Counting on COVID-19 Vaccine: Insights into the Current Strategies, Progress and Future Challenges
Abstract
:1. Introduction
2. Prospects of Vaccination against COVID-19
3. Strategies to Develop Vaccines against COVID-19
3.1. DNA Vaccine
3.2. mRNA Vaccine
3.3. Protein Subunit Vaccine
3.4. Recombinant Viral Vector Vaccine
3.5. Live Attenuated Vaccine
3.6. Whole Killed Vaccine/ Inactivated Virus Vaccine
3.7. Virus-Like Particles
3.8. Oral Mucosal Vaccine
4. Vaccines Approved for Public Use
4.1. Vaccines Approved by WHO for Global Application
4.1.1. Pfizer-BioNTech (mRNA Vaccine)
4.1.2. Astrazeneca/University of Oxford (Viral Vector Vaccine)
4.1.3. Johnson and Johnson (Viral Vector Vaccine)
4.1.4. Moderna (mRNA Vaccine)
4.1.5. Sinopharm (Inactivated Virus Vaccine)
4.1.6. Sinovac Biotech (Inactivated Virus Vaccine)
4.2. Vaccines Approved Regionally
4.2.1. Sputnik V (Viral Vector Vaccine)
4.2.2. EpiVacCorona (Protein Subunit Vaccine)
4.2.3. Bharat Biotech (Inactivated Virus Vaccine)
4.2.4. Cansino Biologics (Viral Vector Vaccine)
4.2.5. Zydus Cadila (Plasmid-DNA Vaccine)
4.3. Success of Approved Vaccines
4.4. Untoward Effects
5. Vaccines under Development: A Brief Overview of Current Forerunners
6. Possibility of Reinfection: What Goes Around, Can Come Back Around?
7. Stories of Some Unsuccessful Vaccine Development
8. Discussions: Lessons Learned towards Future Directions
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACE2 | Angiotensin Converting Enzyme 2 |
| CART | Chimeric Antigen Receptor T cell |
| CD | Cluster of Differentiation |
| CI | Confidence Interval |
| CoV | Corona Virus |
| GVHD | Graft Versus Host Disease |
| HCT | Haematopoietic Cell Transplantaion |
| IFN | Interferon |
| Ig | Immunoglobulin |
| IL | Interleukin |
| LRT | Lower respiratory tract |
| MERS | Middel-East Respiratory Syndrome |
| MHC | Major Histocompatibility Complex |
| NK | Natural Killer |
| PRNT | Plaque Reduction Neutralization Antibody |
| RBD | Receptor Binding Domain |
| SARS | Severe Acute Respiratory Syndrome |
| STL | Serum Mast Cell Tryptase Level |
| Th1 | T helper cell type 1 |
| TLR | Toll Like Receptor |
| TNF-α | Tumor Necrosis Factor α |
References
- Kalra, R.S.; Tomar, D.; Meena, A.S.; Kandimalla, R. SARS-CoV-2, ACE2, and Hydroxychloroquine: Cardiovascular Complications, Therapeutics, and Clinical Readouts in the Current Settings. Pathogens 2020, 9, 546. [Google Scholar] [CrossRef] [PubMed]
- Kandimalla, R.; Dewanjee, S.; Kalra, R.S.; Valupadas, C.; Vallamkondu, J.; Kolli, V.; Reddy, P.A.; Reddy, P.H. COVID-19 and Rheumatoid Arthritis Crosstalk: Emerging association, therapeutic options, and challenges. OSF Preprints 2021. [Google Scholar] [CrossRef]
- Kalra, R.S.; Kandimalla, R. Engaging the spikes: Heparan sulfate facilitates SARS-CoV-2 spike protein binding to ACE2 and potentiates viral infection. Signal Transduct. Target. Ther. 2021, 6, 39. [Google Scholar] [CrossRef] [PubMed]
- Dhanjal, J.K.; Kumar, V.; Garg, S.; Subramani, C.; Agarwal, S.; Wang, J.; Zhang, H.; Kaul, A.; Kalra, R.S.; Kaul, S.C.; et al. Molecular mechanism of anti-SARS-CoV2 activity of Ashwagandha-derived withanolides. Int. J. Biol. Macromol. 2021, 184, 297–312. [Google Scholar] [CrossRef] [PubMed]
- Kalra, R.S.; Kumar, V.; Dhanjal, J.K.; Garg, S.; Li, X.; Kaul, S.C.; Sundar, D.; Wadhwa, R. COVID19-inhibitory activity of withanolides involves targeting of the host cell surface receptor ACE2: Insights from computational and biochemical assays. J. Biomol. Struct. Dyn. 2021, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Asselah, T.; Durantel, D.; Pasmant, E.; Lau, G.; Schinazi, R.F. COVID-19: Discovery, diagnostics and drug development. J. Hepatol. 2020, 74, 168–184. [Google Scholar] [CrossRef] [PubMed]
- Our World Data. Coronavirus (COVID-19) Vaccinations. Available online: https://ourworldindata.org/covid-vaccinations; (accessed on 29 October 2021).
- WHO. Tracking-SARS-CoV-2-Variants. Available online: https://www.who.int/en/activities/tracking-SARS-CoV-2-variants; (accessed on 25 October 2021).
- Matta, S.; Rajpal, S.; Chopra, K.; Arora, V. COVID-19 vaccines and new mutant strains impacting the pandemic. Indian J. Tuberc. 2021, 68, 171–173. [Google Scholar] [CrossRef]
- Xia, S.; Liu, M.; Wang, C.; Xu, W.; Lan, Q.; Feng, S.; Qi, F.; Bao, L.; Du, L.; Liu, S.; et al. Inhibition of SARS-CoV-2 (previously 2019-nCoV) infection by a highly potent pan-coronavirus fusion inhibitor targeting its spike protein that harbors a high capacity to mediate membrane fusion. Cell Res. 2020, 30, 343–355. [Google Scholar] [CrossRef] [Green Version]
- dos Santos, W.G. Impact of virus genetic variability and host immunity for the success of COVID-19 vaccines. Biomed. Pharmacother. 2021, 136, 111272. [Google Scholar] [CrossRef]
- Penna, C.; Mercurio, V.; Tocchetti, C.G.; Pagliaro, P. Sex-related differences in COVID-19 lethality. Br. J. Pharmacol. 2020, 177, 4375–4385. [Google Scholar] [CrossRef]
- To, K.K.-W.; Hung, I.F.-N.; Chan, K.-H.; Yuan, S.; To, W.-K.; Tsang, D.N.-C.; Cheng, V.C.-C.; Chen, Z.; Kok, K.-H.; Yuen, K.-Y. Serum Antibody Profile of a Patient With Coronavirus Disease 2019 Reinfection. Clin. Infect. Dis. 2020, 72, e659–e662. [Google Scholar] [CrossRef]
- Wu, A.; Peng, Y.; Huang, B.; Ding, X.; Wang, X.; Niu, P.; Meng, J.; Zhu, Z.; Zhang, Z.; Wang, J.; et al. Genome Composition and Divergence of the Novel Coronavirus (2019-nCoV) Originating in China. Cell Host Microbe 2020, 27, 325–328. [Google Scholar] [CrossRef] [Green Version]
- Wan, Y.; Shang, J.; Graham, R.; Baric, R.S.; Li, F. Receptor Recognition by the Novel Coronavirus from Wuhan: An Analysis Based on Decade-Long Structural Studies of SARS Coronavirus. J. Virol. 2020, 94, e00127-20. [Google Scholar] [CrossRef] [Green Version]
- Wrapp, D.; Wang, N.; Corbett, K.S.; Goldsmith, J.A.; Hsieh, C.-L.; Abiona, O.; Graham, B.S.; McLellan, J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 2020, 367, 1260–1263. [Google Scholar] [CrossRef] [Green Version]
- Casalino, L.; Gaieb, Z.; Goldsmith, J.A.; Hjorth, C.K.; Dommer, A.C.; Harbison, A.M.; Fogarty, C.A.; Barros, E.P.; Taylor, B.C.; McLellan, J.S.; et al. Beyond Shielding: The Roles of Glycans in the SARS-CoV-2 Spike Protein. ACS Central Sci. 2020, 6, 1722–1734. [Google Scholar] [CrossRef] [PubMed]
- Masihi, K.N. Fighting infection using immunomodulatory agents. Expert Opin. Biol. Ther. 2001, 1, 641–653. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Verma, H.; Singhvi, N.; Sood, U.; Gupta, V.; Singh, M.; Kumari, R.; Hira, P.; Nagar, S.; Talwar, C.; et al. Comparative Genomic Analysis of Rapidly Evolving SARS-CoV-2 Reveals Mosaic Pattern of Phylogeographical Distribution. mSystems 2020, 5, e00505-20. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gayle, A.A.; Wilder-Smith, A.; Rocklöv, J. The reproductive number of COVID-19 is higher compared to SARS coronavirus. J. Travel Med. 2020, 27, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Broder, S. The development of antiretroviral therapy and its impact on the HIV-1/AIDS pandemic. Antivir. Res. 2010, 85, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forni, G.; Mantovani, A. COVID-19 vaccines: Where we stand and challenges ahead. Cell Death Differ. 2021, 28, 626–639. [Google Scholar] [CrossRef]
- Leitner, W.W.; Ying, H.; Restifo, N.P. DNA and RNA-based vaccines: Principles, progress and prospects. Vaccine 1999, 18, 765–777. [Google Scholar] [CrossRef] [Green Version]
- Awasthi, A.; Vishwas, S.; Corrie, L.; Kumar, R.; Khursheed, R.; Kaur, J.; Kumar, R.; Arya, K.; Gulati, M.; Kumar, B.; et al. OUTBREAK of novel corona virus disease (COVID-19): Antecedence and aftermath. Eur. J. Pharmacol. 2020, 884, 173381. [Google Scholar] [CrossRef]
- Yang, Z.-Y.; Kong, W.-P.; Huang, Y.; Roberts, A.; Murphy, B.R.; Subbarao, K.; Nabel, G.J. A DNA vaccine induces SARS coronavirus neutralization and protective immunity in mice. Nature 2004, 428, 561–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hobernik, D.; Bros, M. DNA Vaccines—How Far From Clinical Use? Int. J. Mol. Sci. 2018, 19, 3605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pardi, N.; Hogan, M.; Porter, F.W.; Weissman, D. mRNA vaccines—A new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; Maruggi, G.; Shan, H.; Li, J. Advances in mRNA Vaccines for Infectious Diseases. Front. Immunol. 2019, 10, 594. [Google Scholar] [CrossRef] [Green Version]
- Teijaro, J.R.; Farber, D.L. COVID-19 vaccines: Modes of immune activation and future challenges. Nat. Rev. Immunol. 2021, 21, 195–197. [Google Scholar] [CrossRef]
- Chakraborty, C.; Sharma, A.R.; Bhattacharya, M.; Lee, S.-S. From COVID-19 to Cancer mRNA Vaccines: Moving From Bench to Clinic in the Vaccine Landscape. Front. Immunol. 2021, 12, 679344. [Google Scholar] [CrossRef]
- Li, Y.; Ma, M.-L.; Lei, Q.; Wang, F.; Hong, W.; Lai, D.-Y.; Hou, H.; Xu, Z.-W.; Zhang, B.; Chen, H.; et al. Linear epitope landscape of the SARS-CoV-2 Spike protein constructed from 1,051 COVID-19 patients. Cell Rep. 2021, 34, 108915. [Google Scholar] [CrossRef]
- Bisht, H.; Roberts, A.; Vogel, L.; Bukreyev, A.; Collins, P.L.; Murphy, B.R.; Subbarao, K.; Moss, B. Severe acute respiratory syndrome coronavirus spike protein expressed by attenuated vaccinia virus protectively immunizes mice. Proc. Natl. Acad. Sci. USA 2004, 101, 6641–6646. [Google Scholar] [CrossRef] [Green Version]
- Sahin, U.; Muik, A.; Vogler, I.; Derhovanessian, E.; Kranz, L.M.; Vormehr, M.; Quandt, J.; Bidmon, N.; Ulges, A.; Baum, A.; et al. BNT162b2 vaccine induces neutralizing antibodies and poly-specific T cells in humans. Nature 2021, 595, 572–577. [Google Scholar] [CrossRef]
- Tai, W.; Zhang, X.; He, Y.; Jiang, S.; Du, L. Identification of SARS-CoV RBD-targeting monoclonal antibodies with cross-reactive or neutralizing activity against SARS-CoV-2. Antivir. Res. 2020, 179, 104820. [Google Scholar] [CrossRef]
- Krammer, F. SARS-CoV-2 vaccines in development. Nature 2020, 586, 516–527. [Google Scholar] [CrossRef] [PubMed]
- Pang, H.; Liu, Y.; Han, X.; Xu, Y.; Jiang, F.; Wu, D.; Kong, X.; Bartlam, M.; Rao, Z. Protective humoral responses to severe acute respiratory syndrome-associated coronavirus: Implications for the design of an effective protein-based vaccine. J. Gen. Virol. 2004, 85, 3109–3113. [Google Scholar] [CrossRef] [PubMed]
- Gralinski, L.E.; Menachery, V.D. Return of the Coronavirus: 2019-nCoV. Viruses 2020, 12, 135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graham, B.S. Rapid COVID-19 vaccine development. Science 2020, 368, 945–946. [Google Scholar] [CrossRef] [PubMed]
- Humphreys, I.R.; Sebastian, S. Novel viral vectors in infectious diseases. Immunology 2017, 153, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Pan, C.; Yue, H.; Zhu, L.; Ma, G.-H.; Wang, H.-L. Prophylactic vaccine delivery systems against epidemic infectious diseases. Adv. Drug Deliv. Rev. 2021, 176, 113867. [Google Scholar] [CrossRef]
- Jiang, S.; Hillyer, C.; Du, L. Neutralizing Antibodies against SARS-CoV-2 and Other Human Coronaviruses. Trends Immunol. 2020, 41, 355–359. [Google Scholar] [CrossRef]
- Gao, W.; Tamin, A.; Soloff, A.; D’Aiuto, L.; Nwanegbo, E.; Robbins, P.D.; Bellini, W.J.; Boyes, S.B.; Gambotto, A. Effects of a SARS-associated coronavirus vaccine in monkeys. Lancet 2003, 362, 1895–1896. [Google Scholar] [CrossRef] [Green Version]
- O’Connell, A.K.; Douam, F. Humanized Mice for Live-Attenuated Vaccine Research: From Unmet Potential to New Promises. Vaccines 2020, 8, 36. [Google Scholar] [CrossRef] [Green Version]
- DeDiego, M.L.; Álvarez, E.; Almazán, F.; Rejas, M.T.; Lamirande, E.; Roberts, A.; Shieh, W.-J.; Zaki, S.R.; Subbarao, K.; Enjuanes, L. A Severe Acute Respiratory Syndrome Coronavirus That Lacks the E Gene Is Attenuated In Vitro and In Vivo. J. Virol. 2007, 81, 1701–1713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamirande, E.W.; DeDiego, M.L.; Roberts, A.; Jackson, J.P.; Alvarez, E.; Sheahan, T.; Shieh, W.-J.; Zaki, S.R.; Baric, R.; Enjuanes, L.; et al. A Live Attenuated Severe Acute Respiratory Syndrome Coronavirus Is Immunogenic and Efficacious in Golden Syrian Hamsters. J. Virol. 2008, 82, 7721–7724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandey, S.C.; Pande, V.; Sati, D.; Upreti, S.; Samant, M. Vaccination strategies to combat novel corona virus SARS-CoV-2. Life Sci. 2020, 256, 117956. [Google Scholar] [CrossRef]
- Tsunetsugu-Yokota, Y. Large-Scale Preparation of UV-Inactivated SARS Coronavirus Virions for Vaccine. Antigen. 2008, 454, 119–126. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.-H.; Lu, J.-H.; Wang, Y.-F.; Zheng, H.-Y.; Xiong, S.; Zhang, M.-Y.; Liu, X.-J.; Li, J.-X.; Wan, Z.-Y.; Yan, X.-G.; et al. Immune responses in Balb/c mice induced by a candidate SARS-CoV inactivated vaccine prepared from F69 strain. Vaccine 2005, 23, 3196–3201. [Google Scholar] [CrossRef]
- Kyriakidis, N.C.; López-Cortés, A.; González, E.V.; Grimaldos, A.B.; Prado, E.O. SARS-CoV-2 vaccines strategies: A comprehensive review of phase 3 candidates. NPJ Vaccines 2021, 6, 28. [Google Scholar] [CrossRef] [PubMed]
- Syomin, B.V.; Ilyin, Y.V. Virus-Like Particles as an Instrument of Vaccine Production. Mol. Biol. 2019, 53, 323–334. [Google Scholar] [CrossRef]
- Ashraf, M.; Kim, Y.; Kumar, S.; Seo, D.; Ashraf, M.; Bae, Y.-S. COVID-19 Vaccines (Revisited) and Oral-Mucosal Vector System as a Potential Vaccine Platform. Vaccines 2021, 9, 171. [Google Scholar] [CrossRef]
- U.S. National Institutes of Health. ClinicalTrials.gov Safety and Immunogenicity Trial of an Oral SARS-CoV-2 Vaccine (VXA-CoV2-1) for Prevention of COVID-19 in Healthy Adults. Official Title: A Phase 1 Open-Label, Dose-Ranging Trial to Determine the Safety and Immunogenicity of an Adenoviral-Vector Based Vaccine (VXA-CoV2-1) Expressing a SARS-CoV-2 Antigen and dsRNA Adjuvant Administered Orally to Healthy Adult Volunteers. Identifier: NCT04563702. Available online: https://clinicaltrials.gov/ct2/show/NCT04563702 (accessed on 28 August 2021).
- WHO. The Pfizer BioNTech (BNT162b2) COVID-19 Vaccine: What You Need to Know. 2021. Available online: https://www.who.int/news-room/feature-stories/detail/who-can-take-the-pfizer-biontech-covid-19--vaccine (accessed on 9 September 2021).
- Lustig, Y.; Sapir, E.; Regev-Yochay, G.; Cohen, C.; Fluss, R.; Olmer, L.; Indenbaum, V.; Mandelboim, M.; Doolman, R.; Amit, S.; et al. BNT162b2 COVID-19 vaccine and correlates of humoral immune responses and dynamics: A prospective, single-centre, longitudinal cohort study in health-care workers. Lancet Respir. Med. 2021, 9, 999–1009. [Google Scholar] [CrossRef]
- Salvagno, G.L.; Henry, B.M.; Pighi, L.; De Nitto, S.; Gianfilippi, G.L.; Lippi, G. Three-month analysis of total humoral response to Pfizer BNT162b2 mRNA COVID-19 vaccination in healthcare workers. J. Infect. 2021, 83, e4–e5. [Google Scholar] [CrossRef] [PubMed]
- Pfizer and BioNTech Provide Update on Booster Program in Light of the Delta-Variant New York and Mainz, Germany. Available online: https://cdn.pfizer.com/pfizercom/2021-07/Delta_Variant_Study_Press_Statement_Final_7.8.21.pdf (accessed on 28 August 2021).
- Prendecki, M.; Clarke, C.; Brown, J.; Cox, A.; Gleeson, S.; Guckian, M.; Randell, P.; Pria, A.D.; Lightstone, L.; Xu, X.-N.; et al. Effect of previous SARS-CoV-2 infection on humoral and T-cell responses to single-dose BNT162b2 vaccine. Lancet 2021, 397, 1178–1181. [Google Scholar] [CrossRef]
- Zimmermann, P.; Curtis, N. Factors That Influence the Immune Response to Vaccination. Clin. Microbiol. Rev. 2019, 32, e00084-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Connors, M.; Graham, B.S.; Lane, H.C.; Fauci, A.S. SARS-CoV-2 Vaccines: Much Accomplished, Much to Learn. Ann. Intern. Med. 2021, 174, 687–690. [Google Scholar] [CrossRef]
- Kaur, S.P.; Gupta, V. COVID-19 Vaccine: A comprehensive status report. Virus Res. 2020, 288, 198114. [Google Scholar] [CrossRef]
- Angeli, F.; Spanevello, A.; Reboldi, G.; Visca, D.; Verdecchia, P. SARS-CoV-2 vaccines: Lights and shadows. Eur. J. Intern. Med. 2021, 88, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wise, J. COVID-19: New data on Oxford AstraZeneca vaccine backs 12 week dosing interval. BMJ 2021, 372, n326. [Google Scholar] [CrossRef]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: A pooled analysis of four randomised trials. Lancet 2021, 397, 881–891. [Google Scholar] [CrossRef]
- Mahase, E. COVID-19: South Africa pauses use of Oxford vaccine after study casts doubt on efficacy against variant. BMJ 2021, 372, n372. [Google Scholar] [CrossRef]
- Lumley, S.F.; Rodger, G.; Constantinides, B.; Sanderson, N.; Chau, K.K.; Street, T.L.; O’Donnell, D.; Howarth, A.; Hatch, S.B.; Marsden, B.D.; et al. An Observational Cohort Study on the Incidence of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection and B.1.1.7 Variant Infection in Healthcare Workers by Antibody and Vaccination Status. Clin. Infect. Dis. 2021, ciab608. [Google Scholar] [CrossRef] [PubMed]
- WHO. The Janssen Ad26.COV2.S COVID-19 Vaccine: What You Need to Know. 2021. Available online: https://www.who.int/news-room/feature-stories/detail/the-j-j-covid-19-vaccine-what-you-need-to-know (accessed on 11 September 2021).
- Bos, R.; Rutten, L.; van der Lubbe, J.E.M.; Bakkers, M.J.G.; Hardenberg, G.; Wegmann, F.; Zuijdgeest, D.; de Wilde, A.H.; Koornneef, A.; Verwilligen, A.; et al. Ad26 vector-based COVID-19 vaccine encoding a prefusion-stabilized SARS-CoV-2 Spike immunogen induces potent humoral and cellular immune responses. NPJ Vaccines 2020, 5, 1–11. [Google Scholar] [CrossRef]
- Mukhopadhyay, L.; Yadav, P.D.; Gupta, N.; Mohandas, S.; Patil, D.Y.; Shete-Aich, A.; Panda, S.; Bhargava, B. Comparison of the immunogenicity & protective efficacy of various SARS-CoV-2 vaccine candidates in non-human primates. Indian J. Med. Res. 2021, 153, 93–114. [Google Scholar] [CrossRef] [PubMed]
- Mercado, N.B.; Zahn, R.; Wegmann, F.; Loos, C.; Chandrashekar, A.; Yu, J.; Liu, J.; Peter, L.; McMahan, K.; Tostanoski, L.H.; et al. Single-shot Ad26 vaccine protects against SARS-CoV-2 in rhesus macaques. Nat. Cell Biol. 2020, 586, 1–11. [Google Scholar] [CrossRef]
- van der Lubbe, J.E.M.; Huber, S.K.R.; Vijayan, A.; Dekking, L.; van Huizen, E.; Vreugdenhil, J.; Choi, Y.; Baert, M.R.M.; Boer, K.F.-D.; Gil, A.I.; et al. Ad26.COV2.S protects Syrian hamsters against G614 spike variant SARS-CoV-2 and does not enhance respiratory disease. NPJ Vaccines 2021, 6, 39. [Google Scholar] [CrossRef]
- He, X.; Chandrashekar, A.; Zahn, R.; Wegmann, F.; Yu, J.; Mercado, N.B.; McMahan, K.; Martinot, A.J.; Piedra-Mora, C.; Beecy, S.; et al. Low-dose Ad26.COV2.S protection against SARS-CoV-2 challenge in rhesus macaques. Cell 2021, 184, 3467–3473.e11. [Google Scholar] [CrossRef]
- Oliver, S.E.; Gargano, J.W.; Marin, M.; Wallace, M.; Curran, K.G.; Chamberland, M.; McClung, N.; Campos-Outcalt, D.; Morgan, R.L.; Mbaeyi, S.; et al. The Advisory Committee on Immunization Practices’ Interim Recommendation for Use of Moderna COVID-19 Vaccine—United States, December 2020. MMWR. Morb. Mortal. Wkly. Rep. 2021, 69, 1653–1656. [Google Scholar] [CrossRef] [PubMed]
- Sharma, O.; Sultan, A.A.; Ding, H.; Triggle, C.R. A Review of the Progress and Challenges of Developing a Vaccine for COVID-19. Front. Immunol. 2020, 11, 585354. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Mahase, E. COVID-19: Moderna vaccine is nearly 95% effective, trial involving high risk and elderly people shows. BMJ 2020, 371, m4471. [Google Scholar] [CrossRef]
- Corbett, K.S.; Flynn, B.; Foulds, K.E.; Francica, J.R.; Boyoglu-Barnum, S.; Werner, A.P.; Flach, B.; O’Connell, S.; Bock, K.W.; Minai, M.; et al. Evaluation of the mRNA-1273 Vaccine against SARS-CoV-2 in Nonhuman Primates. N. Engl. J. Med. 2020, 383, 1544–1555. [Google Scholar] [CrossRef]
- Corbett, K.S.; Edwards, D.K.; Leist, S.R.; Abiona, O.M.; Boyoglu-Barnum, S.; Gillespie, R.A.; Himansu, S.; Schäfer, A.; Ziwawo, C.T.; DiPiazza, A.T.; et al. SARS-CoV-2 mRNA vaccine design enabled by prototype pathogen preparedness. Nature 2020, 586, 567–571. [Google Scholar] [CrossRef] [PubMed]
- WHO. The Sinovac-CoronaVac COVID-19 Vaccine: What You Need to Know. 2021. Available online: https://www.who.int/news-room/feature-stories/detail/the-sinovac-covid-19-vaccine-what-you-need-to-know (accessed on 9 September 2021).
- U.S. National Institutes of Health. ClinicalTrials.gov Efficacy, Immunogenicity and Safety of BBIBP-CorV Vaccine against Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection. (ECOVA-01). Official Title: A Phase 3, Ran-domized, Observer-blind, Controlled Trial to Assess the Efficacy, Immunogenicity and Safety of BBIBP-CorV Vaccine Against Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection. Identifier: NCT04984408. Available online: https://clinicaltrials.gov/ct2/show/NCT04984408 (accessed on 30 August 2021).
- BBIBP-CorV, Sinopharm COVID-19 Vaccine. New Drug Approvals. 2021. Available online: https://newdrugapprovals.org/2021/03/23/bbibp-corv-sinopharm-covid-19-vaccine/ (accessed on 1 September 2021).
- Wang, H.; Zhang, Y.; Huang, B.; Deng, W.; Quan, Y.; Wang, W.; Xu, W.; Zhao, Y.; Li, N.; Zhang, J.; et al. Development of an Inactivated Vaccine Candidate, BBIBP-CorV, with Potent Protection against SARS-CoV-2. Cell 2020, 182, 713–721.e9. [Google Scholar] [CrossRef] [PubMed]
- Palacios, R.; Batista, A.P.; Albuquerque, C.S.N.; Patiño, E.G.; Santos, J.D.P. Efficacy and Safety of a COVID-19 Inactivated Vaccine in Healthcare Professionals in Brazil: The PROFISCOV Study. SSRN 2021, 66. [Google Scholar] [CrossRef]
- Zhang, Y.; Zeng, G.; Pan, H.; Li, C.; Hu, Y.; Chu, K.; Han, W.; Chen, Z.; Tang, R.; Yin, W.; et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: A randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect. Dis. 2020, 21, 181–192. [Google Scholar] [CrossRef]
- Logunov, D.Y.; Dolzhikova, I.V.; Shcheblyakov, D.V.; Tukhvatulin, A.I.; Zubkova, O.V.; Dzharullaeva, A.S.; Kovyrshina, A.V.; Lubenets, N.L.; Grousova, D.M.; Erokhova, A.S.; et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: An interim analysis of a randomised controlled phase 3 trial in Russia. Lancet 2021, 397, 671–681. [Google Scholar] [CrossRef]
- Barouch, D.H.; Kik, S.V.; Weverling, G.J.; Dilan, R.; King, S.L.; Maxfield, L.F.; Clark, S.; Ng’Ang’A, D.; Brandariz, K.L.; Abbink, P.; et al. International seroepidemiology of adenovirus serotypes 5, 26, 35, and 48 in pediatric and adult populations. Vaccine 2011, 29, 5203–5209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, I.; Roy, P. Sputnik V COVID-19 vaccine candidate appears safe and effective. Lancet 2021, 397, 642–643. [Google Scholar] [CrossRef]
- Lawton, G. Sputnik V vaccine goes global. New Sci. 2021, 250, 10–11. [Google Scholar] [CrossRef]
- Balakrishnan, V.S. The arrival of Sputnik V. Lancet Infect. Dis. 2020, 20, 1128. [Google Scholar] [CrossRef]
- Burki, T.K. The Russian vaccine for COVID-19. Lancet Respir. Med. 2020, 8, e85–e86. [Google Scholar] [CrossRef]
- Logunov, D.Y.; Dolzhikova, I.V.; Zubkova, O.V.; Tukhvatullin, A.I.; Shcheblyakov, D.V.; Dzharullaeva, A.S.; Grousova, D.M.; Erokhova, A.S.; Kovyrshina, A.V.; Botikov, A.G.; et al. Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: Two open, non-randomised phase 1/2 studies from Russia. Lancet 2020, 396, 887–897. [Google Scholar] [CrossRef]
- Günl, F.; Mecate-Zambrano, A.; Rehländer, S.; Hinse, S.; Ludwig, S.; Brunotte, L. Shooting at a Moving Target—Effectiveness and Emerging Challenges for SARS-CoV-2 Vaccine Development. Vaccines 2021, 9, 1052. [Google Scholar] [CrossRef]
- Ryzhikov, A.B.; Ryzhikov, E.A.; Bogryantseva, M.P.; Danilenko, E.D.; Imatdinov, I.R.; Nechaeva, E.A.; Pyankov, O.V.; Pyankova, O.G.; Susloparov, I.M.; Taranov, O.S.; et al. Immunogenicity and protectivity of the peptide vaccine against SARS-CoV-2. Ann. Russ. Acad. Med Sci. 2021, 76, 5–19. [Google Scholar] [CrossRef]
- U.S. National Institutes of Health. ClinicalTrials.gov Study of the Tolerability, Safety, Immunogenicity and Preventive Efficacy of the EpiVacCorona Vaccine for the Prevention of COVID-19. Official Title: Multicenter Double-blind Place-bo-controlled Comparative Randomized Study of the Tolerability, Safety, Immunogenicity and Prophylactic Efficacy of the EpiVacCorona Peptide Antigen-Based Vaccine for the Prevention of COVID-19, with the Participation of 3000 Volunteers Aged 18 Years and above (Phase III-IV). Identifier: NCT04780035. Available online: https://clinicaltrials.gov/ct2/show/NCT04780035 (accessed on 30 August 2021).
- U.S. National Institutes of Health. ClinicalTrials.gov Study of the Safety, Reactogenicity and Immunogenicity of “EpiVac-Corona” Vaccine for the Prevention of COVID-19 (EpiVacCorona). Official Title: Simple, Blind, Placebo-controlled, Ran-domized Study of the Safety, Reactogenicity and Immunogenicity of Vaccine Based on Peptide Antigens for the Prevention of COVID-19 (EpiVacCorona), in Volunteers Aged 18-60 Years (I–II Phase). Identifier: NCT04527575. Available online: https://clinicaltrials.gov/ct2/show/NCT04527575 (accessed on 10 September 2021).
- Thiagarajan, K. What do we know about India’s Covaxin vaccine? BMJ 2021, 373, n997. [Google Scholar] [CrossRef]
- Ganneru, B.; Jogdand, H.; Dharam, V.K.; Molugu, N.R.; Prasad, S.D.; Vellimudu, S.; Ella, K.M.; Ravikrishnan, R.; Awasthi, A.; Jose, J.; et al. Evaluation of Safety and Immunogenicity of an Adjuvanted, TH-1 Skewed, Whole Virion Inactivat-edSARS-CoV-2 Vaccine—BBV152. bioRxiv 2020. [Google Scholar] [CrossRef]
- Peshimam, G.N.; Farooq, U. CanSinoBIO’s COVID-19 Vaccine 65.7% Effective in Global Trials, Pakistan Official Says; Reuters Healthcare & Pharma, 8 February 2021. Available online: https://www.google.com.hk/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwjs7qb1o4r0AhUEr1YBHe2SDfwQFnoECAUQAQ&url=https%3A%2F%2Fwww.reuters.com%2Fworld%2Fchina%2Fcansinobios-covid-19-vaccine-657-effective-global-trials-pakistan-official-says-2021-02-08%2F&usg=AOvVaw2AqQ6u2XzqSkUjADK166Te (accessed on 10 September 2021).
- Wu, S.; Zhong, G.; Zhang, J.; Shuai, L.; Zhang, Z.; Wen, Z.; Wang, B.; Zhao, Z.; Song, X.; Chen, Y.; et al. A single dose of an adenovirus-vectored vaccine provides protection against SARS-CoV-2 challenge. Nat. Commun. 2020, 11, 4081. [Google Scholar] [CrossRef]
- Kumar, V.M.; Pandi-Perumal, S.R.; Trakht, I.; Thyagarajan, S.P. Strategy for COVID-19 vaccination in India: The country with the second highest population and number of cases. NPJ Vaccines 2021, 6, 60. [Google Scholar] [CrossRef]
- ZyCoV-D Continues India’s Habit of Approving COVID Vaccines with Invisible Data. Available online: https://science.thewire.in/health/zydus-cadila-zycov-d-dna-plasmid-covid-vaccine-missing-data-dcgi-approval/ (accessed on 12 August 2021).
- Dey, A.; Rajanathan, T.C.; Chandra, H.; Pericherla, H.P.; Kumar, S.; Choonia, H.S.; Bajpai, M.; Singh, A.K.; Sinha, A.; Saini, G.; et al. Immunogenic potential of DNA vaccine candidate, ZyCoV-D against SARS-CoV-2 in animal models. Vaccine 2021, 39, 4108–4116. [Google Scholar] [CrossRef] [PubMed]
- Yadav, P.; Kumar, S.; Agarwal, K.; Jain, M.; Patil, D.; Maithal, K.; Mathapati, B.; Giri, S.; Mohandas, S.; Shete, A. Assessment of immunogenicity and protective efficacy of ZyCoV-D DNA vaccine candidates in Rhesus macaques against SARS-CoV-2 in-fection. BioRxiv 2021. [Google Scholar] [CrossRef]
- Momin, T.; Kansagra, K.; Patel, H.; Sharma, S.; Sharma, B.; Patel, J.; Mittal, R.; Sanmukhani, J.; Maithal, K.; Dey, A.; et al. Safety and Immunogenicity of a DNA SARS-CoV-2 vaccine (ZyCoV-D): Results of an open-label, non-randomized phase I part of phase I/II clinical study by intradermal route in healthy subjects in India. EClinicalMedicine 2021, 38, 101020. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Marc, G.P.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Shi, P.-Y.; Xie, X.; Zou, J.; Fontes-Garfias, C.; Xia, H.; Swanson, K.; Cutler, M.; Cooper, D.; Menachery, V.; Weaver, S.; et al. Neutralization of N501Y mutant SARS-CoV-2 by BNT162b2 vaccine-elicited sera. bioRxiv 2021. [Google Scholar] [CrossRef]
- Supasa, P.; Zhou, D.; Dejnirattisai, W.; Liu, C.; Mentzer, A.J.; Ginn, H.M.; Zhao, Y.; Duyvesteyn, H.M.E.; Nutalai, R.; Tuekprakhon, A.; et al. Reduced neutralization of SARS-CoV-2 B.1.1.7 variant by convalescent and vaccine sera. Cell 2021, 184, 2201–2211.e7. [Google Scholar] [CrossRef] [PubMed]
- Amit, S.; Regev-Yochay, G.; Afek, A.; Kreiss, Y.; Leshem, E. Early rate reductions of SARS-CoV-2 infection and COVID-19 in BNT162b2 vaccine recipients. Lancet 2021, 397, 875–877. [Google Scholar] [CrossRef]
- Levine-Tiefenbrun, M.; Yelin, I.; Katz, R.; Herzel, E.; Golan, Z.; Schreiber, L.; Wolf, T.; Nadler, V.; Ben-Tov, A.; Kuint, J.; et al. Initial report of decreased SARS-CoV-2 viral load after inoculation with the BNT162b2 vaccine. Nat. Med. 2021, 27, 790–792. [Google Scholar] [CrossRef]
- Chodick, G.; Tene, L.; Patalon, T.; Gazit, S.; Ben Tov, A.; Cohen, D.; Muhsen, K. Assessment of Effectiveness of 1 Dose of BNT162b2 Vaccine for SARS-CoV-2 Infection 13 to 24 Days After Immunization. JAMA Netw. Open 2021, 4, e2115985. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Liu, Y.; Liu, J.; Zhang, X.; Zou, J.; Fontes-Garfias, C.R.; Xia, H.; Swanson, K.A.; Cutler, M.; Cooper, D.; et al. Neutralization of SARS-CoV-2 spike 69/70 deletion, E484K and N501Y variants by BNT162b2 vaccine-elicited sera. Nat. Med. 2021, 27, 620–621. [Google Scholar] [CrossRef] [PubMed]
- Muik, A.; Wallisch, A.-K.; Sänger, B.; Swanson, K.A.; Mühl, J.; Chen, W.; Cai, H.; Maurus, D.; Sarkar, R.; Türeci, O.; et al. Neutralization of SARS-CoV-2 lineage B.1.1.7 pseudovirus by BNT162b2 vaccine–elicited human sera. Science 2021, 371, 1152–1153. [Google Scholar] [CrossRef] [PubMed]
- Kustin, T.; Harel, N.; Finkel, U.; Perchik, S.; Harari, S.; Tahor, M.; Caspi, I.; Levy, R.; Leshchinsky, M.; Dror, S.K.; et al. Evidence for increased breakthrough rates of SARS-CoV-2 variants of concern in BNT162b2-mRNA-vaccinated individuals. Nat. Med. 2021, 27, 1379–1384. [Google Scholar] [CrossRef]
- Ducloux, D.; Colladant, M.; Chabannes, M.; Yannaraki, M.; Courivaud, C. Humoral response after 3 doses of the BNT162b2 mRNA COVID-19 vaccine in patients on hemodialysis. Kidney Int. 2021, 100, 702–704. [Google Scholar] [CrossRef]
- Morales-Núñez, J.; Muñoz-Valle, J.; Meza-López, C.; Wang, L.-F.; Sulbarán, A.M.; Torres-Hernández, P.; Bedolla-Barajas, M.; De la O-Gómez, B.; Balcázar-Félix, P.; Hernández-Bello, J. Neutralizing Antibodies Titers and Side Effects in Response to BNT162b2 Vaccine in Healthcare Workers with and without Prior SARS-CoV-2 Infection. Vaccines 2021, 9, 742. [Google Scholar] [CrossRef] [PubMed]
- Mariani, M.; Acquila, M.; Tripodi, G.; Spiazzi, R.; Castagnola, E. Antibodies against Receptor Binding Domain of SARS-CoV-2 spike protein induced by BNT162b2 vaccine: Results from a pragmatic, real-life study. J. Infect. Public Health 2021, 14, 1560–1562. [Google Scholar] [CrossRef] [PubMed]
- Rosman, Y.; Lavi, N.; Meir-Shafrir, K.; Lachover-Roth, I.; Cohen-Engler, A.; Mekori, Y.A.; Confino-Cohen, R. Safety of BNT162b2 mRNA COVID-19 vaccine in patients with mast cell disorders. J. Allergy Clin. Immunol. Pract. 2021, 9, 3487–3489. [Google Scholar] [CrossRef] [PubMed]
- Ramos, A.; Cardoso, M.J.; Norton, P.; Sarmento, A.; Guimarães, J.T. Serological response to a single dose of a SARS-CoV-2 mRNA vaccine. J. Virol. Methods 2021, 296, 114223. [Google Scholar] [CrossRef]
- Ben-Tov, A.; Banon, T.; Chodick, G.; Kariv, R.; Assa, A.; Gazit, S. BNT162b2 Messenger RNA COVID-19 Vaccine Effectiveness in Patients With Inflammatory Bowel Disease: Preliminary Real-World Data During Mass Vaccination Campaign. Gastroenterology 2021, 161, 1715–1717.e1. [Google Scholar] [CrossRef]
- Pottegård, A.; Lund, L.C.; Karlstad, Ø.; Dahl, J.; Andersen, M.; Hallas, J.; Lidegaard, Ø.; Tapia, G.; Gulseth, H.L.; Ruiz, P.L.-D.; et al. Arterial events, venous thromboembolism, thrombocytopenia, and bleeding after vaccination with Oxford-AstraZeneca ChAdOx1-S in Denmark and Norway: Population based cohort study. BMJ 2021, 373, n1114. [Google Scholar] [CrossRef] [PubMed]
- Ram, R.; Hagin, D.; Kikozashvilli, N.; Freund, T.; Amit, O.; Bar-On, Y.; Beyar-Katz, O.; Shefer, G.; Moshiashvili, M.M.; Karni, C.; et al. Safety and Immunogenicity of the BNT162b2 mRNA COVID-19 Vaccine in Patients after Allogeneic HCT or CD19-based CART therapy—A Single-Center Prospective Cohort Study. Transplant. Cell. Ther. 2021, 27, 788–794. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.; Xia, H.; Zhang, X.; Zou, J.; Fontes-Garfias, C.R.; Weaver, S.C.; Swanson, K.A.; Cai, H.; Sarkar, R.; et al. BNT162b2-Elicited Neutralization against New SARS-CoV-2 Spike Variants. N. Engl. J. Med. 2021, 385, 472–474. [Google Scholar] [CrossRef]
- Bernal, J.L.; Andrews, N.; Gower, C.; Gallagher, E.; Simmons, R.; Thelwall, S.; Stowe, J.; Tessier, E.; Groves, N.; Dabrera, G.; et al. Effectiveness of COVID-19 Vaccines against the B.1.617.2 (Delta) Variant. N. Engl. J. Med. 2021, 385, 585–594. [Google Scholar] [CrossRef]
- Lospinoso, K.; Nichols, C.S.; Malachowski, S.J.; Mochel, M.C.; Nutan, F. A case of severe cutaneous adverse reaction following administration of the Janssen Ad26.COV2.S COVID-19 vaccine. JAAD Case Rep. 2021, 13, 134–137. [Google Scholar] [CrossRef] [PubMed]
- Rashidi-Alavijeh, J.; Frey, A.; Passenberg, M.; Korth, J.; Zmudzinski, J.; Anastasiou, O.; Saner, F.; Jahn, M.; Lange, C.; Willuweit, K. Humoral Response to SARS-CoV-2 Vaccination in Liver Transplant Recipients–A Single-Center Experience. Vaccines 2021, 9, 738. [Google Scholar] [CrossRef]
- Jahn, M.; Korth, J.; Dorsch, O.; Anastasiou, O.; Sorge-Hädicke, B.; Tyczynski, B.; Gäckler, A.; Witzke, O.; Dittmer, U.; Dolff, S.; et al. Humoral Response to SARS-CoV-2-Vaccination with BNT162b2 (Pfizer-BioNTech) in Patients on Hemodialysis. Vaccines 2021, 9, 360. [Google Scholar] [CrossRef]
- Strengert, M.; Becker, M.; Morilla Ramos, G.; Dulovic, A.; Gruber, J.; Juengling, J.; Luerken, K.; Beigel, A.; Wrenger, E.; Lon-nemann, G.; et al. Cellular and humoral immunogenicity of a SARS-CoV-2 mRNA vaccine in patients on hemodialysis. medRxiv 2021. [Google Scholar] [CrossRef]
- Maneikis, K.; Šablauskas, K.; Ringelevičiūtė, U.; Vaitekėnaitė, V.; Čekauskienė, R.; Kryžauskaitė, L.; Naumovas, D.; Banys, V.; Pečeliūnas, V.; Beinortas, T.; et al. Immunogenicity of the BNT162b2 COVID-19 mRNA vaccine and early clinical outcomes in patients with haematological malignancies in Lithuania: A national prospective cohort study. Lancet Haematol. 2021, 8, e583–e592. [Google Scholar] [CrossRef]
- Terpos, E.; Trougakos, I.P.; Gavriatopoulou, M.; Papassotiriou, I.; Sklirou, A.D.; Ntanasis-Stathopoulos, I.; Papanagnou, E.-D.D.; Fotiou, D.; Kastritis, E.; Dimopoulos, M.A. Low neutralizing antibody responses against SARS-CoV-2 in older patients with myeloma after the first BNT162b2 vaccine dose. Blood 2021, 137, 3674–3676. [Google Scholar] [CrossRef]
- Azzi, L.; Dalla Gasperina, D.; Veronesi, G.; Shallak, M.; Ietto, G.; Iovino, D.; Baj, A.; Gianfagna, F.; Focosi, D.; Maggi, F.; et al. Mucosal Immune Response in BNT162b2 COVID-19 Vaccine Recipients. SSRN Preprint 2021. [Google Scholar] [CrossRef]
- Knoll, M.D.; Wonodi, C. Oxford–AstraZeneca COVID-19 vaccine efficacy. Lancet 2020, 397, 72–74. [Google Scholar] [CrossRef]
- Østergaard, S.D.; Schmidt, M.; Horváth-Puhó, E.; Thomsen, R.W.; Sørensen, H.T. Thromboembolism and the Oxford–AstraZeneca COVID-19 vaccine: Side-effect or coincidence? Lancet 2021, 397, 1441–1443. [Google Scholar] [CrossRef]
- Bernal, J.L.; Andrews, N.; Gower, C.; Robertson, C.; Stowe, J.; Tessier, E.; Simmons, R.; Cottrell, S.; Roberts, R.; O’Doherty, M.; et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on COVID-19 related symptoms, hospital admissions, and mortality in older adults in England: Test negative case-control study. BMJ 2021, 373, n1088. [Google Scholar] [CrossRef]
- Iacobucci, G. COVID-19: Single dose of Pfizer and Oxford vaccines cuts risk of hospital admission by 80% in over 80s, data suggest. BMJ 2021, 372, n612. [Google Scholar] [CrossRef]
- Folegatti, P.; Ewer, K.J.; Aley, P.K.; Angus, B.; Becker, S.; Belij-Rammerstorfer, S.; Bellamy, D.; Bibi, S.; Bittaye, M.; Clutterbuck, E.A.; et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: A preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet 2020, 396, 467–478. [Google Scholar] [CrossRef]
- Ramasamy, M.N.; Minassian, A.M.; Ewer, K.J.; Flaxman, A.L.; Folegatti, P.M.; Owens, D.R.; Voysey, M.; Aley, P.K.; Angus, B.; Babbage, G.; et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): A single-blind, randomised, controlled, phase 2/3 trial. Lancet 2020, 396, 1979–1993. [Google Scholar] [CrossRef]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2020, 397, 99–111. [Google Scholar] [CrossRef]
- Hung, I.F.N.; Poland, G.A. Single-dose Oxford-AstraZeneca COVID-19 vaccine followed by a 12-week booster. Lancet 2021, 397, 854–855. [Google Scholar] [CrossRef]
- Eyre, D.W.; Lumley, S.F.; Wei, J.; Cox, S.; James, T.; Justice, A.; Jesuthasan, G.; O’Donnell, D.; Howarth, A.; Hatch, S.B.; et al. Quantitative SARS-CoV-2 anti-spike responses to Pfizer–BioNTech and Oxford–AstraZeneca vaccines by previous infection status. Clin. Microbiol. Infect. 2021, 27, 1516.e7–1516.e14. [Google Scholar] [CrossRef]
- Griffin, S. COVID-19: AstraZeneca vaccine prevents 79% of symptomatic disease and 100% of severe disease, US study finds. BMJ 2021, 372, n793. [Google Scholar] [CrossRef] [PubMed]
- Sadoff, J.; Le Gars, M.; Shukarev, G.; Heerwegh, D.; Truyers, C.; de Groot, A.M.; Stoop, J.; Tete, S.; Van Damme, W.; Leroux-Roels, I.; et al. Interim Results of a Phase 1–2a Trial of Ad26.COV2.S COVID-19 Vaccine. N. Engl. J. Med. 2021, 384, 1824–1835. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, K.E.; Le Gars, M.; Sadoff, J.; de Groot, A.M.; Heerwegh, D.; Truyers, C.; Atyeo, C.; Loos, C.; Chandrashekar, A.; McMahan, K.; et al. Immunogenicity of the Ad26.COV2.S Vaccine for COVID-19. JAMA 2021, 325, 1535. [Google Scholar] [CrossRef]
- Yu, J.; Tostanoski, L.H.; Mercado, N.B.; McMahan, K.; Liu, J.; Jacob-Dolan, C.; Chandrashekar, A.; Atyeo, C.; Martinez, D.R.; Anioke, T.; et al. Protective efficacy of Ad26.COV2.S against SARS-CoV-2 B.1.351 in macaques. Nature 2021, 596, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Jongeneelen, M.; Kaszas, K.; Veldman, D.; Huizingh, J.; van der Vlugt, R.; Schouten, T.; Zuijdgeest, D.; Uil, T.; van Roey, G.; Guimera, N.; et al. Ad26. COV2. S elicited neutralizing activity against Delta and other SARS-CoV-2 variants of concern. bioRxiv 2021. [Google Scholar] [CrossRef]
- Moore, P.; Moyo, T.; Hermanus, T.; Kgagudi, P.; Ayres, F.; Makhado, Z.; Sadoff, J.; Le Gars, M.; van Roey, G.; Crowther, C.; et al. Neutralizing antibodies elicited by the Ad26.COV2.S COVID-19 vaccine show reduced activity against 501Y.V2 (B.1.351), despite protection against severe disease by this variant. bioRxiv 2021. [Google Scholar] [CrossRef]
- Anderson, E.J.; Rouphael, N.G.; Widge, A.T.; Jackson, L.A.; Roberts, P.C.; Makhene, M.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; Pruijssers, A.J.; et al. Safety and Immunogenicity of SARS-CoV-2 mRNA-1273 Vaccine in Older Adults. N. Engl. J. Med. 2020, 383, 2427–2438. [Google Scholar] [CrossRef]
- Jackson, L.A.; Anderson, E.J.; Rouphael, N.G.; Roberts, P.C.; Makhene, M.; Coler, R.N.; McCullough, M.P.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; et al. An mRNA Vaccine against SARS-CoV-2—Preliminary Report. N. Engl. J. Med. 2020, 383, 1920–1931. [Google Scholar] [CrossRef] [PubMed]
- USFDA. Vaccines and Related Biological Products Advisory Committee Meeting. 2021. Available online: https://www.fda.gov/advisory-committees/advisory-committee-calendar/vaccines-and-related-biological-products-advisory-committee-february-26-2021-meeting-announcement (accessed on 15 August 2021).
- Tré-Hardy, M.; Cupaiolo, R.; Wilmet, A.; Beukinga, I.; Blairon, L. Waning antibodies in SARS-CoV-2 naïve vaccinees: Results of a three-month interim analysis of ongoing immunogenicity and efficacy surveillance of the mRNA-1273 vaccine in healthcare workers. J. Infect. 2021, 83, 381–412. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, S.S.; Ramsey, A.; Staicu, M.L. Administration of a Second Dose of the Moderna COVID-19 Vaccine after an Immediate Hypersensitivity Reaction with the First Dose: Two Case Reports. Ann. Intern. Med. 2021, 174, 1177–1178. [Google Scholar] [CrossRef]
- Banerji, A.; Wickner, P.G.; Saff, R.; Stone, C.A.; Robinson, L.B.; Long, A.A.; Wolfson, A.R.; Williams, P.; Khan, D.A.; Phillips, E.; et al. mRNA Vaccines to Prevent COVID-19 Disease and Reported Allergic Reactions: Current Evidence and Suggested Approach. J. Allergy Clin. Immunol. Pract. 2020, 9, 1423–1437. [Google Scholar] [CrossRef]
- Widge, A.T.; Rouphael, N.G.; Jackson, L.A.; Anderson, E.J.; Roberts, P.C.; Makhene, M.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; Pruijssers, A.J.; et al. Durability of Responses after SARS-CoV-2 mRNA-1273 Vaccination. N. Engl. J. Med. 2021, 384, 80–82. [Google Scholar] [CrossRef]
- Krammer, F.; Srivastava, K.; Alshammary, H.; Amoako, A.A.; Awawda, M.H.; Beach, K.F.; Bermúdez-González, M.C.; Bielak, D.A.; Carreño, J.M.; Chernet, R.L.; et al. Antibody Responses in Seropositive Persons after a Single Dose of SARS-CoV-2 mRNA Vaccine. N. Engl. J. Med. 2021, 384, 1372–1374. [Google Scholar] [CrossRef]
- Xia, S.; Duan, K.; Zhang, Y.; Zhao, D.; Zhang, H.; Xie, Z.; Li, X.; Peng, C.; Zhang, Y.-B.; Zhang, W.; et al. Effect of an Inactivated Vaccine Against SARS-CoV-2 on Safety and Immunogenicity Outcomes. JAMA 2020, 324, 951. [Google Scholar] [CrossRef]
- Chen, R.E.; Zhang, X.; Case, J.B.; Winkler, E.S.; Liu, Y.; VanBlargan, L.A.; Liu, J.; Errico, J.M.; Xie, X.; Suryadevara, N.; et al. Resistance of SARS-CoV-2 variants to neutralization by monoclonal and serum-derived polyclonal antibodies. Nat. Med. 2021, 27, 717–726. [Google Scholar] [CrossRef]
- Kerr, S.; Shepherd, C. COVID vaccine from China’s Sinopharm is 86% effective, says UAE. Financial Times, 9 December 2020. [Google Scholar]
- Baraniuk, C. What do we know about China’s COVID-19 vaccines? BMJ 2021, 373, n912. [Google Scholar] [CrossRef] [PubMed]
- Loo, K.-Y.; Letchumanan, V.; Ser, H.-L.; Teoh, S.; Law, J.; Tan, L.; Ab Mutalib, N.-S.; Chan, K.-G.; Lee, L.-H. COVID-19: Insights into Potential Vaccines. Microorganisms 2021, 9, 605. [Google Scholar] [CrossRef] [PubMed]
- Hatmal, M.; Al-Hatamleh, M.; Olaimat, A.; Hatmal, M.; Alhaj-Qasem, D.; Olaimat, T.; Mohamud, R. Side Effects and Perceptions Following COVID-19 Vaccination in Jordan: A Randomized, Cross-Sectional Study Implementing Machine Learning for Predicting Severity of Side Effects. Vaccines 2021, 9, 556. [Google Scholar] [CrossRef]
- Huang, B.; Dai, L.; Wang, H.; Hu, Z.; Yang, X.; Tan, W.; Gao, G.F. Neutralization of SARS-CoV-2 VOC 501Y.V2 by human antisera elicited by both inactivated BBIBP-CorV and recombinant dimeric RBD ZF2001 vaccines. bioRxiv 2021. [Google Scholar] [CrossRef]
- Jeewandara, C.; Aberathna, I.S.; Pushpakumara, P.D.; Kamaladasa, A.; Guruge, D.; Jayathilaka, D.; Gunesekara, B.; Tanussiya, S.; Kuruppu, H.; Ranasinghe, T.; et al. Antibody and T cell responses to Sinopharm/BBIBP-CorV in naïve and previously infected individuals in Sri Lanka. medRxiv 2021. [Google Scholar] [CrossRef]
- Wu, Z.; Hu, Y.; Xu, M.; Chen, Z.; Yang, W.; Jiang, Z.; Li, M.; Jin, H.; Cui, G.; Chen, P.; et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine (CoronaVac) in healthy adults aged 60 years and older: A randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect. Dis. 2021, 21, 803–812. [Google Scholar] [CrossRef]
- Chen, Y.; Shen, H.; Huang, R.; Tong, X.; Wu, C. Serum neutralising activity against SARS-CoV-2 variants elicited by CoronaVac. Lancet Infect. Dis. 2021, 21, 1071–1072. [Google Scholar] [CrossRef]
- Hitchings, M.D.; Ranzani, O.T.; Torres, M.S.S.; de Oliveira, S.B.; Almiron, M.; Said, R.; Borg, R.; Schulz, W.L.; de Oliveira, R.D.; da Silva, P.V.; et al. Effectiveness of CoronaVac among healthcare workers in the setting of high SARS-CoV-2 Gamma variant transmission in Manaus, Brazil: A test-negative case-control study. Lancet Reg. Health-Am. 2021, 1, 100025. [Google Scholar] [CrossRef]
- Ranzani, O.T.; Hitchings, M.D.T.; Dorion, M.; D’Agostini, T.L.; de Paula, R.C.; de Paula, O.F.P.; Villela, E.F.D.M.; Torres, M.S.S.; de Oliveira, S.B.; Schulz, W.; et al. Effectiveness of the CoronaVac vaccine in older adults during a gamma variant associated epidemic of COVID-19 in Brazil: Test negative case-control study. BMJ 2021, 374, n2015. [Google Scholar] [CrossRef]
- Han, B.; Song, Y.; Li, C.; Yang, W.; Ma, Q.; Jiang, Z.; Li, M.; Lian, X.; Jiao, W.; Wang, L.; et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine (CoronaVac) in healthy children and adolescents: A double-blind, randomised, controlled, phase 1/2 clinical trial. Lancet. Infect. Dis. 2021. [Google Scholar] [CrossRef]
- Faria, E.; Guedes, A.R.; Oliveira, M.S.; Moreira, M.V.G.; Maia, F.L.; Barboza, A.S.; Leme, M.D.; Letaif, L.S.H.; Miethke-Morais, A.; Bonfa, E.; et al. Performance of vaccination with CoronaVac in a cohort of healthcare workers (HCW)-preliminary report. medRxiv 2021. [Google Scholar] [CrossRef]
- Bayram, A.; Demirbakan, H.; Karadeniz, P.G.; Erdoğan, M.; Koçer, I. Quantitation of antibodies against SARS-CoV-2 spike protein after two doses of CoronaVac in healthcare workers. J. Med Virol. 2021, 93, 5560–5567. [Google Scholar] [CrossRef]
- Estofolete, C.; Banho, C.; Campos, G.; Marques, B.; Sacchetto, L.; Ullmann, L.; Possebon, F.; Machado, L.; Syrio, J.; Junior, J.A.; et al. Case Study of Two Post Vaccination SARS-CoV-2 Infections with P1 Variants in CoronaVac Vaccinees in Brazil. Viruses 2021, 13, 1237. [Google Scholar] [CrossRef] [PubMed]
- Kemal, R.A.; Sari, D.K.; Paulin, A.J. Antibody Response to CoronaVac Vaccine in Indonesian COVID-19 Survivor. medRxiv 2021. [Google Scholar] [CrossRef]
- Calil, V.M.L.T.; Palmeira, P.; Zheng, Y.; Krebs, V.L.J.; de Carvalho, W.B.; Carneiro-Sampaio, M. CoronaVac can induce the production of anti-SARS-CoV-2 IgA antibodies in human milk. Clinics 2021, 76, e3185. [Google Scholar] [CrossRef] [PubMed]
- Akpolat, T.; Uzun, O. Reduced mortality rate after coronavac vaccine among healthcare workers. J. Infect. 2021, 83, e20–e21. [Google Scholar] [CrossRef]
- Tanriover, M.D.; Doğanay, H.L.; Akova, M.; Güner, H.R.; Azap, A.; Akhan, S.; Köse, S.; Erdinç, F.; Akalın, E.H.; Tabak, F.; et al. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): Interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet 2021, 398, 213–222. [Google Scholar] [CrossRef]
- Pagotto, V.; Ferloni, A.; Soriano, M.M.; Díaz, M.; Golde, N.B.; González, M.I.; Asprea, V.; Staneloni, M.I.; Zingoni, P.; Vidal, G.; et al. Active monitoring of early safety of Sputnik V vaccine in Buenos Aires, Argentina. Medicina 2021, 81, 408–414. [Google Scholar]
- Ikegame, S.; Siddiquey, M.N.A.; Hung, C.-T.; Haas, G.; Brambilla, L.; Oguntuyo, K.Y.; Kowdle, S.; Chiu, H.-P.; Stevens, C.S.; Vilardo, A.E.; et al. Neutralizing activity of Sputnik V vaccine sera against SARS-CoV-2 variants. Nat. Commun. 2021, 12, 1–11. [Google Scholar] [CrossRef]
- Ikegame, S.; Siddiquey, M.N.; Hung, C.T.; Haas, G.; Brambilla, L.; Oguntuyo, K.Y.; Kowdle, S.; Vilardo, A.E.; Edelstein, A.; Perandones, C.; et al. Qualitatively distinct modes of Sputnik V vaccine-neutralization escape by SARS-CoV-2 Spike variants. medRxiv 2021. [Google Scholar] [CrossRef]
- Gushchin, V.; Dolzhikova, I.; Shchetinin, A.; Odintsova, A.; Siniavin, A.; Nikiforova, M.; Pochtovyi, A.; Shidlovskaya, E.; Kuznetsova, N.; Burgasova, O.; et al. Neutralizing Activity of Sera from Sputnik V-Vaccinated People against Variants of Concern (VOC: B.1.1.7, B.1.351, P.1, B.1.617.2, B.1.617.3) and Moscow Endemic SARS-CoV-2 Variants. Vaccines 2021, 9, 779. [Google Scholar] [CrossRef]
- COVAXIN®–India’s First Indigenous COVID-19 Vaccine. Available online: https://www.bharatbiotech.com/covaxin.html (accessed on 12 September 2021).
- Sapkal, G.N.; Yadav, P.D.; Ella, R.; Deshpande, G.R.; Sahay, R.R.; Gupta, N.; Vadrevu, K.M.; Abraham, P.; Panda, S.; Bhargava, B. Inactivated COVID-19 vaccine BBV152/COVAXIN effectively neutralizes recently emerged B.1.1.7 variant of SARS-CoV-2. J. Travel Med. 2021, 28, taab051. [Google Scholar] [CrossRef]
- Ella, R.; Reddy, S.; Jogdand, H.; Sarangi, V.; Ganneru, B.; Prasad, S.; Das, D.; Raju, D.; Praturi, U.; Sapkal, G.; et al. Safety and immunogenicity clinical trial of an inactivated SARS-CoV-2 vaccine, BBV152 (a phase 2, double-blind, randomised controlled trial) and the persistence of immune responses from a phase 1 follow-up report. medRxiv 2020. [Google Scholar] [CrossRef]
- Ella, R.; Vadrevu, K.M.; Jogdand, H.; Prasad, S.; Reddy, S.; Sarangi, V.; Ganneru, B.; Sapkal, G.; Yadav, P.; Abraham, P.; et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152: A double-blind, randomised, phase 1 trial. Lancet Infect. Dis. 2021, 21, 637–646. [Google Scholar] [CrossRef]
- Srivastava, R.; Ish, P. Safdarjung COVID-19 Vaccination group the initial experience of COVID-19 vaccination from a tertiary care centre of India. Monaldi Arch. Chest Dis. 2021. [Google Scholar] [CrossRef]
- Zhu, F.-C.; Li, Y.-H.; Guan, X.-H.; Hou, L.-H.; Wang, W.-J.; Li, J.-X.; Wu, S.-P.; Wang, B.-S.; Wang, Z.; Wang, L.; et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: A dose-escalation, open-label, non-randomised, first-in-human trial. Lancet 2020, 395, 1845–1854. [Google Scholar] [CrossRef]
- O’Brien, E. COVID Antibodies Endure Over Six Months in China Trial Subjects. 2020. Available online: https://www.bloomberg.com/news/articles/2020-09-25/covid-antibodies-endure-over-six-months-in-china-trial-subjects (accessed on 10 September 2021).
- Zhu, F.-C.; Guan, X.-H.; Li, Y.-H.; Huang, J.-Y.; Jiang, T.; Hou, L.-H.; Li, J.-X.; Yang, B.-F.; Wang, L.; Wang, W.-J.; et al. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 2020, 396, 479–488. [Google Scholar] [CrossRef]
- Clinical Trials Registry-India. Novel Corona Virus-2019-nCov Vaccine by Intradermal Route in Healthy Subjects. Official Title: A Prospective, Randomized, Adaptive, Phase I/II Clinical Study to Evaluate the Safety and Immunogenicity of Novel Corona Virus-2019-nCov Vaccine Candidate of M/s Cadila Healthcare Limited by Intradermal Route in Healthy Subjects. CTRI Number: CTRI/2020/07/026352. Available online: http://ctri.nic.in/Clinicaltrials/showallp.php?mid1=45306&EncHid=&userName=Zydus (accessed on 10 September 2021).
- Clinical Trials Registry-India. Novel Corona Virus-2019-nCov Vaccine by Intradermal Route in Healthy Subjects. Official Title: A Phase III, Randomized, Multi-Centre, Double Blind, Placebo Controlled, Study to Evaluate Efficacy, Safety and Immunogenicity of Novel Corona Virus-2019-nCov vaccine candidate of M/s Cadila Healthcare Limited. CTRI Number: CTRI/2021/01/030416. Available online: http://ctri.nic.in/Clinicaltrials/showallp.php?mid1=51254&EncHid=&userName=ZyCoV-D (accessed on 10 September 2021).
- de Vrieze, J. Pfizer’s vaccine raises allergy concerns. Science 2021, 371, 10–11. [Google Scholar] [CrossRef]
- Rodríguez, N.O.; Berasategui, M.A.; Caballer, B.D.L.H.; Santiago, A.V. The Century of mRNA Vaccines: COVID-19 Vaccines and Allergy. J. Investig. Allergol. Clin. Immunol. 2021, 31, 89–91. [Google Scholar] [CrossRef]
- Troelnikov, A.; Perkins, G.; Yuson, C.; Ahamdie, A.; Balouch, S.; Hurtado, P.R.; Hissaria, P. Basophil reactivity to BNT162b2 is mediated by PEGylated lipid nanoparticles in patients with PEG allergy. J. Allergy Clin. Immunol. 2021, 148, 91–95. [Google Scholar] [CrossRef]
- Shemer, A.; Pras, E.; Einan-Lifshitz, A.; Dubinsky-Pertzov, B.; Hecht, I. Association of COVID-19 Vaccination and Facial Nerve Palsy. JAMA Otolaryngol. Neck Surg. 2021, 147, 739. [Google Scholar] [CrossRef]
- Nevet, A. Acute myocarditis associated with anti-COVID-19 vaccination. Clin. Exp. Vaccine Res. 2021, 10, 196–197. [Google Scholar] [CrossRef]
- European Medicines Agency. COVID-19 Vaccines: Update on Ongoing Evaluation of Myocarditis and Pericarditis. Available online: https://www.ema.europa.eu/en/news/covid-19-vaccines-update-ongoing-evaluation-myocarditis-pericarditis (accessed on 11 August 2021).
- Perera, R.; Fletcher, J. Thromboembolism and the Oxford-AstraZeneca vaccine. BMJ 2021, 373, n1159. [Google Scholar] [CrossRef]
- Tobaiqy, M.; Elkout, H.; MacLure, K. Analysis of Thrombotic Adverse Reactions of COVID-19 AstraZeneca Vaccine Reported to EudraVigilance Database. Vaccines 2021, 9, 393. [Google Scholar] [CrossRef] [PubMed]
- Nawwar, A.A.; Searle, J.; Singh, R.; Lyburn, I.D. Oxford-AstraZeneca COVID-19 vaccination induced lymphadenopathy on [18F] Choline PET/CT-not only an FDG finding. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2657–2658. [Google Scholar] [CrossRef] [PubMed]
- Garnier, M.; Curado, A.; Billoir, P.; Barbay, V.; Demeyere, M.; Dacher, J.-N. Imaging of Oxford/AstraZeneca® COVID-19 vaccine-induced immune thrombotic thrombocytopenia. Diagn. Interv. Imaging 2021, 102, 649–650. [Google Scholar] [CrossRef]
- WHO. Guidance for Clinical Case Management of Thrombosis with Thrombocytopenia Syndrome (TTS) Following Vaccination to Prevent Coronavirus Disease (COVID-19). Available online: https://apps.who.int/iris/bitstream/handle/10665/342999/WHO-2019-nCoV-TTS-2021.1-eng.pdf (accessed on 28 August 2021).
- European Medicines Agency. EMA Confirmed Capillary Leak Syndrome as a Potential Side Effect of ChAdOx1nCoV-19. Available online: https://www.ema.europa.eu/en/medicines/dhpc/vaxzevria-previously-covid-19-vaccine-astrazeneca-contraindication-individuals-previous-capillary (accessed on 22 August 2021).
- European Medicines Agency. COVID-19 Vaccine Janssen: Guillain-Barré Syndrome Listed as a Very Rare SIDE Effect. Available online: https://www.ema.europa.eu/en/news/covid-19-vaccine-janssen-guillain-barre-syndrome-listed-very-rare-side-effect (accessed on 22 August 2021).
- Yocum, A.; Simon, E.L. Thrombotic Thrombocytopenic Purpura after Ad26.COV2-S Vaccination. Am. J. Emerg. Med. 2021, 49, 441.e3–441.e4. [Google Scholar] [CrossRef]
- Nishizawa, Y.; Hoshina, Y.; Baker, V. Bell’s palsy following the Ad26.COV2.S COVID-19 vaccination. Qjm: Int. J. Med. 2021, hcab143. [Google Scholar] [CrossRef]
- Mahase, E. COVID-19: Unusual blood clots are “very rare side effect” of Janssen vaccine, says EMA. BMJ 2021, 373, n1046. [Google Scholar] [CrossRef]
- Takuva, S.; Takalani, A.; Garrett, N.; Goga, A.; Peter, J.; Louw, V.; Opie, J.; Jacobson, B.; Sanne, I.; Gail-Bekker, L.; et al. Thromboembolic Events in the South African Ad26.COV2.S Vaccine Study. N. Engl. J. Med. 2021, 385, 570–571. [Google Scholar] [CrossRef]
- MacNeil, J.R.; Su, J.R.; Broder, K.R.; Guh, A.Y.; Gargano, J.W.; Wallace, M.; Hadler, S.C.; Scobie, H.M.; Blain, A.E.; Moulia, D.; et al. Updated Recommendations from the Advisory Committee on Immunization Practices for Use of the Janssen (Johnson & Johnson) COVID-19 Vaccine After Reports of Thrombosis with Thrombocytopenia Syndrome Among Vaccine Recipients—United States, April 2021. MMWR. Morb. Mortal. Wkly. Rep. 2021, 70, 651–656. [Google Scholar] [CrossRef] [PubMed]
- See, I.; Su, J.R.; Lale, A.; Woo, E.J.; Guh, A.Y.; Shimabukuro, T.T.; Streiff, M.B.; Rao, A.K.; Wheeler, A.P.; Beavers, S.F.; et al. US Case Reports of Cerebral Venous Sinus Thrombosis With Thrombocytopenia After Ad26.COV2.S Vaccination, March 2 to April 21, 2021. JAMA 2021, 325, 2448–2456. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Vaxzevria: EMA Advises Against Use in People with History of Capillary Leak Syndrome. Available online: https://www.ema.europa.eu/en/news/vaxzevria-ema-advises-against-use-people-history-capillary-leak-syndrome (accessed on 15 August 2021).
- Allergic Reactions Including Anaphylaxis After Receipt of the First Dose of Moderna COVID-19 Vaccine—United States, December 21, 2020–January 10, 2021. MMWR. Morb. Mortal. Wkly. Rep. 2021, 70, 125–129. [CrossRef]
- McNeil, M.M.; Weintraub, E.S.; Duffy, J.; Sukumaran, L.; Jacobsen, S.; Klein, N.P.; Hambidge, S.J.; Lee, G.M.; Jackson, L.A.; Irving, S.; et al. Risk of anaphylaxis after vaccination in children and adults. J. Allergy Clin. Immunol. 2015, 137, 868–878. [Google Scholar] [CrossRef] [Green Version]
- Wei, N.; Fishman, M.; Wattenberg, D.; Gordon, M.; Lebwohl, M. “COVID arm”: A reaction to the Moderna vaccine. JAAD Case Rep. 2021, 10, 92–95. [Google Scholar] [CrossRef]
- Subramony, R.; Lin, L.C.; Knight, D.K.; Aminlari, A.; Belovarski, I. Bilateral Retinal Detachments in a Healthy 22-year-old Woman After Moderna SARS-CoV-2 Vaccination. J. Emerg. Med. 2021. [Google Scholar] [CrossRef]
- European Medicines Agency. Signal Assessment Report on Myocarditis and Pericarditis with Spikevax (Previously COVID-19 Vaccine Moderna). Available online: https://www.ema.europa.eu/en/documents/prac-recommendation/signal-assessment-report-myocarditis-pericarditis-spikevax-previously-covid-19-vaccine-moderna_en.pdf (accessed on 15 August 2021).
- Xia, S.; Zhang, Y.; Wang, Y.; Wang, H.; Yang, Y.; Gao, G.F.; Tan, W.; Wu, G.; Xu, M.; Lou, Z.; et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: A randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect. Dis. 2021, 21, 39–51. [Google Scholar] [CrossRef]
- Abu-Hammad, O.; Alduraidi, H.; Abu-Hammad, S.; Alnazzawi, A.; Babkair, H.; Abu-Hammad, A.; Nourwali, I.; Qasem, F.; Dar-Odeh, N. Side Effects Reported by Jordanian Healthcare Workers Who Received COVID-19 Vaccines. Vaccines 2021, 9, 577. [Google Scholar] [CrossRef]
- Riad, A.; Sağıroğlu, D.; Üstün, B.; Pokorná, A.; Klugarová, J.; Attia, S.; Klugar, M. Prevalence and Risk Factors of CoronaVac Side Effects: An Independent Cross-Sectional Study among Healthcare Workers in Turkey. J. Clin. Med. 2021, 10, 2629. [Google Scholar] [CrossRef]
- Orenay, O.; Balta, I.; Yigit, D.; Eksioglu, M. Systemic drug-related intertriginous and flexural exanthema like eruption after CoronaVac vaccine. J. Eur. Acad. Dermatol. Venereol. 2021, 35, e634–e635. [Google Scholar] [CrossRef] [PubMed]
- Akdaş, E.; Ilter, N.; Öğüt, B.; Erdem, Ö. Pityriasis rosea following CoronaVac COVID-19 vaccination: A case report. J. Eur. Acad. Dermatol. Venereol. 2021, 35, e491–e493. [Google Scholar] [CrossRef]
- Thai Clinical Trials Registry. Neurological Complication after Injection of Inactivated COVID-19 Vaccine in Srinagarind Hospital, Thailand: The Case Series. Trial ID: Ictrp-TCTR20210610004. Available online: https://pesquisa.bvsalud.org/global-literature-on-novel-coronavirus-2019-ncov/resource/en/ictrp-TCTR20210610004 (accessed on 5 September 2021).
- Cebeci, F.; Kartal, I. Petechial skin rash associated with CoronaVac vaccination: First cutaneous side effect report before phase 3 results. Eur. J. Hosp. Pharm. 2021. [Google Scholar] [CrossRef]
- Onsun, N.; Kaya, G.; Işık, B.G.; Güneş, B. A generalized pustular psoriasis flare after CoronaVac COVID-19 vaccination: Case report. Heal. Promot. Perspect. 2021, 11, 261–262. [Google Scholar] [CrossRef] [PubMed]
- Özdemir, I.H.; Özlek, B.; Özen, M.B.; Gündüz, R.; Bayturan, Ö. Type 1 Kounis Syndrome Induced by Inactivated SARS-CoV-2 Vaccine. J. Emerg. Med. 2021. [Google Scholar] [CrossRef]
- An, Q.-J.; Qin, D.-A.; Pei, J.-X. Reactive arthritis after COVID-19 vaccination. Hum. Vaccines Immunother. 2021, 17, 2954–2956. [Google Scholar] [CrossRef]
- Moutinho, S.; Wadman, M. Brazil and Russia face off over vaccine contamination charge. Science 2021, 372, 551–554. [Google Scholar] [CrossRef]
- Jarynowski, A.; Semenov, A.; Kamiński, M.; Belik, V. Mild Adverse Events of Sputnik V Vaccine Extracted from Russian Language Telegram Posts via BERT Deep Learning Model. medRxiv 2021. [Google Scholar] [CrossRef]
- Montalti, M.; Soldà, G.; Di Valerio, Z.; Salussolia, A.; Lenzi, J.; Forcellini, M.; Barvas, E.; Guttman, S.; Messina, R.; Poluzzi, E.; et al. ROCCA study protocol and interim analysis on safety of Sputnik V vaccine (Gam-COVID-Vac) in the Republic of San Marino: An observational study using active surveillance. medRxiv 2021. [Google Scholar] [CrossRef]
- Mulligan, M.J.; Lyke, K.E.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Raabe, V.; Bailey, R.; Swanson, K.A.; et al. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nat. Cell Biol. 2020, 586, 589–593. [Google Scholar] [CrossRef]
- Walsh, E.E.; Frenck, R.W.; Falsey, A.R.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Mulligan, M.J.; Bailey, R.; et al. Safety and Immunogenicity of Two RNA-Based COVID-19 Vaccine Candidates. N. Engl. J. Med. 2020, 383, 2439–2450. [Google Scholar] [CrossRef]
- U.S. National Institutes of Health. ClinicalTrials.gov A Global Phase III Clinical Trial of Recombinant COVID- 19 Vaccine (Sf9 Cells). Official Title: A Global Multicenter, Randomized, Double-blind, Placebo-controlled, Phase III Clinical Trial to Evaluate the Efficacy, Safety, and Immunogenicity of Recombinant COVID-19 Vaccine (Sf9 Cells), for the Prevention of COVID-19 in Adults Aged 18 Years and Older. Identifier: NCT04904471. Available online: https://clinicaltrials.gov/ct2/show/NCT04904471 (accessed on 28 August 2021).
- U.S. National Institutes of Health. ClinicalTrials.gov A Phase III Clinical Study of a SARS-CoV-2 Messenger Ribonucleic Acid (mRNA) Vaccine Candidate Against COVID-19 in Population Aged 18 Years and above. Official Title: A Global, Multi-Center, Randomized, Double-Blind, Placebo-Controlled, Phase III Clinical Trial to Evaluate the Protective Efficacy, Safety, and Immunogenicity of a SARS-CoV-2 Messenger Ribonucleic Acid (mRNA) Vaccine Candidate in Population Aged 18 Years and Above. Identifier: NCT04847102. Available online: https://clinicaltrials.gov/ct2/show/NCT04847102 (accessed on 28 August 2021).
- Shinde, V.; Bhikha, S.; Hoosain, Z.; Archary, M.; Bhorat, Q.; Fairlie, L.; Lalloo, U.; Masilela, M.S.; Moodley, D.; Hanley, S.; et al. Efficacy of NVX-CoV2373 COVID-19 Vaccine against the B.1.351 Variant. N. Engl. J. Med. 2021, 384, 1899–1909. [Google Scholar] [CrossRef]
- U.S. National Institutes of Health. ClinicalTrials.gov Study of a Recombinant Coronavirus-like Particle COVID-19 Vaccine in Adults. Official Title: Randomized, Observer-Blind, Placebo-Controlled, Phase 2/3 Study to Assess the Safety, Efficacy, and Immunogenicity of a Recombinant Coronavirus-Like Particle COVID-19 Vaccine in Adults 18 Years of Age or Older. Identifier: NCT04636697. Available online: https://clinicaltrials.gov/ct2/show/NCT04636697 (accessed on 28 August 2021).
- Medicago and GSK Announce Positive Interim Phase 2 Results for Adjuvanted COVID-19 Vaccine Candidate. Available online: https://www.medicago.com/en/media-room/medicago-and-gsk-announce-positive-interim-phase-2-results-for-adjuvanted-covid-19-vaccine-candidate/ (accessed on 28 August 2021).
- USFDA. COVID-19 Vaccines: The FDA Has Regulatory Processes in Place to Facilitate the Development of COVID-19 Vaccines That Meet the FDA’s Rigorous Scientific Standards. Available online: https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/covid-19-vaccines (accessed on 28 August 2021).
- Valneva. Valneva Reports Positive Phase 3 Results for Inactivated, Adjuvanted COVID-19 Vaccine Candidate VLA2001. Available online: https://valneva.com/press-release/valneva-reports-positive-phase-3-results-for-inactivated-adjuvanted-covid-19-vaccine-candidate-vla2001/ (accessed on 28 August 2021).
- U.S. National Institutes of Health. ClinicalTrials.gov Study to Compare the Immunogenicity against COVID-19, of VLA2001 Vaccine To AZD1222 Vaccine (COV-COMPARE). Official Title: A Randomized, Observer-Blind, Controlled, Superiority Study to Compare the Immunogenicity Against COVID-19, of VLA2001 Vaccine to AZD1222 Vaccine, in Adults. Identifier: NCT04864561. Available online: https://clinicaltrials.gov/ct2/show/NCT04864561 (accessed on 28 August 2021).
- Clinical Trials Registry-India. Biological E’s Novel COVID-19 Vaccine of SARS-CoV-2 for Protection against COVID-19 Disease. Official Title: A Prospective Open Label Randomised Phase-I Seamlessly Followed by Phase-II Study to Assess the Safety, Reactogenicity and Immunogenicity of Biological E’s Novel COVID-19 Vaccine Containing Receptor Binding Domain of SARS-CoV-2 for Protection against Covid-19 Disease When Administered Intramuscularly in a Two Dose Schedule (0, 28D) to Healthy Volunteers. CTRI Number: CTRI/2020/11/029032. Available online: http://ctri.nic.in/Clinicaltrials/pdf_generate.php?trialid=48329&EncHid=&modid=&compid=%27,%2748329det%27 (accessed on 10 September 2021).
- Sanofi and GSK COVID-19 Vaccine Candidate Demonstrates Strong Immune Responses across All Adult Age Groups in Phase 2 Trial. Available online: https://www.sanofi.com/en/media-room/press-releases/2021/2021-05-17-07-30-00-2230312 (accessed on 10 September 2021).
- Sanofi and GSK Initiate Global Phase 3 Clinical Efficacy Study of COVID-19 Vaccine Candidate. Available online: https://www.sanofi.com/en/media-room/press-releases/2021/2021-05-27-07-30-00-2236989 (accessed on 10 September 2021).
- News Medical Life Sciences. Phase 1/2 Trial Results for Nanocovax, Protein Subunit SARS-CoV-2 Vaccine from Vietnam. Available online: https://www.news-medical.net/news/20210729/Phase-12-trial-results-for-Nanocovax-protein-subunit-SARS-CoV-2-vaccine-from-Vietnam.aspx (accessed on 10 September 2021).
- U.S. National Institutes of Health. ClinicalTrials.gov Study to Evaluate the Safety, Immunogenicity, and Efficacy of Nanocovax Vaccine against COVID-19. Official Title: A Phase 3, Adaptive, Multicenter, Randomized, Double-Blind, Placebo-Controlled Study to Evaluate the Safety, Immunogenicity, and Efficacy of the Nanocovax Vaccine Against COVID-19 in Volunteer Subjects 18 Years of Age and Older. Identifier: NCT04922788. Available online: https://clinicaltrials.gov/ct2/show/NCT04922788 (accessed on 10 September 2021).
- Chinese Clinical Trials Registry. A Randomized, Double-Blind, Placebo-Controlled Phase I Clinical Trial to Evaluate the Safety and Immunogenicity of Re-combinant SARS-CoV-2 Fusion Protein Vaccine (V-01) in Healthy Subjects. Trial ID: Ictrp-ChiCTR2100045108. Available online: https://www.chictr.org.cn/historyversionpuben.aspx?regno=ChiCTR2100045108 (accessed on 10 September 2021).
- Chinese Clinical Trials Registry. A Randomized, Double-Blind, Placebo-controlled Phase II Clinical Trial to Evaluate the Immunogenicity and Safety of Re-Combinant SARS-CoV-2 Fusion Protein Vaccine (V-01) in Healthy Subjects. Trial ID: Ictrp-ChiCTR2100045107. Available online: https://www.chictr.org.cn/historyversionpuben.aspx?regno=ChiCTR2100045107 (accessed on 10 September 2021).
- Iranian Registry of Clinical Trials. Comparison of the Safety and Efficacy of Razi SARS-CoV-2 Recombinant Spike Protein (Razi Cov Pars) and Sinopharm Vaccines. Official Title: Comparison of the Safety and Efficacy of Razi SARS-CoV-2 Recombinant Spike Protein (Razi Cov Pars) and Sinopharm Vaccines in Adults Aged 18 and Over, a Phase III Randomised, Double Blind, Non-Inferiority Clinical Trial. Trial id: IRCT20201214049709N3. Available online: http://en.irct.ir/trial/58143 (accessed on 10 September 2021).
- U.S. National Institutes of Health. ClinicalTrials.gov Safety and Immunogenicity Study of SARS-CoV-2 Nanoparticle Vaccine (GBP510) Adjuvanted with or Without AS03 (COVID-19). Official Title: A 2-Stage, Phase I/II, Placebo-controlled, Randomized, Observer-blinded, Dose-finding Study to Assess the Safety, Reactogenicity, and Immunogenicity of a SARS-CoV-2 Recom-binant Protein Nanoparticle Vaccine (GBP510) Adjuvanted with or Without AS03 in Healthy Younger and Older Adults. Identifier: NCT04750343. Available online: https://clinicaltrials.gov/ct2/show/NCT04750343 (accessed on 10 September 2021).
- U.S. National Institutes of Health. ClinicalTrials.gov Immunogenicity and Safety Study of SK SARS-CoV-2 Recombinant Nanoparticle Vaccine (GBP510) Adjuvanted with AS03 (COVID-19). Official Title: A Phase III, Randomized, Active-Controlled, Observer-Blind, Parallel-Group, Multi-Center Study to Assess the Immunogenicity and Safety of SK SARS-CoV-2 Recombinant Nanoparticle Vaccine Adjuvanted with AS03 (GBP510) in Adults Aged 18 Years and Older. Identifier: NCT05007951. Available online: https://clinicaltrials.gov/ct2/show/NCT05007951 (accessed on 10 September 2021).
- Weinreich, D.M.; Sivapalasingam, S.; Norton, T.; Ali, S.; Gao, H.; Bhore, R.; Musser, B.J.; Soo, Y.; Rofail, D.; Im, J.; et al. REGN-COV2, a Neutralizing Antibody Cocktail, in Outpatients with COVID-19. N. Engl. J. Med. 2021, 384, 238–251. [Google Scholar] [CrossRef]
- Tass. Russia’s CoviVac Jab Prevents Severe Coronavirus Cases, Developer Assures. Available online: https://tass.com/society/1341561 (accessed on 12 September 2021).
- Edridge, A.W.D.; Kaczorowska, J.; Hoste, A.C.R.; Bakker, M.; Klein, M.; Loens, K.; Jebbink, M.F.; Matser, A.; Kinsella, C.M.; Rueda, P.; et al. Seasonal coronavirus protective immunity is short-lasting. Nat. Med. 2020, 26, 1691–1693. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.Y.; Gandhi, R.T. Reinfection with Severe Acute Respiratory Syndrome Coronavirus 2: What Goes Around May Come Back Around. Clin. Infect. Dis. 2020, 73, e3009–e3012. [Google Scholar] [CrossRef] [PubMed]
- Burton, D.R.; Topol, E.J. Toward superhuman SARS-CoV-2 immunity? Nat. Med. 2020, 27, 5–6. [Google Scholar] [CrossRef] [PubMed]
- Radbruch, A.; Chang, H.-D. A long-term perspective on immunity to COVID. Nature 2021, 595, 359–360. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Rowland-Jones, S.; Gea-Mallorquí, E. Will SARS-CoV-2 Infection Elicit Long-Lasting Protective or Sterilising Immunity? Implications for Vaccine Strategies (2020). Front. Immunol. 2020, 11, 571481. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Han, J.; Lichtfouse, E. Backward transmission of COVID-19 from humans to animals may propagate reinfections and induce vaccine failure. Environ. Chem. Lett. 2021, 19, 763–768. [Google Scholar] [CrossRef]
- Baldo, A.; Leunda, A.; Willemarck, N.; Pauwels, K. Environmental Risk Assessment of Recombinant Viral Vector Vaccines against SARS-CoV-2. Vaccines 2021, 9, 453. [Google Scholar] [CrossRef] [PubMed]
- U.S. National Institutes of Health. ClinicalTrials.gov Clinical Trial to Evaluate the Safety and Immunogenicity of the COVID-19 Vaccine (COVID-19-101). Official Title: A Randomized, Placebo-Controlled Trial, to Evaluate the Safety and Immunogenicity of the COVID-19 Vaccine, a Measles Vector-Based Vaccine Candidate against COVID-19 in Healthy Volunteers Consisting of an Unblinded Dose Escalation and a Blinded Treatment Phase. ClinicalTrials.gov Identifier: NCT04497298. Available online: https://clinicaltrials.gov/ct2/show/NCT04497298 (accessed on 27 October 2021).
- U.S. National Institutes of Health. ClinicalTrials.gov Dose Ranging Trial to Assess Safety and Immunogenicity of V590 (COVID-19 Vaccine) in Healthy adults (V590-001). Official Title: A Phase 1, Randomized, Double-Blind, Placebo-Controlled, Dose-Ranging Trial to Evaluate the Safety and Immunogenicity of V590 in Healthy Adults. ClinicalTrials.gov Identifier: NCT04569786. Available online: https://clinicaltrials.gov/ct2/show/NCT04569786 (accessed on 27 October 2021).
- Merck Stops Developing Both of Its COVID-19 Vaccine Candidates. Available online: https://www.herald.co.zw/merck-stops-developing-both-of-its-covid-19-vaccine-candidates/ (accessed on 27 October 2021).
- Merck and IAVI Discontinue Development of COVID-19 Vaccine Candidate V590. Available online: www.iavi.org/news-resources/press-releases/2021/merck-and-iavi-discontinue-development-of-covid-19-vaccine-candidate-v590 (accessed on 27 October 2021).
- Imperial Vaccine Tech to Target COVID Mutations and Booster Doses. Available online: https://www.imperial.ac.uk/news/213313/imperial-vaccine-tech-target-covid-mutations/ (accessed on 27 October 2021).
- Altimmune and the University of Alabama at Birmingham (UAB) Announce Positive Preclinical Results for Intranasal COVID-19 Vaccine Candidate, AdCOVID™. Available online: https://www.globenewswire.com/news-release/2020/07/13/2061103/0/en/Altimmune-and-the-University-of-Alabama-at-Birmingham-UAB-Announce-Positive-Preclinical-Results-for-Intranasal-COVID-19-Vaccine-Candidate-AdCOVID.html (accessed on 27 October 2021).
- U.S. National Institutes of Health. ClinicalTrials.gov Safety and Immunogenicity of AdCOVID in Healthy Adults (COVID-19 Vaccine Study). Official Title: A Phase 1, Double-blind, Randomized, Placebo-controlled, First-in-Human Study of the Safety and Immunogenicity of AdCOVID Administered as One or Two Doses. ClinicalTrials.gov Identifier: NCT04679909. Available online: https://clinicaltrials.gov/ct2/show/NCT04679909 (accessed on 27 October 2021).
- Kalnin, K.V.; Plitnik, T.; Kishko, M.; Zhang, J.; Zhang, D.; Beauvais, A.; Anosova, N.G.; Tibbitts, T.; DiNapoli, J.; Ulinski, G.; et al. Immunogenicity and efficacy of mRNA COVID-19 vaccine MRT5500 in preclinical animal models. NPJ Vaccines 2021, 6, 61. [Google Scholar] [CrossRef]
- U.S. National Institutes of Health. ClinicalTrials.gov Study of mRNA Vaccine Formulation against COVID-19 in Healthy Adults 18 Years of Age and Older (VAW00001). Official Title: Immunogenicity and Safety of the First-in-Human SARS-CoV-2 mRNA Vaccine Formulation in Healthy Adults 18 Years of Age and Older. Identifier: NCT04798027. Available online: https://clinicaltrials.gov/ct2/show/NCT04798027 (accessed on 27 October 2021).
- Sanofi to Focus Its COVID-19 Development Efforts on the Recombinant Vaccine Candidate. Available online: https://www.sanofi.com/en/media-room/press-releases/2021/2021-09-28-18-44-47-2304800 (accessed on 27 October 2021).
- European Medicines Aagency. EMA ends rolling review of CVnCoV COVID-19 vaccine following withdrawal by CureVac AG. Available online: https://www.ema.europa.eu/en/news/ema-ends-rolling-review-cvncov-covid-19-vaccine-following-withdrawal-curevac-ag (accessed on 27 October 2021).
- BBC News. COVID-19: Netherlands Suspends Use of Astrazeneca Vaccine. Available online: https://www.bbc.com/news/world-europe-56397157 (accessed on 29 October 2021).
- U.S. National Institutes of Health. ClinicalTrials.gov a Study on the Safety, Tolerability and Immune Response of SARS-CoV-2 Sclamp (COVID-19) Vaccine in Healthy Adults. Official Title: A Phase 1, Randomised, Double-Blind, Placebo-Controlled, Dosage-Escalation, Single Centre Study to Evaluate the Safety and Immunogenicity of an Adjuvanted SARS-CoV-2 Sclamp Protein Subunit Vaccine in Healthy Adults Aged 18 to 55 Years Old and Healthy Older Adults, Aged 56 Years and Over. ClinicalTrials.gov Identifier: NCT04495933. Available online: https://clinicaltrials.gov/ct2/show/NCT04495933 (accessed on 29 October 2021).
- The University of Queensland. Update on UQ COVID-19 Vaccine. Available online: https://www.uq.edu.au/news/article/2020/12/update-uq-covid-19-vaccine (accessed on 29 October 2021).
- University of Queensland COVID-19 Vaccine Still in Redevelopment, but Won’t Be Available Soon. Available online: https://www.abc.net.au/news/health/2021-04-26/university-queensland-covid-19-vaccine-research-molecular-clamp/100050240 (accessed on 29 October 2021).
- The New York Times. Iran, Turning More to Imports, Plans to Abandon One of Its Homegrown COVID Vaccines. Available online: https://www.nytimes.com/2021/10/20/world/middleeast/iran-covid-vaccine-fakhravac.html (accessed on 29 October 2021).
- Cromer, D.; Juno, J.A.; Khoury, D.; Reynaldi, A.; Wheatley, A.K.; Kent, S.J.; Davenport, M.P. Prospects for durable immune control of SARS-CoV-2 and prevention of reinfection. Nat. Rev. Immunol. 2021, 21, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Harvey, W.T.; Carabelli, A.M.; Jackson, B.; Gupta, R.K.; Thomson, E.C.; Harrison, E.M.; Ludden, C.; Reeve, R.; Rambaut, A.; Peacock, S.J.; et al. SARS-CoV-2 variants, spike mutations and immune escape. Nat. Rev. Genet. 2021, 19, 409–424. [Google Scholar] [CrossRef]
- Rogliani, P.; Chetta, A.; Cazzola, M.; Calzetta, L. SARS-CoV-2 Neutralizing Antibodies: A Network Meta-Analysis across Vaccines. Vaccines 2021, 9, 227. [Google Scholar] [CrossRef] [PubMed]
- Britton, A.; Slifka, K.M.J.; Edens, C.; Nanduri, S.A.; Bart, S.M.; Shang, N.; Harizaj, A.; Armstrong, J.; Xu, K.; Ehrlich, H.Y.; et al. Effectiveness of the Pfizer-BioNTech COVID-19 Vaccine Among Residents of Two Skilled Nursing Facilities Experiencing COVID-19 Outbreaks—Connecticut, December 2020–February 2021. MMWR. Morb. Mortal. Wkly. Rep. 2021, 70, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Tenforde, M.W.; Olson, S.M.; Self, W.H.; Talbot, H.K.; Lindsell, C.J.; Steingrub, J.S.; Shapiro, N.I.; Ginde, A.A.; Douin, D.J.; Prekker, M.E.; et al. Effectiveness of Pfizer-BioNTech and Moderna Vaccines Against COVID-19 Among Hospitalized Adults Aged ≥ 65 Years—United States, January–March 2021. MMWR. Morb. Mortal. Wkly. Rep. 2021, 70, 674–679. [Google Scholar] [CrossRef]
- Thompson, M.G.; Burgess, J.L.; Naleway, A.L.; Tyner, H.L.; Yoon, S.K.; Meece, J.; Olsho, L.E.; Caban-Martinez, A.J.; Fowlkes, A.; Lutrick, K.; et al. Interim Estimates of Vaccine Effectiveness of BNT162b2 and mRNA-1273 COVID-19 Vaccines in Preventing SARS-CoV-2 Infection Among Health Care Personnel, First Responders, and Other Essential and Frontline Workers—Eight U.S. Locations, December 2020–March 2021. MMWR. Morb. Mortal. Wkly. Rep. 2021, 70, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Karron, R.A.; Key, N.S.; Sharfstein, J.M. Assessing a Rare and Serious Adverse Event Following Administration of the Ad26.COV2.S Vaccine. JAMA 2021, 325, 2445. [Google Scholar] [CrossRef]
- Dewanjee, S.; Vallamkondu, J.; Kalra, R.S.; Puvvada, N.; Kandimalla, R.; Reddy, P.H. Emerging COVID-19 Neurological Manifestations: Present Outlook and Potential Neurological Challenges in COVID-19 Pandemic. Mol. Neurobiol. 2021, 58, 4694–4715. [Google Scholar] [CrossRef] [PubMed]
- Kalra, R.S.; Dhanjal, J.K.; Meena, A.S.; Kalel, V.C.; Dahiya, S.; Singh, B.; Dewanjee, S.; Kandimalla, R. COVID-19, Neuropathology, and Aging: SARS-CoV-2 Neurological Infection, Mechanism, and Associated Complications. Front. Aging Neurosci. 2021, 13, 662786. [Google Scholar] [CrossRef] [PubMed]
- COVID-19 vaccines: Comparison of biological, pharmacological characteristics and adverse effects of Pfizer/BioNTech and Moderna Vaccines. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 1663–1669. [CrossRef]
- Wang, G.-L.; Wang, Z.-Y.; Duan, L.-J.; Meng, Q.-C.; Jiang, M.-D.; Cao, J.; Yao, L.; Zhu, K.-L.; Cao, W.-C.; Ma, M.-J. Susceptibility of Circulating SARS-CoV-2 Variants to Neutralization. N. Engl. J. Med. 2021, 384, 2354–2356. [Google Scholar] [CrossRef]
- Muena, N.A.; García-Salum, T.; Pardo-Roa, C.; Serrano, E.F.; Levican, J.; Avendaño, M.J.; Almonacid, L.I.; Valenzuela, G.; Poblete, E.; Strohmeier, S. Long-lasting neutralizing antibody responses in SARS-CoV-2 seropositive individuals are robustly boosted by immunization with the CoronaVac and BNT162b2 vaccines. medRxiv 2021. [Google Scholar] [CrossRef]
- Ulhaq, Z.S.; Soraya, G.V.; Indriana, K. Breakthrough COVID-19 case after full-dose administration of CoronaVac vaccine. Indian J. Med. Microbiol. 2021. [Google Scholar] [CrossRef]
- Calzetta, L.; Ritondo, B.; Coppola, A.; Matera, M.; Di Daniele, N.; Rogliani, P. Factors Influencing the Efficacy of COVID-19 Vaccines: A Quantitative Synthesis of Phase III Trials. Vaccines 2021, 9, 341. [Google Scholar] [CrossRef]
- Reuters. Volunteers Break Rank to Raise Doubts in Trial of Russia’s Second COVID-19 Vaccine. Available online: https://www.reuters.com/article/us-health-coronavirus-russia-vaccine-let-idUSKBN2BI25B (accessed on 24 August 2021).
- Dobrovidova, O. Latest Russian vaccine comes with a big dose of mystery. Science 2021, 372, 116–117. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tenchov, R.; Smoot, J.; Liu, C.; Watkins, S.; Zhou, Q. A Comprehensive Review of the Global Efforts on COVID-19 Vaccine Development. ACS Central Sci. 2021, 7, 512–533. [Google Scholar] [CrossRef] [PubMed]
- Farrera-Soler, L.; Daguer, J.-P.; Barluenga, S.; Vadas, O.; Cohen, P.; Pagano, S.; Yerly, S.; Kaiser, L.; Vuilleumier, N.; Winssinger, N. Identification of immunodominant linear epitopes from SARS-CoV-2 patient plasma. PLoS ONE 2020, 15, e0238089. [Google Scholar] [CrossRef] [PubMed]
- Bhuyan, A. India begins COVID-19 vaccination amid trial allegations. Lancet 2021, 397, 264. [Google Scholar] [CrossRef]
- Singh, A.K.; Phatak, S.; Singh, N.K.; Gupta, A.; Sharma, A.; Bhattacharjee, K.; Singh, R. Antibody Response after First-dose of ChAdOx1-nCOV (Covishield) and BBV-152 (Covaxin) amongst Health Care Workers in India: Preliminary Results of Cross-sectional Coronavirus Vaccine-induced Antibody Titre (COVAT) study. medRxiv 2021. [Google Scholar] [CrossRef]
- Singh, A.K.; Phatak, S.R.; Singh, R.; Bhattacharjee, K.; Singh, N.K.; Gupta, A.; Sharma, A. Antibody response after first and second-dose of ChAdOx1-nCOV (CovishieldTM®) and BBV-152 (CovaxinTM®) among health care workers in India: The final results of cross-sectional coronavirus vaccine-induced antibody titre (COVAT) study. Vaccine 2021, 39, 6492–6509. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xing, M.; Zhou, D. Coronavirus disease-19 vaccine development utilizing promising technology. Curr. Opin. HIV AIDS 2021, 15, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Yale Medicine. Yale Medicine Doctors Answer Common Questions about Merck’s New COVID-19 Treatment. Available online: https://www.yalemedicine.org/news/9-things-to-know-about-covid-pill (accessed on 30 October 2021).
- Merck. Merck and Ridgeback’s Investigational Oral Antiviral Molnupiravir Reduced the Risk of Hospitalization or Death by Approximately 50 Percent Compared to Placebo for Patients with Mild or Moderate COVID-19 in Positive Interim Analysis of Phase 3 Study. Available online: https://www.merck.com/news/merck-and-ridgebacks-investigational-oral-antiviral-molnupiravir-reduced-the-risk-of-hospitalization-or-death-by-approximately-50-percent-compared-to-placebo-for-patients-with-mild-or-moderat/ (accessed on 30 October 2021).
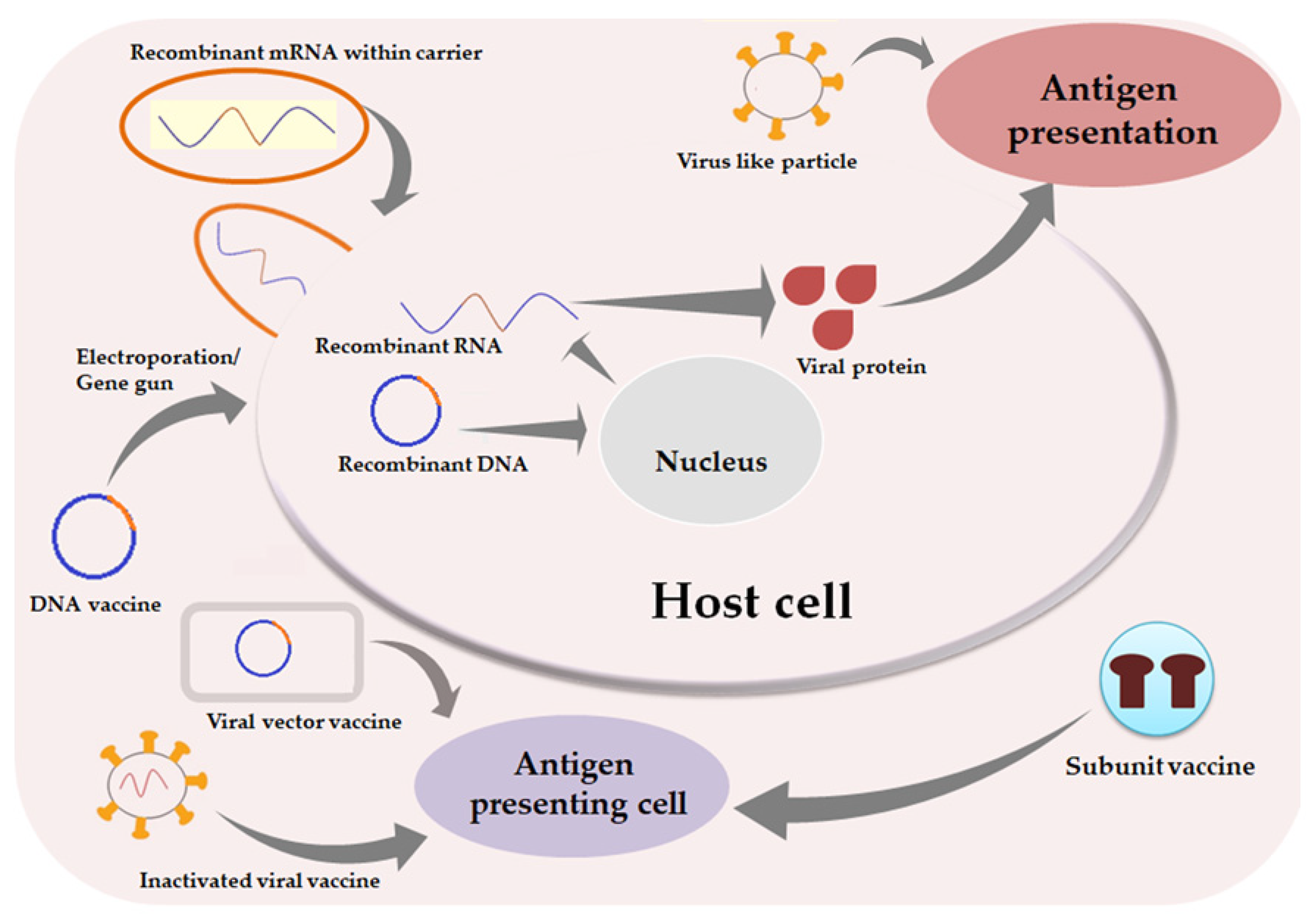

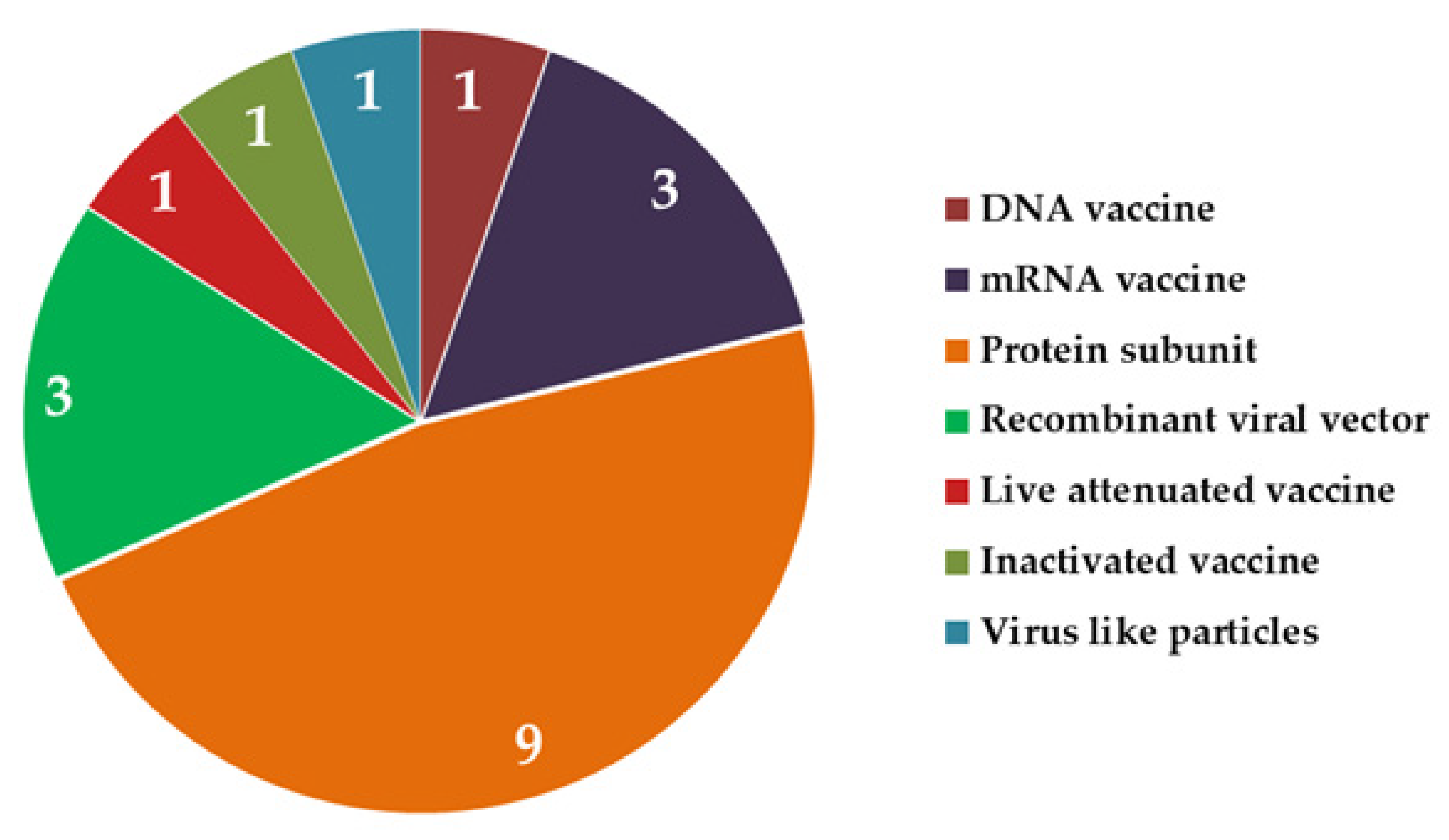

| S. No. | Strategies | Advantages | Limitations | Leading Candidates |
|---|---|---|---|---|
| 1 | DNA vaccine | Stable, cost effective, induces cellular, humoral and neutralizing antibody response | Immunogenicity lower than viral vaccines | Zycov-D, INO-4800 |
| 2 | mRNA vaccine | Easy to design, lower risk of accidental infection than viral vaccines | Carrier required to stabilize and pack naked RNA | BNT162b2, mRNA-1273, ARCoV, CureVac (Tübingen, Germany) |
| 3 | Protein subunit vaccine | Non-infectious, pure antigens easily elicit immunogenic response | Comparatively costlier | Corbevax, Sanofi (Paris, France) NVX-CoV2373, UB612, SCB-2019, EpiVacCorona (Federal Budgetary Research Institution State Research Center of Virology and Biotechnology, Koltsovo, Russia), Nanocovax (Nanogen Pharmaceutical, Ho Chi Minh City, Vietnam) |
| 4 | Recombinant viral vector vaccine | Efficient design easily elicits immunogenicity to desired level, fast and reusable platform | Possibility of undesirable reactions, possibility of Th2 bias | AZD1222, Janssen (Bersee, Belgium), Immunity Bio (Culver City, CA, USA), GRAd-COV2, Sputnik V (Gamaleya Research Institute, Moscow, Russia), Convidicea (Cansino Biologics, Tianjin, China), OraPro-COVID-19™ (IosBio, Somarset, UK and Biocell Corporation, Auckland, New Zealand) |
| 5 | Live attenuated vaccine | Presents entire viral antigen to immune system, strong and long-lasting immune response | Risk of infection going out of control, not suitable for immunocompromised individuals | Covi-Vac, BCG (repurposing) |
| 6 | Whole killed vaccine | Rapid development, can elicit very good immunogenic response, broad antigenic profile | Th2 bias | BBIBP-CorV, CoronaVac, VLA2001, BBV152 |
| 7 | Virus like particles vaccine | Non-infectious, broad antigenic profile | Weaker immunogenicity | Medicago (Quebec City, Canada) |
| S. No. | Vaccines | Types | Carriers | Doses |
|---|---|---|---|---|
| 1 | Oxford-AstraZeneca (ChAdOx1nCoV-19, AZD1222) (University of Oxford, Oxford, UK) | Viral vector, targeted towards S protein | Modified Chimpanzee Adenovirus ChAdOx1 | 2 doses 8 to 12 weeks apart, i.m. |
| 2 | Pfizer-BioNTech (BNT162b2) | Nucleoside modified mRNA | Lipid nanoparticles | 2 doses 21 to 28 days apart, i.m. |
| 3 | Johnson and Johnson (Ad26.COV2.S, Janssen) | S protein of SARS-CoV-2 WA1/2020 strain | Recombinant, replication incompetent adenovirus Ad26 | Single dose, i.m. |
| 4 | Moderna (mRNA-1273) | Nucleoside modified mRNA | Lipid nanoparticles | 2 doses, 4 to 6 weeks apart, i.m. |
| 5 | Sinopharm (BBIBP-CorV) | Inactivated virus (2019-CoV) | Inactivated virus + adjuvant | 2 doses, 3–4 weeks apart, i.m. |
| 6 | CoronaVac (Sinovac) | Inactivated virus | Inactivated virus + adjuvant | 2 doses, 2–4 weeks apart, i.m. |
| S. No. | Vaccines | Country of Origin | Trial Phase | Types | Potential Promises |
|---|---|---|---|---|---|
| 1 | Vidprevtyn | USA | III | Recombinant protein with adjuvant | Interim phase II result reported 95–100% seroconversion, 3rd phase trial started in May 2021 |
| 2 | CVnCoV (CureVac) | Germany and Belgium | IIb/III | Non-chemically modified mRNA (CBnCOV) | Protection against B.1.951 variant in mice, 48% efficacy in phase IIb/III trial |
| 3 | BCG vaccine (repurposing) | Australia, The Netherlands | II/III | Live attenuated virus | Reduced COVID-19 related clinical symptoms, not impressive enough for confirmatory decision |
| 4 | NVX-CoV2373 | Australia | III | Protein nanoparticles | About 90% efficacy reported in various trials, approval sought in Australia, USA, Canada, Europe |
| 5 | ARCoV | China | III | mRNA (encoding receptor binding domain) lipid nanoparticles | Phase III trial on the way |
| 6 | Unnamed (Medicago) | Canada | III | Virus like particle along with plant based adjuvants | High antibody titers with tolerable safety profile |
| 7 | VLA2001 | UK | III | Inactivated vaccine | Safe, well tolerated as per phase I/II trial reports in April 2021 |
| 8 | Corbevax | India | III | Protein subunit with CpG1018 as adjuvant | Very positive results from phase I/II, 3rd phase announced on April 2021 |
| 9 | Nanocovax | Vietnam | III | Glycosylated recombinant S protein | Displayed in vitro neutralizing activity, in phase II, all non-placebo recipients developed antibodies |
| 10 | BNT162 | USA | I/II/III | mRNA vaccine | BNT162b2 already approved by WHO, BNT162b1 displayed similar efficacy with altered adverse reaction profile |
| 11 | INO-4800 | USA | II/III | Intradermal DNA vaccine (plasmid) | Phase II portion declared INO-4800 as safe and well tolerated in May 2021 |
| 12 | Unnamed (Immunity Bio, Culver City, CA, USA) | USA | II/III | Adenovirus based vaccine targeting S protein and nucleocapsid DNA | Reported CD4+ and CD8+ antigen specific T cell response in mice, no serious adverse events reported in human receiving low dose |
| 13 | UB612 | Taiwan | II/III | Multitope peptide vaccine | Well tolerated, CD4+/CD8+ T cell response |
| 14 | GRAd-COV2 | Italy | II/III | Defective gorilla adenovirus encoding prefusion stabilized full length S protein of SARS-CoV-2 | Reported as safe and well tolerated in phase I on November 2020 |
| 15 | SCB-2019 | China | II/III | Protein subunit with adjuvants | Adjuvant optimized for formulation, robust cellular and humoral immune response reported along with strong neutralizing activity |
| 16 | Unnamed (West Bank Biopharma) | China | III | Recombinant vaccine (Sf9 cells) targeting receptor binding domain using Baculovirus vector | 3rd Phase trial is enrolling by invitation |
| 17 | V-01 | China | III | Recombinant fusion protein | Induces immune response, good safety profile in phase 2 trial |
| 18 | Razi Cov Pars | Iran | III | Recombinant S protein | Phase III trial started after potential promise shown in earlier phases |
| 19 | GBP510 | Korea | III | Nanoparticles (key regions of viral S protein) | Phase III trial approved based on immune response and safety profile in earlier phases |
| S. No. | Vaccine Types | Approved Vaccines | Adverse Events | |
|---|---|---|---|---|
| Mild | Severe | |||
| 1 | mRNA vaccines | BNT162b2, mRNA-1273 | Pain, tenderness, redness and swelling at injection site, fatigue, headache, fever, nausea, chills, COVID arm | Allergy-like reactions, Bell’s palsy, acute myocarditis, pericarditis, arthralgia (grade 3 and above), anaphylactic reactions, bilateral retinal detachment |
| 2 | Protein subunit vaccines | EpiVacCorona | Pain at injection site | - |
| 3 | Recombinant viral vector vaccines | AZD1222, Ad26.COV2.S, Sputnik V, Convidicea | Pain and tenderness at injection site, exhaustion, discomfort, headache, pyrexia, fatigue, muscle pain, diarrhoea, asthenia, joint pain | Clotting events, venous and arterial thromboembolism, cerebral venous thrombosis, pulmonary thromboembolism, acute stroke, capillary leak syndrome, Guillain–Barré syndrome, thrombotic thrombocytopenic purpura, cutaneous rash |
| 4 | Whole killed vaccines | BBIBP-CorV, CoronaVac, BBV152 | Pain at injection site, fever, fatigue, myalgia, dizziness, arm numbness, headache, ear symptoms, joint pain, itchy rash, pityriasis rosea, pustular psoriasis, nausea, chest pain | Neurological complications, type I Kounis syndrome, reactive arthritis |
| 5 | DNA vaccines | ZyCoV-D | Tenderness at injection site, fever, itching, joint pain, diarrhoea | Enteric fever |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kandimalla, R.; Chakraborty, P.; Vallamkondu, J.; Chaudhary, A.; Samanta, S.; Reddy, P.H.; De Feo, V.; Dewanjee, S. Counting on COVID-19 Vaccine: Insights into the Current Strategies, Progress and Future Challenges. Biomedicines 2021, 9, 1740. https://doi.org/10.3390/biomedicines9111740
Kandimalla R, Chakraborty P, Vallamkondu J, Chaudhary A, Samanta S, Reddy PH, De Feo V, Dewanjee S. Counting on COVID-19 Vaccine: Insights into the Current Strategies, Progress and Future Challenges. Biomedicines. 2021; 9(11):1740. https://doi.org/10.3390/biomedicines9111740
Chicago/Turabian StyleKandimalla, Ramesh, Pratik Chakraborty, Jayalakshmi Vallamkondu, Anupama Chaudhary, Sonalinandini Samanta, P. Hemachandra Reddy, Vincenzo De Feo, and Saikat Dewanjee. 2021. "Counting on COVID-19 Vaccine: Insights into the Current Strategies, Progress and Future Challenges" Biomedicines 9, no. 11: 1740. https://doi.org/10.3390/biomedicines9111740
APA StyleKandimalla, R., Chakraborty, P., Vallamkondu, J., Chaudhary, A., Samanta, S., Reddy, P. H., De Feo, V., & Dewanjee, S. (2021). Counting on COVID-19 Vaccine: Insights into the Current Strategies, Progress and Future Challenges. Biomedicines, 9(11), 1740. https://doi.org/10.3390/biomedicines9111740









