Correlation of Periodontal Bacteria with Chronic Inflammation Present in Patients with Metabolic Syndrome
Abstract
:1. Introduction
2. Materials and Methods
2.1. Body Analysis of Patients with MS
2.1.1. Anthropometric Tests
2.1.2. Tracking Metabolic Parameters
2.1.3. Paraclinical Analyses
2.2. Micro-IDent Test
3. Results
3.1. Evolution of Parameters
3.1.1. Results (Evolution) of Anthropometric Analyses
3.1.2. The Result of Paraclinical Analyzes
3.1.3. Micro-IDent Tests Results
3.1.4. Pearson Correlation
4. Discussion
5. Conclusions
6. Patents
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wendland, N.; Opydo-Szymaczek, J.; Mizgier, M.; Jarząbek-Bielecka, G. Subgingival microflora in adolescent females with polycystic ovary syndrome and its association with oral hygiene, gingivitis, and selected metabolic and hormonal parameters. Clin. Oral Investig. 2021, 25, 1485–1496. [Google Scholar] [CrossRef]
- Ramos-Lopez, O.; Riezu-Boj, J.I.; Milagro, F.I.; Cuervo, M.; Goni, L.; Martinez, J.A. Interplay of an Obesity-Based Genetic Risk Score with Dietary and Endocrine Factors on Insulin Resistance. Nutrients 2019, 12, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Dyke, T.E.; Bartold, P.M.; Reynolds, E.C. The nexus between periodontal inflammation and dysbiosis. Front. Immunol. 2020, 11, 511. [Google Scholar] [CrossRef] [PubMed]
- Kamer, A.R.; Craig, R.G.; Dasanayake, A.P.; Brys, M.; Glodzik-Sobanska, L.; de Leon, M.J. Inflammation and Alzheimer’s disease: Possible role of periodontal diseases. Alzheimer Dement. 2008, 4, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Harrandah, A.M.; Chukkapalli, S.S.; Bhattacharyya, I.; Progulske-Fox, A.; Chan, E.K. Fusobacteria modulate oral carcinogenesis and promote cancer progression. J. Oral Microbiol. 2021, 13, 1849493. [Google Scholar] [CrossRef]
- Van der Weijden, F.; Rijnen, M.; Valkenburg, C. Comparison of three qPCR-based commercial tests for detection of periodontal pathogens. Sci. Rep. 2021, 11, 6141. [Google Scholar]
- Cantore, S.; Mirgaldi, R.; Ballini, A.; Coscia, M.F.; Scacco, S.; Papa, F.; Inchingolo, F.; Dipalma, G.; de Vito, D. Cytokine Gene Polymorphisms Associate with Microbiogical Agents in Periodontal Disease: Our Experience. Int. J. Med. Sci. 2014, 11, 674–679. [Google Scholar] [CrossRef] [Green Version]
- Tonetti, M.S.; Greenwell, H.; Kornman, K.S. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J. Periodontol. 2018, 89 (Suppl. 1), S159–S172. [Google Scholar] [CrossRef] [Green Version]
- Caton, J.G.; Armitage, G.; Berglundh, T.; Chapple, I.L.C.; Jepsen, S.; Kornman, K.S.; Mealey, B.L.; Papapanou, P.N.; Sanz, M.; Tonetti, M.S. A new classification scheme for periodontal and peri-implant diseases and conditions—Introduction and key changes from the 1999 classification. J. Periodontol. 2018, 89 (Suppl. 1), S1–S8. [Google Scholar] [CrossRef]
- Kawamoto, R.; Tabara, Y.; Kohara, K.; Miki, T.; Kusunoki, T.; Takayama, S.; Abe, M.; Katoh, T.; Ohtsuka, N. Relationships between lipid profiles and metabolic syndrome, insulin resistance and serum high molecular adiponectin in Japanese community-dwelling adults. Lipids Health Dis. 2011, 10, 79. [Google Scholar] [CrossRef] [Green Version]
- Zaha, D.C.; Vesa, C.; Uivarosan, D.; Bratu, O.; Fratila, O.; Tit, D.M.; Pantis, C.; Diaconu, C.C.; Bungau, S. Influence of inflammation and adipocyte biochemical markers on the components of metabolic syndrome. Exp. Ther. Med. 2020, 20, 121–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Golbidi, S.; Mesdaghinia, A.; Laher, I. Exercise in the metabolic syndrome. Oxid. Med. Cell Longev. 2012, 2012, 349710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabbaa, M.; Golubic, M.; Roizen, M.F.; Bernstein, A.M. Docosahexaenoic acid, inflammation, and bacterial dysbiosis in relation to periodontal disease, inflammatory bowel disease, and the metabolic syndrome. Nutrients 2013, 5, 3299–3310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milovanovic, T.; Pantic, I.; Dragasevic, S.; Lugonja, S.; Dumic, I.; Rajilic-Stojanovic, M. The Interrelationship Among Non-Alcoholic Fatty Liver Disease, Colonic Diverticulosis and Metabolic Syndrome. J. Gastrointestin. Liver Dis. 2021, 30, 274–282. [Google Scholar] [CrossRef]
- Saltzman, E.T.; Palacios, T.; Thomsen, M.; Vitetta, L. Intestinal microbiome shifts, dysbiosis, inflammation, and non-alcoholic fatty liver disease. Front. Microbiol. 2018, 9, 61. [Google Scholar] [CrossRef]
- Jeganathan, N.A.; Davenport, E.R.; Yochum, G.S.; Koltun, W.A. The microbiome of diverticulitis. Curr. Opin. Physiol. 2021, 22, 100452. [Google Scholar] [CrossRef]
- Jabłonowska-Lietz, B.; Wrzosek, M.; Włodarczyk, M.; Nowicka, G. New indexes of body fat distribution, visceral adiposity index, body adiposity index, waist-to-height ratio, and metabolic disturbances in the obese. Kardiol. Pol. 2017, 75, 1185–1191. [Google Scholar] [CrossRef] [Green Version]
- Mboowa, G.; Ocheng, F.; Okeng, A.; Bwanga, F. Periodontopathogenic bacterial species among patients with periodontal diseases at Mulago Hospital Dental Clinic in Kampala, Uganda: A cross-section study. J. Dent. Oral Hyg. 2014, 6, 58–63. [Google Scholar]
- Dekker, J.M.; Girman, C.; Rhodes, T.; Nijpels, G.; Stehouwer, C.D.; Bouter, L.M.; Heine, R.J. Metabolic syndrome and 10-year cardiovascular disease risk in the Hoorn Study. Circulation 2005, 112, 666–673. [Google Scholar] [CrossRef]
- Dimitrov, B.D.; Bahchevanov, K.M.; Atanassova, P.A.; Mitkov, M.D.; Massaldjieva, R.I.; Chompalov, K.A.; Hadzhipetrov, G.K. Metabolic syndrome severity score: Range and associations with cardiovascular risk factors. Arch. Med. Sci. Atheroscler. Dis. 2016, 1, e90–e97. [Google Scholar] [CrossRef]
- Nazare, J.A.; Smith, J.; Borel, A.L.; Aschner, P.; Barter, P.; Van Gaal, L.; Tan, C.E.; Wittchen, H.U.; Matsuzawa, Y.; Kadowaki, T.; et al. Usefulness of measuring both body mass index and waist circumference for the estimation of visceral adiposity and related cardiometabolic risk profile (from the INSPIRE ME IAA study). Am. J. Cardiol. 2015, 115, 307–315. [Google Scholar] [CrossRef]
- Ghitea, T.C.; El-Kharoubi, A.; Ganea, M.; Bimbo-Szuhai, E.; Nemeth, T.S.; Ciavoi, G.; Foghis, M.; Dobjanschi, L.; Pallag, A.; Micle, O. The Antimicrobial Activity of Origanum vulgare L. Correlated with the Gastrointestinal Perturbation in Patients with Metabolic Syndrome. Molecules 2021, 26, 283. [Google Scholar] [CrossRef]
- Lapik, I.A.; Sharafetdinov, K.K.; Plotnikova, O.A.; Semenchenko, I.I. Influence of Dietotherapy on Body Composition in Patients With Obesity and Diabetes Mellitus Type 2. Vopr. Pitan. 2013, 82, 53–58. [Google Scholar] [PubMed]
- Bogdanov, A.R.; Derbeneva, S.A. Influence of dietotherapy enriched with conjugated linoleic acid on anthropometrical indicators and body composite structure in patients with an overweight. Vopr. Pitan. 2013, 82, 55–62. [Google Scholar]
- Spencer, L.; Rollo, M.; Hauck, Y.; MacDonald-Wicks, L.; Wood, L.; Hutchesson, M.; Giglia, R.; Smith, R.; Collins, C. The effect of weight management interventions that include a diet component on weight-related outcomes in pregnant and postpartum women: A systematic review protocol. JBI Database Syst. Rev. Implement Rep. 2015, 13, 88–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corb Aron, R.A.; Abid, A.; Vesa, C.M.; Nechifor, A.C.; Behl, T.; Ghitea, T.C.; Munteanu, M.A.; Fratila, O.; Andronie-Cioara, F.L.; Toma, M.M. Recognizing the benefits of pre-/probiotics in metabolic syndrome and type 2 diabetes mellitus considering the influence of akkermansia muciniphila as a key gut bacterium. Microorganisms 2021, 9, 618. [Google Scholar] [CrossRef]
- Kassaian, N.; Feizi, A.; Aminorroaya, A.; Amini, M. Probiotic and synbiotic supplementation could improve metabolic syndrome in prediabetic adults: A randomized controlled trial. Diabetes Metab. Syndr. 2019, 13, 2991–2996. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Wei, H.; Ao, J.; Long, G.; Peng, J.; Björkroth, J. Inclusion of Konjac Flour in the Gestation Diet Changes the Gut Microbiota, Alleviates Oxidative Stress, and Improves Insulin Sensitivity in Sows. Appl. Environ. Microbiol. 2016, 82, 5899–5909. [Google Scholar] [CrossRef] [Green Version]
- Verkhnyatskaya, S.; Ferrari, M.; de Vos, P.; Walvoort, M.T. Shaping the infant microbiome with non-digestible carbohydrates. Front. Microbiol. 2019, 10, 343. [Google Scholar] [CrossRef] [Green Version]
- Del Giudice, M.M.; Indolfi, C.; Strisciuglio, C. Vitamin D: Immunomodulatory aspects. J. Clin. Gastroenterol. 2018, 52, S86–S88. [Google Scholar] [CrossRef]
- Lopez-Siles, M.; Enrich-Capó, N.; Aldeguer, X.; Sabat-Mir, M.; Duncan, S.H.; Garcia-Gil, L.J.; Martinez-Medina, M. Alterations in the abundance and co-occurrence of Akkermansia muciniphila and Faecalibacterium prausnitzii in the colonic mucosa of inflammatory bowel disease subjects. Front. Cell. Infect. Microbiol. 2018, 8, 281. [Google Scholar] [CrossRef]
- Ciavoi, G.; Dobjanschi, L.; Jurca, T.; Osser, G.; Scrobota, I.; Pallag, A.; Muresan, M.E.; Vicaș, L.G.; Marian, E.; Bechir, F. Comparative Effectiveness of a Commercial Mouthwash and an Herbal Infusion in Oral Health Care. Appl. Sci. 2021, 11, 3008. [Google Scholar] [CrossRef]
- Schmidt-Trucksäss, A. The metabolic syndrome and sports. MMW Fortschr. Med. 2006, 148, 30–32. [Google Scholar] [PubMed]
- Bird, S.R.; Hawley, J.A. Update on the effects of physical activity on insulin sensitivity in humans. BMJ Open Sport Exerc. Med. 2016, 2, e000143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ostman, C.; Smart, N.A.; Morcos, D.; Duller, A.; Ridley, W.; Jewiss, D. The effect of exercise training on clinical outcomes in patients with the metabolic syndrome: A systematic review and meta-analysis. Cardiovasc. Diabetol. 2017, 16, 110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghitea, T.C.; Vlad, S.; Birle, D.; Tit, D.M.; Lazar, L.; Nistor-Cseppento, C.; Behl, T.; Bungau, S. The influence of diet therapeutic intervention on the sarcopenic index of patients with metabolic syndrome. Acta Endocrinol. (Bucharest) 2020, 16, 470–478. [Google Scholar] [CrossRef]
- Nibali, L.; Tatarakis, N.; Needleman, I.; Tu, Y.-K.; D’Aiuto, F.; Rizzo, M.; Donos, N. Association Between Metabolic Syndrome and Periodontitis: A Systematic Review and Meta-analysis. J. Clin. Endocrinol. Metab. 2013, 98, 913–920. [Google Scholar] [CrossRef] [Green Version]
- Miranda, T.S.; de Freitas Figueiredo, N.; Figueiredo, L.C.; da Silva, H.D.P.; Rocha, F.R.G.; Duarte, P.M. Cytokine profiles of healthy and diseased sites in individuals with periodontitis. Arch. Oral Biol. 2020, 120, 104957. [Google Scholar] [CrossRef]
- Kawamoto, D.; Amado, P.P.L.; Albuquerque-Souza, E.; Bueno, M.R.; Vale, G.C.; Saraiva, L.; Mayer, M.P.A. Chemokines and cytokines profile in whole saliva of patients with periodontitis. Cytokine 2020, 135, 155197. [Google Scholar] [CrossRef]
- Das, U.N. Is obesity an inflammatory condition? Nutrition 2001, 17, 953–966. [Google Scholar] [CrossRef]
- Mitsides, N.; Cornelis, T.; Broers, N.J.H.; Diederen, N.M.P.; Brenchley, P.; van der Sande, F.M.; Schalkwijk, C.G.; Kooman, J.P.; Mitra, S. Extracellular overhydration linked with endothelial dysfunction in the context of inflammation in haemodialysis dependent chronic kidney disease. PLoS ONE 2017, 12, e0183281. [Google Scholar] [CrossRef]
- Serra-Prat, M.; Lorenzo, I.; Palomera, E.; Ramírez, S.; Yébenes, J.C. Total Body Water and Intracellular Water Relationships with Muscle Strength, Frailty and Functional Performance in an Elderly Population. A Cross-Sectional Study. J. Nutr. Health Aging 2018, 23, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Andre, T.; Jean-Pierre, D. Pathophysiology of human visceral obesity: An update. Physiol. Rev. 2013, 93, 359–404. [Google Scholar] [CrossRef]
- Su, X.; Xu, J.; Zheng, C. The relationship between non-alcoholic fatty liver and skeletal muscle mass to visceral fat area ratio in women with type 2 diabetes. BMC Endocr. Disord. 2019, 19, 76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ballak, D.B.; Li, S.; Cavalli, G.; Stahl, J.L.; Tengesdal, I.W.; van Diepen, J.A.; Klück, V.; Swartzwelter, B.; Azam, T.; Tack, C.J. Interleukin-37 treatment of mice with metabolic syndrome improves insulin sensitivity and reduces pro-inflammatory cytokine production in adipose tissue. J. Biol. Chem. 2018, 293, 14224–14236. [Google Scholar] [CrossRef] [Green Version]
- Woelber, J.P.; Tennert, C. Diet and periodontal diseases. Impact Nutr. Diet Oral Health 2020, 28, 125–133. [Google Scholar]
- Ghitea, T.C.; Aron, R.A.C.; Lazar, L.; Bungau, S. The Influence Of Dietary Interventions On Paraclinical Parameters In Patients With Metabolic Syndrome. Arch. Balk. Med. Union 2020, 55, 592–600. [Google Scholar] [CrossRef]
- Butera, A.; Lovati, E.; Simone, R.; Segù, M.; Scribante, A.; Lanteri, V.; Chiesa, A.; Granata, M.; Rodriguez y Baena, R. Professional and Home-Management in non -surgical periodontal therapy to evaluate the percentage of glycated hemoglobin in type 1 diabetes patients. Int. J. Clin. Dent. 2021, 14, 41–53. [Google Scholar]
- Hasebe, A.; Yoshimura, A.; Into, T.; Kataoka, H.; Tanaka, S.; Arakawa, S.; Ishikura, H.; Golenbock, D.T.; Sugaya, T.; Tsuchida, N. Biological activities of Bacteroides forsythus lipoproteins and their possible pathological roles in periodontal disease. Infect. Immun. 2004, 72, 1318–1325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dongari-Bagtzoglou, A.; Ebersole, J. Production of inflammatory mediators and cytokines by human gingival fibroblasts following bacterial challenge. J. Periodontal Res. 1996, 31, 90–98. [Google Scholar] [CrossRef]
- Bostanci, N.; Allaker, R.; Belibasakis, G.; Rangarajan, M.; Curtis, M.; Hughes, F.; McKay, I. Porphyromonas gingivalis antagonises Campylobacter rectus induced cytokine production by human monocytes. Cytokine 2007, 39, 147–156. [Google Scholar] [CrossRef]
- van Winkel, R.; Rutten, B.P.; Peerbooms, O.; Peuskens, J.; van Os, J.; De Hert, M. MTHFR and risk of metabolic syndrome in patients with schizophrenia. Schizophr. Res. 2010, 121, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-I.; Choi, C.-H.; Chung, K.-H. No Association between Metabolic Syndrome and Periodontitis in Korean Postmenopausal Women. Int. J. Environ. Res. Public Health 2021, 18, 11110. [Google Scholar] [CrossRef]
- Chooi, Y.C.; Ding, C.; Magkos, F. The epidemiology of obesity. Metabolism 2019, 92, 6–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mihaela, M.; Cristian, I.D.; Ramona, D.A.; Condurache, G. Assessment of local risk factors in the etiology and evolution of periodontal diseases. Rom. J. Oral Rehabil. 2019, 11, 115–121. [Google Scholar]

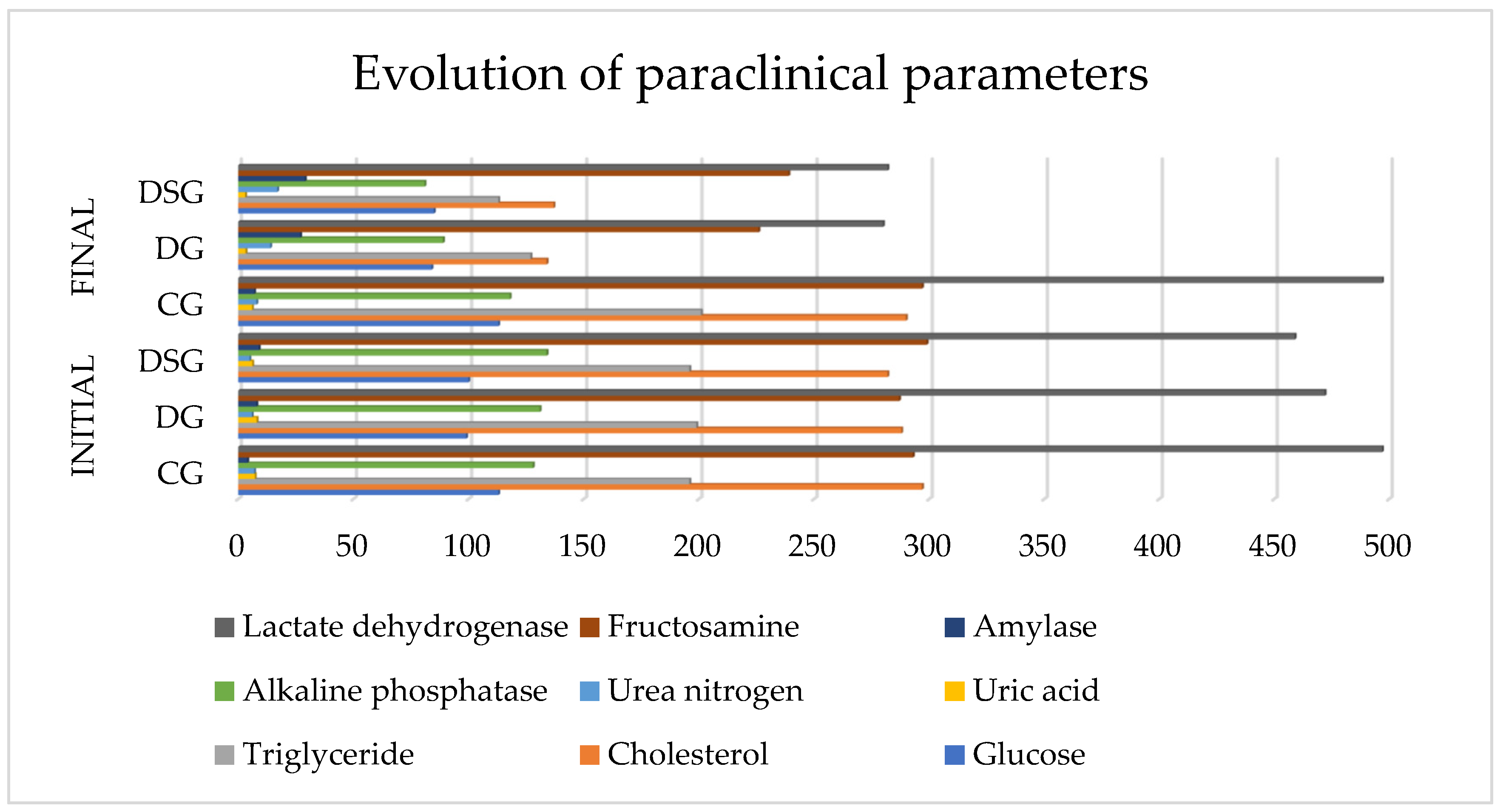
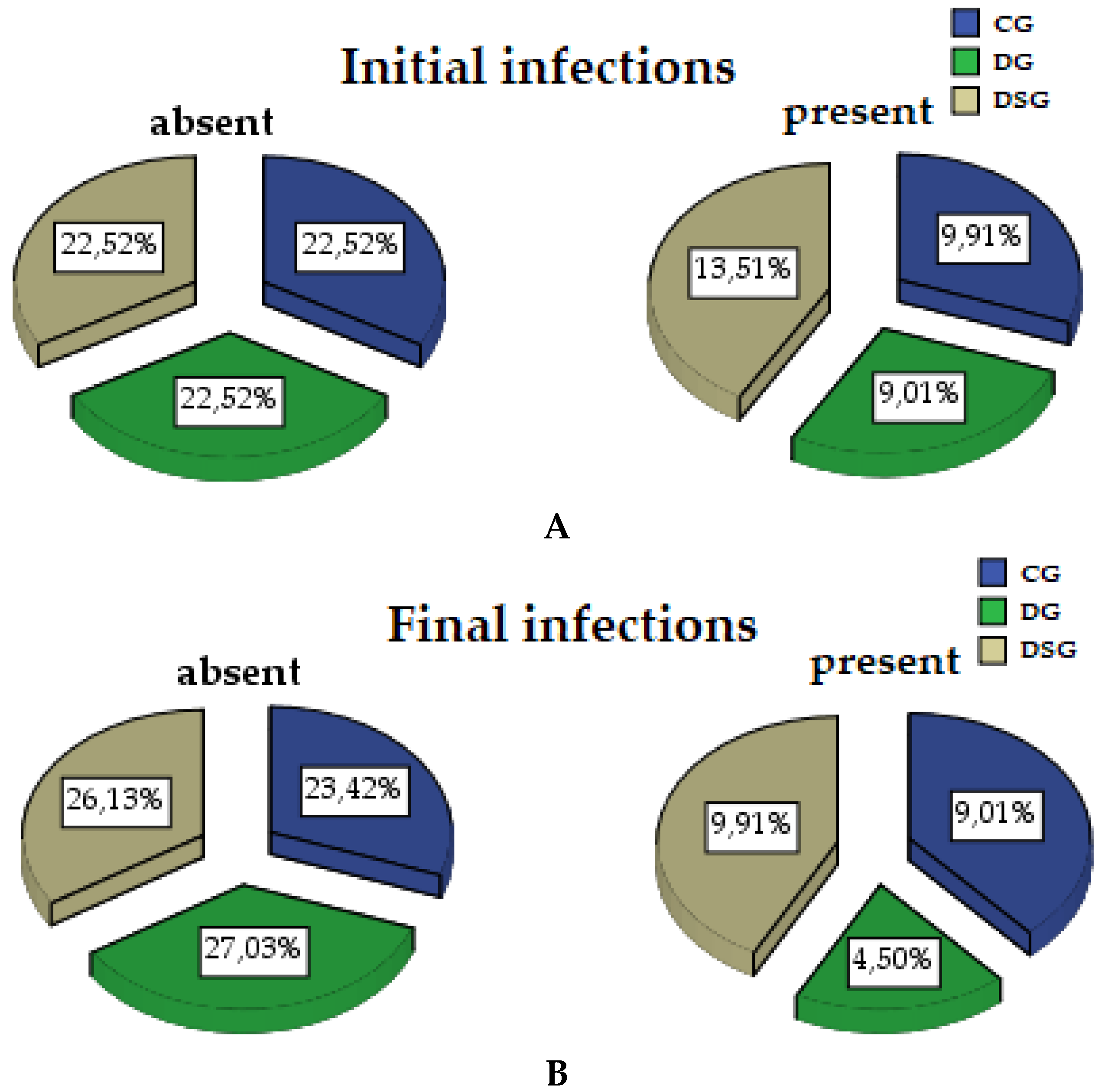



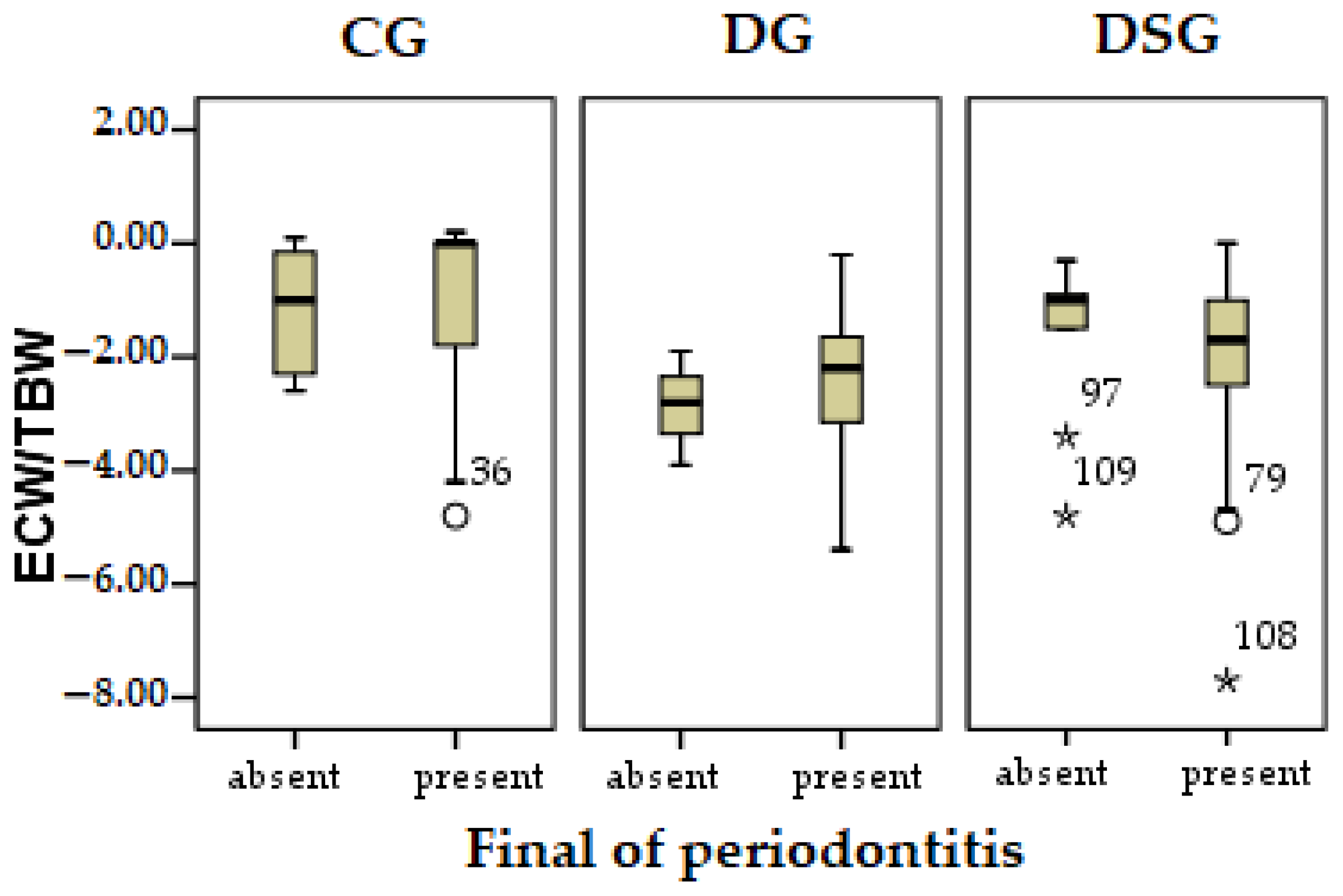
| Initial Parameters | Groups | ||||||
|---|---|---|---|---|---|---|---|
| CG | DG | DSG | |||||
| N | % | N | % | N | % | ||
| Gender | Men | 28 | 77.77 | 29 | 82.86 | 30 | 75.00 |
| Women | 8 | 22.22 | 6 | 17.14 | 10 | 25.00 | |
| Area of provenience | Urban | 12 | 33.33 | 14 | 40.00 | 22 | 55.00 |
| Rural | 24 | 66.66 | 21 | 60.00 | 18 | 45.00 | |
| Periodontopathogens | Absent | 25 | 69.44 | 25 | 71.43 | 25 | 62.50 |
| Present | 11 | 30.55 | 10 | 28.57 | 15 | 37.50 | |
| Initial Parameters | Groups | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| CG | DG | DSG | |||||||
| N | Mean | SD | N | Mean | SD | N | Mean | SD | |
| Age | 36 | 42.67 | 15.64 | 35 | 42.31 | 17.95 | 40 | 34.05 | 9.40 |
| Initial BMI | 36 | 29.29 | 5.60 | 35 | 30.95 | 6.50 | 40 | 31.42 | 9.95 |
| Initial fat mass | 36 | 32.27 | 7.82 | 35 | 33.71 | 7.10 | 40 | 26.92 | 9.67 |
| Initial visceral fat | 36 | 8.78 | 4.87 | 35 | 9.37 | 6.70 | 40 | 4.90 | 3.36 |
| Initial ECW/TBW | 36 | 43.21 | 2.83 | 35 | 43.69 | 3.13 | 40 | 41.85 | 3.31 |
| Groups | Mean | N | Std. Deviation | Correlation | t | Sig. | ||
|---|---|---|---|---|---|---|---|---|
| CG | Pair 1 | Initial BMI | 29.2889 | 36 | 5.59682 | 0.999 ** | −3.897 | 0.001 |
| Final BMI | 29.4667 | 36 | 5.79300 | |||||
| Pair 2 | Initial fat mass | 32.2667 | 36 | 7.82407 | 1.000 ** | −4.799 | 0.001 | |
| Final fat mass | 32.3222 | 36 | 7.82023 | |||||
| Pair 3 | Initial visceral fat | 8.7778 | 36 | 4.87038 | 0.999 ** | −2.092 | 0.044 | |
| Final visceral fat | 8.8889 | 36 | 5.11456 | |||||
| Pair 4 | Initial ECW/TBW | 43.2056 | 36 | 2.82539 | 0.865 ** | 3.741 | 0.001 | |
| Final ECW/TBW | 42.3000 | 36 | 2.76106 | |||||
| DG | Pair 1 | Initial BMI | 30.9497 | 35 | 6.49928 | 0.862 ** | 8.903 | 0.001 |
| Final BMI | 24.7031 | 35 | 3.08141 | |||||
| Pair 2 | Initial fat mass | 33.7057 | 35 | 7.10435 | 0.719 ** | 7.118 | 0.001 | |
| Final fat mass | 27.7371 | 35 | 5.58633 | |||||
| Pair 3 | Initial visceral fat | 9.3714 | 35 | 6.69981 | 0.989 ** | 7.242 | 0.001 | |
| Final visceral fat | 7.7429 | 35 | 5.73607 | |||||
| Pair 4 | Initial ECW/TBW | 43.6886 | 35 | 3.13320 | 0.941 ** | 12.180 | 0.001 | |
| Final ECW/TBW | 41.2686 | 35 | 3.45805 | |||||
| DSG | Pair 1 | Initial BMI | 31.4205 | 40 | 9.95106 | 0.840 ** | 5.636 | 0.001 |
| Final BMI | 24.7725 | 40 | 3.21248 | |||||
| Pair 2 | Initial fat mass | 26.9160 | 40 | 9.66844 | 0.835 ** | 6.315 | 0.001 | |
| Final fat mass | 21.4950 | 40 | 6.97593 | |||||
| Pair 3 | Initial visceral fat | 4.9000 | 40 | 3.35735 | 0.897 ** | 4.210 | 0.001 | |
| Final visceral fat | 3.9000 | 40 | 2.79009 | |||||
| Pair 4 | Initial ECW/TBW | 41.8475 | 40 | 3.31012 | 0.894 ** | 7.758 | 0.001 | |
| Final ECW/TBW | 39.9725 | 40 | 3.33459 | |||||
| Periodontopathogens | Final Periodontopathogens | ||
|---|---|---|---|
| Absent | Present | ||
| N | N | ||
| Groups | CG | 26 | 10 |
| DG | 30 | 5 | |
| DSG | 29 | 11 | |
| Pearson Correlation | Periodontopathogens | |
|---|---|---|
| BMI | r | 0.102 |
| Sig. | 0.286 | |
| N | 111 | |
| Fat mass difference | r | −0.023 |
| Sig. | 0.813 | |
| N | 111 | |
| Visceral fat difference | r | −0.133 |
| Sig. | 0.164 | |
| N | 111 | |
| ECW/TBW difference | r | −0.069 |
| Sig. | 0.471 | |
| N | 111 | |
| Groups | Periodontopathogens | ||
|---|---|---|---|
| CG | Fat mass difference | r | 0.166 |
| Sig. | 0.332 | ||
| Visceral fat difference | r | 0.273 | |
| Sig. | 0.107 | ||
| ECW/TBW difference | r | −0.452 ** | |
| Sig. | 0.006 | ||
| Patients | N | 36 | |
| DG | Fat mass difference | r | 0.062 |
| Sig. | 0.724 | ||
| Visceral fat difference | r | 0.009 | |
| Sig. | 0.960 | ||
| ECW/TBW difference | r | −0.197 | |
| Sig. | 0.258 | ||
| Patients | N | 35 | |
| DSG | Fat mass difference | r | 0.076 |
| Sig. | 0.640 | ||
| Visceral fat difference | r | 0.156 | |
| Sig. | 0.337 | ||
| ECW/TBW difference | r | −0.056 | |
| Sig. | 0.733 | ||
| Patients | N | 40 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghitea, T.C. Correlation of Periodontal Bacteria with Chronic Inflammation Present in Patients with Metabolic Syndrome. Biomedicines 2021, 9, 1709. https://doi.org/10.3390/biomedicines9111709
Ghitea TC. Correlation of Periodontal Bacteria with Chronic Inflammation Present in Patients with Metabolic Syndrome. Biomedicines. 2021; 9(11):1709. https://doi.org/10.3390/biomedicines9111709
Chicago/Turabian StyleGhitea, Timea Claudia. 2021. "Correlation of Periodontal Bacteria with Chronic Inflammation Present in Patients with Metabolic Syndrome" Biomedicines 9, no. 11: 1709. https://doi.org/10.3390/biomedicines9111709
APA StyleGhitea, T. C. (2021). Correlation of Periodontal Bacteria with Chronic Inflammation Present in Patients with Metabolic Syndrome. Biomedicines, 9(11), 1709. https://doi.org/10.3390/biomedicines9111709







