Analysis of Parainflammation in Chronic Glaucoma Using Vitreous-OCT Imaging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Collection
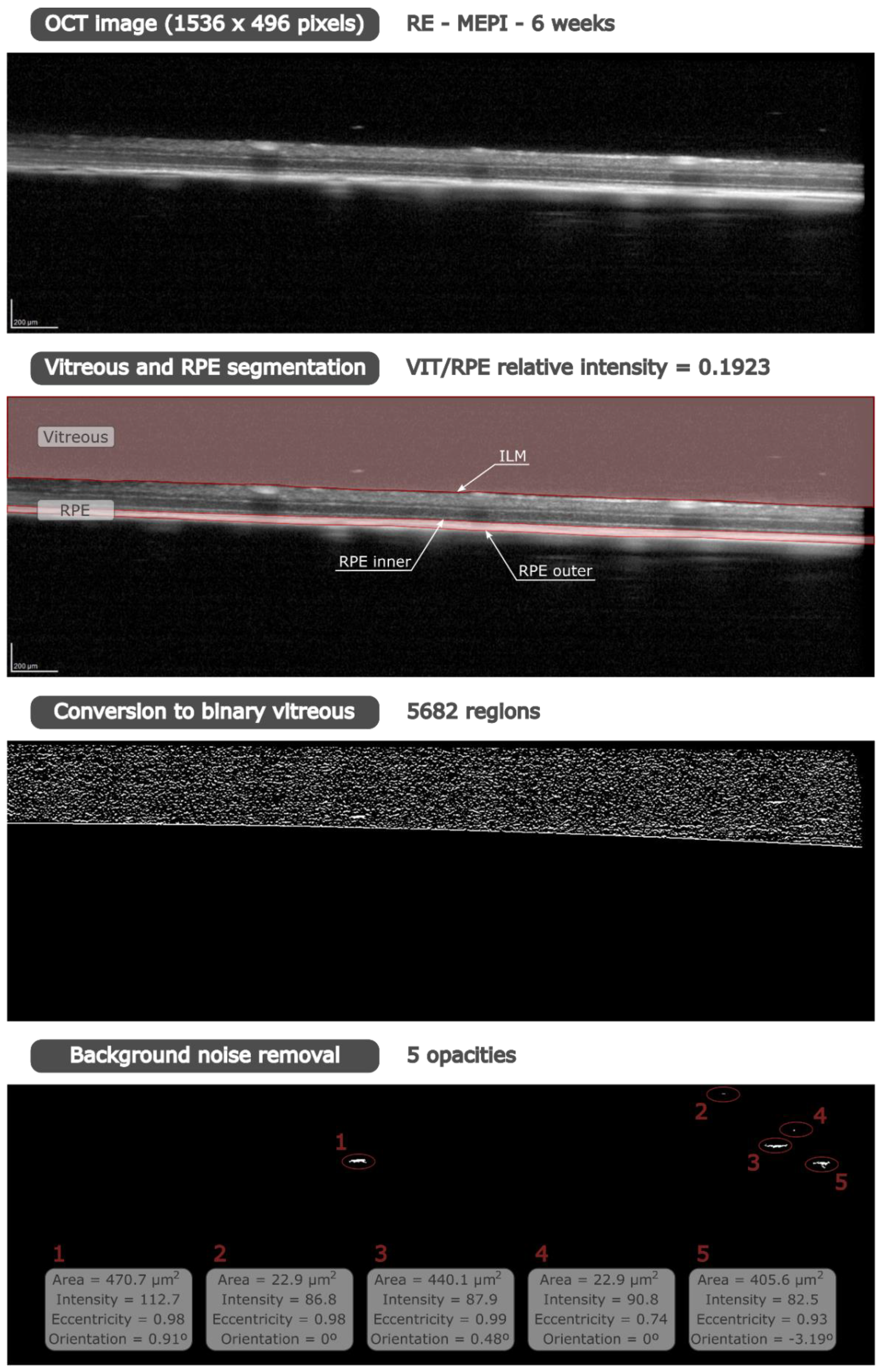
2.2. Image Analysis
2.3. Histological Analysis
2.4. Statistical Analysis
3. Results
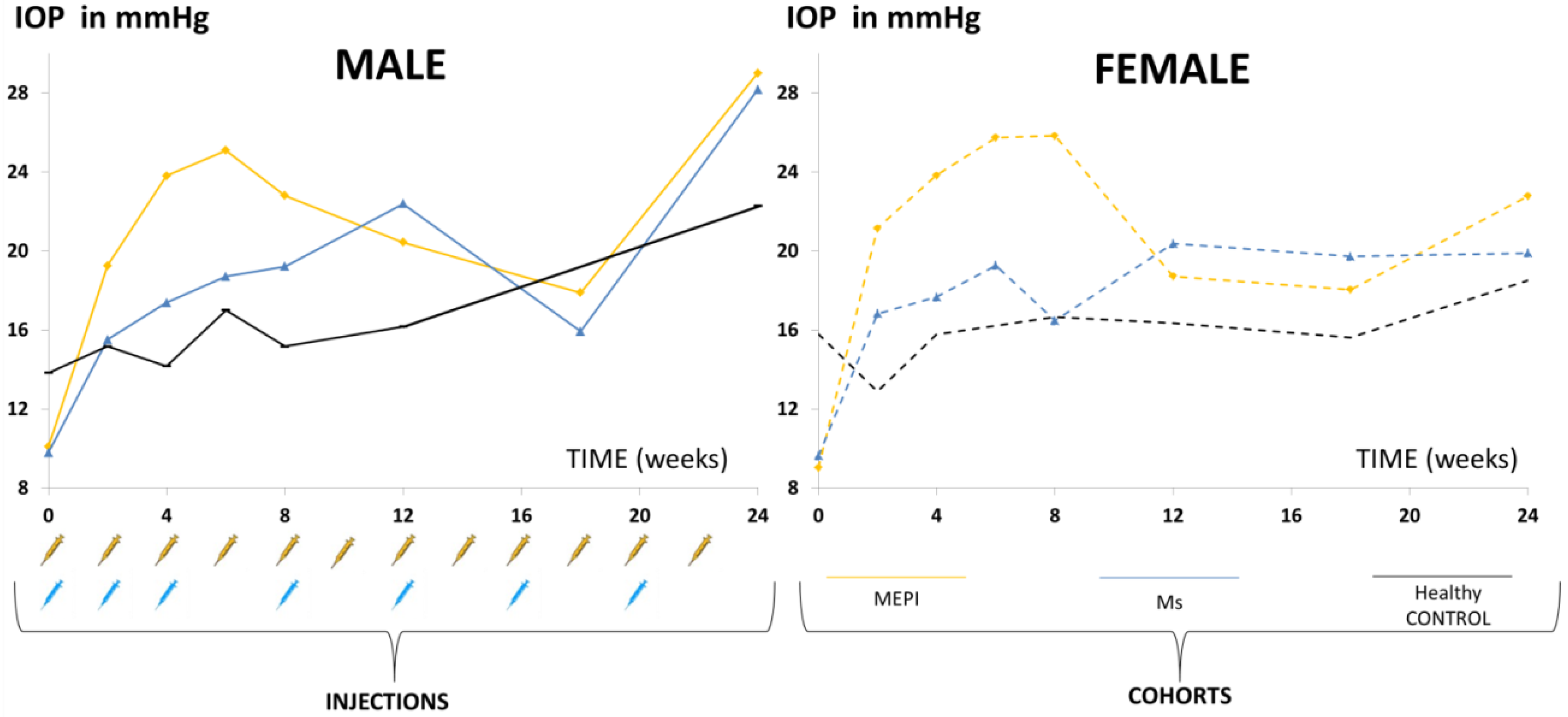
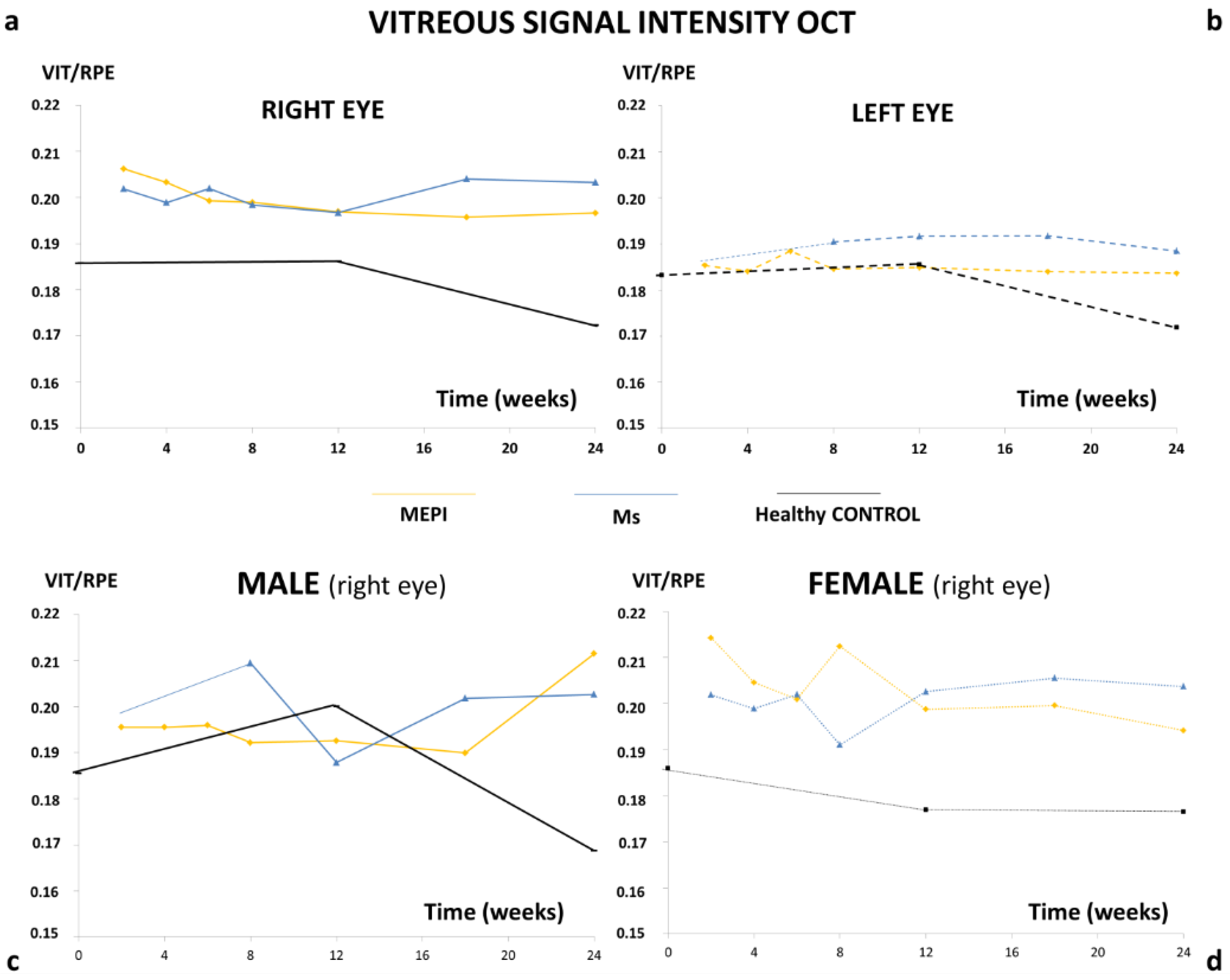
3.1. Descriptive Data
3.2. Descriptive Data
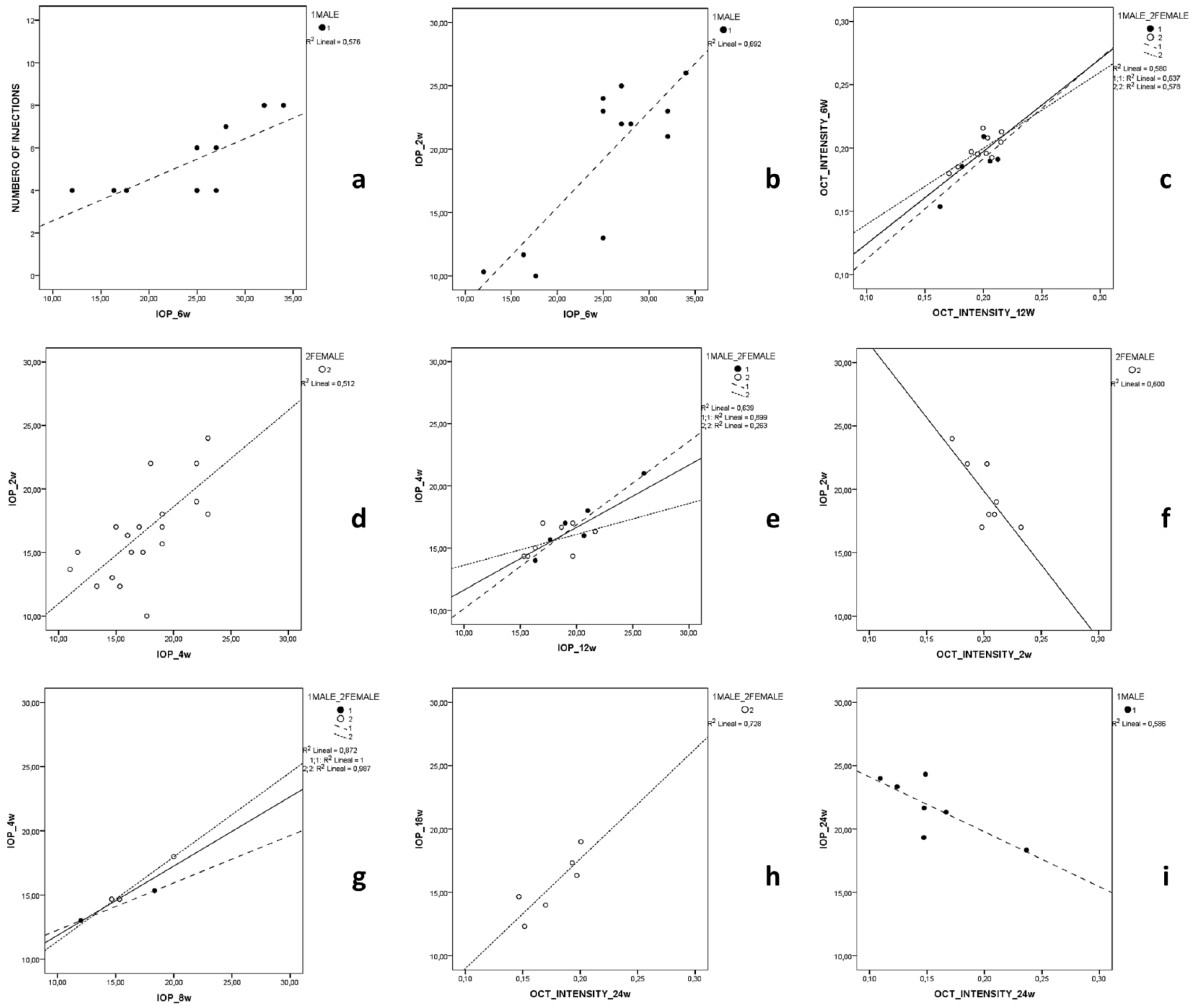
3.3. Correlation Analysis
3.3.1. MEPI Model
3.3.2. Ms Model
3.3.3. Healthy Control Cohort
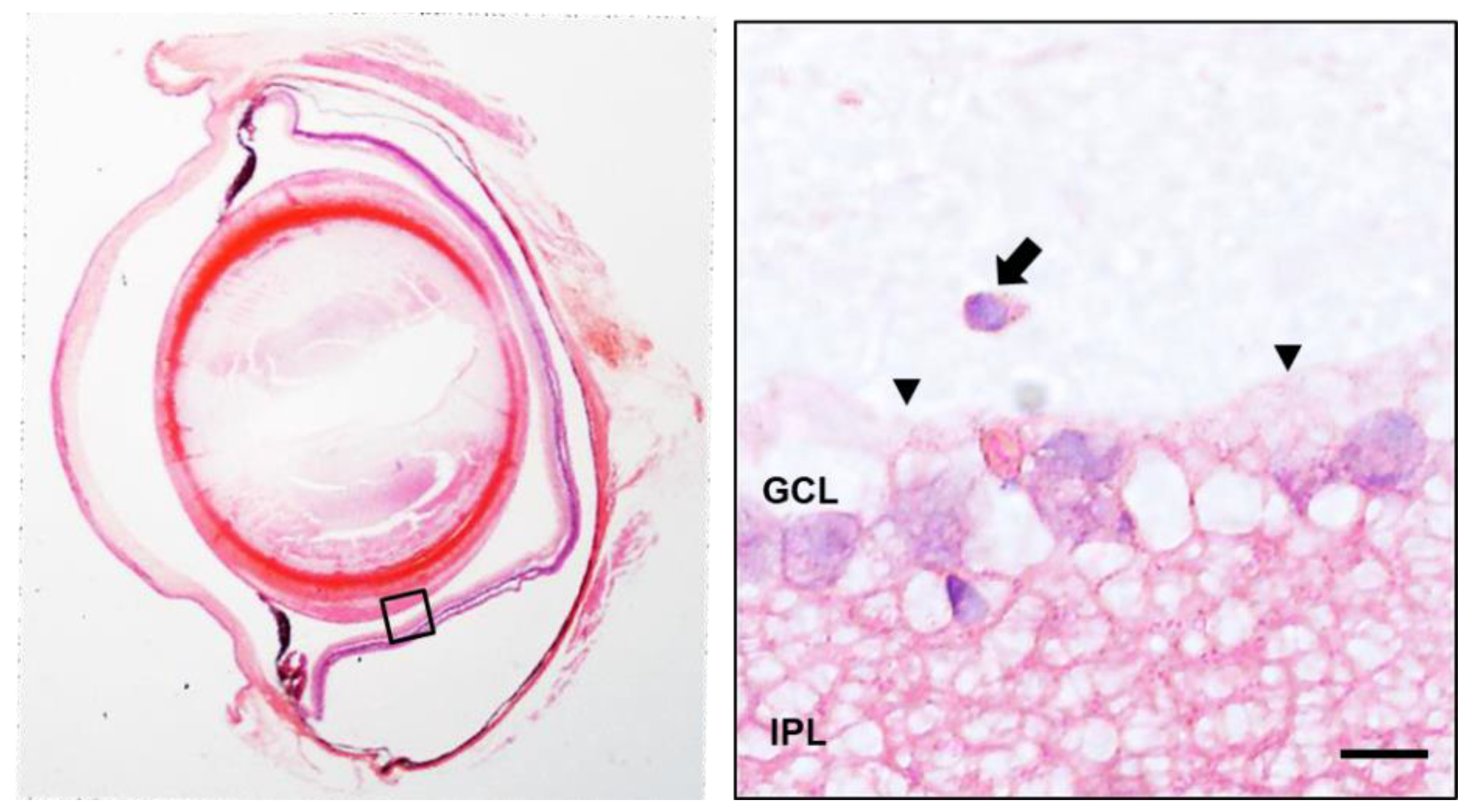
3.4. Histology
3.5. In Vivo Analysis of Vitreous Immunity Detected Using OCT
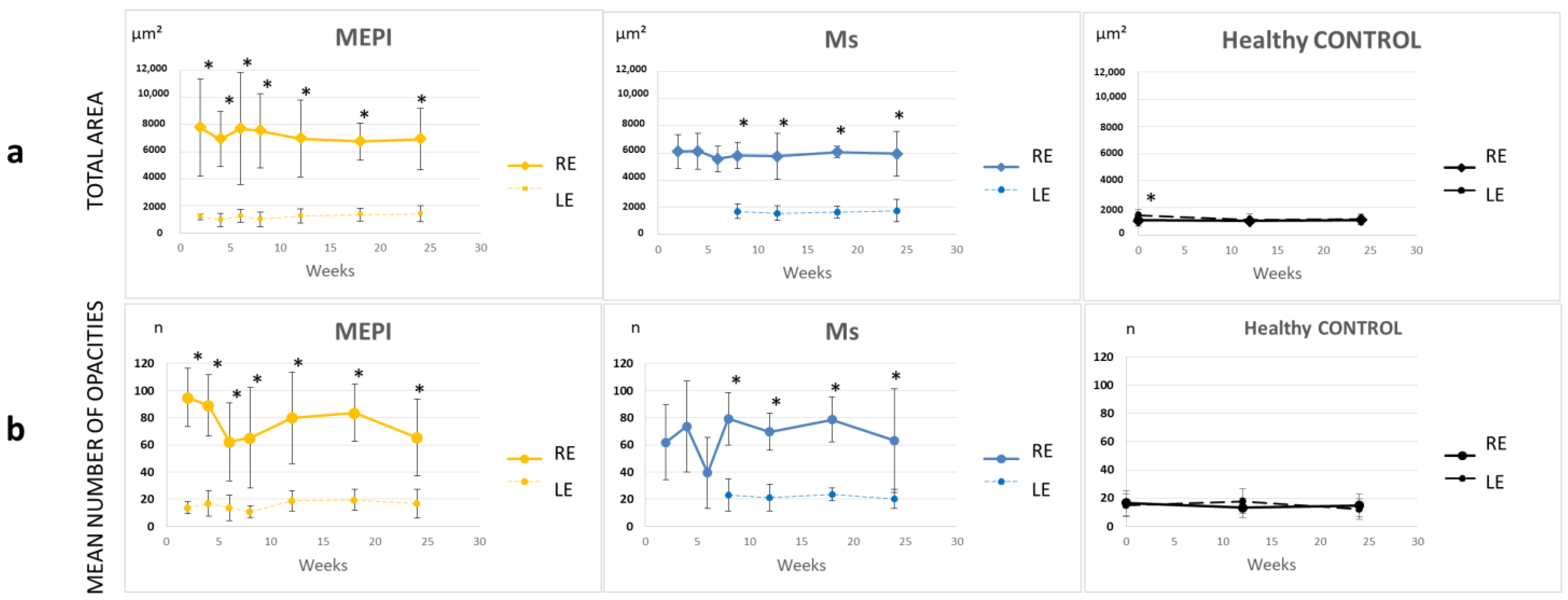
3.5.1. Quantification of the Mean Number of Opacities/Cells
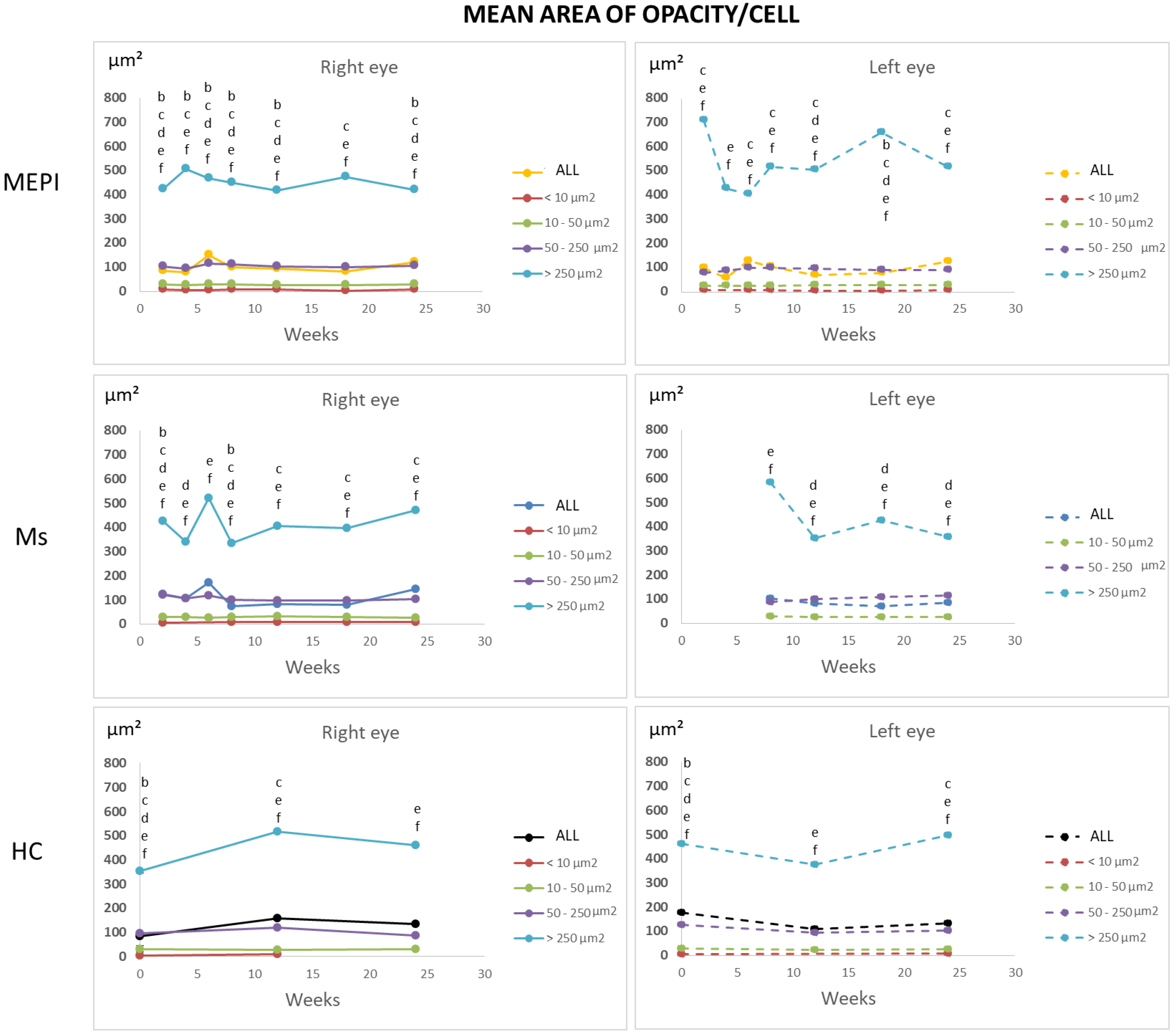
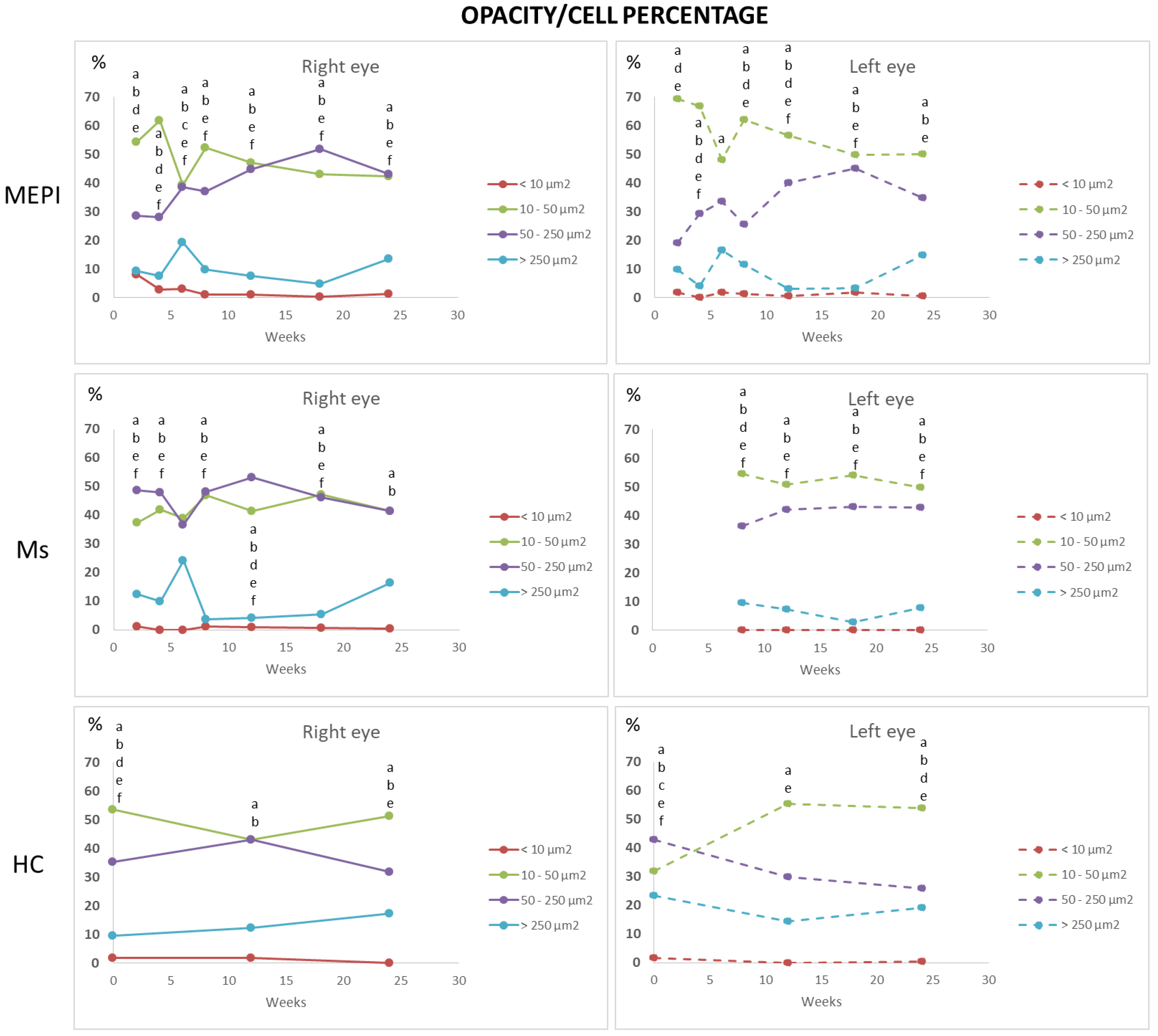
3.5.2. Percentage of Opacities/Cells by Size
3.5.3. Mean Intensity of Opacities/Cells
3.5.4. Average Eccentricity of the Opacities/Cells
3.5.5. Mean Orientation of the Opacities/Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Quigley, H.; Broman, A.T. The number of people with glaucoma worldwide in 2010 and 2020. Br. J. Ophthalmol. 2006, 90, 262–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- AGIS Investigators. The advanced glaucoma intervention study (AGIS): 7. the relationship between control of intraocular pressure and visual field deterioration. Am. J. Ophthalmol. 2000, 130, 429–440. [Google Scholar] [CrossRef]
- Jonas, J.B.; Aung, T.; Bourne, R.R.; Bron, A.M.; Ritch, R.; Panda-Jonas, S. Glaucoma. Lancet 2017, 390, 2183–2193. [Google Scholar] [CrossRef]
- Lau, L.I.; Liu, C.J.L.; Chou, J.C.K.; Hsu, W.M.; Liu, J.H. Patterns of visual field defects in chronic angle-closure glaucoma with different disease severity. Ophthalmology 2003, 110, 1890–1894. [Google Scholar] [CrossRef]
- Schlamp, C.L.; Li, Y.; Dietz, J.A.; Janssen, K.T.; Nickells, R.W. Progressive ganglion cell loss and optic nerve degeneration in DBA/2J mice is variable and asymmetric. BMC Neurosci. 2006, 7, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, B.; Smith, R.S.; Hawes, N.L.; Anderson, M.G.; Zabaleta, A.; Savinova, O.; Roderick, T.H.; Heckenlively, J.R.; Davisson, M.T.; John, S.W.M. Interacting loci cause severe iris atrophy and glaucoma in DBA/2J mice. Nat. Genet. 1999, 21, 405–409. [Google Scholar] [CrossRef]
- Lucas, D.R.; Newhouse, J.P. The Toxic Effect of Sodium L-Glutamate on the Inner Layers of the Retina. Arch. Ophthalmol. 1957, 58, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Neufeld, A.H. Nitric Oxide: A Potential Mediator of Retinal Ganglion Cell Damage in Glaucoma. Surv. Ophthalmol. 1999, 43, S129–S135. [Google Scholar] [CrossRef]
- Dey, A.; Manthey, A.L.; Chiu, K.; Do, C.W. Methods to Induce Chronic Ocular Hypertension: Reliable Rodent Models as a Platform for Cell Transplantation and Other Therapies. Cell Transplant. 2018, 27, 213–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgan, J.E.; Tribble, J.R. Microbead models in glaucoma. Exp. Eye Res. 2015, 141, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Morrison, J.C.; Moore, C.G.; Deppmeier, L.M.H.; Gold, B.G.; Meshul, C.K.; Johnson, E.C. A rat model of chronic pressure-induced optic nerve damage. Exp. Eye Res. 1997, 64, 85–96. [Google Scholar] [CrossRef]
- Sapienza, A.; Raveu, A.-L.; Reboussin, E.; Roubeix, C.; Boucher, C.; Dégardin, J.; Godefroy, D.; Rostène, W.; Reaux-Le Goazigo, A.; Baudouin, C.; et al. Bilateral neuroinflammatory processes in visual pathways induced by unilateral ocular hypertension in the rat. J. Neuroinflamm. 2016, 13, 44. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Cho, K.S.; Vu, T.H.K.; Shen, C.H.; Kaur, M.; Chen, G.; Mathew, R.; McHam, M.L.; Fazelat, A.; Lashkari, K.; et al. Commensal microflora-induced T cell responses mediate progressive neurodegeneration in glaucoma. Nat. Commun. 2018, 9, 3209. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.; Reinehr, S.; Maliha, A.M.; Joachim, S.C. Immune Mediated Degeneration and Possible Protection in Glaucoma. Front. Neurosci. 2019, 13, 931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beutgen, V.M.; Perumal, N.; Pfeiffer, N.; Grus, F.H. Autoantibody Biomarker Discovery in Primary Open Angle Glaucoma Using Serological Proteome Analysis (SERPA). Front. Immunol. 2019, 10, 381. [Google Scholar] [CrossRef]
- Streilein, J.W. Ocular immune privilege: The eye takes a dim but practical view of immunity and inflammation. J. Leukoc. Biol. 2003, 74, 179–185. [Google Scholar] [CrossRef]
- Streilein, J.W.; Stein-Streilein, J. Does innate immune privilege exist? J. Leukoc. Biol. 2000, 67, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Kametani, M.; Chen, D.F. Adaptive Immunity: New Aspects of Pathogenesis Underlying Neurodegeneration in Glaucoma and Optic Neuropathy. Front. Immunol. 2020, 11, 65. [Google Scholar] [CrossRef] [Green Version]
- Forrester, J.V.; Xu, H. Good news–bad news: The Yin and Yang of immune privilege in the eye. Front. Immunol. 2012, 3, 338. [Google Scholar] [CrossRef] [Green Version]
- Nussenblatt, R.B.; Liu, B.; Wei, L.; Sen, H.N. The Immunological Basis of Degenerative Diseases of the Eye. Int. Rev. Immunol. 2013, 32, 97–112. [Google Scholar] [CrossRef]
- Cruz-Guilloty, F.; Perez, V.L. Defence against oxidative damage. Nature 2011, 478, 42–43. [Google Scholar] [CrossRef]
- Geyer, O.; Levo, Y. Glaucoma is an autoimmune disease. Autoimmun. Rev. 2020, 19, 102535. [Google Scholar] [CrossRef]
- Gala, A. Observations on the hydrogen ion concentration in the vitreous body of the eye with reference to glaucoma. Br. J. Ophthalmol. 1925, 9, 516–519. [Google Scholar] [CrossRef] [Green Version]
- Walsh, M.M.; Yi, H.; Friedman, J.; Cho, K.I.; Tserentsoodol, N.; McKinnon, S.; Searle, K.; Yeh, A.; Ferreira, P.A. Gene and protein expression pilot profiling and biomarkers in an experimental mouse model of hypertensive glaucoma. Exp. Biol. Med. 2009, 234, 918–930. [Google Scholar] [CrossRef] [Green Version]
- Carter-Dawson, L.; Crawford, M.L.J.; Harwerth, R.S.; Smith, E.L.; Feldman, R.; Shen, F.F.; Mitchell, C.K.; Whitetree, A. Vitreal glutamate concentration in monkeys with experimental glaucoma. Investig. Ophthalmol. Vis. Sci. 2002, 43, 2633–2637. [Google Scholar]
- Tong, Y.; Zhou, Y.L.; Zheng, Y.; Biswal, M.; Zhao, P.Q.; Wang, Z.Y. Analyzing cytokines as biomarkers to evaluate severity of glaucoma. Int. J. Ophthalmol. 2017, 10, 925–930. [Google Scholar] [CrossRef]
- Gramlich, O.W.; Beck, S.; Hohenstein-Blaul, N.V.T.U.; Boehm, N.; Ziegler, A.; Vetter, J.M.; Pfeiffer, N.; Grus, F.H. Enhanced Insight into the Autoimmune Component of Glaucoma: IgG Autoantibody Accumulation and Pro-Inflammatory Conditions in Human Glaucomatous Retina. PLoS ONE 2013, 8, e57557. [Google Scholar] [CrossRef]
- Wei, X.; Cho, K.-S.; Thee, E.F.; Jager, M.J.; Chen, D.F. Neuroinflammation and microglia in glaucoma: Time for a paradigm shift. J. Neurosci. Res. 2019, 97, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Gallego, B.I.; Salazar, J.J.; de Hoz, R.; Rojas, B.; Ramírez, A.I.; Salinas-Navarro, M.; Ortín-Martínez, A.; Valiente-Soriano, F.J.; Avilés-Trigueros, M.; Villegas-Perez, M.P.; et al. IOP induces upregulation of GFAP and MHC-II and microglia reactivity in mice retina contralateral to experimental glaucoma. J. Neuroinflamm. 2012, 9, 586. [Google Scholar] [CrossRef] [Green Version]
- Gramlich, O.W.; Teister, J.; Neumann, M.; Tao, X.; Beck, S.; von Pein, H.D.; Pfeiffer, N.; Grus, F.H. Immune response after intermittent minimally invasive intraocular pressure elevations in an experimental animal model of glaucoma. J. Neuroinflamm. 2016, 13, 82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kozlowski, C.; Weimer, R.M. An Automated Method to Quantify Microglia Morphology and Application to Monitor Activation State Longitudinally In Vivo. PLoS ONE 2012, 7, e31814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kettenmann, H.; Hanisch, U.-K.; Noda, M.; Verkhratsky, A. Physiology of Microglia. Physiol. Rev. 2011, 91, 461–553. [Google Scholar] [CrossRef] [PubMed]
- Bosco, A.; Romero, C.O.; Ambati, B.K.; Vetter, M.L. In Vivo Dynamics of Retinal Microglial Activation During Neurodegeneration: Confocal Ophthalmoscopic Imaging and Cell Morphometry in Mouse Glaucoma. J. Vis. Exp. 2015, 2015, 52731. [Google Scholar] [CrossRef]
- Kezic, J.M.; Chrysostomou, V.; Trounce, I.A.; McMenamin, P.G.; Crowston, J.G. Effect of anterior chamber cannulation and acute IOP elevation on retinal macrophages in the adult mouse. Investig. Ophthalmol. Vis. Sci. 2013, 54, 3028–3036. [Google Scholar] [CrossRef] [PubMed]
- Bosco, A.; Romero, C.O.; Breen, K.T.; Chagovetz, A.A.; Steele, M.R.; Ambati, B.K.; Vetter, M.L. Neurodegeneration severity can be predicted from early microglia alterations monitored in vivo in a mouse model of chronic glaucoma. Dis. Model. Mech. 2015, 8, 443–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holekamp, N.M. The Vitreous Gel: More than Meets the Eye. Am. J. Ophthalmol. 2010, 149, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Siegfried, C.J.; Shui, Y.B. Intraocular Oxygen and Antioxidant Status: New Insights on the Effect of Vitrectomy and Glaucoma Pathogenesis. Am. J. Ophthalmol. 2019, 203, 12–25. [Google Scholar] [CrossRef] [Green Version]
- Sebag, J. Imaging vitreous. Eye 2002, 16, 429–439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boehm, M.R.R.; Oellers, P.; Thanos, S. Inflammation and immunology of the vitreoretinal compartment. Inflamm. Allergy Drug Targets 2011, 10, 283–309. [Google Scholar] [CrossRef]
- Sakamoto, T.; Ishibashi, T. Hyalocytes: Essential cells of the vitreous cavity in vitreoretinal pathophysiology? Retina 2011, 31, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Vagaja, N.N.; Chinnery, H.R.; Binz, N.; Kezic, J.M.; Rakoczy, E.P.; McMenamin, P.G. Changes in murine hyalocytes are valuable early indicators of ocular disease. Investig. Ophthalmol. Vis. Sci. 2012, 53, 1445–1451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahendradas, P.; Madhu, S.; Kawali, A.; Roy, A.S.; Vala, R.; Vinekar, A.; Shetty, R. Enhanced Vitreous Imaging in Uveitis. Ocul. Immunol. Inflamm. 2019, 27, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Mehta, M.; Rasheed, R.A.; Duker, J.; Reichel, E.; Feinberg, E.; Husain, D.; Foster, C.S.; Laver, N.V. Vitreous evaluation: A diagnostic challenge. Ophthalmology 2015, 122, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Keane, P.A.; Karampelas, M.; Sim, D.A.; Sadda, S.R.; Tufail, A.; Sen, H.N.; Nussenblatt, R.B.; Dick, A.D.; Lee, R.W.; Murray, P.I.; et al. Objective measurement of vitreous inflammation using optical coherence tomography. Ophthalmology 2014, 121, 1706–1714. [Google Scholar] [CrossRef] [Green Version]
- Uji, A.; Yoshimura, N. Microarchitecture of the Vitreous Body: A High-Resolution Optical Coherence Tomography Study. Am. J. Ophthalmol. 2016, 168, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Sreekantam, S.; Macdonald, T.; Keane, P.A.; Sim, D.A.; Murray, P.I.; Denniston, A.K. Quantitative analysis of vitreous inflammation using optical coherence tomography in patients receiving sub-Tenon’s triamcinolone acetonide for uveitic cystoid macular oedema. Br. J. Ophthalmol. 2017, 101, 175–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chu, C.J.; Herrmann, P.; Carvalho, L.S.; Liyanage, S.E.; Bainbridge, J.W.B.; Ali, R.R.; Dick, A.D.; Luhmann, U.F.O. Assessment and In Vivo Scoring of Murine Experimental Autoimmune Uveoretinitis Using Optical Coherence Tomography. PLoS ONE 2013, 8, e63002. [Google Scholar] [CrossRef] [Green Version]
- Korot, E.; Comer, G.; Steffens, T.; Antonetti, D.A. Algorithm for the Measure of Vitreous Hyperreflective Foci in Optical Coherence Tomographic Scans of Patients with Diabetic Macular Edema. JAMA Ophthalmol. 2016, 134, 15–20. [Google Scholar] [CrossRef]
- Rodrigo, M.J.; Del Palomar, A.P.; Montolío, A.; Mendez-Martinez, S.; Subias, M.; Cardiel, M.J.; Martinez-Rincon, T.; Cegoñino, J.; Fraile, J.M.; Vispe, E.; et al. Monitoring new long-lasting intravitreal formulation for glaucoma with vitreous images using optical coherence tomography. Pharmaceutics 2021, 13, 217. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, M.J.; Garcia-Herranz, D.; Subias, M.; Martinez-Rincón, T.; Mendez-Martínez, S.; Bravo-Osuna, I.; Carretero, A.; Ruberte, J.; Garcia-Feijoo, J.; Pablo, L.E.; et al. Chronic Glaucoma Using Biodegradable Microspheres to Induce Intraocular Pressure Elevation. Six-Month Follow-Up. Biomedicines 2021, 9, 682. [Google Scholar] [CrossRef]
- Garcia-Herranz, D.; Rodrigo, M.J.; Subias, M.; Martinez-Rincon, T.; Mendez-Martinez, S.; Bravo-Osuna, I.; Bonet, A.; Ruberte, J.; Garcia-Feijoo, J.; Pablo, L.; et al. Novel Use of PLGA Microspheres to Create an Animal Model of Glaucoma with Progressive Neuroretinal Degeneration. Pharmaceutics 2021, 13, 237. [Google Scholar] [CrossRef]
- Liu, X.; Wang, C.-H.; Dai, C.; Camesa, A.; Zhang, H.F.; Jiao, S. Effect of Contact Lens on Optical Coherence Tomography Imaging of Rodent Retina. Curr. Eye Res. 2013, 38, 1235. [Google Scholar] [CrossRef] [Green Version]
- Ogawa, K. Scanning electron microscopic study of hyalocytes in the guinea pig eye. Arch. Histol. Cytol. 2002, 65, 263–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otsu, N. A Threshold Selection Method from Gray-Level Histograms. IEEE Trans. Syst. Man. Cybern. 1979, 9, 62–66. [Google Scholar] [CrossRef] [Green Version]
- Liba, O.; Lew, M.D.; Sorelle, E.D.; Dutta, R.; Sen, D.; Moshfeghi, D.M.; Chu, S.; De La Zerda, A. Speckle-modulating optical coherence tomography in living mice and humans. Nat. Commun. 2017, 8, 15845. [Google Scholar] [CrossRef] [Green Version]
- London, A.; Itskovich, E.; Benhar, I.; Kalchenko, V.; Mack, M.; Jung, S.; Schwartz, M. Neuroprotection and progenitor cell renewal in the injured adult murine retina requires healing monocyte-derived macrophages. J. Exp. Med. 2011, 208, 23–39. [Google Scholar] [CrossRef]
- Jacobs, A.H.; Tavitian, B. Noninvasive Molecular Imaging of Neuroinflammation. J. Cereb. Blood Flow Metab. 2012, 32, 1393–1415. [Google Scholar] [CrossRef] [Green Version]
- Ajami, B.; Bennett, J.L.; Krieger, C.; Tetzlaff, W.; Rossi, F.M.V. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat. Neurosci. 2007, 10, 1538–1543. [Google Scholar] [CrossRef] [PubMed]
- Ajami, B.; Bennett, J.L.; Krieger, C.; McNagny, K.M.; Rossi, F.M.V. Infiltrating monocytes trigger EAE progression, but do not contribute to the resident microglia pool. Nat. Neurosci. 2011, 14, 1142–1150. [Google Scholar] [CrossRef]
- Damisah, E.C.; Hill, R.A.; Rai, A.; Chen, F.; Rothlin, C.V.; Ghosh, S.; Grutzendler, J. Astrocytes and microglia play orchestrated roles and respect phagocytic territories during neuronal corpse removal in vivo. Sci. Adv. 2020, 6, eaba3239. [Google Scholar] [CrossRef]
- Tay, T.L.; Béchade, C.; D’Andrea, I.; St-Pierre, M.K.; Henry, M.S.; Roumier, A.; Tremblay, M.E. Microglia gone rogue: Impacts on psychiatric disorders across the lifespan. Front. Mol. Neurosci. 2018, 10, 421. [Google Scholar] [CrossRef] [Green Version]
- Rodrigo, M.J.; Martinez-Rincon, T.; Subias, M.; Mendez-Martinez, S.; Luna, C.; Pablo, L.E.; Polo, V.; Garcia-Martin, E. Effect of age and sex on neurodevelopment and neurodegeneration in the healthy eye: Longitudinal functional and structural study in the Long–Evans rat. Exp. Eye Res. 2020, 200, 108208. [Google Scholar] [CrossRef]
- Medawar, P.B. Immunity to homologous grafted skin; the fate of skin homografts. Br. J. Exp. Pathol. 1948, 29, 58–69. [Google Scholar]
- Kehlet, S.N.; Willumsen, N.; Armbrecht, G.; Dietzel, R.; Brix, S.; Henriksen, K.; Karsdal, M.A. Age-related collagen turnover of the interstitial matrix and basement membrane: Implications of age- and sex-dependent remodeling of the extracellular matrix. PLoS ONE 2018, 13, e0194458. [Google Scholar] [CrossRef] [Green Version]
- Perez, V.L.; Caspi, R.R. Immune mechanisms in inflammatory and degenerative eye disease. Trends Immunol. 2015, 36, 354–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, M.; Provis, J.M.; Penfold, P.L. The human hyaloid system: Cellular phenotypes and inter-relationships. Exp. Eye Res. 1999, 68, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Qiao, H.; Hisatomi, T.; Sonoda, K.H.; Kura, S.; Sassa, Y.; Kinoshita, S.; Nakamura, T.; Sakamoto, T.; Ishibashi, T. The characterisation of hyalocytes: The origin, phenotype, and turnover. Br. J. Ophthalmol. 2005, 89, 513–517. [Google Scholar] [CrossRef] [PubMed]
- Imai, Y.; Ibata, I.; Ito, D.; Ohsawa, K.; Kohsaka, S. A novel gene iba1 in the major histocompatibility complex class III region encoding an EF hand protein expressed in a monocytic lineage. Biochem. Biophys. Res. Commun. 1996, 224, 855–862. [Google Scholar] [CrossRef]
- Ahmed, Z.; Shaw, G.; Sharma, V.P.; Yang, C.; McGowan, E.; Dickson, D.W. Actin-binding proteins coronin-1a and IBA-1 are effective microglial markers for immunohistochemistry. J. Histochem. Cytochem. 2007, 55, 687–700. [Google Scholar] [CrossRef]
- Santos, A.M.; Martin-Oliva, D.; Ferrer-Martãn, R.M.; Tassi, M.; Calvente, R.; Sierra, A.; Carrasco, M.-C.; Marin-Teva, J.L.; Navascues, J.; Cuadros, M.A. Microglial response to light-induced photoreceptor degeneration in the mouse retina. J. Comp. Neurol. 2010, 518, 477–492. [Google Scholar] [CrossRef] [PubMed]
- Noda, Y.; Hata, Y.; Hisatomi, T.; Nakamura, Y.; Hirayama, K.; Miura, M.; Nakao, S.; Fujisawa, K.; Sakamoto, T.; Ishibashi, T. Functional properties of hyalocytes under PDGF-rich conditions. Investig. Ophthalmol. Vis. Sci. 2004, 45, 2107–2114. [Google Scholar] [CrossRef] [Green Version]
- Castanos, M.V.; Zhou, D.B.; Linderman, R.E.; Allison, R.; Milman, T.; Carroll, J.; Migacz, J.; Rosen, R.B.; Chui, T.Y.P. Imaging of Macrophage-Like Cells in Living Human Retina Using Clinical OCT. Investig. Ophthalmol. Vis. Sci. 2020, 61, 48. [Google Scholar] [CrossRef]
- Staurenghi, G.; Sadda, S.; Chakravarthy, U.; Spaide, R.F. Proposed lexicon for anatomic landmarks in normal posterior segment spectral-domain optical coherence tomography: The IN•OCT consensus. Ophthalmology 2014, 121, 1572–1578. [Google Scholar] [CrossRef] [PubMed]
- Choudhry, N.; Duker, J.S.; Freund, K.B.; Kiss, S.; Querques, G.; Rosen, R.; Sarraf, D.; Souied, E.H.; Stanga, P.E.; Staurenghi, G.; et al. Classification and Guidelines for Widefield Imaging: Recommendations from the International Widefield Imaging Study Group. Ophthalmol. Retin. 2019, 3, 843–849. [Google Scholar] [CrossRef]
- Ramírez, A.I.; Fernández-Albarral, J.A.; de Hoz, R.; López-Cuenca, I.; Salobrar-García, E.; Rojas, P.; Valiente-Soriano, F.J.; Avilés-Trigueros, M.; Villegas-Pérez, M.P.; Vidal-Sanz, M.; et al. Microglial changes in the early aging stage in a healthy retina and an experimental glaucoma model. In Progress in Brain Research; Elsevier: Amsterdam, The Netherlands, 2020; Volume 256, pp. 125–149. ISBN 9780128211069. [Google Scholar]
- Saito, M.; Barbazetto, I.A.; Spaide, R.F. Intravitreal Cellular Infiltrate Imaged as Punctate Spots by Spectral-Domain Optical Coherence Tomography in Eyes with Posterior Segment Inflammatory Disease. Retina 2013, 33, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Keane, P.A.; Balaskas, K.; Sim, D.A.; Aman, K.; Denniston, A.K.; Aslam, T.; Aslam, T. Automated analysis of vitreous inflammation using spectral-domain optical coherence tomography. Transl. Vis. Sci. Technol. 2015, 4, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zarranz-Ventura, J.; Keane, P.A.; Sim, D.A.; Llorens, V.; Tufail, A.; Sadda, S.R.; Dick, A.D.; Lee, R.W.; Pavesio, C.; Denniston, A.K.; et al. Evaluation of Objective Vitritis Grading Method Using Optical Coherence Tomography: Influence of Phakic Status and Previous Vitrectomy. Am. J. Ophthalmol. 2016, 161, 172–180. [Google Scholar] [CrossRef] [Green Version]
- Russo, R.; Varano, G.P.; Adornetto, A.; Nucci, C.; Corasaniti, M.T.; Bagetta, G.; Morrone, L.A. Retinal ganglion cell death in glaucoma: Exploring the role of neuroinflammation. Eur. J. Pharmacol. 2016, 787, 134–142. [Google Scholar] [CrossRef]
- Tezel, G. The immune response in glaucoma: A perspective on the roles of oxidative stress. Exp. Eye Res. 2011, 93, 178–186. [Google Scholar] [CrossRef] [Green Version]
- Taylor, A.W.; Ng, T.F. Negative regulators that mediate ocular immune privilege. J. Leukoc. Biol. 2018, 103, 1179–1187. [Google Scholar] [CrossRef]
- Gordon, S.; Plüddemann, A.; Martinez Estrada, F. Macrophage heterogeneity in tissues: Phenotypic diversity and functions. Immunol. Rev. 2014, 262, 36–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forrester, J.V.; Xu, H.; Kuffová, L.; Dick, A.D.; McMenamin, P.G. Dendritic cell physiology and function in the eye. Immunol. Rev. 2010, 234, 282–304. [Google Scholar] [CrossRef]
- Skeie, J.M.; Mahajan, V.B. Dissection of Human Vitreous Body Elements for Proteomic Analysis. J. Vis. Exp. 2011, 47, 2455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, L.Q.; Streilein, J.W. Immune Privilege Extended to Allogeneic Tumor Cells in the Vitreous Cavity. Investig. Ophthalmol. Vis. Sci. 1991, 32, 224–228. [Google Scholar]
- Bain, C.C.; Gibson, D.A.; Steers, N.J.; Boufea, K.; Louwe, P.A.; Doherty, C.; González-Huici, V.; Gentek, R.; Magalhaes-Pinto, M.; Shaw, T.; et al. Rate of replenishment and microenvironment contribute to the sexually dimorphic phenotype and function of peritoneal macrophages. Sci. Immunol. 2020, 5, eabc4466. [Google Scholar] [CrossRef]
- Hanamsagar, R.; Bilbo, S.D. Sex differences in neurodevelopmental and neurodegenerative disorders: Focus on microglial function and neuroinflammation during development. J. Steroid Biochem. Mol. Biol. 2016, 160, 127–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klein, S.L.; Flanagan, K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016, 16, 626–638. [Google Scholar] [CrossRef] [PubMed]
- Ngo, S.T.; Steyn, F.J.; McCombe, P.A. Gender differences in autoimmune disease. Front. Neuroendocrinol. 2014, 35, 347–369. [Google Scholar] [CrossRef] [Green Version]
- Rodrigo, M.J.; Martinez-Rincon, T.; Subias, M.; Mendez-Martinez, S.; Pablo, L.E.; Polo, V.; Aragon-Navas, A.; Garcia-Herranz, D.; Feijoo, J.G.; Osuna, I.B.; et al. Influence of Sex on Neuroretinal Degeneration: Six-Month Follow-Up in Rats with Chronic Glaucoma. Investig. Ophthalmol. Vis. Sci. 2021, 62, 9. [Google Scholar] [CrossRef] [PubMed]
- Zanon-Moreno, V.; Raga-Cervera, J.; García-Medina, J.J.; Benitez-del-Castillo, J.; Vinuesa-Silva, I.; Torregrosa, S.; Pinazo-Durán, M.D. Nuevos horizontes para el tratamiento del glaucoma. I: Neuroinflamación e inflamasomas. Arch. Soc. Esp. Oftalmol. 2018, 93, e7–e9. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, Z.; Weinreb, R.N.; Xu, G.; Lindsey, J.D.; Ye, C.; Yung, W.; Pang, C.-P.; Lam, D.S.C.; Leung, C.K. Tracking Retinal Microgliosis in Models of Retinal Ganglion Cell Damage. Investig. Opthalmol. Vis. Sci. 2012, 53, 6254. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Caspi, R.R. Ocular immune privilege. F1000 Biol. Rep. 2010, 2, 3. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, A.I.; de Hoz, R.; Fernández-Albarral, J.A.; Salobrar-Garcia, E.; Rojas, B.; Valiente-Soriano, F.J.; Avilés-Trigueros, M.; Villegas-Pérez, M.P.; Vidal-Sanz, M.; Triviño, A.; et al. Time course of bilateral microglial activation in a mouse model of laser-induced glaucoma. Sci. Rep. 2020, 10, 4890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, W.; Li, X.; Wang, W.; Mo, J.S.; Kaplan, H.; Cooper, N.G.F. Early Involvement of Immune/Inflammatory Response Genes in Retinal Degeneration in DBA/2J Mice. Ophthalmol. Eye Dis. 2010, 1, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashimoto, D.; Chow, A.; Noizat, C.; Teo, P.; Beasley, M.B.; Leboeuf, M.; Becker, C.D.; See, P.; Price, J.; Lucas, D.; et al. Tissue-Resident Macrophages Self-Maintain Locally throughout Adult Life with Minimal Contribution from Circulating Monocytes. Immunity 2013, 38, 792–804. [Google Scholar] [CrossRef] [Green Version]
- Ling, E.A. Transformation of monocytes into amoeboid microglia and into microglia in the corpus callosum of postnatal rats, as shown by labelling monocytes by carbon particles. J. Anat. 1979, 128, 847–858. [Google Scholar]
- O’Koren, E.G.; Mathew, R.; Saban, D.R. Fate mapping reveals that microglia and recruited monocyte-derived macrophages are definitively distinguishable by phenotype in the retina. Sci. Rep. 2016, 6, 20636. [Google Scholar] [CrossRef]
- Liu, B.; Hong, J.-S. Role of Microglia in Inflammation-Mediated Neurodegenerative Diseases: Mechanisms and Strategies for Therapeutic Intervention. J. Pharmacol. Exp. Ther. 2003, 304, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Venneti, S.; Lopresti, B.J.; Wiley, C.A. Molecular imaging of microglia/macrophages in the brain. Glia 2013, 61, 10–23. [Google Scholar] [CrossRef] [Green Version]
- Coric, D.; Ometto, G.; Montesano, G.; Keane, P.A.; Balk, L.J.; Uitdehaag, B.M.J.; Petzold, A.; Crabb, D.P.; Denniston, A.K. Objective quantification of vitreous haze on optical coherence tomography scans: No evidence for relationship between uveitis and inflammation in multiple sclerosis. Eur. J. Neurol. 2020, 27, 144. [Google Scholar] [CrossRef] [PubMed]

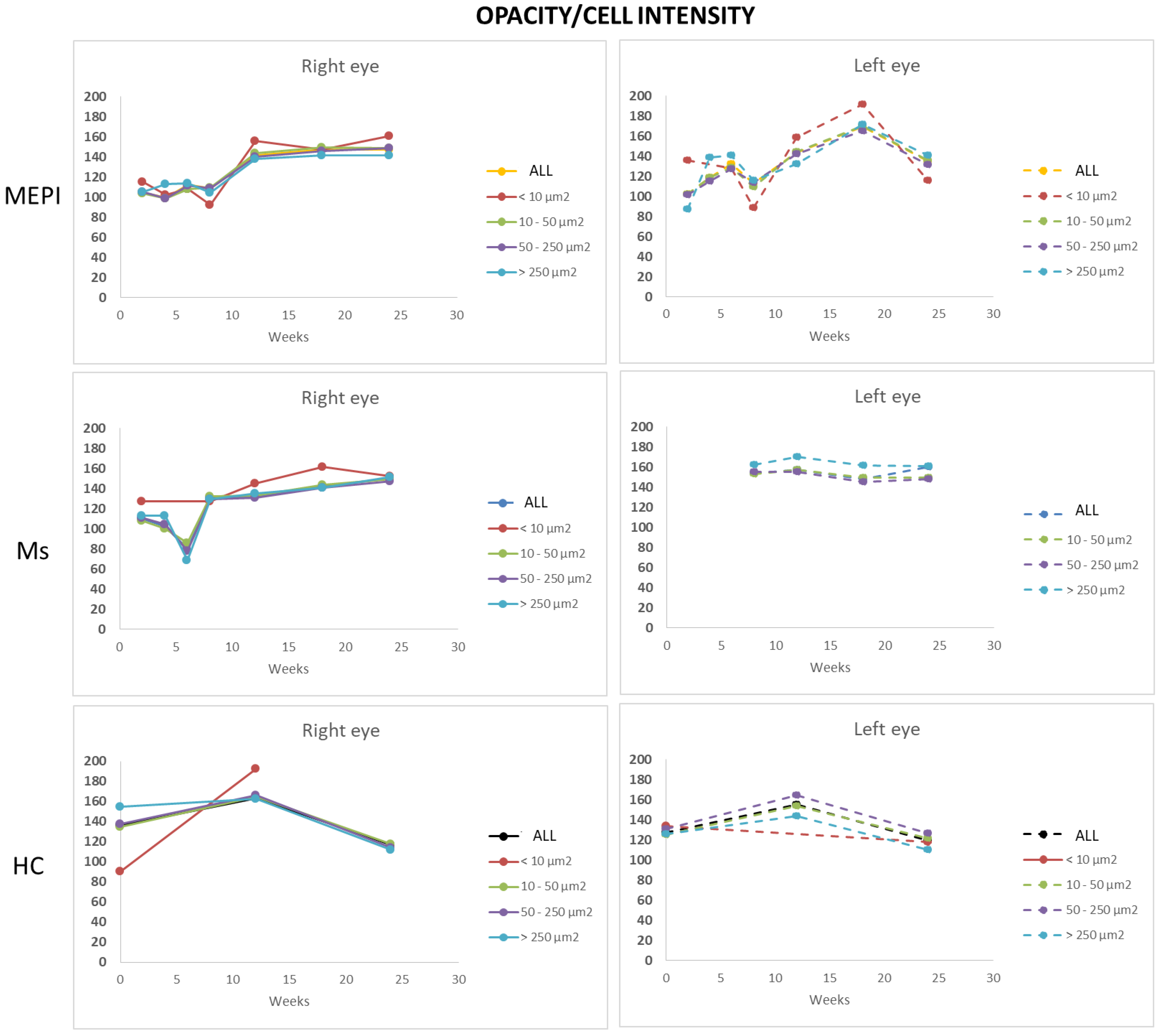
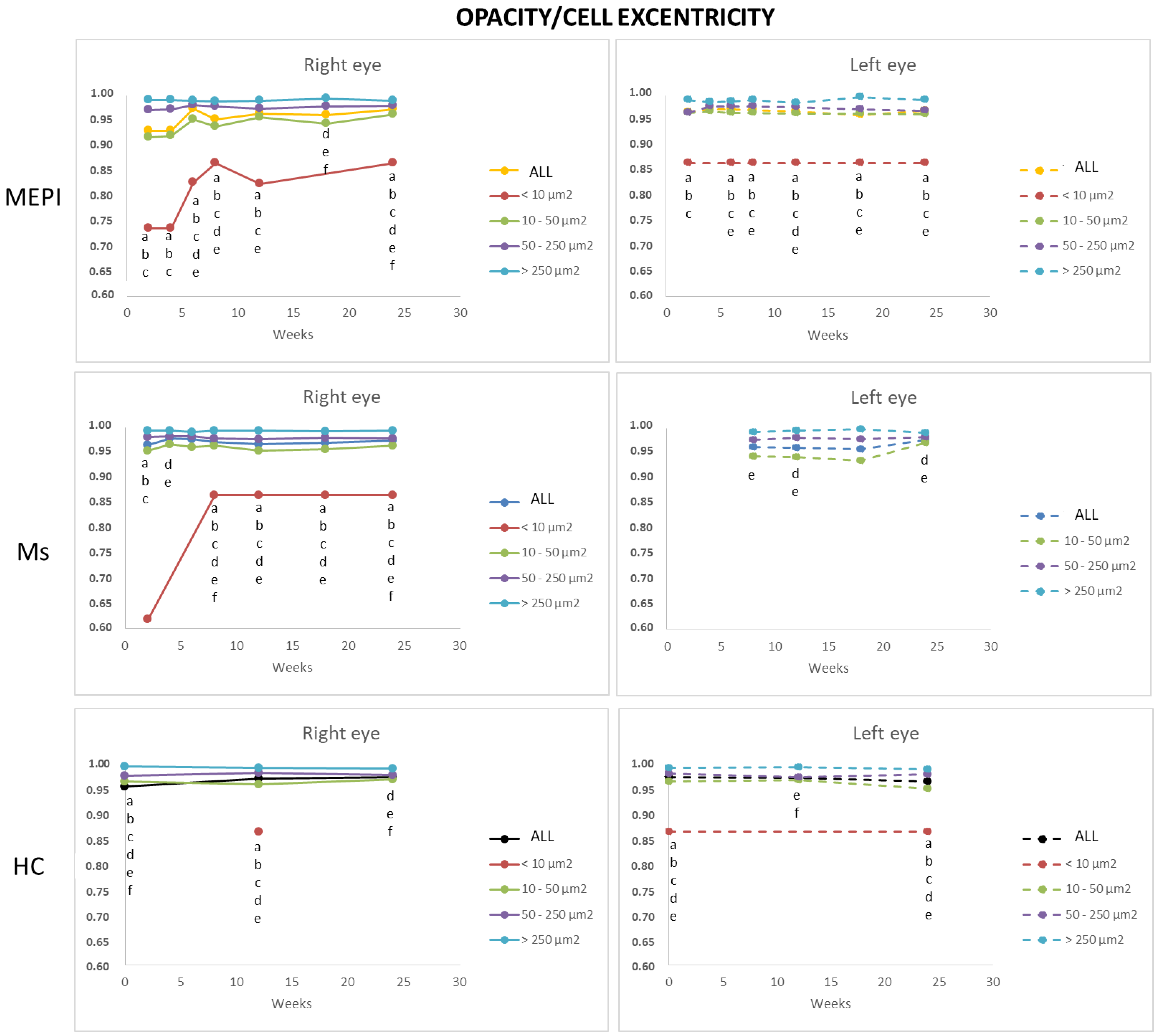
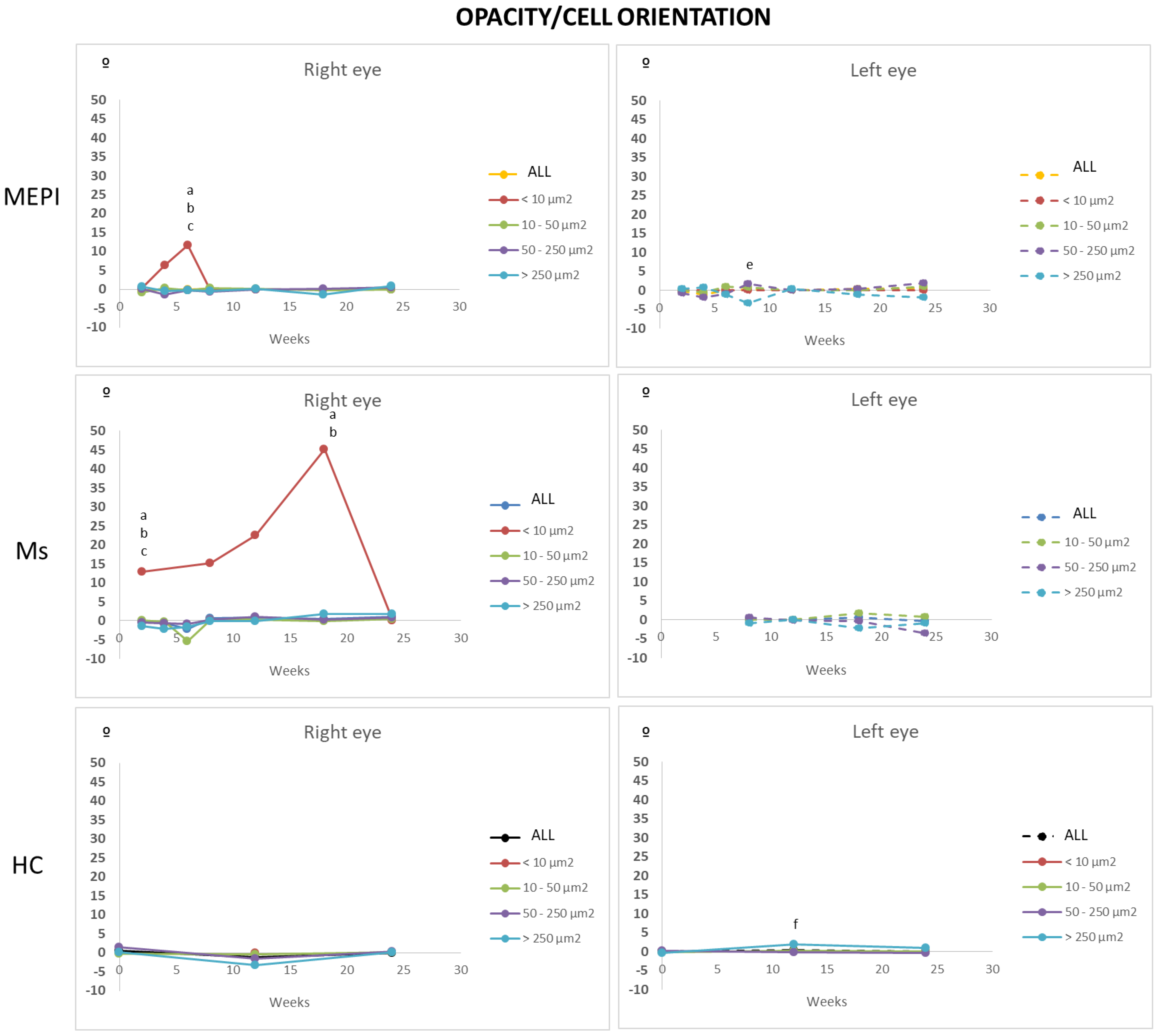
| RIGHT EYE | LEFT EYE | |||||
|---|---|---|---|---|---|---|
| MEPI | Ms | HC | MEPI | Ms | HC | |
| INJ/IOP | Inj/6 w (m) | Inj/8 w (m) | ||||
| IOP/IOP | 0 w/8 w (im) 2 w/4–6–8 w (m) 2 w/24 w (r = 0.816, p = 0.025) | 2 w/4 w (m) 8 w/12 w (m) 8 w/24 w (r = 0.917, p = 0.028) | 4 w/8 w (r = 0.934, p = 0.020) | 0 w/8 w (im) | 4 w/12 w (r = 0.800, p = 0.004) | |
| IOP/OCT | 2 w/2 w (r = 0.812, p = 0.027) | 0 w/18 w (r = −0.979 p = 0.004) 24 w/24 w (r = 0.885, p = 0.008) | 18 w/18 w (r = 0.890 p = 0.043) | |||
| OCT/OCT | 6 w/12 w (m) (r = 0.762, p = 0.001) | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigo, M.J.; Subías, M.; Montolío, A.; Méndez-Martínez, S.; Martínez-Rincón, T.; Arias, L.; García-Herranz, D.; Bravo-Osuna, I.; Garcia-Feijoo, J.; Pablo, L.; et al. Analysis of Parainflammation in Chronic Glaucoma Using Vitreous-OCT Imaging. Biomedicines 2021, 9, 1792. https://doi.org/10.3390/biomedicines9121792
Rodrigo MJ, Subías M, Montolío A, Méndez-Martínez S, Martínez-Rincón T, Arias L, García-Herranz D, Bravo-Osuna I, Garcia-Feijoo J, Pablo L, et al. Analysis of Parainflammation in Chronic Glaucoma Using Vitreous-OCT Imaging. Biomedicines. 2021; 9(12):1792. https://doi.org/10.3390/biomedicines9121792
Chicago/Turabian StyleRodrigo, María Jesús, Manuel Subías, Alberto Montolío, Silvia Méndez-Martínez, Teresa Martínez-Rincón, Lorena Arias, David García-Herranz, Irene Bravo-Osuna, Julian Garcia-Feijoo, Luis Pablo, and et al. 2021. "Analysis of Parainflammation in Chronic Glaucoma Using Vitreous-OCT Imaging" Biomedicines 9, no. 12: 1792. https://doi.org/10.3390/biomedicines9121792
APA StyleRodrigo, M. J., Subías, M., Montolío, A., Méndez-Martínez, S., Martínez-Rincón, T., Arias, L., García-Herranz, D., Bravo-Osuna, I., Garcia-Feijoo, J., Pablo, L., Cegoñino, J., Herrero-Vanrell, R., Carretero, A., Ruberte, J., Garcia-Martin, E., & Pérez del Palomar, A. (2021). Analysis of Parainflammation in Chronic Glaucoma Using Vitreous-OCT Imaging. Biomedicines, 9(12), 1792. https://doi.org/10.3390/biomedicines9121792






