Repetitive Trans Spinal Magnetic Stimulation Improves Functional Recovery and Tissue Repair in Contusive and Penetrating Spinal Cord Injury Models in Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Care and Use Statement
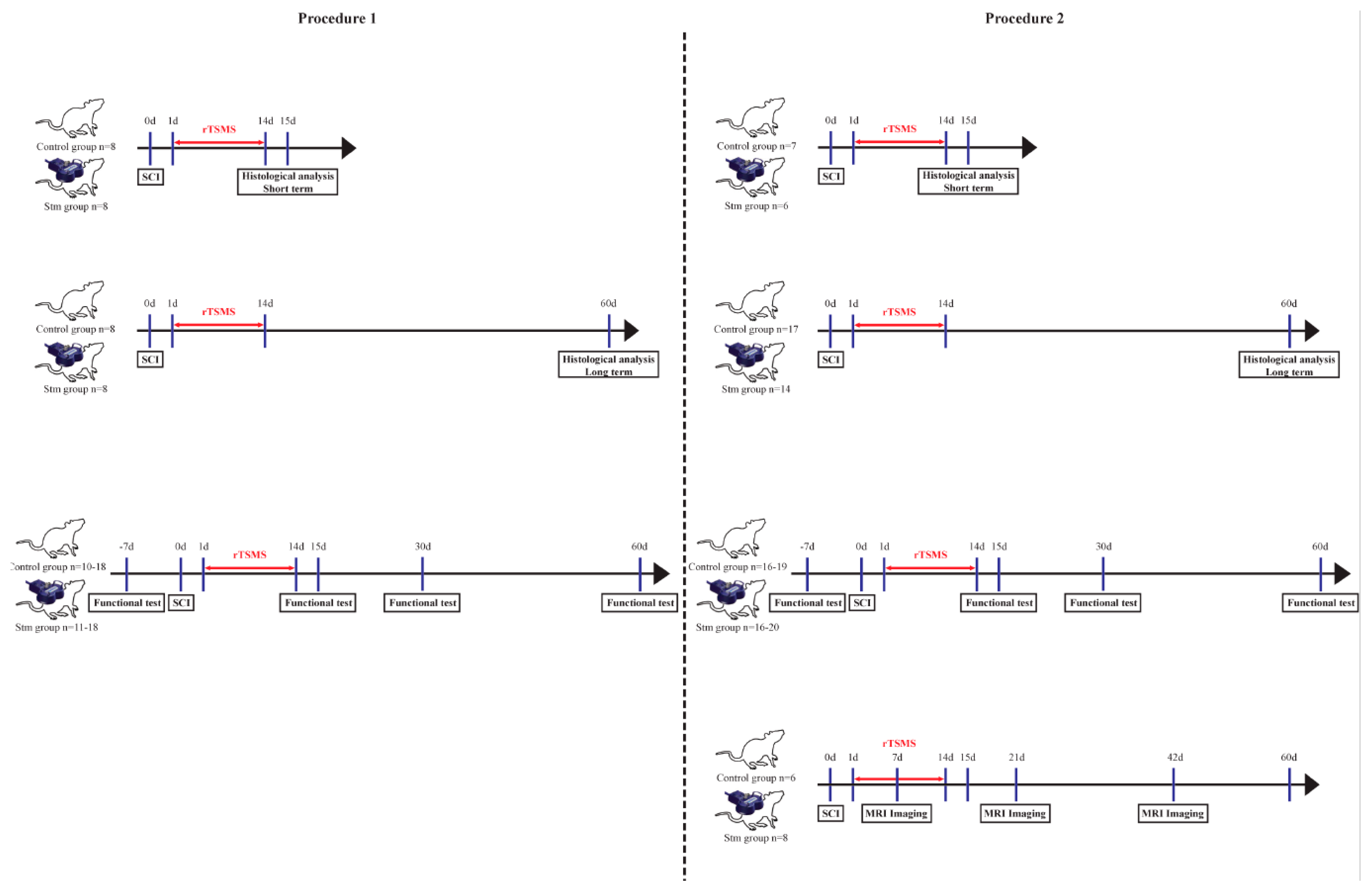
2.2. Experimental Design
2.2.1. Procedure 1
Surgical Procedure
- - Two groups of 8 rats for the immunohistological experiments 15 days after SCI;
- - Two groups of 20 rats for functional tests and for immunohistological experiments 60 days after SCI.
2.2.2. Procedure 2
Surgical Procedure
2.3. rTSMS Treatment
2.4. Functional Test
2.4.1. Locotronic Test: Foot Misplacement Apparatus
2.4.2. Hargreaves Apparatus
2.5. MRI Imaging
- ParaVision software (Paravision 5.1, Bruker Biospin MRI, Ettlingen, Germany) to determine the surface quantification of hyposignal and hypersignal, but also identify tissues structure on sagittal images;
- Osirix software (OsiriX 11, Pixmeo SARL, Geneva, Switzerland), to determine the volume of spinal cord section and realize a 3D representation on axial images.
2.6. Tissue Preparation and Sectioning
2.7. Immunohistochemistry
2.8. Image Acquisition Analysis
2.9. Quantification of Immunohistochemically Stained Areas
2.10. Statistical Analysis
3. Results
3.1. Procedure 1
3.1.1. rTSMS Treatment Induces Locomotor Recovery after a Complete Transection of the Spinal Cord in Rats
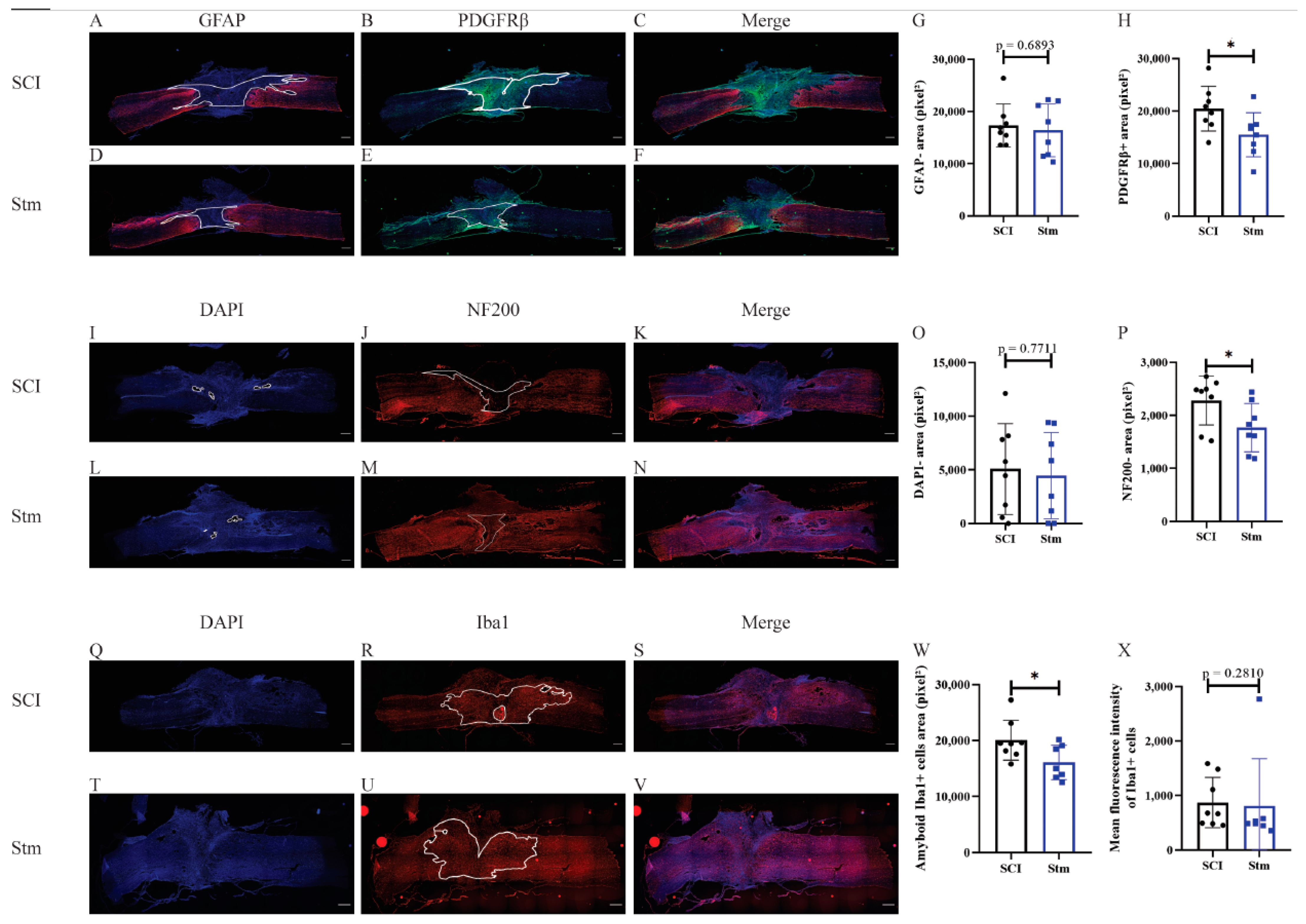
3.1.2. rTSMS Treatment Enhances Tissue Repair after a Complete Transection of the Spinal Cord in Rats
3.2. Procedure 2
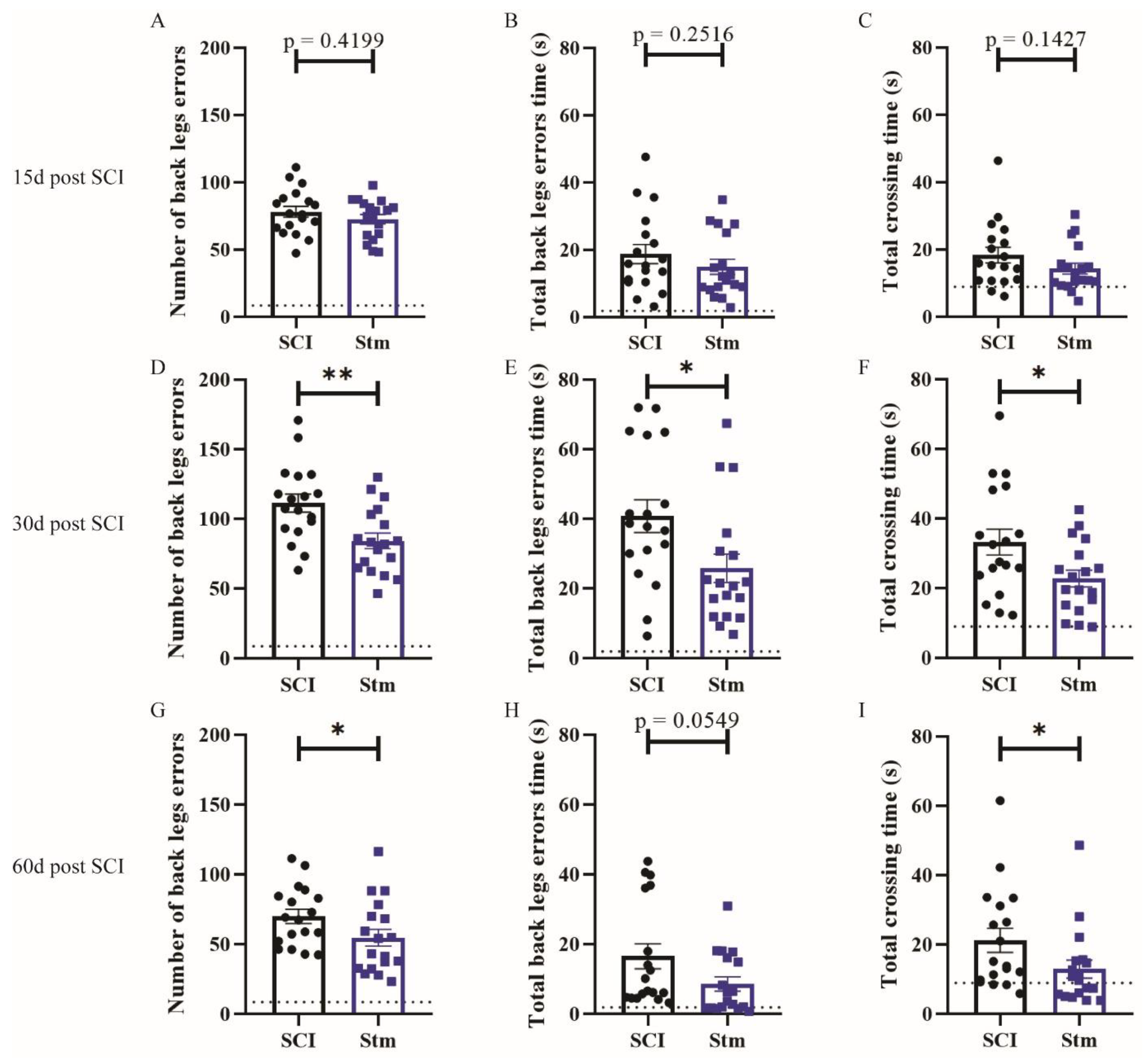
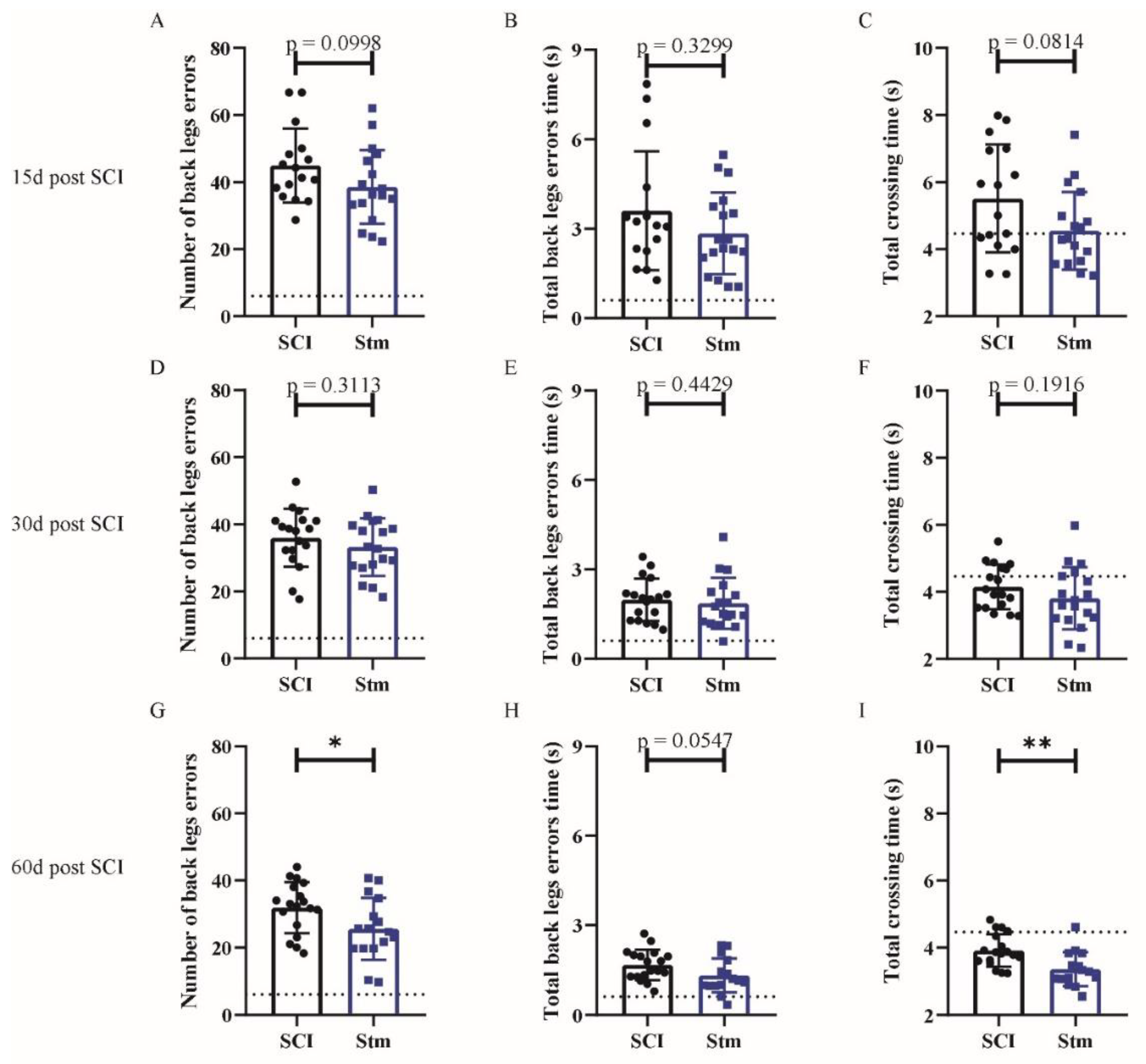
3.2.1. rTSMS Treatment Induces Functional Recovery after Contusive SCI in Rats
3.2.2. MRI Analyses Show That rTSMS Treatment Decreases Cystic Cavities and Increases Spinal Cord Spared Tissue
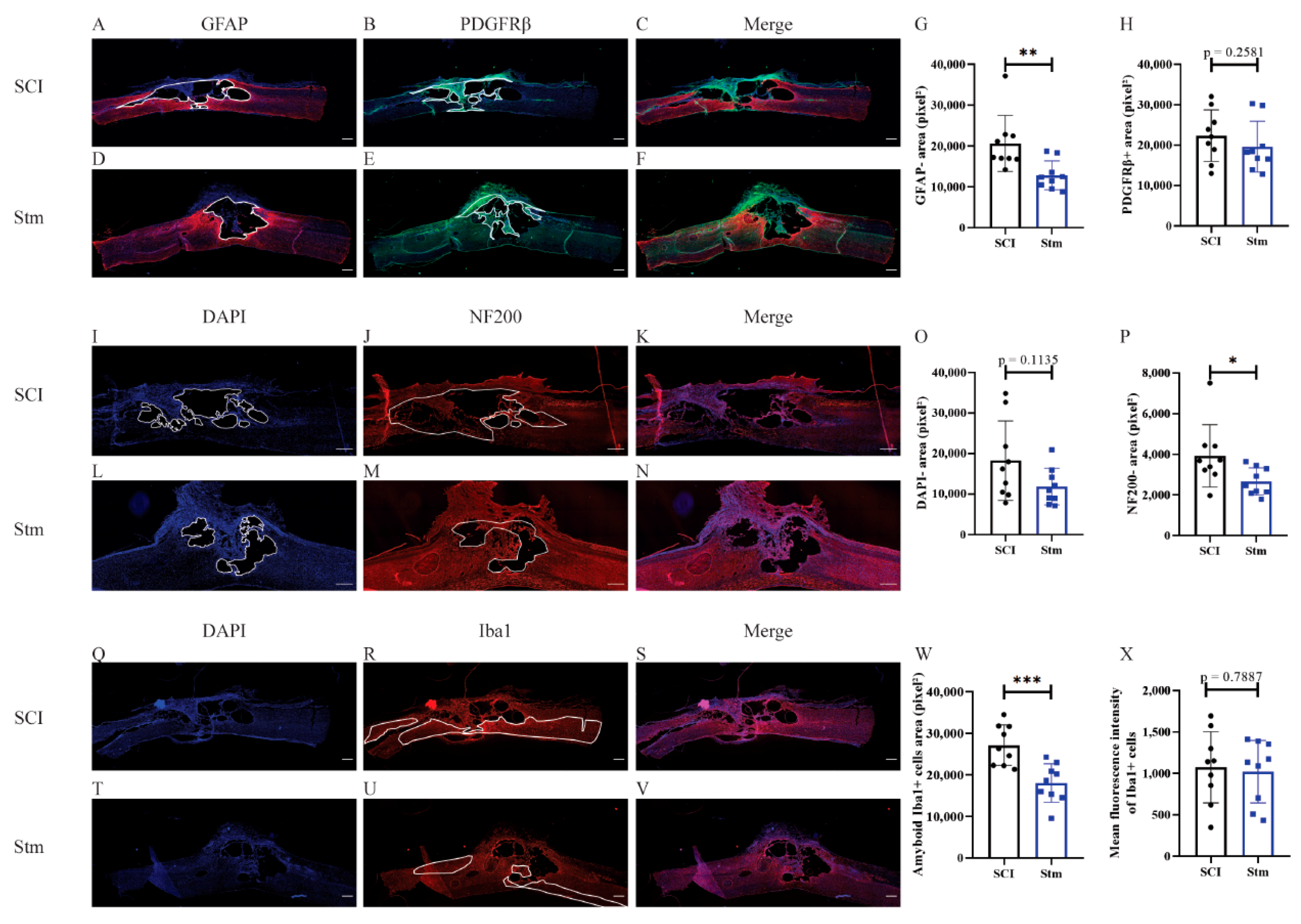
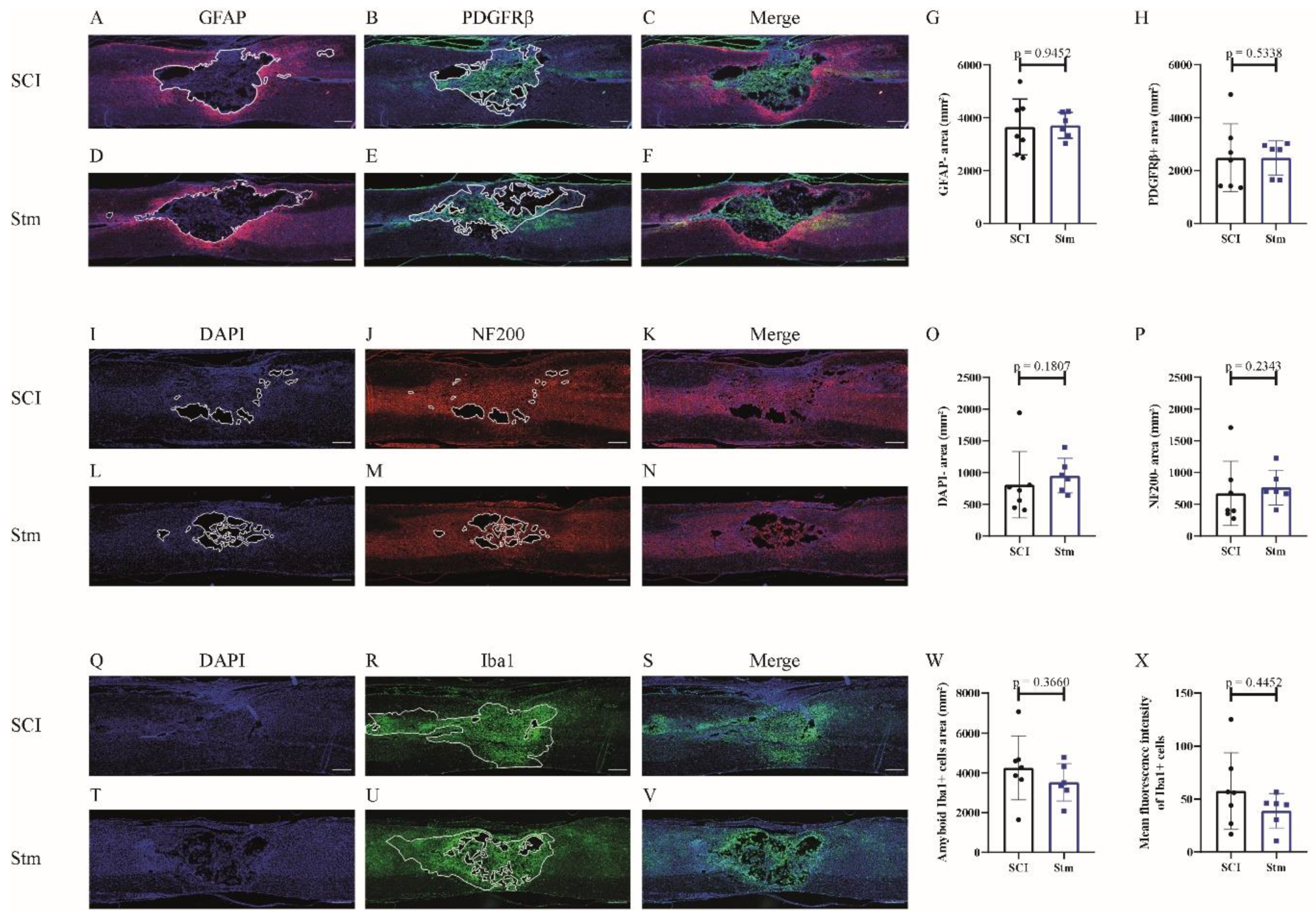
3.2.3. rTSMS Treatment Enhances Tissue Repair after Contusive SCI in Rats 60 Days after SCI
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guérout, N. Plasticity of the injured spinal cord. Cells 2021, 10, 1886. [Google Scholar] [CrossRef] [PubMed]
- Hejrati, N.; Fehlings, M.G. A review of emerging neuroprotective and neuroregenerative therapies in traumatic spinal cord injury. Curr. Opin. Pharmacol. 2021, 60, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Brockie, S.; Hong, J.; Fehlings, M.G. The role of microglia in modulating neuroinflammation after spinal cord injury. Int. J. Mol. Sci. 2021, 22, 9706. [Google Scholar] [CrossRef]
- Grégoire, C.-A.; Goldenstein, B.L.; Floriddia, E.M.; Barnabé-Heider, F.; Fernandes, K.J.L. Endogenous neural stem cell responses to stroke and spinal cord injury. Glia 2015, 63, 1469–1482. [Google Scholar] [CrossRef] [PubMed]
- Göritz, C.; Dias, D.O.; Tomilin, N.; Barbacid, M.; Shupliakov, O.; Frisén, J. A pericyte origin of spinal cord scar tissue. Science 2011, 333, 238–242. [Google Scholar] [CrossRef]
- Sabelström, H.; Stenudd, M.; Frisén, J. Neural stem cells in the adult spinal cord. Exp. Neurol. 2014, 260, 44–49. [Google Scholar] [CrossRef]
- Sabelström, H.; Stenudd, M.; Réu, P.; Dias, D.O.; Elfineh, M.; Zdunek, S.; Damberg, P.; Göritz, C.; Frisén, J. Resident neural stem cells restrict tissue damage and neuronal loss after spinal cord injury in mice. Science 2013, 342, 637–640. [Google Scholar] [CrossRef] [Green Version]
- Hussein, R.K.; Mencio, C.P.; Katagiri, Y.; Brake, A.M.; Geller, H.M. Role of chondroitin sulfation following spinal cord injury. Front. Cell. Neurosci. 2020, 14, 208. [Google Scholar] [CrossRef] [PubMed]
- Chalfouh, C.; Guillou, C.; Hardouin, J.; Delarue, Q.; Li, X.; Duclos, C.; Schampan, D.; Marie, J.-P.; Cosette, P.; Guérout, N. The regenerative effect of trans-spinal magnetic stimulation after spinal cord injury: Mechanisms and pathways underlying the effect. Neurotherapeutics 2020, 17, 2069–2088. [Google Scholar] [CrossRef]
- Chedly, J.; Soares, S.; Montembault, A.; Von Boxberg, Y.; Veron-Ravaille, M.; Mouffle, C.; Benassy, M.-N.; Taxi, J.; David, L.; Nothias, F. Physical chitosan microhydrogels as scaffolds for spinal cord injury restoration and axon regeneration. Biomaterials 2017, 138, 91–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delarue, Q.; Mayeur, A.; Chalfouh, C.; Honoré, A.; Duclos, C.; Di Giovanni, M.; Li, X.; Salaun, M.; Dampierre, J.; Vaudry, D.; et al. Inhibition of ADAMTS-4 expression in olfactory ensheathing cells enhances recovery after transplantation within spinal cord injury. J. Neurotrauma 2020, 37, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Kobashi, S.; Terashima, T.; Katagi, M.; Nakae, Y.; Okano, J.; Suzuki, Y.; Urushitani, M.; Kojima, H. Transplantation of M2-deviated microglia promotes recovery of motor function after spinal cord injury in mice. Mol. Ther. 2020, 28, 254–265. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Fan, L.; Liu, C.; Wen, H.; Wang, S.; Guan, P.; Chen, D.; Ning, C.; Zhou, L.; Tan, G. An injectable, self-healing, electroconductive extracellular matrix-based hydrogel for enhancing tissue repair after traumatic spinal cord injury. Bioact Mater. 2021, 7, 98–111. [Google Scholar] [CrossRef]
- Courtine, G.; Sofroniew, M.V. Spinal cord repair: Advances in biology and technology. Nat. Med. 2019, 25, 898–908. [Google Scholar] [CrossRef] [PubMed]
- Watzlawick, R.; Rind, J.; Sena, E.S.; Brommer, B.; Zhang, T.; Kopp, M.A.; Dirnagl, U.; Macleod, M.R.; Howells, D.W.; Schwab, J.M. Olfactory ensheathing cell transplantation in experimental spinal cord injury: Effect size and reporting bias of 62 experimental treatments: A systematic review and meta-analysis. PLoS Biol. 2016, 14, e1002468. [Google Scholar] [CrossRef] [PubMed]
- Delarue, Q.; Robac, A.; Massardier, R.; Marie, J.-P.; Guérout, N. Comparison of the effects of two therapeutic strategies based on olfactory ensheathing cell transplantation and repetitive magnetic stimulation after spinal cord injury in female mice. J. Neurosci. Res. 2021, 99, 1835–1849. [Google Scholar] [CrossRef]
- Delarue, Q.; Chalfouh, C.; Guérout, N. Spinal cord injury: Can we repair spinal cord non-invasively by using magnetic stimulation? Neural. Regen. Res. 2021, 16, 2429–2430. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ma, S.; Ke, X.; Yi, Y.; Yu, H.; Yu, D.; Li, Q.; Shang, Y.; Lu, Y.; Pei, L. The mechanism of Annexin A1 to modulate TRPV1 and nociception in dorsal root ganglion neurons. Cell Biosci. 2021, 11, 167. [Google Scholar] [CrossRef]
- Algahtany, M.; McFaull, S.; Chen, L.; Zhang, S.; Saarela, O.; Alqahtani, F.; Cusimano, M.D. The changing etiology and epidemiology of traumatic spinal injury: A population-based study. World Neurosurg. 2021, 149, e116–e127. [Google Scholar] [CrossRef]
- Hofstetter, C.P.; Holmström, N.A.; Lilja, J.A.; Schweinhardt, P.; Hao, J.; Spenger, C.; Wiesenfeld-Hallin, Z.; Kurpad, S.N.; Frisén, J.; Olson, L. Allodynia limits the usefulness of intraspinal neural stem cell grafts; directed differentiation improves outcome. Nat. Neurosci. 2005, 8, 346–353. [Google Scholar] [CrossRef]
- Nakhjavan-Shahraki, B.; Yousefifard, M.; Rahimi-Movaghar, V.; Baikpour, M.; Nasirinezhad, F.; Safari, S.; Yaseri, M.; Jafari, A.M.; Ghelichkhani, P.; Tafakhori, A.; et al. Transplantation of olfactory ensheathing cells on functional recovery and neuropathic pain after spinal cord injury; systematic review and meta-analysis. Sci. Rep. 2018, 8, 325. [Google Scholar] [CrossRef] [Green Version]
- Soderblom, C.; Luo, X.; Blumenthal, E.; Bray, E.; Lyapichev, K.; Ramos, J.; Krishnan, V.; Lai-Hsu, C.; Park, K.K.; Tsoulfas, P.; et al. Perivascular fibroblasts form the fibrotic scar after contusive spinal cord injury. J. Neurosci. 2013, 33, 13882–13887. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Soderblom, C.; Krishnan, V.; Ashbaugh, J.; Bethea, J.R.; Lee, J.K. Hematogenous macrophage depletion reduces the fibrotic scar and increases axonal growth after spinal cord injury. Neurobiol. Dis. 2015, 74, 114–125. [Google Scholar] [CrossRef] [Green Version]
- Dias, D.O.; Kalkitsas, J.; Kelahmetoglu, Y.; Estrada, C.P.; Tatarishvili, J.; Holl, D.; Jansson, L.; Banitalebi, S.; Amiry-Moghaddam, M.; Ernst, A.; et al. Pericyte-derived fibrotic scarring is conserved across diverse central nervous system lesions. Nat. Commun. 2021, 12, 5501. [Google Scholar] [CrossRef]
- Bellver-Landete, V.; Bretheau, F.; Mailhot, B.; Vallières, N.; Lessard, M.; Janelle, M.E.; Vernoux, N.; Tremblay, M.-È.; Fuehrmann, T.; Shoichet, M.S.; et al. Microglia are an essential component of the neuroprotective scar that forms after spinal cord injury. Nat. Commun. 2019, 10, 518. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Ritzel, R.M.; Khan, N.; Cao, T.; He, J.; Lei, Z.; Matyas, J.J.; Sabirzhanov, B.; Liu, S.; Li, H.; et al. Delayed microglial depletion after spinal cord injury reduces chronic inflammation and neurodegeneration in the brain and improves neurological recovery in male mice. Theranostics 2020, 10, 11376–11403. [Google Scholar] [CrossRef] [PubMed]
- Brifault, C.; Gras, M.; Liot, D.; May, V.; Vaudry, D.; Wurtz, O. Delayed pituitary adenylate cyclase-activating polypeptide delivery after brain stroke improves functional recovery by inducing m2 microglia/macrophage polarization. Stroke 2015, 46, 520–528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evans, T.A.; Barkauskas, D.S.; Myers, J.T.; Hare, E.G.; You, J.Q.; Ransohoff, R.M.; Huang, A.Y.; Silver, J. High-resolution intravital imaging reveals that blood-derived macrophages but not resident microglia facilitate secondary axonal dieback in traumatic spinal cord injury. Exp. Neurol. 2014, 254, 109–120. [Google Scholar] [CrossRef] [Green Version]
- Vinhas, A.; Almeida, A.F.; Gonçalves, A.I.; Rodrigues, M.T.; Gomes, M.E. Magnetic stimulation drives macrophage polarization in cell to-cell communication with IL-1β primed tendon cells. Int. J. Mol. Sci. 2020, 21, 5441. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; He, X.; Kawaguchi, R.; Zhang, Y.; Wang, Q.; Monavarfeshani, A.; Yang, Z.; Chen, B.; Shi, Z.; Meng, H.; et al. Microglia-organized scar-free spinal cord repair in neonatal mice. Nature 2020, 587, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Olah, M.; Menon, V.; Habib, N.; Taga, M.F.; Ma, Y.; Yung, C.J.; Cimpean, M.; Khairallah, A.; Coronas- Samano, G.; Sankowski, R.; et al. Single cell RNA sequencing of human microglia uncovers a subset associated with Alzheimer’s disease. Nat. Commun. 2020, 11, 6129. [Google Scholar] [CrossRef] [PubMed]



| TR (ms) | TE (ms) | Thickness (µm) | FOV (cm) | Matrix (pixel) | Acquisition Time | |
|---|---|---|---|---|---|---|
| T2 *-weighted axial section | 2000 | 6.5 20.4 34.3 48.1 62.1 75.9 89.1 103.7 117.5 131.4 | 500 | 4 | 256 | 15 min 56 s |
| T2 *-weighted sagittal section | 2000 | 6.5 | 500 | 4 | 320 | 16 min |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Robac, A.; Neveu, P.; Hugede, A.; Garrido, E.; Nicol, L.; Delarue, Q.; Guérout, N. Repetitive Trans Spinal Magnetic Stimulation Improves Functional Recovery and Tissue Repair in Contusive and Penetrating Spinal Cord Injury Models in Rats. Biomedicines 2021, 9, 1827. https://doi.org/10.3390/biomedicines9121827
Robac A, Neveu P, Hugede A, Garrido E, Nicol L, Delarue Q, Guérout N. Repetitive Trans Spinal Magnetic Stimulation Improves Functional Recovery and Tissue Repair in Contusive and Penetrating Spinal Cord Injury Models in Rats. Biomedicines. 2021; 9(12):1827. https://doi.org/10.3390/biomedicines9121827
Chicago/Turabian StyleRobac, Amandine, Pauline Neveu, Alizée Hugede, Elisabeth Garrido, Lionel Nicol, Quentin Delarue, and Nicolas Guérout. 2021. "Repetitive Trans Spinal Magnetic Stimulation Improves Functional Recovery and Tissue Repair in Contusive and Penetrating Spinal Cord Injury Models in Rats" Biomedicines 9, no. 12: 1827. https://doi.org/10.3390/biomedicines9121827
APA StyleRobac, A., Neveu, P., Hugede, A., Garrido, E., Nicol, L., Delarue, Q., & Guérout, N. (2021). Repetitive Trans Spinal Magnetic Stimulation Improves Functional Recovery and Tissue Repair in Contusive and Penetrating Spinal Cord Injury Models in Rats. Biomedicines, 9(12), 1827. https://doi.org/10.3390/biomedicines9121827







