Differences in Distribution and Biological Effects of F3O4@PEG Nanoparticles in Normotensive and Hypertensive Rats—Focus on Vascular Function and Liver
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
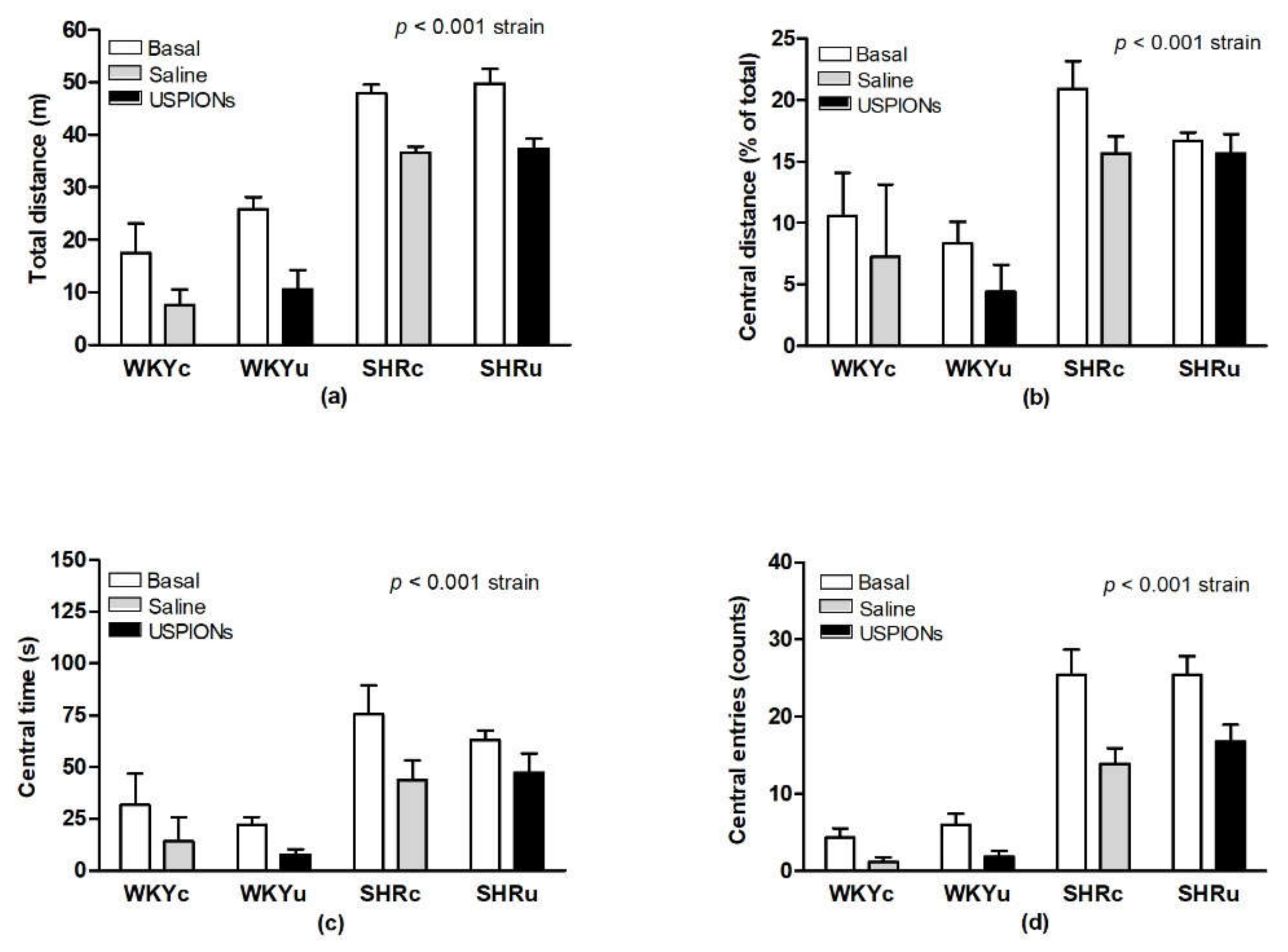
2.2. Open-Field Test
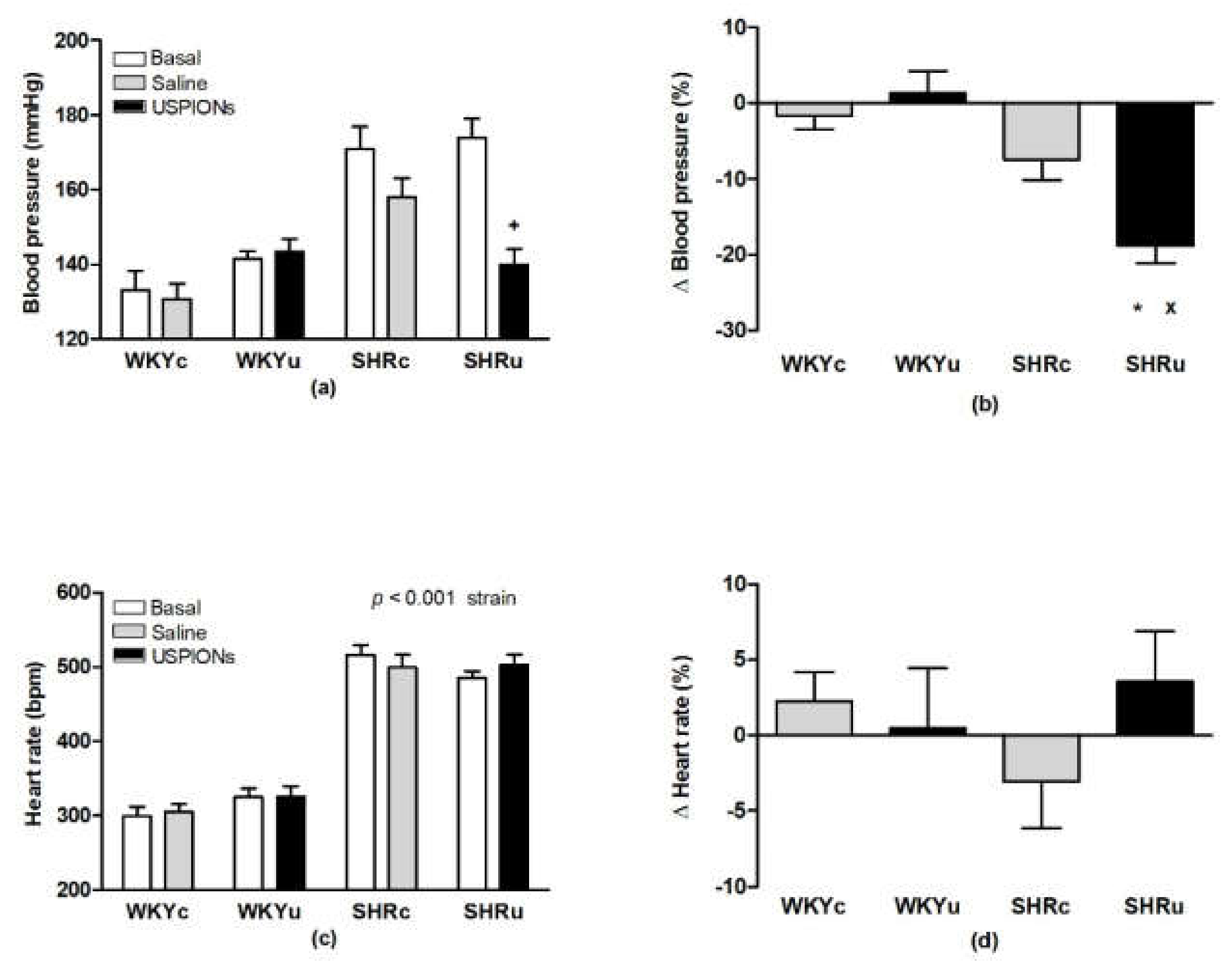
2.3. Systolic Blood Pressure and Heart Rate
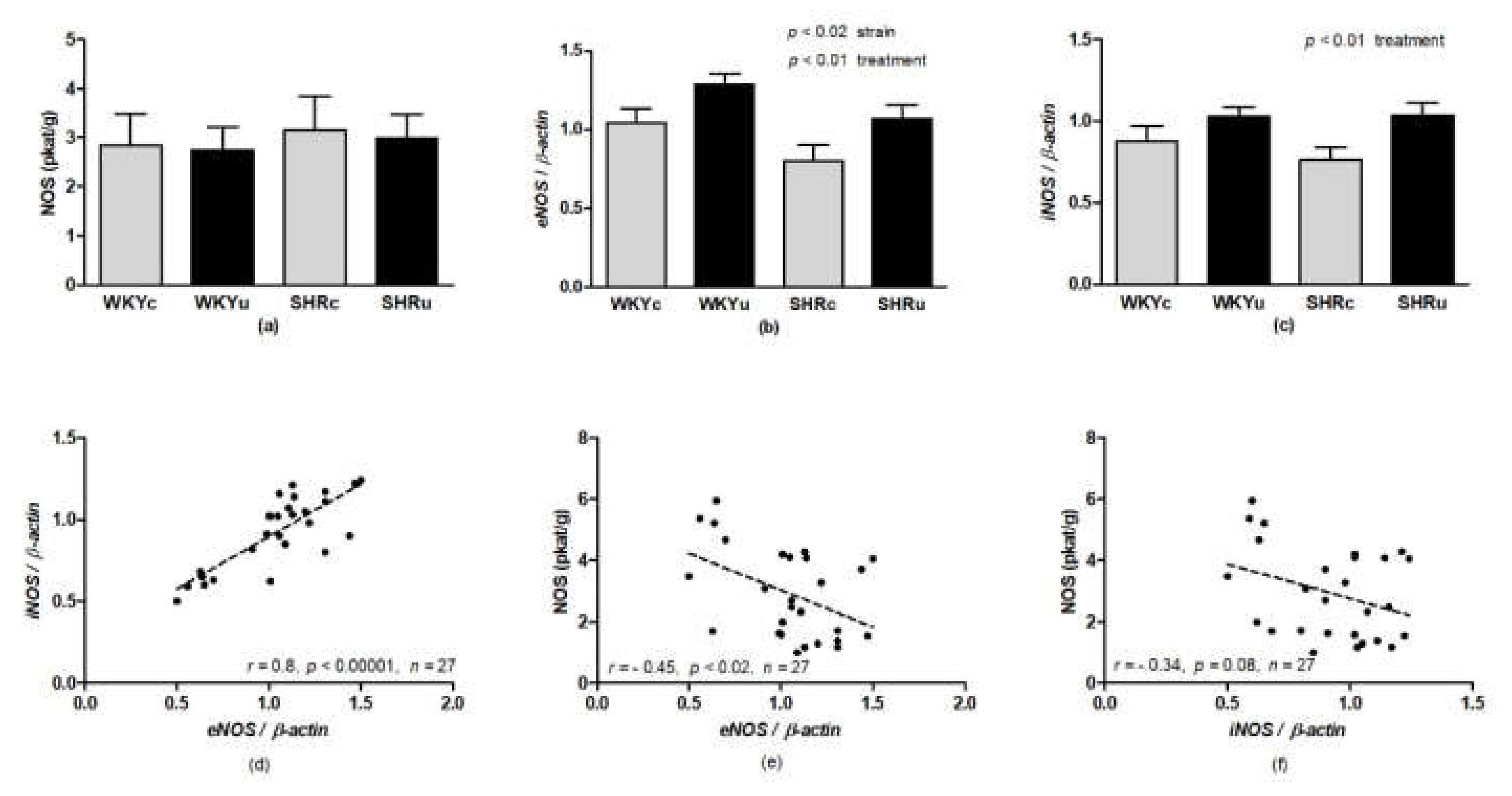
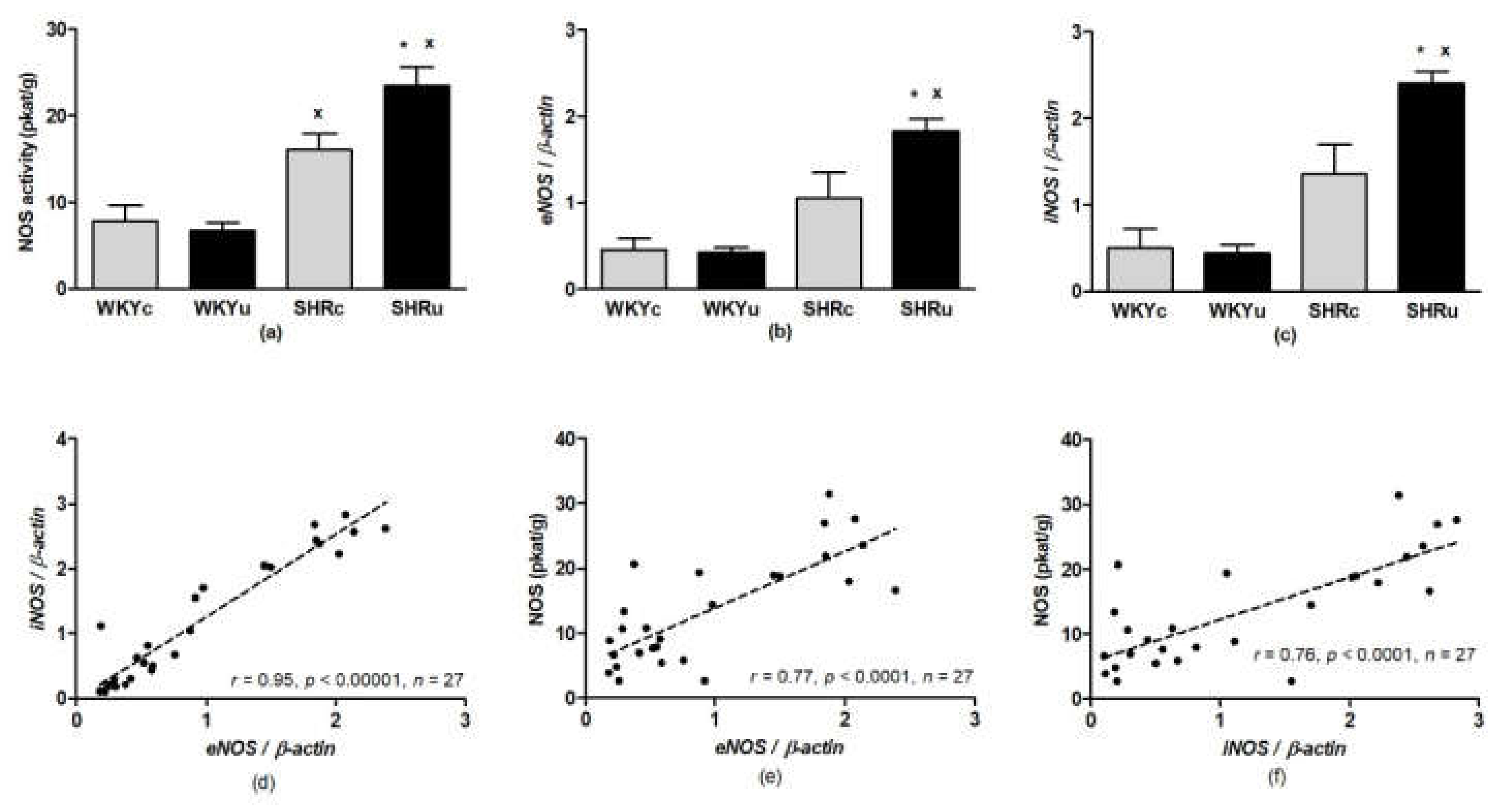
2.4. Nitric Oxide Synthase Activity
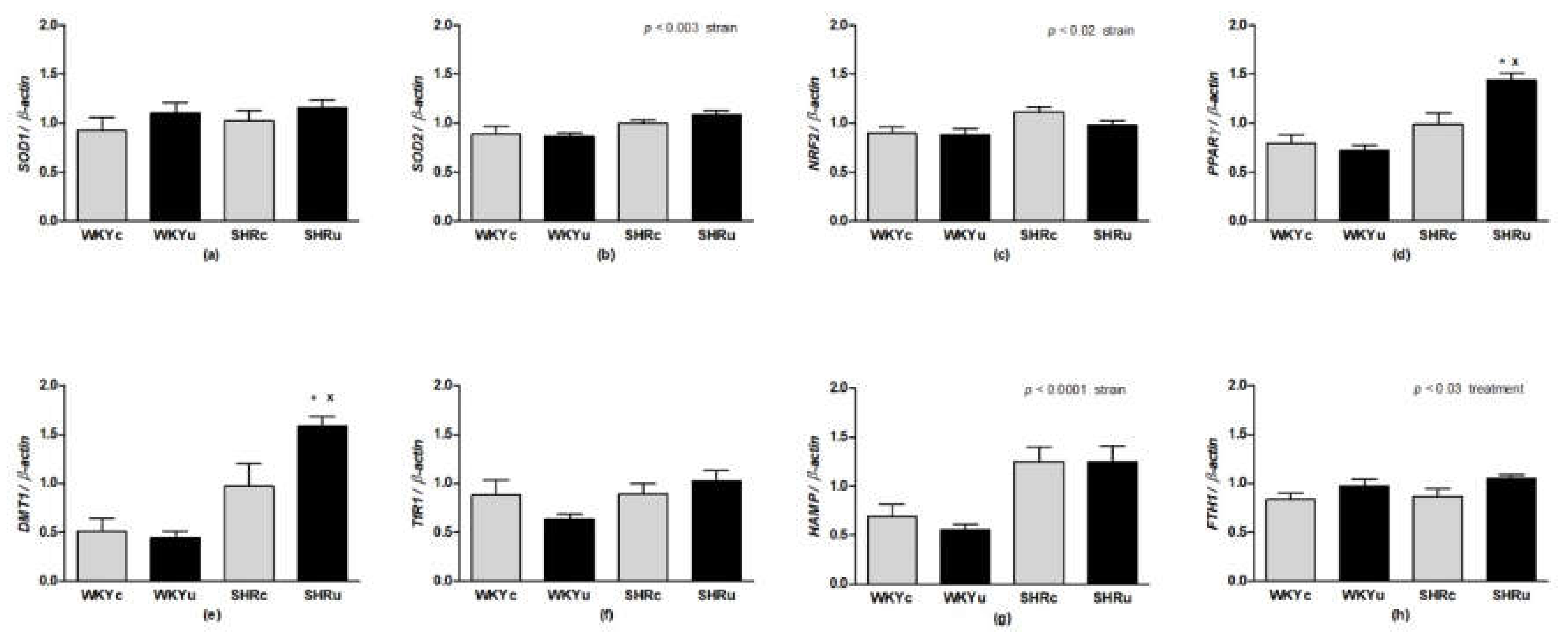
2.5. Gene Expression
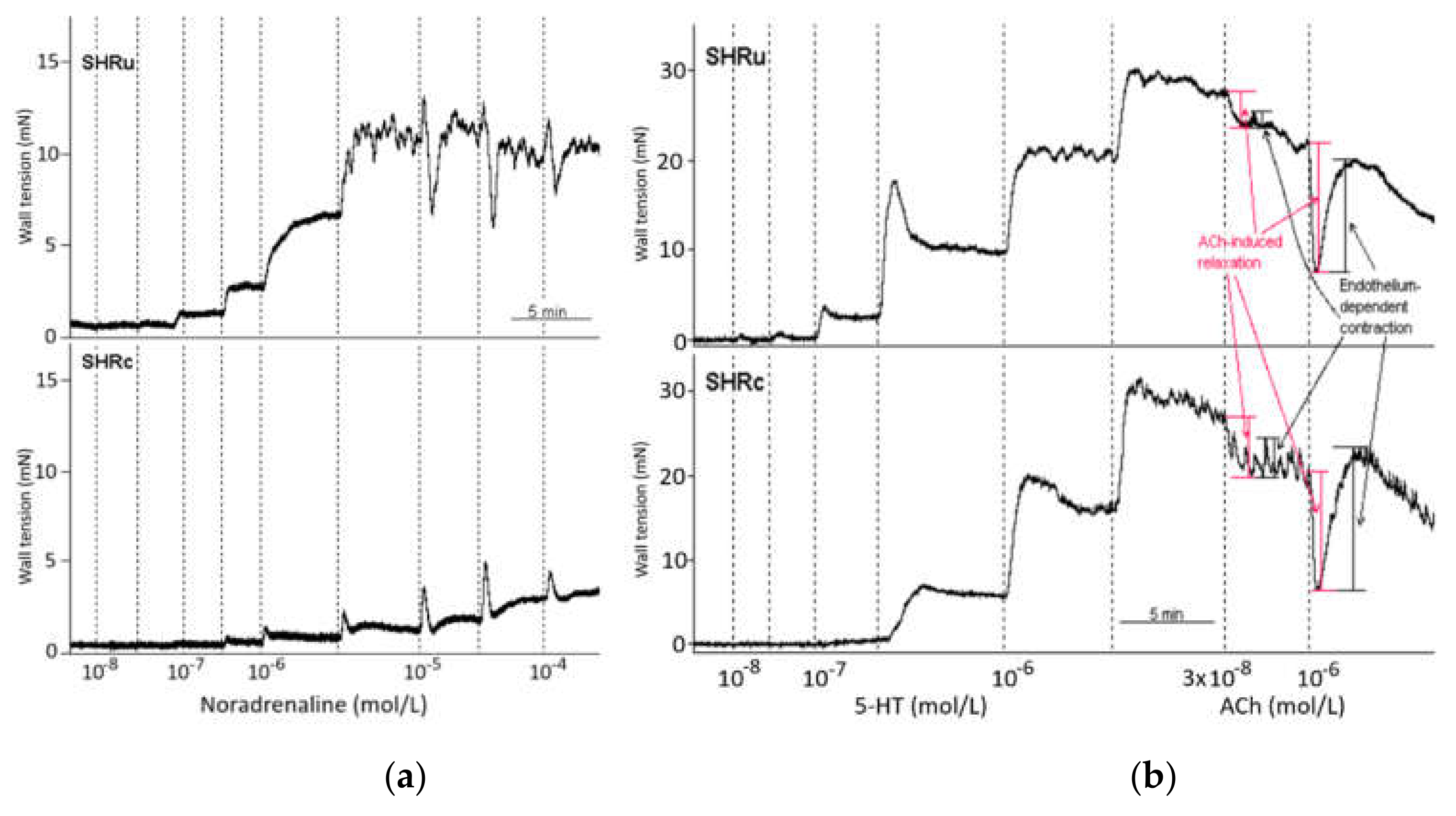
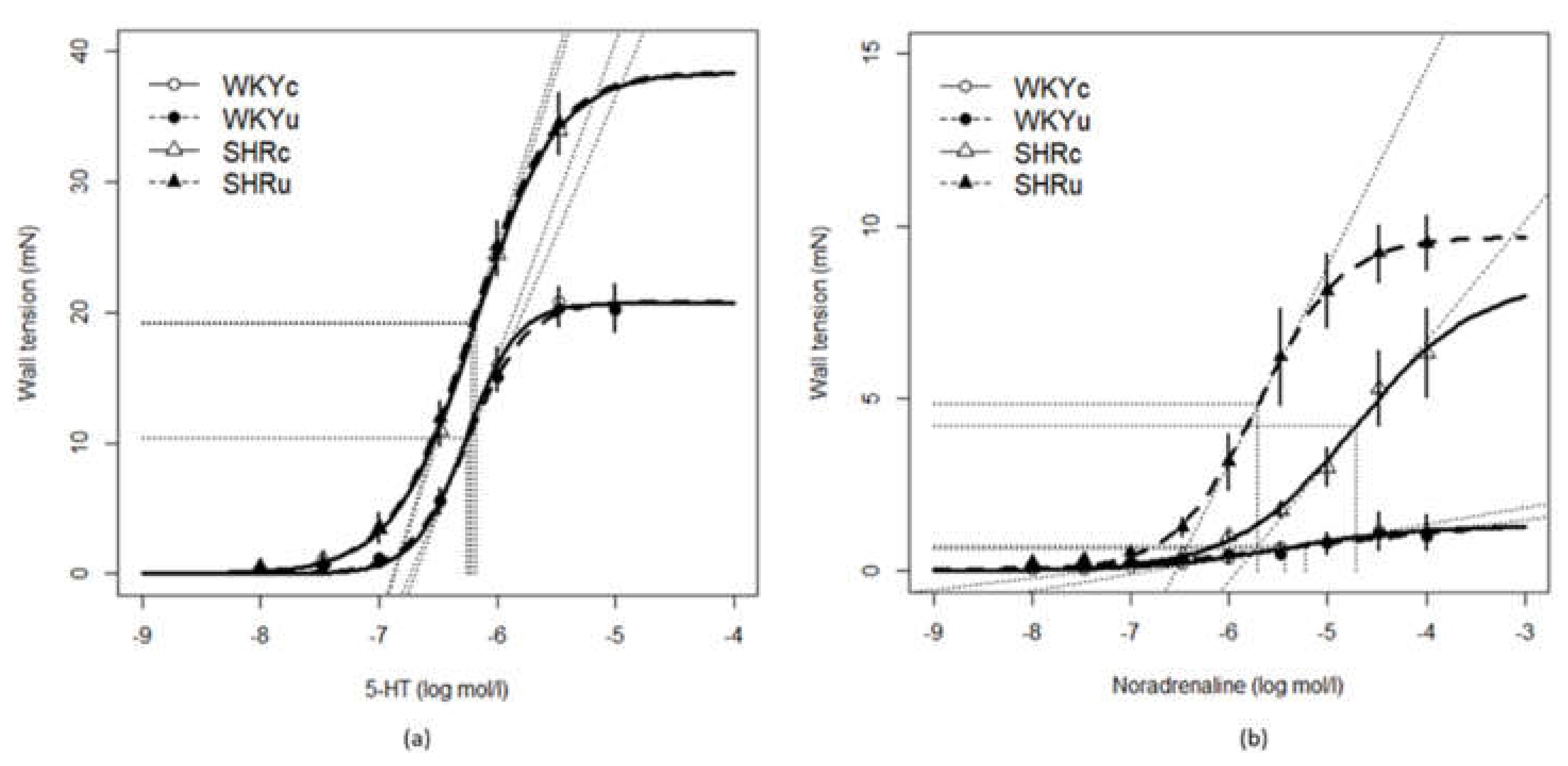
2.6. Vascular Functions
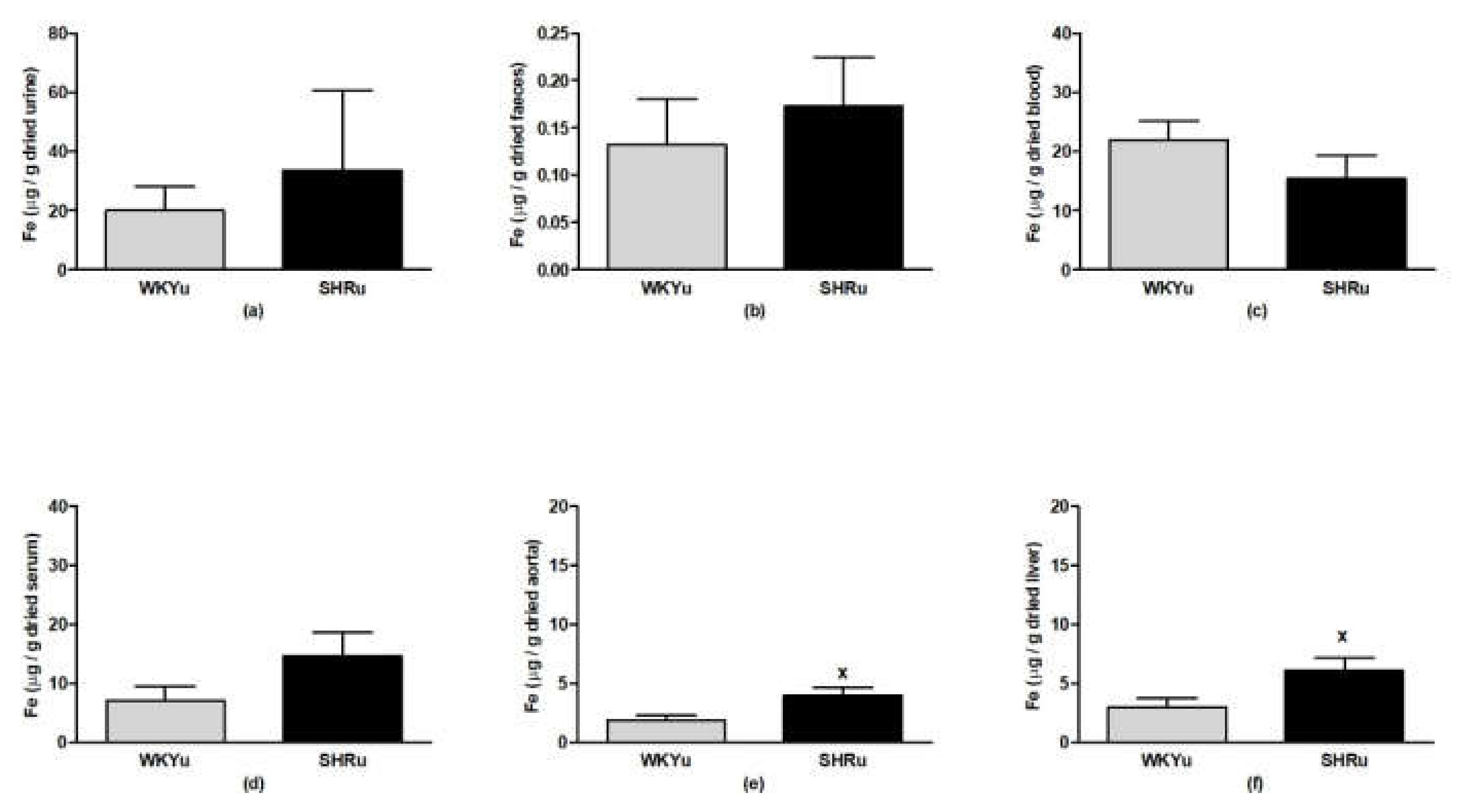
2.7. Determination of the USPIONs Content
2.8. Transmission Electron Microscopy of the Tissues
2.9. Statistical Analysis
3. Results
3.1. Open-Field Behaviour
3.2. Blood Pressure and Heart Rate
3.3. NO Synthase Activity
3.4. Gene Expression
3.5. Vascular Function
3.6. USPION-Originated Iron Content
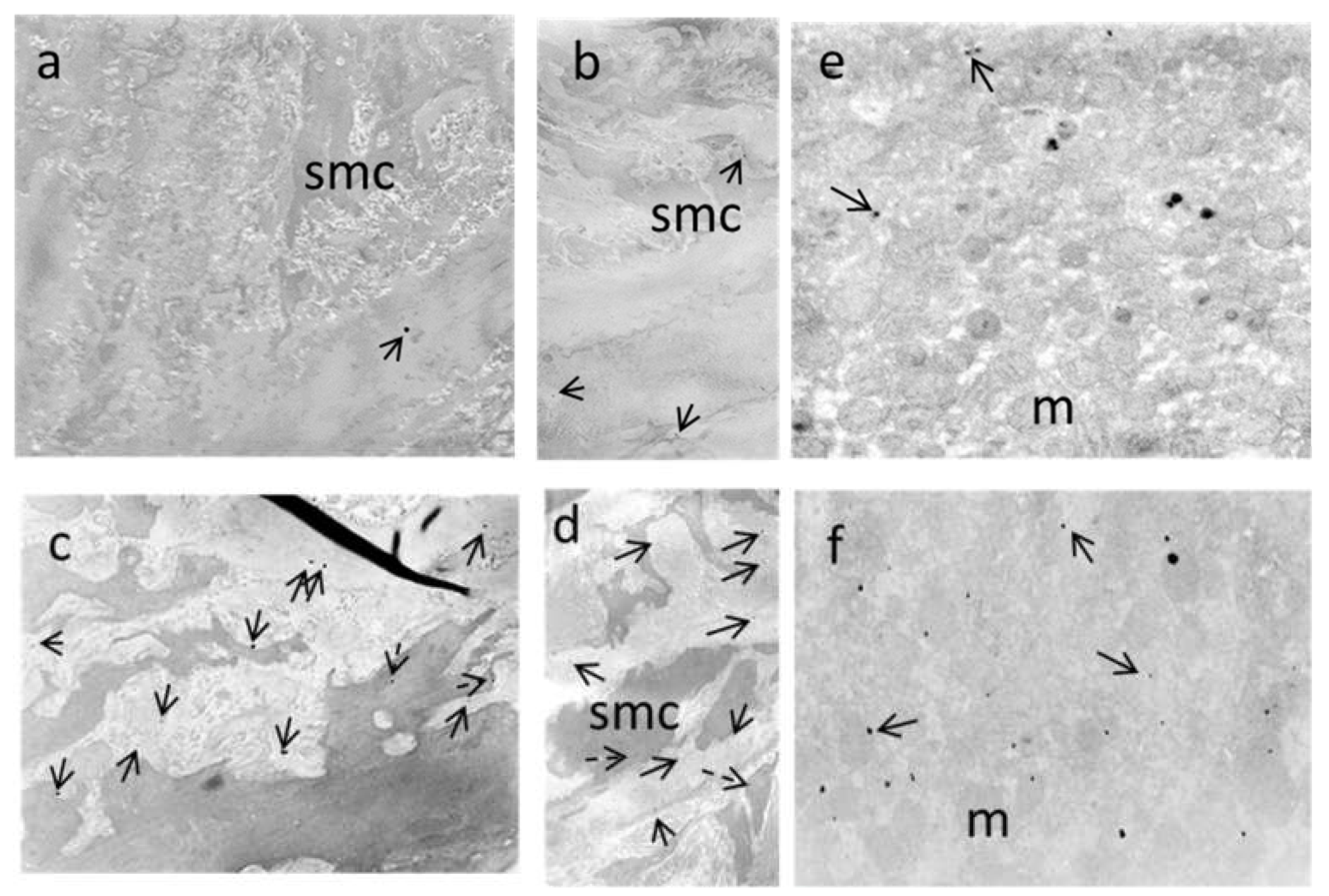
3.7. Electron Microscopy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andrade, R.G.D.; Veloso, S.R.S.; Castanheira, E.M.S. Shape Anisotropic Iron Oxide-Based Magnetic Nanoparticles: Synthesis and Biomedical Applications. Int. J. Mol. Sci. 2020, 21, 2455. [Google Scholar] [CrossRef] [Green Version]
- Poller, W.C.; Pieber, M.; Boehm-Sturm, P.; Ramberger, E.; Karampelas, V.; Möller, K.; Schleicher, M.; Wiekhorst, F.; Löwa, N.; Wagner, S.; et al. Very small superparamagnetic iron oxide nanoparticles: Long-term fate and metabolic processing in atherosclerotic mice. Nanomedicine 2018, 14, 2575–2586. [Google Scholar] [CrossRef] [PubMed]
- Rego, G.N.A.; Nucci, M.P.; Mamani, J.B.; Oliveira, F.A.; Marti, L.C.; Filgueiras, I.S.; Ferreira, J.M.; Real, C.C.; Faria, D.D.P.; Espinha, P.L.; et al. Therapeutic Efficiency of Multiple Applications of Magnetic Hyperthermia Technique in Glioblastoma Using Aminosilane Coated Iron Oxide Nanoparticles: In Vitro and In Vivo Study. Int. J. Mol. Sci. 2020, 21, 958. [Google Scholar] [CrossRef] [Green Version]
- Roca, A.G.; Marco, J.F.; Morales, M.D.P.; Serna, C.J. Effect of Nature and Particle Size on Properties of Uniform Magnetite and Maghemite Nanoparticles. J. Phys. Chem. C 2007, 111, 18577–18584. [Google Scholar] [CrossRef]
- Larsen, E.K.U.; Nielsen, T.; Wittenborn, T.; Rydtoft, L.M.; Lokanathan, A.R.; Hansen, L.; Østergaard, L.; Kingshott, P.; Howard, K.A.; Besenbacher, F.; et al. Accumulation of magnetic iron oxide nanoparticles coated with variably sized polyethylene glycol in murine tumors. Nanoscale 2012, 4, 2352–2361. [Google Scholar] [CrossRef] [PubMed]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, W.-K.; Park, J.-Y.; Jung, S.; Yang, C.W.; Kim, W.-U.; Kim, H.-Y.; Park, J.-H. Preparation and characterization of biodegradable nanoparticles entrapping immunodominant peptide conjugated with PEG for oral tolerance induction. J. Control. Release 2005, 105, 77–88. [Google Scholar] [CrossRef]
- Malhotra, N.; Lee, J.-S.; Liman, R.A.D.; Ruallo, J.M.S.; Villaflores, O.B.; Ger, T.-R.; Hsiao, C.-D. Potential Toxicity of Iron Oxide Magnetic Nanoparticles: A Review. Molecules 2020, 25, 3159. [Google Scholar] [CrossRef] [PubMed]
- Drakesmith, H.; Nemeth, E.; Ganz, T. Ironing out Ferroportin. Cell Metab. 2015, 22, 777–787. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Pantopoulos, K. Regulation of cellular iron metabolism. Biochem. J. 2011, 434, 365–381. [Google Scholar] [CrossRef] [Green Version]
- Cuadrado, A.; Manda, G.; Hassan, A.; Alcaraz, M.J.; Barbas, C.; Daiber, A.; Ghezzi, P.; León, R.; López, M.G.; Oliva, B.; et al. Transcription Factor NRF2 as a Therapeutic Target for Chronic Diseases: A Systems Medicine Approach. Pharmacol. Rev. 2018, 70, 348–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robledinos-Antón, N.; Fernández-Ginés, R.; Manda, G.; Cuadrado, A. Activators and Inhibitors of NRF2: A Review of Their Potential for Clinical Development. Oxidative Med. Cell. Longev. 2019, 2019, 1–20. [Google Scholar] [CrossRef]
- Zhou, S.; Sun, W.; Zhang, Z.; Zheng, Y. The Role of Nrf2-Mediated Pathway in Cardiac Remodeling and Heart Failure. Oxidative Med. Cell. Longev. 2014, 2014, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Kerins, M.J.; Ooi, A. The Roles of NRF2 in Modulating Cellular Iron Homeostasis. Antioxid. Redox Signal. 2018, 29, 1756–1773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, P.J.; Duarte, T.L.; Arezes, J.; Garcia-Santos, D.; Hamdi, A.; Pasricha, S.-R.; Armitage, A.E.; Mehta, H.; Wideman, S.K.; Santos, A.G.; et al. Nrf2 controls iron homoeostasis in haemochromatosis and thalassaemia via Bmp6 and hepcidin. Nat. Metab. 2019, 1, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Lee, C. Collaborative Power of Nrf2 and PPARγ Activators against Metabolic and Drug-Induced Oxidative Injury. Oxidative Med. Cell. Longev. 2017, 2017, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Videla, L.A.; Pettinelli, P. Misregulation of PPAR Functioning and Its Pathogenic Consequences Associated with Nonalcoholic Fatty Liver Disease in Human Obesity. PPAR Res. 2012, 2012, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tailleux, A.; Wouters, K.; Staels, B. Roles of PPARs in NAFLD: Potential therapeutic targets. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2012, 1821, 809–818. [Google Scholar] [CrossRef]
- Líšková, S.; Bališ, P.; Mičurová, A.; Kluknavský, M.; Okuliarová, M.; Puzserová, A.; Škrátek, M.; Sekaj, I.; Maňka, J.; Valovič, P.; et al. Effect of iron oxide nanoparticles on vascular function and nitric oxide production in acute stress-exposed rats. Physiol. Res. 2020, 69, 1067–1083. [Google Scholar] [CrossRef] [PubMed]
- Skrátek, M.; Dvurečenskij, A.; Kluknavský, M.; Barta, A.; Balis, P.; Mičurová, A.; Cigáň, A.; Andicsová, A.E.; Maňka, J.; Bernátová, I. Sensitive SQUID Bio-Magnetometry for Determination and Differentiation of Biogenic Iron and Iron Oxide Nanoparticles in the Biological Samples. Nanomaterials 2020, 10, 1993. [Google Scholar] [CrossRef]
- Bernatova, I. Endothelial Dysfunction in Experimental Models of Arterial Hypertension: Cause or Consequence? BioMed Res. Int. 2014, 2014, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Puzserova, A.; Bernatova, I. Blood Pressure Regulation in Stress: Focus on Nitric Oxide-Dependent Mechanisms. Physiol. Res. 2016, 65, S309–S342. [Google Scholar] [CrossRef]
- El Hadi, H.; Di Vincenzo, A.; Vettor, R.; Rossato, M. Relationship between Heart Disease and Liver Disease: A Two-Way Street. Cells 2020, 9, 567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kluknavsky, M.; Balis, P.; Puzserova, A.; Radosinska, J.; Berenyiova, A.; Drobna, M.; Lukac, S.; Muchova, J.; Bernatova, I. (−)-Epicatechin Prevents Blood Pressure Increase and Reduces Locomotor Hyperactivity in Young Spontaneously Hypertensive Rats. Oxidative Med. Cell. Longev. 2016, 2016, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Puzserova, A.; Slezak, P.; Balis, P.; Bernatova, I. Long-term social stress induces nitric oxide-independent endothelial dysfunction in normotensive rats. Stress 2012, 16, 331–339. [Google Scholar] [CrossRef]
- Radosinska, J.; Jasenovec, T.; Radosinska, D.; Balis, P.; Puzserova, A.; Skratek, M.; Manka, J.; Bernatova, I. Ultra-Small Superparamagnetic Iron-Oxide Nanoparticles Exert Different Effects on Erythrocytes in Normotensive and Hypertensive Rats. Biomedicines 2021, 9, 377. [Google Scholar] [CrossRef] [PubMed]
- Okruhlicová, L.; Cicaková, Z.; Frimmel, K.; Weismann, P.; Krizak, J.; Sotniková, R.; Knezl, V.; Slezák, J. Lipopolysaccharide-induced redistribution of myocardial connexin43 is associated with increased macrophage infiltration in both normotensive and spontaneously hypertensive rats. J. Physiol. Pharm. 2018, 69, 709–717. [Google Scholar]
- Silvia, L.; Miriam, P.; Petr, K.; Jaroslav, K.; Josef, Z. Effects of aging and hypertension on the participation of endothelium-derived constricting factor (EDCF) in norepinephrine-induced contraction of rat femoral artery. Eur. J. Pharm. 2011, 667, 265–270. [Google Scholar] [CrossRef]
- Askri, D.; Ouni, S.; Galai, S.; Arnaud, J.; Chovelon, B.; Lehmann, S.G.; Sturm, N.; Sakly, M.; Sève, M.; Amara, S. Intranasal instillation of iron oxide nanoparticles induces inflammation and perturbation of trace elements and neurotransmitters, but not behavioral impairment in rats. Environ. Sci. Pollut. Res. 2018, 25, 16922–16932. [Google Scholar] [CrossRef] [PubMed]
- Saeidienik, F.; Shahraki, M.R.; Fanaei, H.; Badini, F. The Effects of Iron Oxide Nanoparticles Administration on Depression Symptoms Induced by LPS in Male Wistar Rats. Basic Clin. Neurosci. J. 2018, 9, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Dhakshinamoorthy, V.; Manickam, V.; Perumal, E. Neurobehavioural Toxicity of Iron Oxide Nanoparticles in Mice. Neurotox. Res. 2017, 32, 187–203. [Google Scholar] [CrossRef] [PubMed]
- Sagvolden, T.; Johansen, E.B. Rat models of ADHD. In Behavioral Neuroscience of Attention Deficit Hyperactivity Disorder and Its Treatment. Current Topics in Behavioral Neurosciences; Stanford, C., Tannock, R., Eds.; Springer: Berlin, Heidelberg, 2011; Volume 9, pp. 301–315. [Google Scholar]
- Pires, P.; Ramos, C.M.D.; Matin, N.; Dorrance, A.M. The effects of hypertension on the cerebral circulation. Am. J. Physiol. Circ. Physiol. 2013, 304, H1598–H1614. [Google Scholar] [CrossRef] [PubMed]
- Mayhan, W.G.; Faraci, F.; Heistad, D.D. Disruption of the blood-brain barrier in cerebrum and brain stem during acute hypertension. Am. J. Physiol. Circ. Physiol. 1986, 251, H1171–H1175. [Google Scholar] [CrossRef] [PubMed]
- Iversen, N.K.; Frische, S.; Thomsen, K.; Laustsen, C.; Pedersen, M.; Hansen, P.B.L.; Bie, P.; Fresnais, J.; Berret, J.-F.; Baatrup, E.; et al. Superparamagnetic iron oxide polyacrylic acid coated γ-Fe2O3 nanoparticles do not affect kidney function but cause acute effect on the cardiovascular function in healthy mice. Toxicol. Appl. Pharm. 2013, 266, 276–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oleksa, V.; Bernátová, I.; Patsula, V.; Líšková, S.; Bališ, P.; Radošinská, J.; Mičurová, A.; Kluknavský, M.; Jasenovec, T.; Radošinská, D.; et al. Poly(ethylene glycol)-Alendronate-Coated Magnetite Nanoparticles Do Not Alter Cardiovascular Functions and Red Blood Cells’ Properties in Hypertensive Rats. Nanomaterials 2021, 11, 1238. [Google Scholar] [CrossRef] [PubMed]
- Dlugosova, K.; Okruhlicova, L.; Mitasikova, M.; Sotnikova, R.; Bernatova, I.; Weismann, P.; Slezak, J.; Tribulova, N. Modulation of connexin-43 by omega-3 fatty acids in the aorta of old spontaneously hypertensive rats. J. Physiol. Pharm. 2009, 60, 63–69. [Google Scholar]
- Kaspar, J.W.; Niture, S.K.; Jaiswal, A.K. Nrf2:INrf2 (Keap1) signaling in oxidative stress. Free. Radic. Biol. Med. 2009, 47, 1304–1309. [Google Scholar] [CrossRef] [Green Version]
- Janani, C.; Kumari, B.R. PPAR gamma gene—A review. Diabetes Metab. Syndr. Clin. Res. Rev. 2015, 9, 46–50. [Google Scholar] [CrossRef]



| Day | Procedure |
|---|---|
| −3 | 07:00–07:30 h Open-field test (basal) |
| −2 | 08:30–09:00 h Blood pressure determination (basal) |
| −1 | 13:00–15:00 h Catheterization |
| 0 | 07:00 h Infusion—the first USPION (or saline) dose |
| 1 | 07:00 h Infusion—the second USPION (or saline) dose |
| 2 | 07:00 h Open-field test (end) 09:00 h Blood pressure determination (end) 09:30 h Decapitation |
| Gene | Forward Primer | Reverse Primer | Tm (°C) | Amp (bp) |
|---|---|---|---|---|
| iNOS (NM_012611.3) | AAA CGC TAC ACT TCC AAC GC | TGC TGA GAG CTT TGT TGA GGT C | 59 | 91 |
| eNOS (NM_021838.2) | GAT CCC CCG GAG AAT GGA GA | TCG GAT TTT GTA ACT CTT GTG CT | 60 | 105 |
| NRF2 (NM_021838.2) | TGC CAT TAG TCA GTC GCT CTC | ACC GTG CCT TCA GTG TGC | 60 | 102 |
| SOD1 (NM_017050.1) | CTG AAG GCG AGC ATG GGT TC | TCC AAC ATG CCT CTC TTC ATC C | 60 | 131 |
| SOD2 (NM_017051.2) | GCT GGC CAA GGG AGA TGT TAC | TGC TGT GAT TGA TAT GGC CCC | 60 | 83 |
| PPARγ (NM_013124.3) | CTC ACA ATG CCA TCA GG TTT GG | GCT GGT CGA TAT CAC TGG AGA T | 59 | 84 |
| DMT1 (NM_013173.2) | CTA CTT GGG TTG GCA GTG TTT G | ATC TTC GCT CAG CAG GAC TTT | 60 | 94 |
| TFR1 (NM_022712.1) | GCT ATG AGG AAC CAG ACC GC | CAC TGG ACT TCG CAA CAC CA | 58 | 78 |
| β-actin (NM_031144.3) | CTC TGT GTG GAT TGG TGG CT | CGC AGC TCA GTA ACA GTC CG | 59 | 139 |
| 5-HT | ||||
|---|---|---|---|---|
| WKYc n = 6 | WKYu n = 7 | SHRc n = 7 | SHRu n = 7 | |
| KPSS (mN/mm) + | 14.15 ± 0.89 | 13.88 ± 0.90 | 28.21 ± 1.94 | 26.95 ± 2.88 |
| Emax (%) + | 20.78 ± 1.21 | 20.12 ± 1.53 | 36.26 ± 2.11 | 38.44 ± 2.86 |
| EC50 (log mol/l) + | −6.25 ± 0.05 | −6.24 ± 0.04 | −6.16 ± 0.05 | −6.17 ± 0.03 |
| slope | 25.07 ± 2.62 | 22.89 ± 1.47 | 32.42 ± 2.51 | 30.24 ± 2.31 |
| NA | ||||
| WKYc n = 6 | WKYu n = 6 | SHRc n = 6 | SHRu n = 7 | |
| KPSS (mN/mm) + | 19.08 ± 0.84 | 17.34 ± 0.79 | 26.84 ± 1.73 | 25.15 ± 1.63 |
| Emax (%) | 1.38 ± 0.65 | 1.93 ± 0.36 | 6. 12 ± 1.35 x | 10. 39 ± 0.86 x,* |
| EC50 (log mol/l) | −5.66 ± 0.41 | −5.10 ± 0.34 | −4.63 ± 0.13 x | −5.55 ± 0.34 * |
| slope | 0.60 ± 0.28 | 0.44 ± 0.09 | 2.89 ± 0.50 | 8.33 ± 1.67 * |
| Groups | Aorta | Liver | ||
|---|---|---|---|---|
| Total | Smooth Muscle Cells | Elastic Layers | ||
| WKYu | + | − | + | +, ++ |
| SHRu | +++ | + | +++ | ++,+++ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Micurova, A.; Kluknavsky, M.; Liskova, S.; Balis, P.; Skratek, M.; Okruhlicova, L.; Manka, J.; Bernatova, I. Differences in Distribution and Biological Effects of F3O4@PEG Nanoparticles in Normotensive and Hypertensive Rats—Focus on Vascular Function and Liver. Biomedicines 2021, 9, 1855. https://doi.org/10.3390/biomedicines9121855
Micurova A, Kluknavsky M, Liskova S, Balis P, Skratek M, Okruhlicova L, Manka J, Bernatova I. Differences in Distribution and Biological Effects of F3O4@PEG Nanoparticles in Normotensive and Hypertensive Rats—Focus on Vascular Function and Liver. Biomedicines. 2021; 9(12):1855. https://doi.org/10.3390/biomedicines9121855
Chicago/Turabian StyleMicurova, Andrea, Michal Kluknavsky, Silvia Liskova, Peter Balis, Martin Skratek, Ludmila Okruhlicova, Jan Manka, and Iveta Bernatova. 2021. "Differences in Distribution and Biological Effects of F3O4@PEG Nanoparticles in Normotensive and Hypertensive Rats—Focus on Vascular Function and Liver" Biomedicines 9, no. 12: 1855. https://doi.org/10.3390/biomedicines9121855






