Blood Compatibility of Amphiphilic Phosphorous Dendrons—Prospective Drug Nanocarriers †
Abstract
:1. Introduction
2. Materials, Subjects and Methods
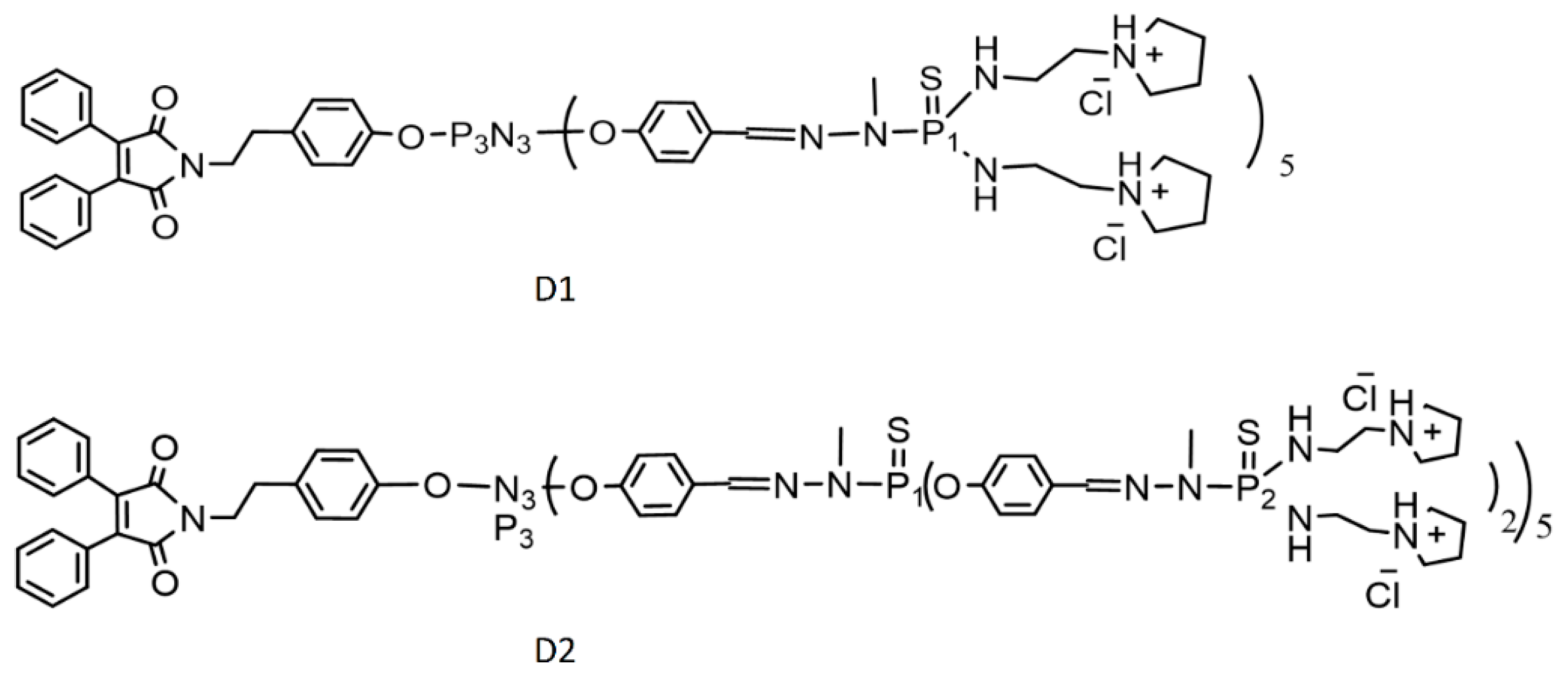
2.1. Chemicals and Dendrons
2.2. Preparation of Liposomes
2.3. Size and Zeta Potential Measurements
2.4. Blood Compatibility Study
2.5. Statistical Analysis
3. Results
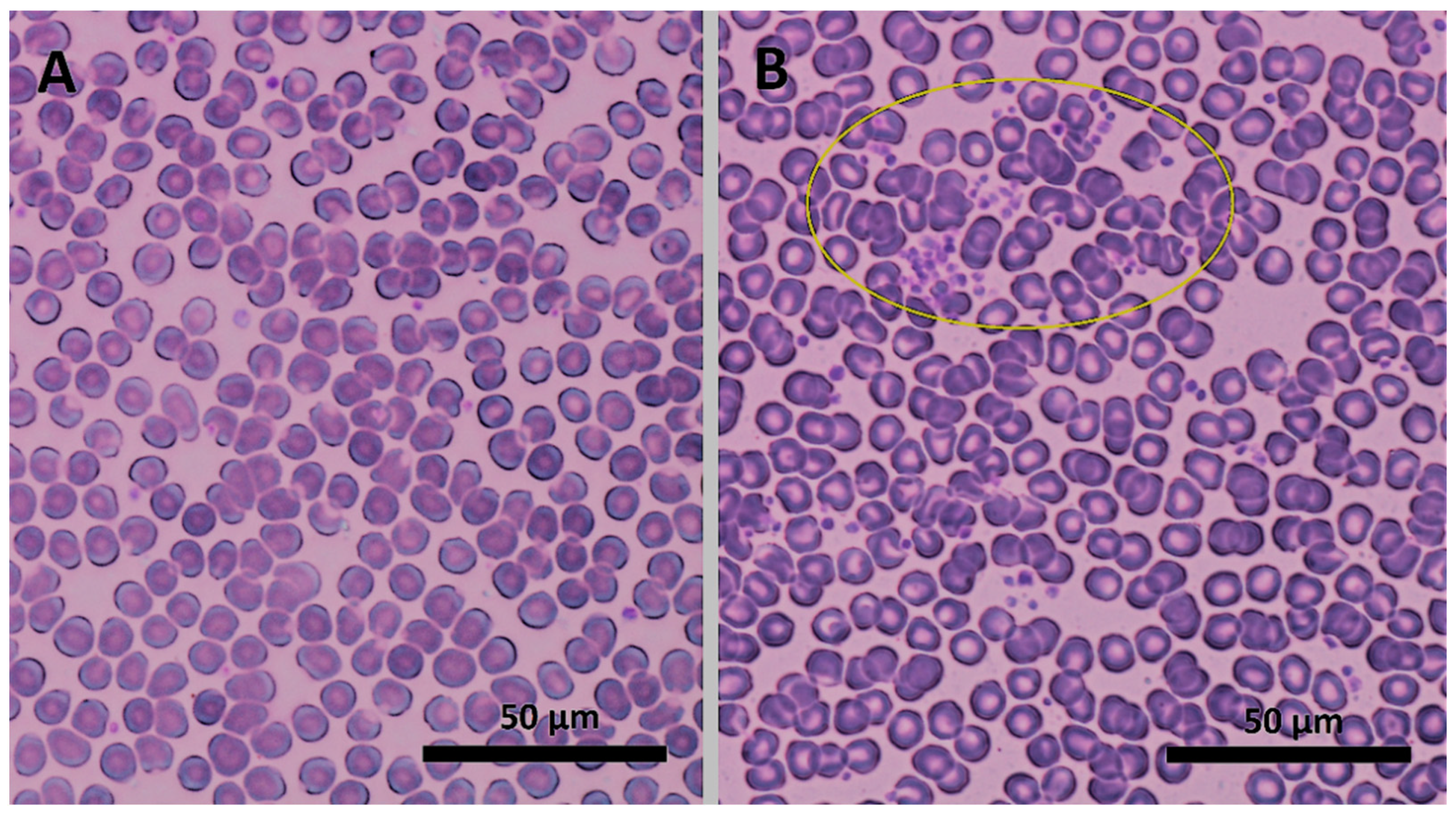
3.1. Dendrons’ Blood Compatibility Assessment
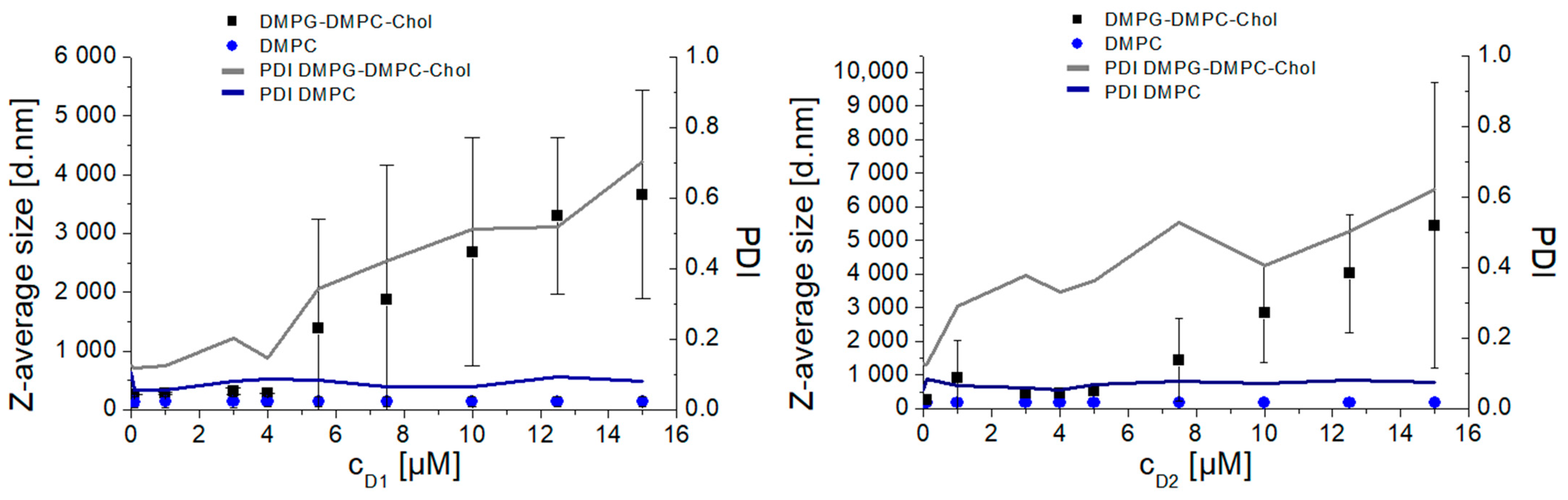
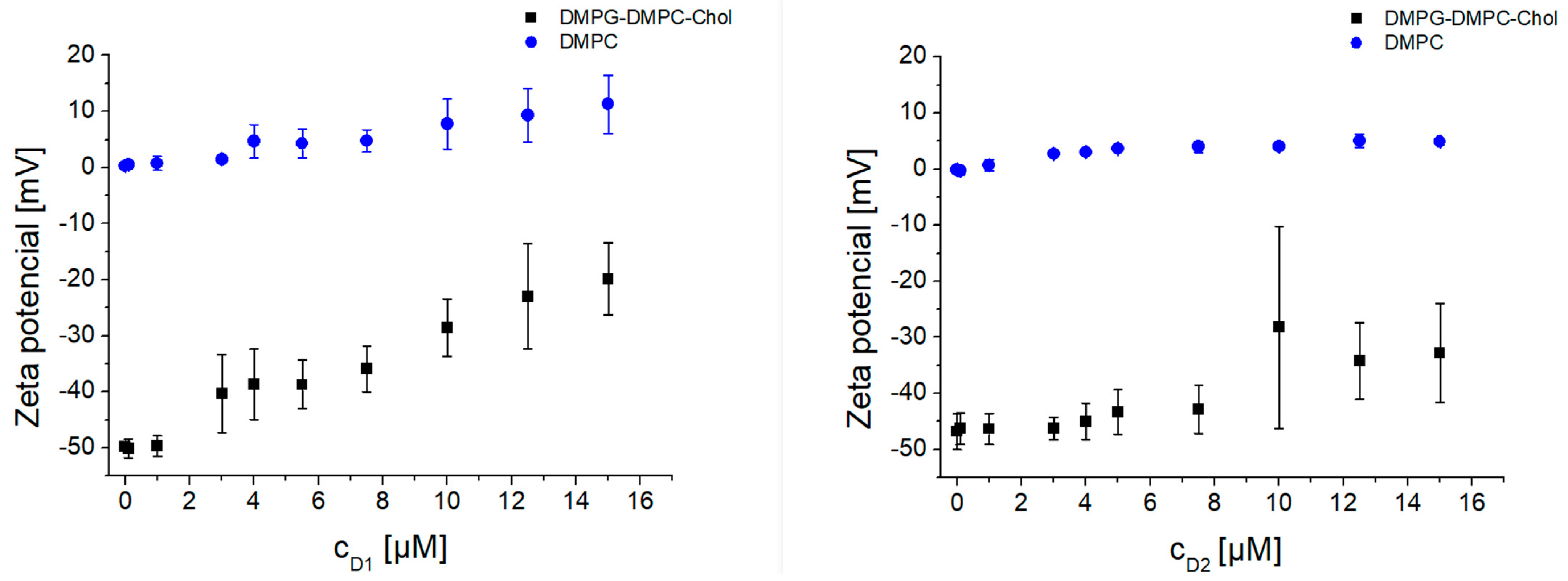
3.2. Interaction of Dendrons with Model Lipid Membranes—Liposomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| D1 | D2 | |||||||
|---|---|---|---|---|---|---|---|---|
| Mean 1 | Mean 2 | Diff | Adjusted p | Mean 1 | Mean 2 | Diff | Adjusted p | |
| RBC (×1012/L) | ||||||||
| Control vs. C1 | 4.80 | 4.89 | −0.088 | 0.0001 | 4.80 | 4.84 | −0.043 | 0.0242 |
| Control vs. C2 | 4.80 | 4.84 | −0.039 | 0.0567 | 4.80 | 4.82 | −0.024 | 0.1979 |
| C1 vs. C2 | 4.89 | 4.84 | 0.049 | 0.0383 | 4.84 | 4.82 | 0.019 | 0.3048 |
| HBG (g/L) | ||||||||
| Control vs. C1 | 141.1 | 144.4 | −3.357 | <0.0001 | 141.1 | 142.2 | −1.143 | 0.0487 |
| Control vs. C2 | 141.1 | 143.6 | −2.500 | <0.0001 | 141.1 | 141.9 | −0.786 | 0.1713 |
| C1 vs. C2 | 144.4 | 143.6 | 0.857 | 0.1388 | 142.2 | 141.9 | 0.357 | 0.4067 |
| PLT (×109/L) | ||||||||
| Control vs. C1 | 225.7 | 227.4 | −1.643 | 0.6465 | 225.7 | 214.2 | 11.50 | 0.1794 |
| Control vs. C2 | 225.7 | 226.4 | −0.714 | 0.9057 | 225.7 | 81.50 | 144.2 | <0.0001 |
| C1 vs. C2 | 227.4 | 226.4 | 0.929 | 0.8488 | 214.2 | 81.50 | 132.7 | <0.0001 |
| MPV (fL) | ||||||||
| Control vs. C1 | 9.31 | 9.41 | −0.100 | 0.2754 | 9.31 | 9.61 | −0.300 | 0.0275 |
| Control vs. C2 | 9.31 | 9.34 | −0.021 | 0.9333 | 9.31 | 11.44 | −2.129 | <0.0001 |
| C1 vs. C2 | 9.41 | 9.34 | 0.079 | 0.4836 | 9.61 | 11.44 | −1.829 | <0.0001 |
| PCT (%) | ||||||||
| Control vs. C1 | 0.209 | 0.213 | −0.0039 | 0.3554 | 0.209 | 0.204 | 0.0050 | 0.6057 |
| Control vs. C2 | 0.209 | 0.209 | −0.0001 | 0.9995 | 0.209 | 0.094 | 0.1158 | <0.0001 |
| C1 vs. C2 | 0.213 | 0.209 | 0.0038 | 0.4495 | 0.204 | 0.094 | 0.1108 | <0.0001 |
| PDW (%) | ||||||||
| Control vs. C1 | 16.88 | 16.91 | −0.029 | 0.879 | 16.88 | 17.11 | −0.229 | 0.0631 |
| Control vs. C2 | 16.88 | 16.84 | 0.036 | 0.8377 | 16.88 | 17.19 | −0.307 | 0.1616 |
| C1 vs. C2 | 16.91 | 16.84 | 0.064 | 0.3207 | 17.11 | 17.19 | −0.079 | 0.8976 |
| TT5 (s) | ||||||||
| Control vs. C1 | 14.37 | 14.31 | 0.064 | 0.7748 | 14.37 | 14.54 | −0.171 | 0.1792 |
| Control vs. C2 | 14.37 | 14.14 | 0.229 | 0.0650 | 14.37 | 10.63 | 3.743 | <0.0001 |
| C1 vs. C2 | 14.31 | 14.14 | 0.164 | 0.0216 | 14.54 | 10.63 | 3.914 | <0.0001 |
| PT-RP (s) | ||||||||
| Control vs. C1 | 11.73 | 11.34 | 0.386 | 0.0003 | 11.73 | 11.29 | 0.443 | 0.0082 |
| Control vs. C2 | 11.73 | 10.96 | 0.764 | 0.0010 | 11.73 | 38.16 | −26.43 | <0.0001 |
| C1 vs. C2 | 11.34 | 10.96 | 0.379 | 0.1227 | 11.29 | 38.16 | −26.87 | <0.0001 |
| aPTT-SS (s) | ||||||||
| Control vs. C1 | 29.77 | 32.80 | −3.029 | <0.0001 | 29.77 | 50.81 | −21.04 | 0.0006 |
| Control vs. C2 | 29.77 | 34.18 | −4.407 | 0.0007 | 29.77 | 152.4 | −122.6 | <0.0001 |
| C1 vs. C2 | 32.80 | 34.18 | −1.379 | 0.1690 | 50.81 | 152.4 | −101.6 | <0.0001 |
| FBG (g/L) | ||||||||
| Control vs. C1 | 2.760 | 2.804 | −0.044 | 0.6181 | 2.760 | 2.786 | −0.026 | 0.8923 |
| Control vs. C2 | 2.760 | 2.701 | 0.059 | 0.3756 | 2.760 | 2.829 | −0.069 | 0.2544 |
| C1 vs. C2 | 2.804 | 2.701 | 0.104 | 0.0375 | 2.786 | 2.829 | −0.043 | 0.6422 |
Appendix B
Interaction of Amphiphilic Phosphorous Dendrons with Model Lipid Membranes—Liposomes—Statistical Analysis
- D1 zeta-average size:
- D2 zeta-average size:
- D1 zeta potential:
- D2 zeta potential:


References
- Tomalia, D.A. Birth of a new macromolecular architecture: Dendrimers as quantized building blocks for nanoscale synthetic polymer chemistry. Prog. Polym. Sci. 2005, 30, 294–324. [Google Scholar] [CrossRef]
- Prashant, K.; Keerti, J.; Narendra, J.K. Dendrimer as nanocarrier for drug delivery. Prog. Polym. Sci. 2014, 39, 268–307. [Google Scholar] [CrossRef]
- Svenson, S. Dendrimers as versatile platform in drug delivery applications. Eur. J. Pharm. Biopharm. 2009, 71, 445–462. [Google Scholar] [CrossRef] [PubMed]
- De La Harpe, K.M.; Kondiah, P.P.D.; Choonara, Y.E.; Marimuthu, T.; Du Toit, L.C.; Pillary, V. The hemocompatibility of nanoparticles: A review of cell-nanoparticle interactions and hemostasis. Cells 2019, 8, 1209. [Google Scholar] [CrossRef] [Green Version]
- Garaiova, Z.; Melikishvili, S.; Michlewska, S.; Ionov, M.; Pedziwiatr-Werbicka, E.; Waczulikova, I.; Hianik, T.; Gomez-Ramirez, R.; Javier De La Mata, F.; Bryszewska, M. Dendronized gold nanoparticles as carriers for gp160 (HIV-1) peptides: Biophysical insight into complex formation. Langmuir 2021, 37, 1542–1550. [Google Scholar] [CrossRef]
- Wagner, J.; Li, L.; Simon, J.; Krutzke, L.; Landfester, K.; Mailänder, V.; Müllen, K.; Ng, D.Y.W.; Wu, Y.; Weil, T. Amphiphilic polyphenylene dendron conjungates for surface remodeling of adenovirus 5. Angew. Chem. Int. Ed. Engl. 2020, 59, 5712–5720. [Google Scholar] [CrossRef] [Green Version]
- Kargl, R.; Kleinschek, K.S. How can we understand the influence of nanoparticles on the coagulation of blood? Nanomedicine 2020, 15, 1923–1926. [Google Scholar] [CrossRef]
- Sanfinsg, E.; Augustsson, C.; Dahlbäck, B.; Linse, S.; Cedervall, T. Size-dependent effects of nanoparticles on enzymes in the blood coagulation cascade. Nano Lett. 2014, 14, 4736–4744. [Google Scholar] [CrossRef]
- Urbán, P.; Liptrott, N.J.; Bremer, S. Overview of the blood compatibility of nanomedicines: A trend analysis of in vitro and in vivo studies. WIREs Nanomed. Nanobiotechnol. 2019, 11, e1546. [Google Scholar] [CrossRef]
- Ziemba, B.; Matuszko, G.; Bryszewska, M.; Klajnert, B. Influence of dendrimers on red blood cells. Cell Mol. Biol. Lett. 2012, 17, 21–35. [Google Scholar] [CrossRef]
- Svenson, S. The dendrimer paradox—High medical expectations but poor clinical translation. Chem. Soc. Rev. 2015, 44, 4131. [Google Scholar] [CrossRef]
- Dobrovolskaia, M.A.; Patri, A.K.; Simak, J.; Hall, J.B.; Semberova, J.; De Paoli Lacerda, S.H.; McNeil, S.E. Nanoparticle size and surface charge determine effects of PAMAM dendrimers on human platelets in vitro. Mol. Pharm. 2011, 9, 382–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, C.F.; Campbell, R.A.; Brooks, A.E.; Assemi, S.; Tadjiki, S.; Thiagarajan, G.; Mulcock, C.; Weyrich, A.S.; Brooks, B.D.; Ghandehari, H.; et al. Cationic PAMAM dendrimers aggressively initiate blood clot formation. ACS Nano 2012, 6, 9900–9910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matus, M.F.; Vilos, C.; Cisterna, B.A.; Fuentes, E.; Palomo, I. Nanotechnology and primary hemostasis: Differential effects of nanoparticles on platelet responses. Vasc. Pharmacol. 2018, 101, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.F.; Campbell, R.A.; Franks, Z.; Gibson, C.C.; Thiagarajan, G.; Vieira-de-Abreu, A.; Sukavaneshvar, S.; Mohammad, S.F.; Li, D.Y.; Ghandehari, H.; et al. Cationic PAMAM dendrimers disrupt key platelet functions. Mol. Pharm. 2012, 9, 1599–1611. [Google Scholar] [CrossRef] [Green Version]
- Ilinskaya, A.N.; Dobrovolskaia, M.A. Nanoparticles and blood coagulation system. Part II: Safety concerns. Nanomedicine 2013, 8, 969–981. [Google Scholar] [CrossRef] [Green Version]
- Qiu, J.; Chen, L.; Zhan, M.; Laurent, R.; Bignon, J.; Mignani, S.; Shi, X.; Caminade, A.M.; Majoral, J.P. Facile synthesis of amphiphilic fluorescent phosphorus dendrons-based micelles as antiproliferative agents: First investigations. Bioconjug. Chem. 2021, 32, 339–349. [Google Scholar] [CrossRef]
- De La Cruz, G.G.; Rodríguez-Fragoso, P.; Reyes-Esparza, J.; Rodríguez-López, A.; Gómez-Cansino, R.; Rodriguez-Fragoso, L. Interaction of nanoparticles with blood components and associated pathophysiological effects. In Unraveling the Safety Profile of Nanoscale Particles and Materials-from Biomedical to Environmental Applications; Gomes, A.C., Sarria, M.P., Eds.; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef] [Green Version]
- Pagana, K.D.; Pagana, T.J.; Pagana, T.N. Mosby’s Diagnostic & Laboratory Test Reference, 14th ed.; Elsevier: St. Louis, MO, USA, 2019; ISBN 9780323609692. [Google Scholar]
- Levi, M. Pathogenesis and diagnosis of disseminated intravascular coagulation. Int. J. Lab. Hematol. 2018, 40, 15–20. [Google Scholar] [CrossRef] [Green Version]
- Serchenya, T.; Shcharbin, D.; Shyrochyna, I.; Sviridov, O.; Terekhova, M.; Dzmitruk, V.; Abashkin, V.; Apartsin, E.; Mignani, S.; Majoral, J.P.; et al. Immunoreactivity changes of human serum albumin and alpha-1-microglobulin induced by their interaction with dendrimers. Colloids Surf. B Biointerfaces 2019, 179, 226–232. [Google Scholar] [CrossRef]
- Janaszewska, A.; Lazniewska, J.; Trzepiński, P.; Marcinkowska, M.; Klajnert-Maculewicz, B. Cytotoxicity of dendrimers. Biomolecules 2019, 9, 330. [Google Scholar] [CrossRef] [Green Version]
- Madaan, K.; Kumar, S.; Poonia, N.; Lather, V.; Pandita, D. Dendrimers in drug delivery and targeting: Drug-dendrimer interactions and toxicity issues. J. Pharm. Bioallied Sci. 2014, 6, 139–150. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suty, S.; Oravczova, V.; Garaiova, Z.; Subjakova, V.; Ionov, M.; Shcharbin, D.; Simonikova, Z.; Bartek, P.; Zvarik, M.; Shi, X.; et al. Blood Compatibility of Amphiphilic Phosphorous Dendrons—Prospective Drug Nanocarriers. Biomedicines 2021, 9, 1672. https://doi.org/10.3390/biomedicines9111672
Suty S, Oravczova V, Garaiova Z, Subjakova V, Ionov M, Shcharbin D, Simonikova Z, Bartek P, Zvarik M, Shi X, et al. Blood Compatibility of Amphiphilic Phosphorous Dendrons—Prospective Drug Nanocarriers. Biomedicines. 2021; 9(11):1672. https://doi.org/10.3390/biomedicines9111672
Chicago/Turabian StyleSuty, Simon, Veronika Oravczova, Zuzana Garaiova, Veronika Subjakova, Maksim Ionov, Dzmitry Shcharbin, Zuzana Simonikova, Peter Bartek, Milan Zvarik, Xiangyang Shi, and et al. 2021. "Blood Compatibility of Amphiphilic Phosphorous Dendrons—Prospective Drug Nanocarriers" Biomedicines 9, no. 11: 1672. https://doi.org/10.3390/biomedicines9111672
APA StyleSuty, S., Oravczova, V., Garaiova, Z., Subjakova, V., Ionov, M., Shcharbin, D., Simonikova, Z., Bartek, P., Zvarik, M., Shi, X., Mignani, S., Majoral, J.-P., Bryszewska, M., Hianik, T., & Waczulikova, I. (2021). Blood Compatibility of Amphiphilic Phosphorous Dendrons—Prospective Drug Nanocarriers. Biomedicines, 9(11), 1672. https://doi.org/10.3390/biomedicines9111672










