The Effectiveness of Compartmentalized Bone Graft Sponges Made Using Complementary Bone Graft Materials and Succinylated Chitosan Hydrogels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Bone Graft Materials (BGMs) Loaded with Succinylated Chitosan (SCS)-Based Hydrogel (SCS-B)
2.3. Characterizations of Synthesized SCS-B
2.4. Cell Viability and Proliferation
2.5. In Vitro Osteogenic Differentiation
2.6. In Vivo Study
2.7. Micro-Computed Tomography (μ-CT) Analysis
2.8. Statistical Analyses
3. Results and Discussion
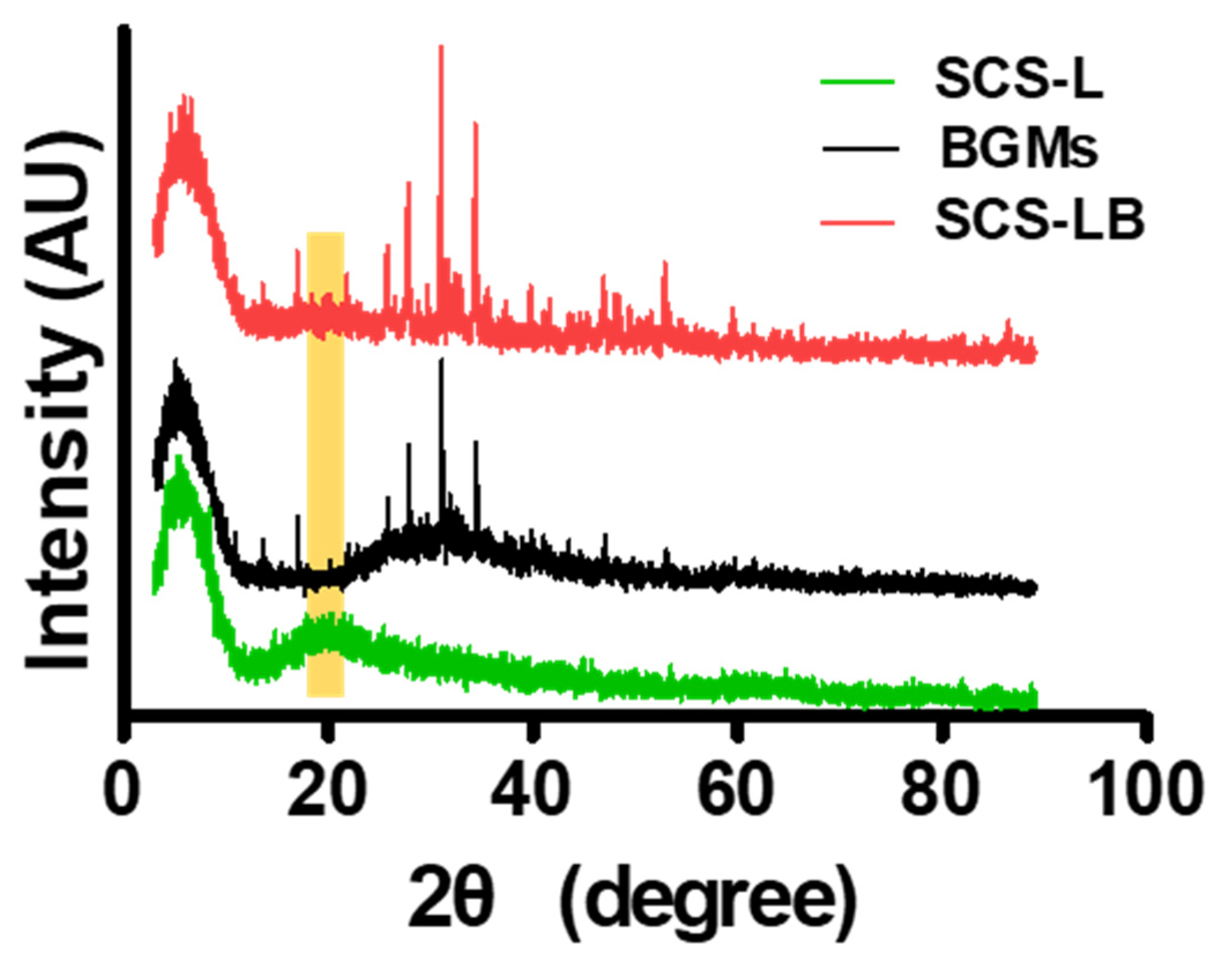
3.1. Preparation and Characterization of SCS-B
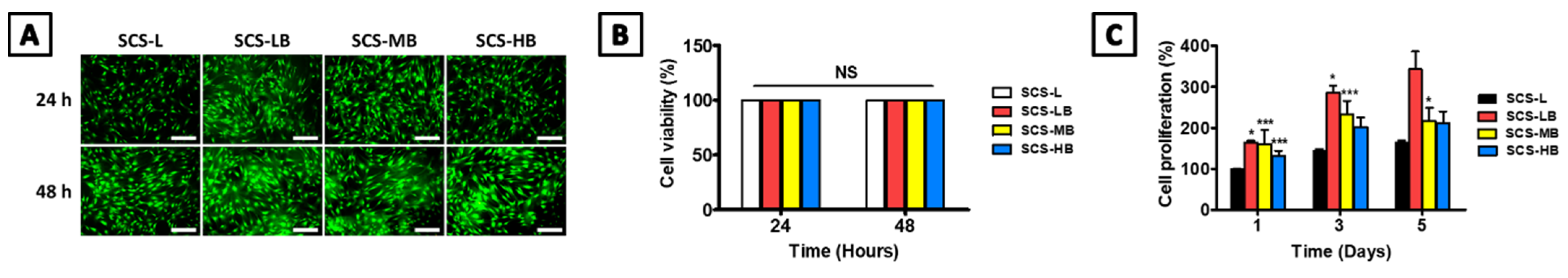
3.2. In Vitro SCS-B Cell Viability and Osteogenic Differentiation
3.3. In Vivo Bone Regeneration of SCS-B
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BGMs | Synthetic bone materials |
| SCS-L | Low-succinlyated chitosan |
| SCS-LB | BGMs-loaded SCS-L |
| SCS-MB | BGMs-loaded SCS-M |
| SCS-HB | BGMs-loaded SCS-H |
References
- Smrke, D.; Rožman, P.; Veselko, M.; Gubina, B. Treatment of Bone Defects—Allogenic Platelet Gel and Autologous Bone Technique. In Regenerative Medicine and Tissue Engineering; IntechOpen: London, UK, 2013. [Google Scholar]
- Maisani, M.; Pezzoli, D.; Chassande, O.; Mantovani, D. Cellularizing hydrogel-based scaffolds to repair bone tissue: How to create a physiologically relevant micro-environment? J. Tissue Eng. 2017, 8, 2041731417712073. [Google Scholar] [CrossRef] [Green Version]
- Ercin, B.S.; Kabakas, F.; Keles, M.K.; Ozcelik, I.B.; Mersa, B.J.P.; Research, A. Olecranon bone grafting for the treatment of nonunion after distal finger replantation. Plast. Aesthetic Res. 2020, 7, 38. [Google Scholar] [CrossRef]
- Huber, E.; Pobloth, A.-M.; Bormann, N.; Kolarczik, N.; Schmidt-Bleek, K.; Schell, H.; Schwabe, P.; Duda, G.N.; Wildemann, B. Demineralized bone matrix as a carrier for bone morphogenetic protein-2: Burst release combined with long-term binding and osteoinductive activity evaluated in vitro and in vivo. Tissue Eng. Part A 2017, 23, 1321–1330. [Google Scholar] [CrossRef]
- Zimmermann, G.; Moghaddam, A. Allograft bone matrix versus synthetic bone graft substitutes. Injury 2011, 42, S16–S21. [Google Scholar] [CrossRef] [PubMed]
- Yun, P.-Y.; Kim, Y.-K.; Jeong, K.-I.; Park, J.-C.; Choi, Y.-J. Influence of bone morphogenetic protein and proportion of hydroxyapatite on new bone formation in biphasic calcium phosphate graft: Two pilot studies in animal bony defect model. J. Cranio-Maxillofac. Surg. 2014, 42, 1909–1917. [Google Scholar] [CrossRef] [PubMed]
- Jang, K.; Lee, J.; Oh, S.; Ham, B.; Chung, S.; Lee, J.K.; Ku, J.K. Bone graft materials for current implant dentistry. J. Dent. Implant. Res. 2020, 39, 1–10. [Google Scholar] [CrossRef]
- Huang, R.-L.; Kobayashi, E.; Liu, K.; Li, Q. Bone graft prefabrication following the in vivo bioreactor principle. EBioMedicine 2016, 12, 43–54. [Google Scholar] [CrossRef] [Green Version]
- Heo, D.N.; Ko, W.-K.; Bae, M.S.; Lee, J.B.; Lee, D.-W.; Byun, W.; Lee, C.H.; Kim, E.-C.; Jung, B.-Y.; Kwon, I.K. Enhanced bone regeneration with a gold nanoparticle–hydrogel complex. J. Mater. Chem. B 2014, 2, 1584–1593. [Google Scholar] [CrossRef]
- Han, X.; Hung, H.-C.; Jain, P.; Sun, F.; Xu, X.; Yang, W.; Bai, T.; Jiang, S. Sterilization, hydration-dehydration and tube fabrication of zwitterionic hydrogels. Biointerphases 2017, 12, 02C411. [Google Scholar] [CrossRef]
- Re, F.; Sartore, L.; Moulisova, V.; Cantini, M.; Almici, C.; Bianchetti, A.; Chinello, C.; Dey, K.; Agnelli, S.; Manferdini, C.; et al. 3D gelatin-chitosan hybrid hydrogels combined with human platelet lysate highly support human mesenchymal stem cell proliferation and osteogenic differentiation. J. Tissue Eng. 2019, 10, 2041731419845852. [Google Scholar] [CrossRef] [Green Version]
- Sohrabi, M.; Eftekhari Yekta, B.; Rezaie, H.; Naimi-Jamal, M.R.; Kumar, A.; Cochis, A.; Miola, M.; Rimondini, L. Enhancing Mechanical Properties and Biological Performances of Injectable Bioactive Glass by Gelatin and Chitosan for Bone Small Defect Repair. Biomedicines 2020, 8, 616. [Google Scholar] [CrossRef]
- Zwingenberger, B.; Vater, C.; Bell, R.L.; Bolte, J.; Mehnert, E.; Brunler, R.; Aibibu, D.; Zwingenberger, S. Treatment of Critical-Size Femoral Bone Defects with Chitosan Scaffolds Produced by a Novel Process from Textile Engineering. Biomedicines 2021, 9, 1015. [Google Scholar] [CrossRef]
- Lee, J.S.; Nah, H.; Moon, H.-J.; Lee, S.J.; Heo, D.N.; Kwon, I.K. Controllable delivery system: A temperature and pH-responsive injectable hydrogel from succinylated chitosan. Appl. Surf. Sci. 2020, 528, 146812. [Google Scholar] [CrossRef]
- Rajas, F.; Gautier-Stein, A.; Mithieux, G. Glucose-6 Phosphate, a Central Hub for Liver Carbohydrate Metabolism. Metabolites 2019, 9, 282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohamed, N.A.; Abd El-Ghany, N.A. Synthesis and antimicrobial activity of some novel terephthaloyl thiourea cross-linked carboxymethyl chitosan hydrogels. Cellulose 2012, 19, 1879–1891. [Google Scholar] [CrossRef]
- Sun, M.; Sun, H.; Wang, Y.; Sánchez-Soto, M.; Schiraldi, D.A. The relation between the rheological properties of gels and the mechanical properties of their corresponding aerogels. Gels 2018, 4, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LogithKumar, R.; KeshavNarayan, A.; Dhivya, S.; Chawla, A.; Saravanan, S.; Selvamurugan, N. A review of chitosan and its derivatives in bone tissue engineering. Carbohydr. Polym. 2016, 151, 172–188. [Google Scholar] [CrossRef]
- Lee, J.S.; Lee, S.J.; Yang, S.B.; Lee, D.; Nah, H.; Heo, D.N.; Moon, H.-J.; Hwang, Y.-S.; Reis, R.L.; Moon, J.-H.; et al. Facile preparation of mussel-inspired antibiotic-decorated titanium surfaces with enhanced antibacterial activity for implant applications. Appl. Surf. Sci. 2019, 496, 143675. [Google Scholar] [CrossRef]
- Saravanan, S.; Leena, R.; Selvamurugan, N. Chitosan based biocomposite scaffolds for bone tissue engineering. Int. J. Biol. Macromol. 2016, 93, 1354–1365. [Google Scholar] [CrossRef]
- Di Martino, A.; Sittinger, M.; Risbud, M.V. Chitosan: A versatile biopolymer for orthopaedic tissue-engineering. Biomaterials 2005, 26, 5983–5990. [Google Scholar] [CrossRef]
- Lee, S.J.; Nah, H.; Heo, D.N.; Kim, K.-H.; Seok, J.M.; Heo, M.; Moon, H.-J.; Lee, D.; Lee, J.S.; An, S.Y.; et al. Induction of osteogenic differentiation in a rat calvarial bone defect model using an In situ forming graphene oxide incorporated glycol chitosan/oxidized hyaluronic acid injectable hydrogel. Carbon 2020, 168, 264–277. [Google Scholar] [CrossRef]
- Seeherman, H.; Li, R.; Wozney, J. A review of preclinical program development for evaluating injectable carriers for osteogenic factors. JBJS 2003, 85, 96–108. [Google Scholar] [CrossRef]
- Ko, W.-K.; Heo, D.N.; Moon, H.-J.; Lee, S.J.; Bae, M.S.; Lee, J.B.; Sun, I.-C.; Jeon, H.B.; Park, H.K.; Kwon, I.K. The effect of gold nanoparticle size on osteogenic differentiation of adipose-derived stem cells. J. Colloid Interface Sci. 2015, 438, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Kurata, S.; Morishita, K.; Kawase, T.; Umemoto, K. Cytotoxic effects of acrylic acid, methacrylic acid, their corresponding saturated carboxylic acids, HEMA, and hydroquinone on fibroblasts derived from human pulp. Dent. Mater. J. 2012, 31, 1203190247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sohn, J.-Y.; Park, J.-C.; Um, Y.-J.; Jung, U.-W.; Kim, C.-S.; Cho, K.-S.; Choi, S.-H. Spontaneous healing capacity of rabbit cranial defects of various sizes. J. Periodontal Implant Sci. 2010, 40, 180–187. [Google Scholar] [CrossRef] [Green Version]
- Xu, A.; Zhuang, C.; Xu, S.; He, F.; Xie, L.; Yang, X.; Gou, Z. Optimized bone regeneration in calvarial bone defect based on biodegradation-tailoring dual-shell biphasic bioactive ceramic microspheres. Sci. Rep. 2018, 8, 1–14. [Google Scholar]
- Wang, W.; Yeung, K.W. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact. Mater. 2017, 2, 224–247. [Google Scholar] [CrossRef]
- Qin, A.; Cheng, T.S.; Lin, Z.; Cao, L.; Chim, S.M.; Pavlos, N.J.; Xu, J.K.; Zheng, M.H.; Dai, K.R. Prevention of Wear Particle-Induced Osteolysis by a Novel V-ATPase Inhibitor Saliphenylhalamide through Inhibition of Osteoclast Bone Resorption. PLoS ONE 2012, 7, e34132. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zhang, M. Cell growth and function on calcium phosphate reinforced chitosan scaffolds. J. Mater. Sci. Mater. Med. 2004, 15, 255–260. [Google Scholar] [CrossRef]
- Fourie, J.; Taute, F.; du Preez, L.; De Beer, D. Chitosan Composite Biomaterials for Bone Tissue Engineering—A Review. Regen. Eng. Transl. Med. 2020, 1–21. [Google Scholar] [CrossRef]
- Zhou, H.; Liang, C.Y.; Wei, Z.; Bai, Y.J.; Bhaduri, S.B.; Webster, T.J.; Bian, L.M.; Yang, L. Injectable biomaterials for translational medicine. Mater. Today 2019, 28, 81–97. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.S.; Kim, H.-S.; Nah, H.; Lee, S.J.; Moon, H.-J.; Bang, J.B.; Lee, J.B.; Do, S.H.; Kwon, I.K.; Heo, D.N. The Effectiveness of Compartmentalized Bone Graft Sponges Made Using Complementary Bone Graft Materials and Succinylated Chitosan Hydrogels. Biomedicines 2021, 9, 1765. https://doi.org/10.3390/biomedicines9121765
Lee JS, Kim H-S, Nah H, Lee SJ, Moon H-J, Bang JB, Lee JB, Do SH, Kwon IK, Heo DN. The Effectiveness of Compartmentalized Bone Graft Sponges Made Using Complementary Bone Graft Materials and Succinylated Chitosan Hydrogels. Biomedicines. 2021; 9(12):1765. https://doi.org/10.3390/biomedicines9121765
Chicago/Turabian StyleLee, Jae Seo, Hyo-Sung Kim, Haram Nah, Sang Jin Lee, Ho-Jin Moon, Jae Beum Bang, Jung Bok Lee, Sun Hee Do, Il Keun Kwon, and Dong Nyoung Heo. 2021. "The Effectiveness of Compartmentalized Bone Graft Sponges Made Using Complementary Bone Graft Materials and Succinylated Chitosan Hydrogels" Biomedicines 9, no. 12: 1765. https://doi.org/10.3390/biomedicines9121765





