylphosphonic Acid Treatment
2.1.1. Wettability
2.1.2. Surface Texture Analysis
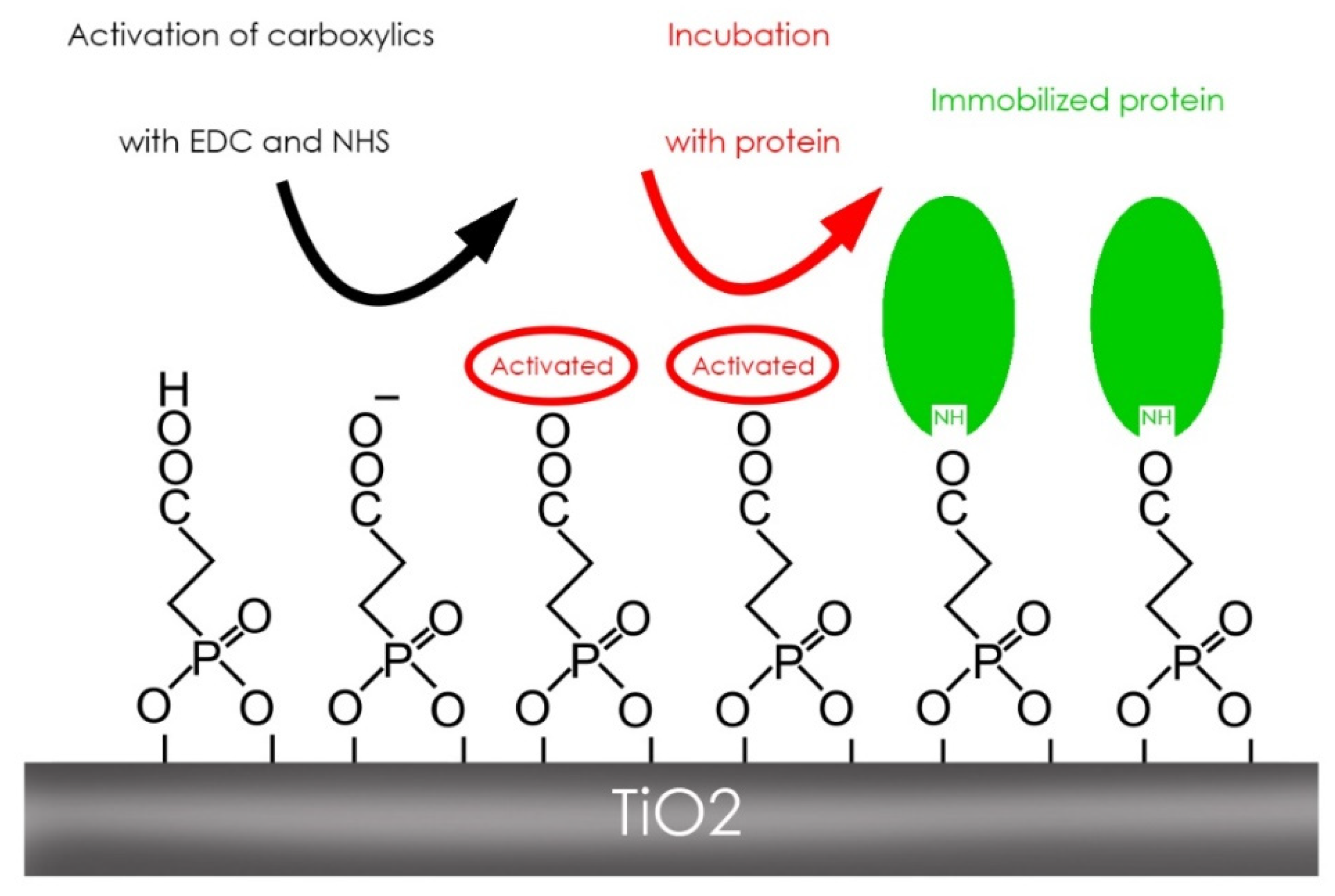
2.1.3. Protein Immobilization
2.2. Cellular Behaviour of Fibroblasts and MSCat Cells
2.2.1. Preparation of the Cultivation Medium
- Amniomax (Life Technologies, Inc., Gibco/BRL, Gaithersburg, MD, USA): According to the manufacturer’s package.
- Medium 199: 9.82 g of M-199 (Life Technologies, Inc., Gibco/BRL, Gaithersburg, MD, USA) was diluted in 1 L of ultrapure water, followed by the addition of sodium bicarbonate, and diluted through a 0.22 µm porous filter (Millipore Ref.4480). Once aliquoted into 100 mL fractions, the culture medium was supplemented with: 20% fetal bovine serum (FCS) (Life Technologies, Inc., Gibco/BRL, Gaithersburg, MD, USA); 1.2 mL of penicillin/streptomycin; 2 Mm/L hydroxyl-ethyl-piperazine-ethane-sulfonic acid (HEPES buffer) (Life Technologies, Inc., Gibco/BRL, Gaithersburg, MD, USA); 2 Mm/L-Glutamine (Gibco BRL); 20 µg/mL endothelial cell growth factor (ECGF) (Sigma Aldrich, San Luis, MO, USA); and 90µg/mL heparin sodium (Roche, Basel, Switzerland).
- Minimum Essentials Medium Eagle-MEM (Sigma Aldrich, San Luis, MO, USA). It was reconstituted with 15% fetal bovine serum (Cultek), 1% L-glutamine (Sigma-Aldrich, San Luis, MO, USA), and 1% antibiotics (penicillin and streptomycin) (Life Technologies, Inc., Gibco/BRL, Gaithersburg, MD, USA) in 1 L of ultrapure water—2.2 g of sodium bicarbonate was added and filtered through a 0.22 µm porous filter (Millipore Ref.4480). The medium was then aliquoted into 100 mL glass bottles, and supplemented with 0.05 lm Amphotericin B (Fungizone, Applied Biological Materials, Richmond, BC, Canada) and 1.2 mL penicillin (10,000 IU/mL)/Streptomycin (10,000 µg/mL) (Life Technologies, Inc., Gibco/BRL, Gaithersburg, MD, USA).
2.2.2. Proteolytic Enzyme Preparation
- Collagenase. 100 mg of collagenase type I CLS (Worthington Biochemical Corporation, Lakewood, New Jersey, USA) was reconstituted in 100 mL of MEM, and 1.5 mL of calcium chloride was added. One the reconstitution was performed, it was sterilized by filters of 0.22 µm (Millipore Ref.4480), and stored at −20 °C in 10 mL aliquots until usage.
- Trypsin. 10 mL of Hanks Balanced Buffer Saline Solution (HBSS) (Life Technologies, Inc., Gibco/BRL, Gaithersburg, MD, USA) was added to 90 mL of sterile distilled water, mixed well, and then, 10 mL of this solution was added to another 10 mL of trypsin EDTA 10X (Life Technologies, Inc., Gibco/BRL, Gaithersburg, MD, USA) to obtain trypsin-EDTA 1X.
2.2.3. Cell Procurement and Cell Expansion in Culture
- Mesenchymal Stem Cells from Adipose Tissue (MSCat). For the extraction of stem cells from rat adipose tissue (MSCat), a sample of subcutaneous adipose tissue was procured from the inguinal area of the rat, and preserved in MEM medium until processing (no more than 24 h). The tissue processing was performed under sterile conditions using sterile materials and a laminar flow cabinet. At first, the tissue was washed with MEM medium, then placed in a Falcon tube with 10 mL of 0.1% collagenase type I, and incubated for 30 min at 37 °C in the bath with maximum agitation.
- Fibroblasts
2.3. Proliferation Study
2.4. Migration Study
2.5. Analysis of Titanium Surface Behavior
- Enzymatic Treatment
- Planting and Study Times. For this part of the procedure, the three study groups were used for each cell lineage (Fibroblasts and MSCat cells):
- ○
- Control group (untreated screw);
- ○
- Screws treated with carboxyethylphosphonic acid;
- ○
- Screws treated with carboxyethylphosphonic acid plus BMP-2.
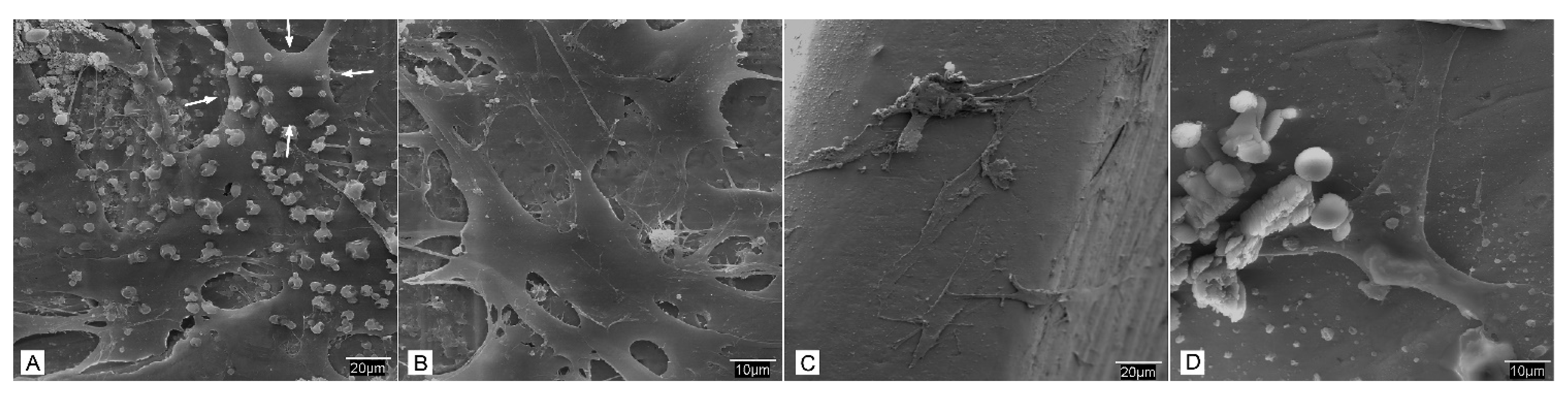
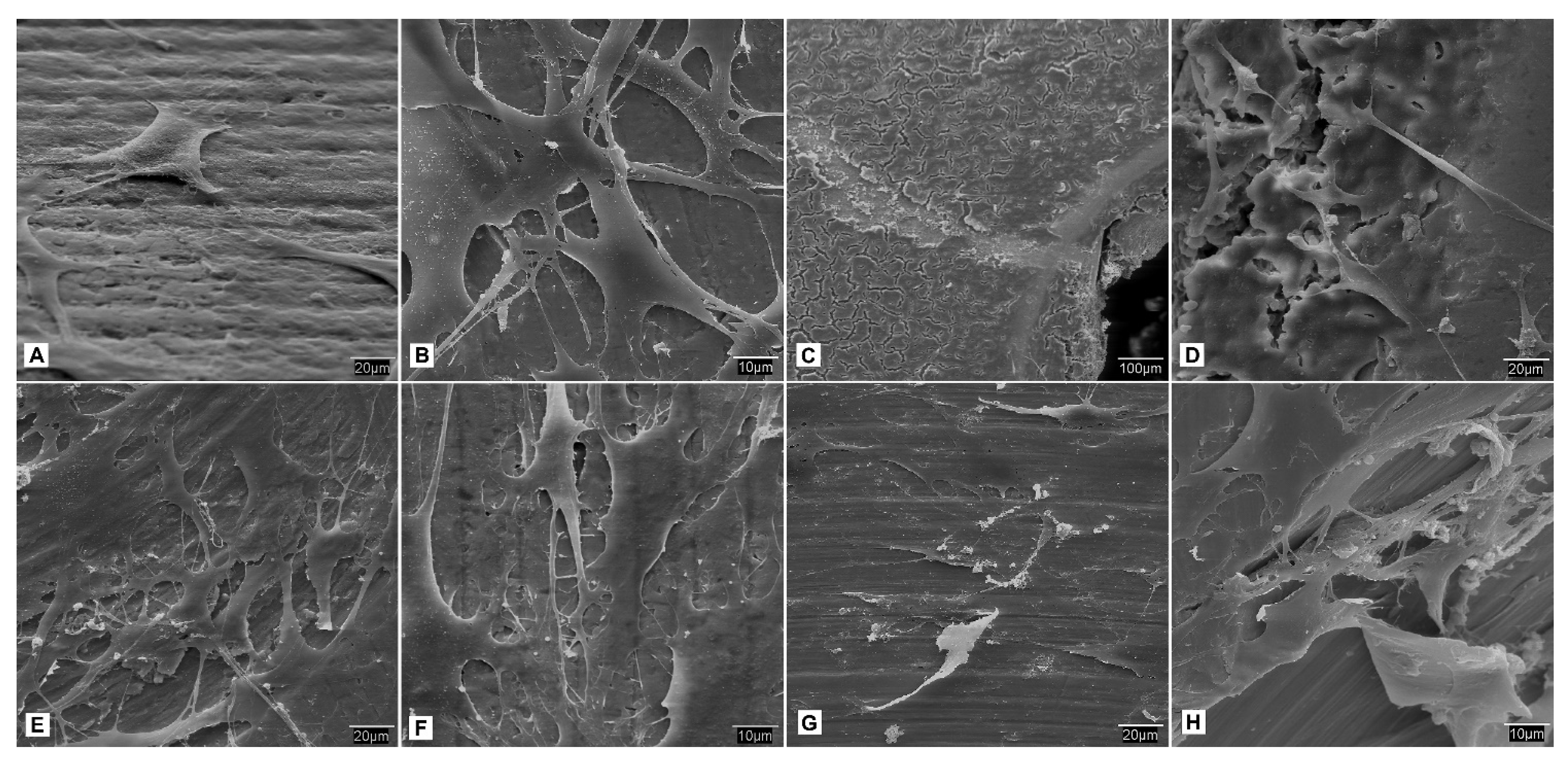
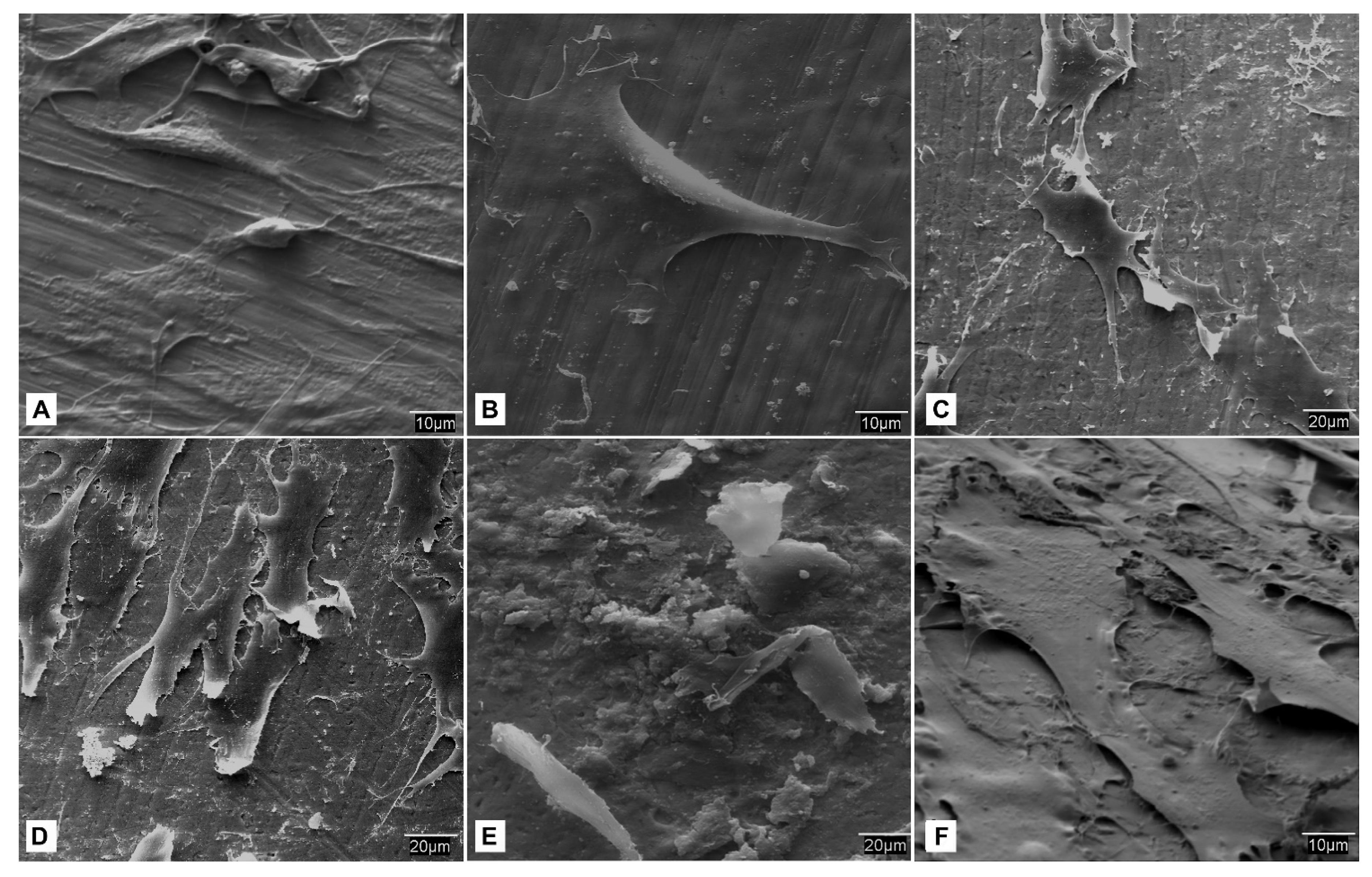
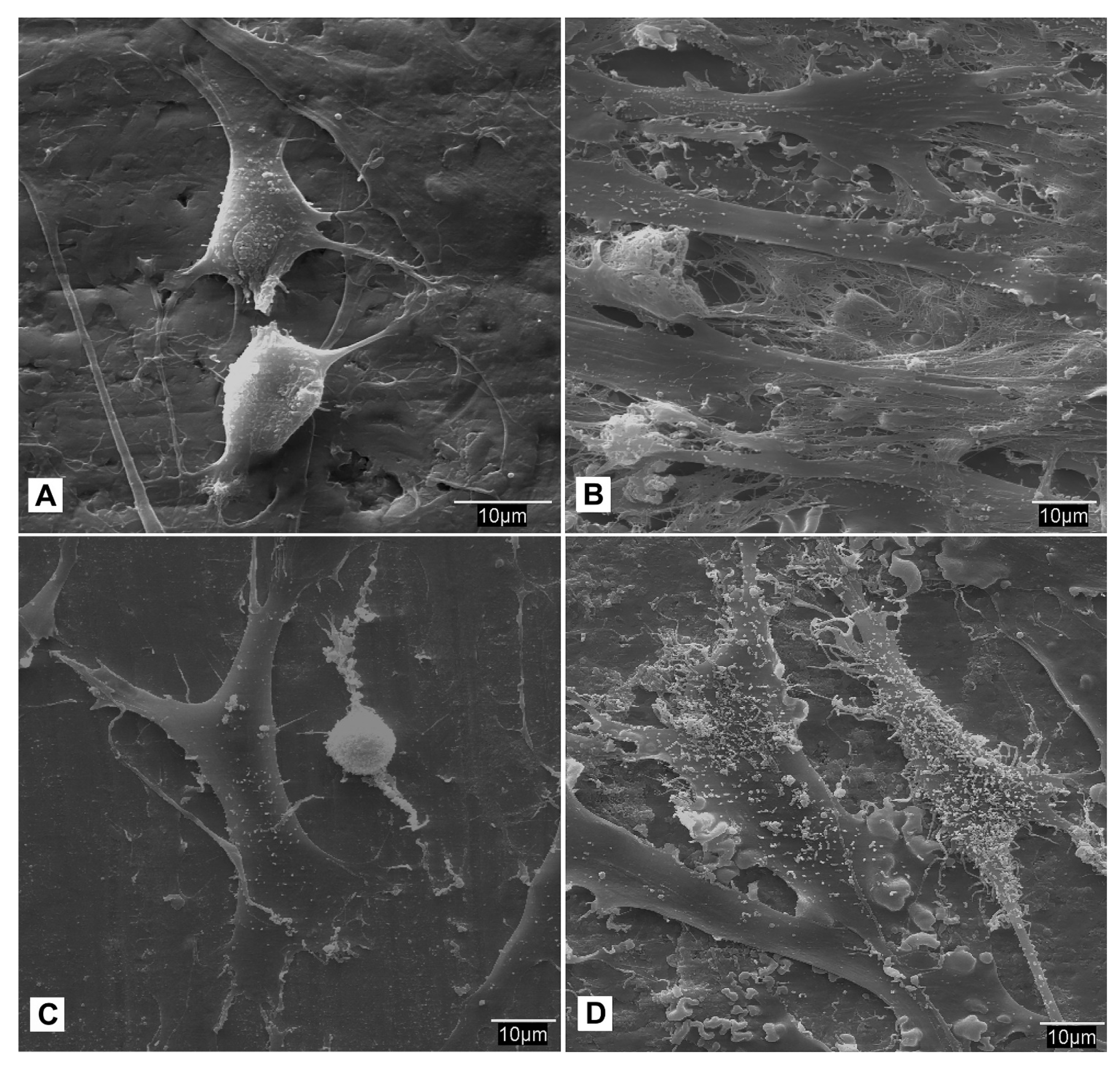
- SEM observation. At each study time interval, four samples were obtained for each cell lineage and study group. Each sample was fixed for 2–4 h in 0.3% glutaraldehyde solution. After the fixation time, the glutaraldehyde residues were removed by immersing samples in a Millionig buffer solution. After the washing process, the samples were processed for observation under the scanning electron microscope. Once fixed, the pieces were dehydrated using increasing series of acetone (30, 50, 70, 90 and 100%) for 10 min per series. Subsequently, the dehydrated parts were mounted in filter paper cases for subjecting them to a critical point in a Polaron E-3000 with CO2. Once completely dried, the pieces were mounted on steel plates (ANAME), and metallized with gold-palladium in a POLARON metallizer. The samples were processed in this manner for observation by SEM on a DSM-950 microscope (Carl-Zeiss). The state of the cells on the surface of the screws belonging to each study group at different time intervals were observed using SEM.
2.6. Identification and Phenotypic Characterization
2.6.1. Immunohistochemical Tests
- Conventional immunohistochemistry. The technique used to detect the antigen-antibody reaction was avidin-biotin labeled with alkaline phosphatase. The labeling was studied with an optical microscope (ZEISS, Jena, Germany), and the same biological material to which the primary antibody had not been added was used as a negative control, being replaced by washing buffer (PBS).
- Immunofluorescence. The marking was performed on tissue fixed with 10 % formaldehyde. Kerosene embedding, microtomy, and deparaffinization of the sections were performed according to the technique used for optical microscopy. The antigen–antibody reaction was detected using a reaction amplification system (TSA TM Plus Fluorescence Systems; Perkin Elmer Life Sciences, Boston, MA, USA), with fluorescein isothiocyanate (FITC) or cyanine 3 (Cy 3). The same biological material to which the primary antibody had not been added was used as a negative control, and was replaced by a buffer solution.
2.6.2. Enzyme-Linked Immunosorbent Assay (ELISA)
2.6.3. Molecular Studies (qRT-PCR)
- In vitro retrotranscription. Retrotranscription (RT) reactions of the messenger RNAs were performed to give rise to the corresponding cDNAs using the kit.
- Real-time PCR. Roche’s commercial Lightcycler FastStart DNA Master SYBR GreenI kit was used to perform the real-time PCR reactions. Once the reaction mixture was generated, it was subjected to a program of PCR cycles performed in the Roche Lightcycler thermocycler.
2.7. Statistical Analysis
3. Results
3.1. Wettability
3.2. Surface Texture Analysis Using SEM
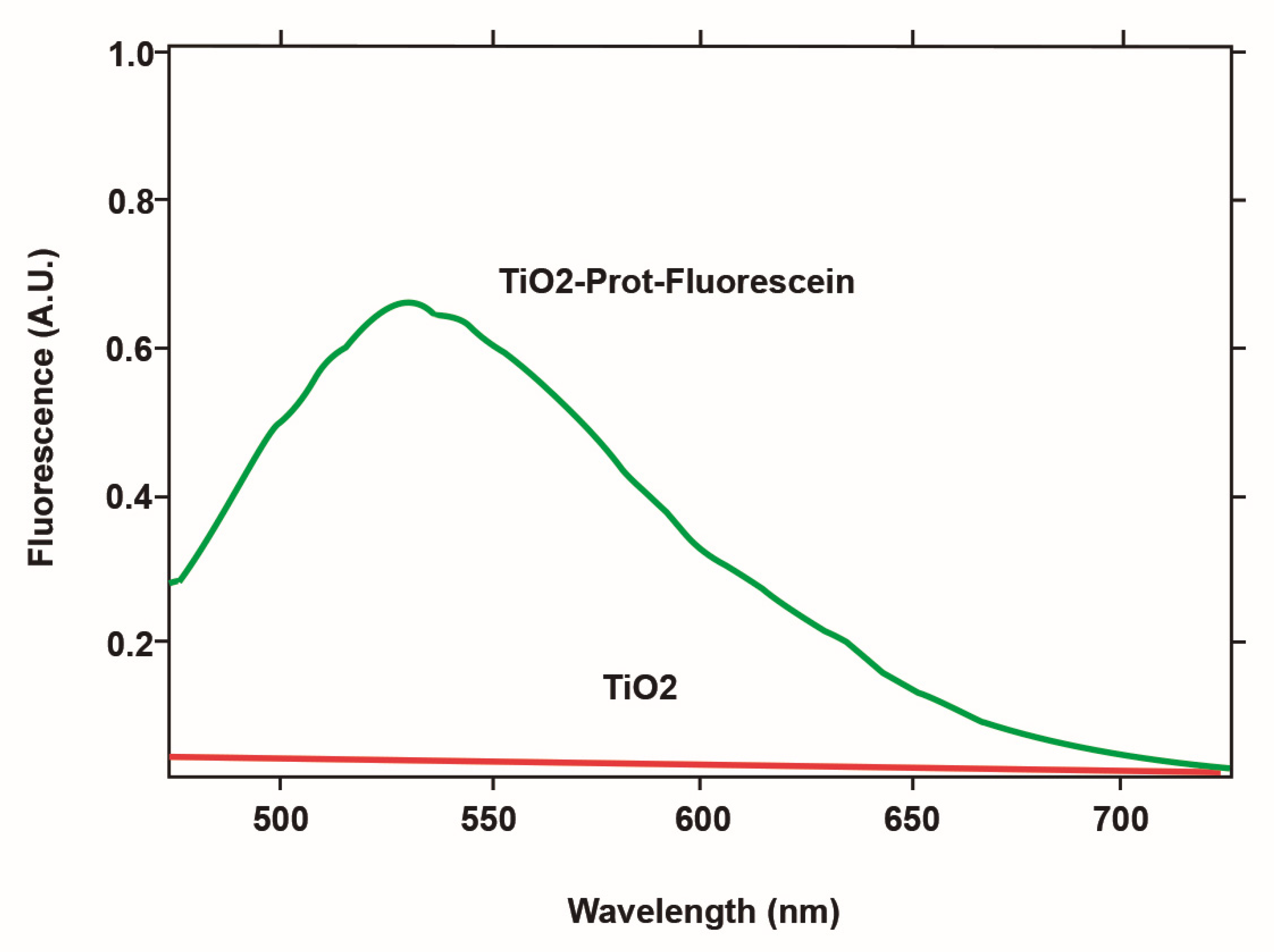
3.3. Protein Immobilization
3.4. Cellular Behavior—Fibroblasts
3.5. Cellular Behavior—MSCat Cells
3.6. Identification and Phenotypic Characterization
3.6.1. Immunohistochemical Tests
3.6.2. Enzyme-Linked Immunosorbent Assay (ELISA)
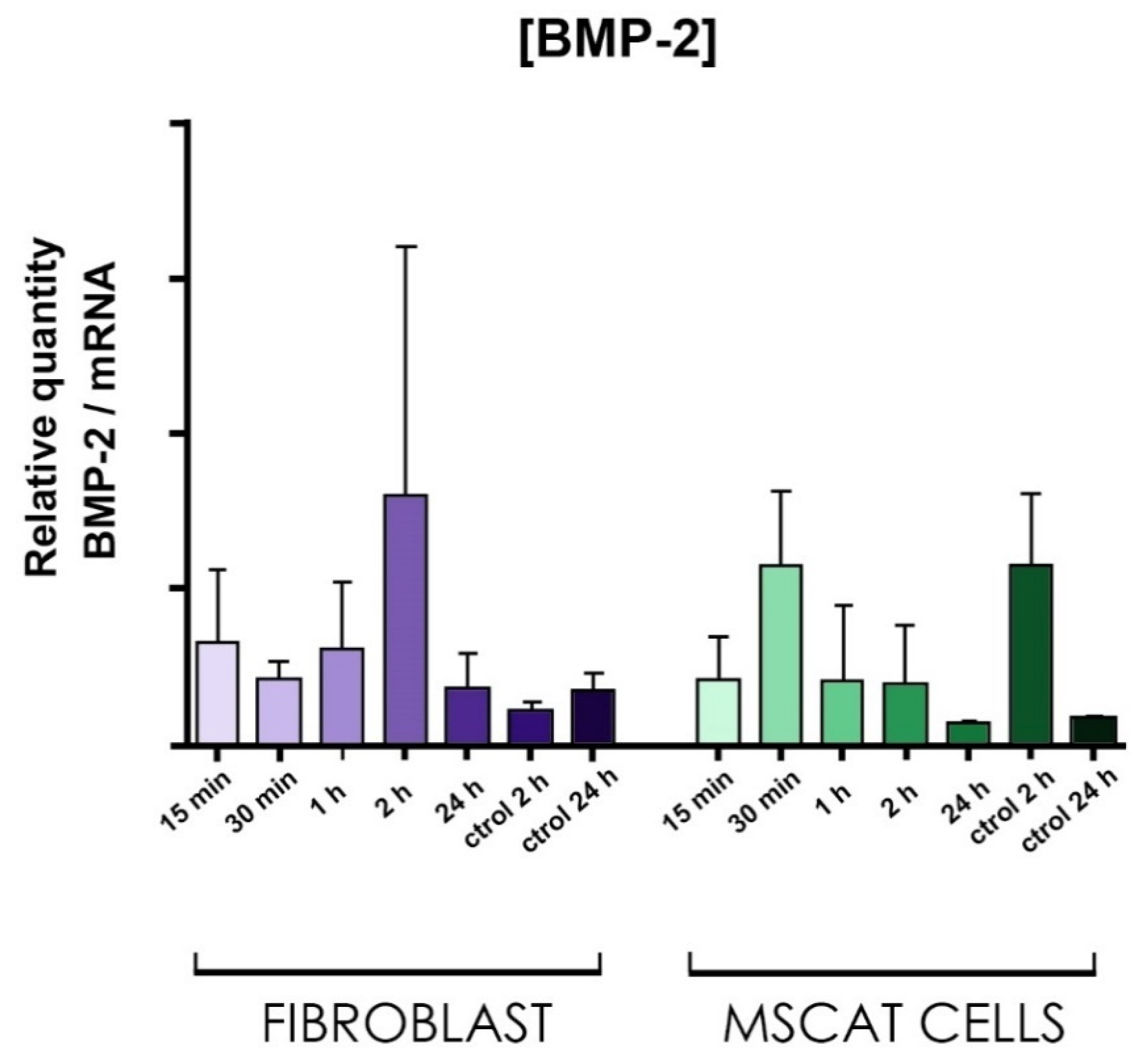
3.6.3. Molecular Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Pjetursson, B.E.; Heimisdottir, K. Dental implants—Are they better than natural teeth? Eur. J. Oral. Sci. 2018, 126, 81–87. [Google Scholar] [CrossRef] [Green Version]
- Branemark, P. Osseointegration and its experimental background. J. Prosthet. Dent. 1983, 50, 399–410. [Google Scholar] [CrossRef]
- Jemat, A.; Ghazali, M.J.; Razali, M.; Otsuka, Y. Surface Modifications and Their Effects on Titanium Dental Implants. BioMed Res. Int. 2015, 2015, 791725. [Google Scholar] [CrossRef] [Green Version]
- Barros, R.R.M.; Novaes, A.B., Jr.; Papalexiou, V.; Souza, S.L.S.; Taba, M., Jr.; Palioto, D.B.; Grisi, M.F. Effect of biofunctionalized implant surface on osseointegration: A histomorphometric study in dogs. Braz. Dent. J. 2009, 20, 91–98. [Google Scholar] [CrossRef] [Green Version]
- Beutner, R.; Michael, J.; Schwenzer, B.; Scharnweber, D. Biological nano-functionalization of titanium-based biomaterial surfaces: A flexible toolbox. J. R. Soc. Interface 2010, 7 (Suppl. 1). Available online: https://royalsocietypublishing.org/doi/10.1098/rsif.2009.0418.focus (accessed on 30 November 2020). [CrossRef] [PubMed] [Green Version]
- Lutz, R.; Srour, S.; Nonhoff, J.; Weisel, T.; Damien, C.J.; Schlegel, K.A. Biofunctionalization of titanium implants with a biomimetic active peptide (P-15) promotes early osseointegration: Biofunctionalization of titanium implants with a biomimetic active peptide. Clin. Oral. Implant. Res. 2010, 21, 726–734. [Google Scholar] [CrossRef] [PubMed]
- Hanawa, T. Biofunctionalization of titanium for dental implant. Jpn. Dent. Sci. Rev. 2010, 46, 93–101. [Google Scholar] [CrossRef] [Green Version]
- Tan, G.; Zhang, L.; Ning, C.; Liu, X.; Liao, J. Preparation and Characterization of APTES Films on Modification Titanium by SAMs. Thin Solid Films 2011, 519, 4997–5001. [Google Scholar] [CrossRef]
- Spori, D.M.; Venkatarman, N.V.; Tosatti, S.G.P.; Durmaz, F.; Spencer, N.D.; Zürcher, S. Influence of Alkyl Chain Length on Phosphate Self-Assembled Monolayers. Langmuir 2007, 23, 8053–8060. [Google Scholar] [CrossRef]
- Mann, J.R.; Nevins, J.S.; Soja, G.R.; Wells, D.D.; Levy, S.C.; Marsh, D.A.; Watson, D.F. Influence of Solvation and the Structure of Adsorbates on the Kinetics and Mechanism of Dimerization- Induced Compositional Changes of Mixed Monolayers on TiO2. Langmuir 2009, 25, 12217–12228. [Google Scholar] [CrossRef]
- Ajami, E.; Aguey-Zinsou, K.F. Formation of OTS Self- Assembled Monolayers at Chemically Treated Titanium Surfaces. J. Mater. Sci. Mater. Med. 2011, 22, 1813–1824. [Google Scholar] [CrossRef]
- Bozzini, S.; Petrini, P.; Tanzi, M.C.; Zürcher, S.; Tosatti, S. Poly(ethylene glycol) and Hydroxy Functionalized Alkane Phosphate Mixed Self-Assembled Monolayers to Control Nonspecific Adsorption of Proteins on Titanium Oxide Surfaces. Langmuir 2010, 26, 6529–6534. [Google Scholar] [CrossRef]
- Ulman, A. Formation and Structure of Self-Assembled Monolayers. Chem. Rev. 1996, 96, 1533–1554. [Google Scholar] [CrossRef] [PubMed]
- Love, J.C.; Estroff, L.A.; Kriebel, J.K.; Nuzzo, R.G.; Whitesides, G.M. Self-Assembled Monolayers of Thiolates on Metals as a Form of Nanotechnology. Chem. Rev. 2005, 105, 1103–1170. [Google Scholar] [CrossRef]
- Liu, Q.; Ding, J.; Mante, F.K.; Wunder, S.L.; Baran, G.R. The role of surface functional groups in calcium phosphate nucleation on titanium foil: A self-assembled monolayer technique. Biomaterials 2002, 23, 3103–3111. [Google Scholar] [CrossRef]
- Liu, D.P.; Majewski, P.; O’Neill, B.K.; Ngothai, Y.; Colby, C.B. The optimal SAM surface functional group for producing a biomimetic HA coating on Ti. J. Biomed. Mater. Res. A 2006, 77, 763–772. [Google Scholar] [CrossRef]
- Shalabi, M.M.; Wolke, J.G.C.; de Ruijter, A.J.E.; Jansen, J.A. Histological evaluation of oral implants inserted with different surgical techniques into the trabecular bone of goats. Clin. Oral. Implant. Res. 2007, 18, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Svensson, S.; Suska, F.; Emanuelsson, L.; Palmquist, A.; Norlindh, B.; Trobos, M.; Bäckros, H.; Persson, L.; Rydja, G.; Ohrlander, M.; et al. Osseointegration of titanium with an antimicrobial nanostructured noble metal coating. Nanomedicine 2013, 9, 1048–1056. [Google Scholar] [CrossRef] [PubMed]
- Hulander, M.; Hong, J.; Andersson, M.; Gerven, F.; Ohrlander, M.; Tengvall, P.; Elwing, H. Blood interactions with noble metals: Coagulation and immune complement activation. ACS Appl. Mater Interfaces 2009, 1, 1053–1062. [Google Scholar] [CrossRef]
- Hulander, M.; Lundgren, A.; Berglin, M.; Ohrlander, M.; Lausmaa, J.; Elwing, H. Immune complement activation is attenuated by surface nanotopo-graphy. Int. J. Nanomed. 2011, 6, 2653–2666. [Google Scholar] [CrossRef] [Green Version]
- Chomczynski, P.; Sacchi, N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987, 162, 156–159. [Google Scholar] [CrossRef]
- Shi, Q.; Qian, Z.; Liu, D.; Liu, H. Surface Modification of Dental Titanium Implant by Layer-by-Layer Electrostatic Self-Assembly. Front. Physiol. 2017, 8, 574. Available online: http://journal.frontiersin.org/article/10.3389/fphys.2017.00574/full (accessed on 5 December 2020). [CrossRef] [PubMed] [Green Version]
- Kumar, S.M.; Balakrishnan, P.K.; Hedge, C.; Dandekeri, S. Self-Assembled Monolayer- A Nano Surface Modification. J. Evol. Med. Dent. Sci. 2020, 9, 1608–1612. [Google Scholar] [CrossRef]
- Anil, S.; Anand, P.S.; Alghamdi, H.; Janse, J.A. Dental Implant Surface Enhancement and Osseointegration. Implant. Dent. A Rapidly Evol. Pract. 2011, 83–108. Available online: http://www.intechopen.com/books/implant-dentistry-a-rapidly-evolving-practice/dental-implant-surface-enhancement-and-osseointegration (accessed on 5 December 2020).
- Lan, W.C.; Huang, T.S.; Cho, Y.C.; Huang, Y.T.; Walinski, C.J.; Chiang, P.C.; Rusilin, M.; Pai, F.T.; Huang, C.C.; Huang, M.S. The Potential of a Nanostructured Titanium Oxide Layer with Self-Assembled Monolayers for Biomedical Applications: Surface Properties and Biomechanical Behaviors. Appl. Sci. 2020, 10, 590. [Google Scholar] [CrossRef] [Green Version]
- Chen, R.S.; Chen, Y.J.; Chen, M.H.; Young, T.H. Cell-surface interactions of rat tooth germ cells on various biomaterials. J. Biomed. Mater. Res. A 2007, 83, 241–248, Erratum in: J. Biomed. Mater. Res. A 2008, 84, 567. [Google Scholar] [CrossRef]
- Boyan, B.D.; Lohmann, C.H.; Dean, D.D.; Sylvia, V.L.; Cochran, D.L.; Schwartz, Z. Mechanisms Involved in Osteoblast Response to Implant Surface Morphology. Annu. Rev. Mater. Res. 2001, 31, 357–371. [Google Scholar] [CrossRef]
- Brett, P.M.; Harle, J.; Salih, V.; Mihoc, R.; Olsen, I.; Jones, F.H.; Tonetti, M. Roughness response genes in osteoblasts. Bone 2004, 35, 124–133. [Google Scholar] [CrossRef]
- Tack, L.; Schickle, K.; Böke, F.; Fischer, H. Immobilization of specific proteins to titanium surface using self-assembled monolayer technique. Dent. Mater. 2015, 31, 1169–1179. [Google Scholar] [CrossRef]
- Viornery, C.; Chevolot, Y.; Léonard, D.; Aronsson, B.-O.; Péchy, P.; Mathieu, H.J.; Descouts, P.; Graetzel, M. Surface Modification of Titanium with Phosphonic Acid to Improve Bone Bonding: Characterization by XPS and ToF-SIMS. Langmuir 2002, 18, 2582–2589. [Google Scholar] [CrossRef]
- Mani, G.; Chandrasekar, B.; Feldman, M.D.; Patel, D.; Agrawal, C.M. Interaction of endothelial cells with self-assembled monolayers for potential use in drug-eluting coronary stents. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 90, 789–801. [Google Scholar] [CrossRef] [PubMed]





| Gene | S Primer Sequence (5′→3′) | AS Primer Sequence (5′→3′) | Banding Temperature |
|---|---|---|---|
| BMP-2 | CCA GGT TAG TGA CTC AGA ACA C | TCA TCT TGG TGC AAA GAC CTG C | 60° |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aresti, A.; Aragoneses, J.; López-Valverde, N.; Suárez, A.; Aragoneses, J.M. Effectiveness of Biofunctionalization of Titanium Surfaces with Phosphonic Acid. Biomedicines 2021, 9, 1663. https://doi.org/10.3390/biomedicines9111663
Aresti A, Aragoneses J, López-Valverde N, Suárez A, Aragoneses JM. Effectiveness of Biofunctionalization of Titanium Surfaces with Phosphonic Acid. Biomedicines. 2021; 9(11):1663. https://doi.org/10.3390/biomedicines9111663
Chicago/Turabian StyleAresti, Ainhoa, Javier Aragoneses, Nansi López-Valverde, Ana Suárez, and Juan Manuel Aragoneses. 2021. "Effectiveness of Biofunctionalization of Titanium Surfaces with Phosphonic Acid" Biomedicines 9, no. 11: 1663. https://doi.org/10.3390/biomedicines9111663
APA StyleAresti, A., Aragoneses, J., López-Valverde, N., Suárez, A., & Aragoneses, J. M. (2021). Effectiveness of Biofunctionalization of Titanium Surfaces with Phosphonic Acid. Biomedicines, 9(11), 1663. https://doi.org/10.3390/biomedicines9111663









