Rewarding Social Interaction in Rats Increases CaMKII in the Nucleus Accumbens
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
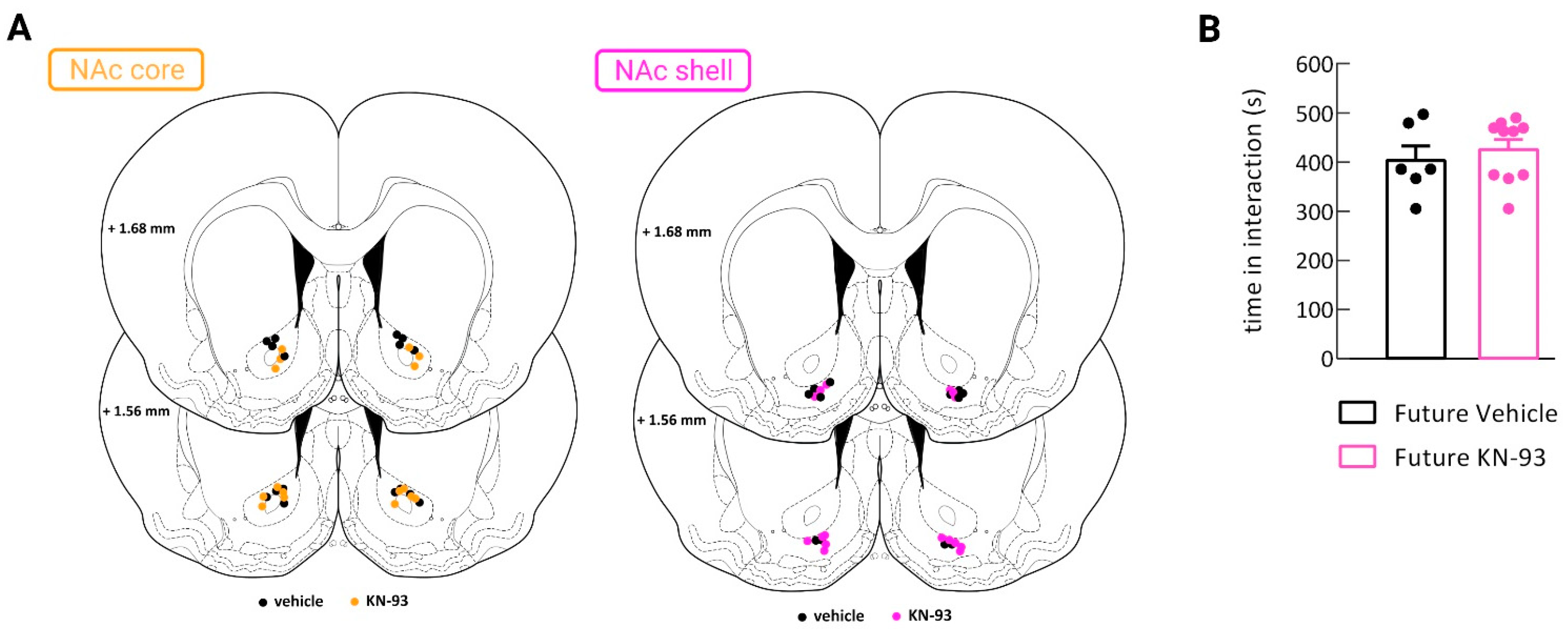
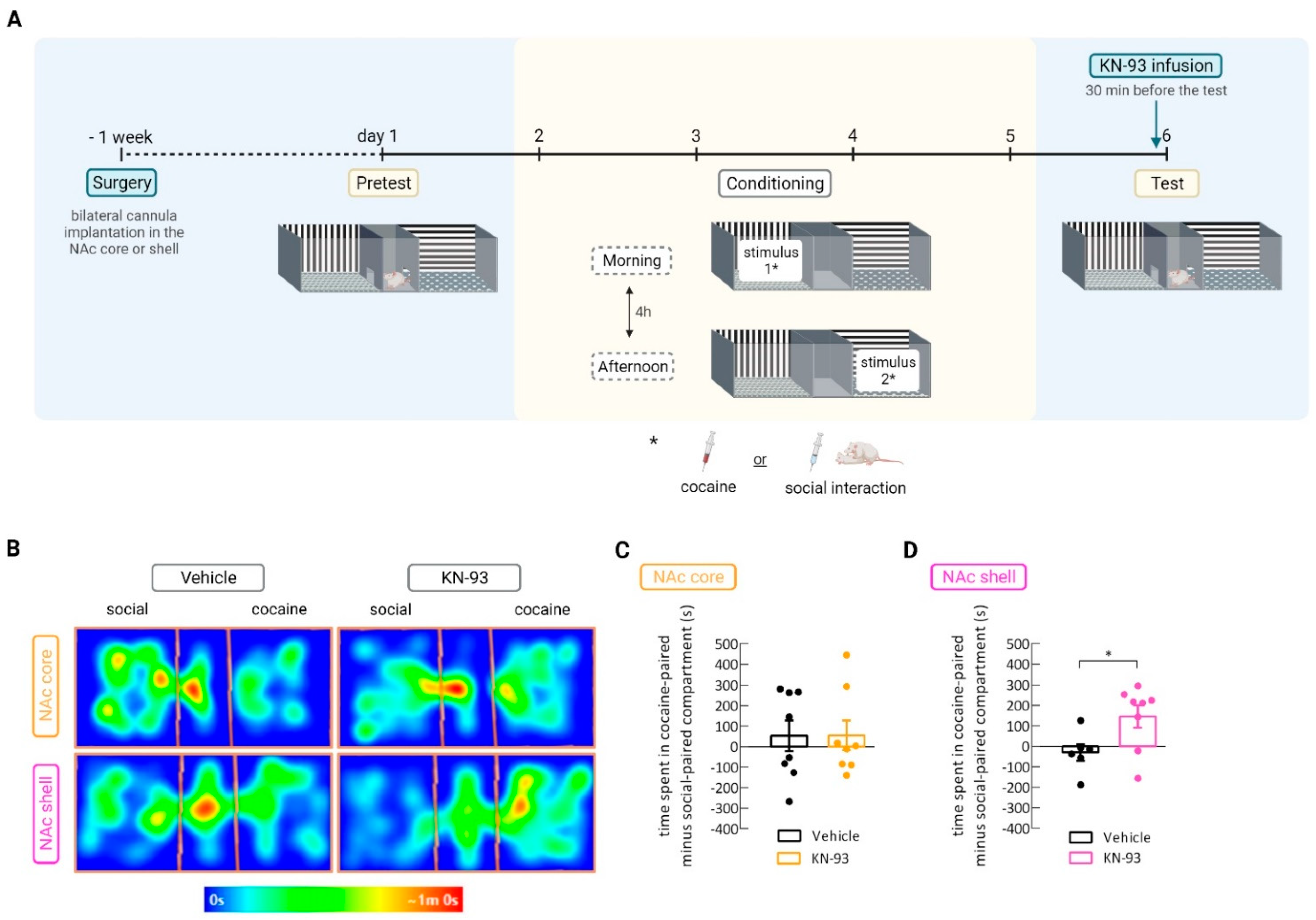
2.2. Stereotaxic Surgery and Intra-NAc Core and Shell Inhibitor Infusion
2.3. Conditioned Place Preference Apparatus
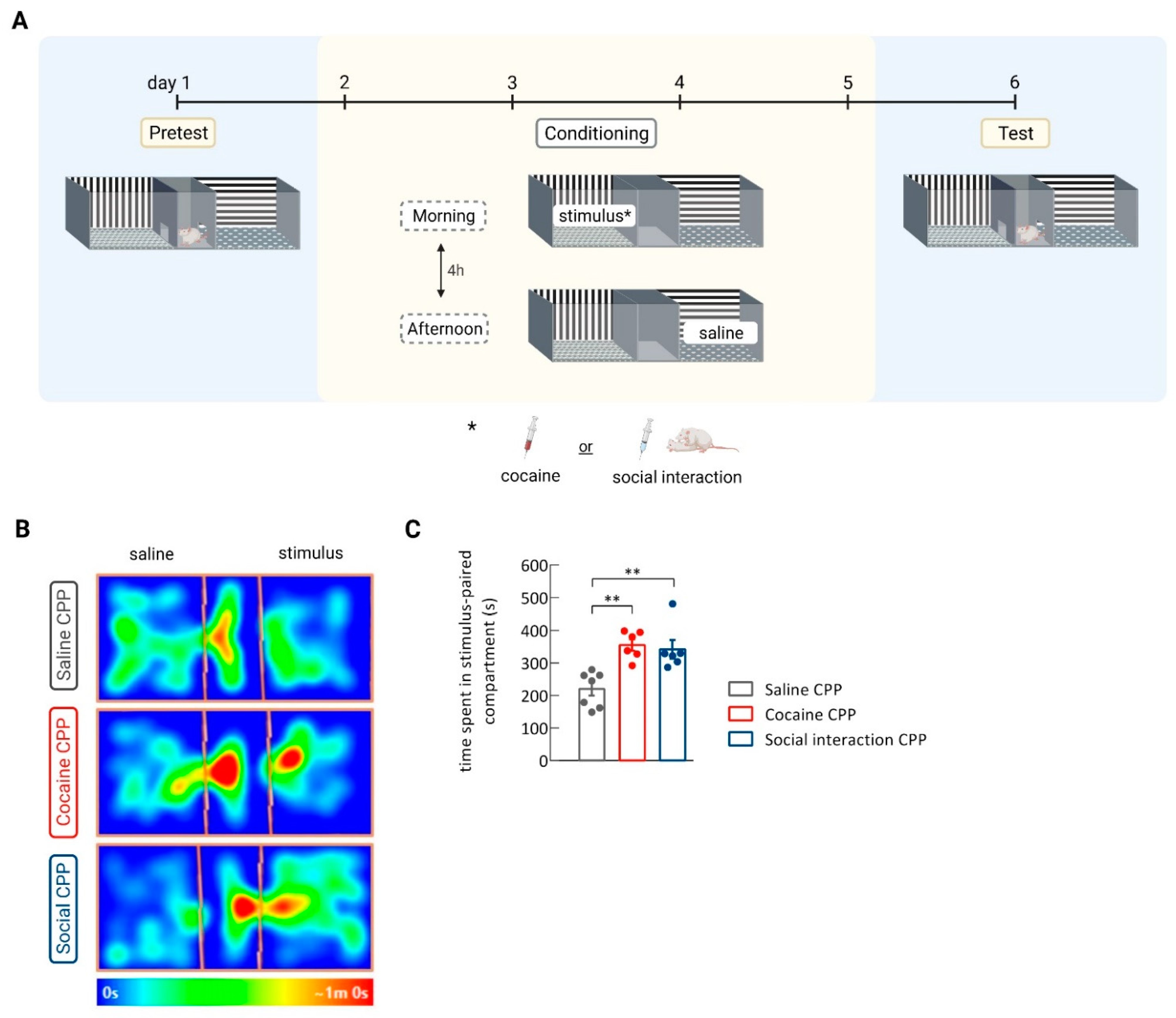
2.4. Conditioned Place Preference Protocol
- Acquisition of CPP
- CPP testing
- Concurrent CPP protocol
2.5. Western Blotting
2.6. Statistical Analyses
3. Results
3.1. Social Interaction CPP Increased CaMKII Activity in the Nucleus Accumbens
3.2. CaMKII Inhibition in the Nucleus Accumbens Shell Shifted Preference toward Cocaine
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Anderson, S.M.; Famous, K.R.; Sadri-Vakili, G.; Kumaresan, V.; Schmidt, H.D.; Bass, C.E.; Terwilliger, E.F.; Cha, J.-H.H.J.; Pierce, R.C. CaMKII: A biochemical bridge linking accumbens dopamine and glutamate systems in cocaine seeking. Nat. Neurosci. 2008, 11, 344–353. [Google Scholar] [CrossRef]
- Robison, A.J.; Vialou, V.; Mazei-Robison, M.; Feng, J.; Kourrich, S.; Collins, M.; Wee, S.; Koob, G.; Turecki, G.; Neve, R.; et al. Behavioral and structural responses to chronic cocaine require a feedforward loop involving Δ FosB and calcium/calmodulin-dependent protein kinase II in the nucleus accumbens shell. J. Neurosci. 2013, 33, 4295–4307. [Google Scholar] [CrossRef]
- Liu, X.; Liu, Y.; Zhong, P.; Wilkinson, B.; Qi, J.; Olsen, C.M.; Bayer, K.U.; Liu, Q.S. CaMKII activity in the ventral tegmental area gates cocaine-induced synaptic plasticity in the nucleus accumbens. Neuropsychopharmacology 2014, 39, 989–999. [Google Scholar] [CrossRef][Green Version]
- Easton, A.C.; Lourdusamy, A.; Havranek, M.; Mizuno, K.; Solati, J.; Golub, Y.; Clarke, T.K.; Vallada, H.; Laranjeira, R.; Desrivières, S.; et al. αCaMKII controls the establishment of cocaine’s reinforcing effects in mice and humans. Transl. Psychiatry 2014, 4. [Google Scholar] [CrossRef]
- Li, C.Y.; Mao, X.; Wei, L. Genes and (common) pathways underlying drug addiction. PLoS Comput. Biol. 2008, 4, 0028–0034. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.-E.E. Impairing the amphetamine conditioning in rats through the inhibition of hippocampal calcium/calmodulin-dependent protein kinase II activity. Neuropharmacology 2002, 42, 540–547. [Google Scholar] [CrossRef]
- Steinkellner, T.; Mus, L.; Eisenrauch, B.; Constantinescu, A.; Leo, D.; Konrad, L.; Rickhag, M.; Sørensen, G.; Efimova, E.V.; Kong, E.; et al. In Vivo Amphetamine Action is Contingent on αCaMKII. Neuropsychopharmacology 2014, 39, 2681–2693. [Google Scholar] [CrossRef] [PubMed]
- Pierce, R.C.; Quick, E.A.; Reeder, D.C.; Morgan, Z.R.; Kalivas, P.W. Calcium-Mediated Second Messengers Modulate the Expression of Behavioral Sensitization to Cocaine. J. Pharmacol. Exp. Ther. 1998, 286, 1171–1176. [Google Scholar]
- Wang, L.; Lv, Z.; Hu, Z.; Sheng, J.; Hui, B.; Sun, J.; Ma, L. Chronic cocaine-induced H3 acetylation and transcriptional activation of CaMKIIα in the nucleus accumbens is critical for motivation for drug reinforcement. Neuropsychopharmacology 2010, 35, 913–928. [Google Scholar] [CrossRef]
- Kourrich, S.; Klug, J.R.; Mayford, M.; Thomas, M.J. AMPAR-independent effect of striatal αCaMKII promotes the sensitization of cocaine reward. J. Neurosci. 2012, 32, 6578–6586. [Google Scholar] [CrossRef]
- Loweth, J.A.; Li, D.; Cortright, J.J.; Wilke, G.; Jeyifous, O.; Neve, R.L.; Bayer, K.U.; Vezina, P. Persistent reversal of enhanced amphetamine intake by transient CaMKII inhibition. J. Neurosci. 2013, 33, 1411–1416. [Google Scholar] [CrossRef]
- Loweth, J.A.; Singer, B.F.; Baker, L.K.; Wilke, G.; Inamine, H.; Bubula, N.; Alexander, J.K.; Carlezon, W.A.; Neve, R.L.; Vezina, P. Transient overexpression of α-Ca2+/calmodulin-dependent protein kinase II in the nucleus accumbens shell enhances behavioral responding to amphetamine. J. Neurosci. 2010, 30, 939–949. [Google Scholar] [CrossRef] [PubMed]
- Loweth, J.A.; Baker, L.K.; Guptaa, T.; Guillory, A.M.; Vezina, P. Inhibition of CaMKII in the nucleus accumbens shell decreases enhanced amphetamine intake in sensitized rats. Neurosci. Lett. 2008, 444, 157–160. [Google Scholar] [CrossRef]
- Licata, S.C.; Schmidt, H.D.; Pierce, R.C. Suppressing calcium/calmodulin-dependent protein kinase II activity in the ventral tegmental area enhances the acute behavioural response to cocaine but attenuates the initiation of cocaine-induced behavioural sensitization in rats. Eur. J. Neurosci. 2004, 19, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Christopher Pierce, R.; Kalivas, P.W. Repeated cocaine modifies the mechanism by which amphetamine releases dopamine. J. Neurosci. 1997, 17, 3254–3261. [Google Scholar] [CrossRef]
- Venniro, M.; Panlilio, L.V.; Epstein, D.H.; Shaham, Y. The protective effect of operant social reward on cocaine self-administration, choice, and relapse is dependent on delay and effort for the social reward. Neuropsychopharmacology 2021, 46, 2350–2357. [Google Scholar] [CrossRef]
- Fritz, M.; El Rawas, R.; Salti, A.; Klement, S.; Bardo, M.T.; Kemmler, G.; Dechant, G.; Saria, A.; Zernig, G. Reversal of cocaine-conditioned place preference and mesocorticolimbic Zif268 expression by social interaction in rats. Addict. Biol. 2011, 16, 273–284. [Google Scholar] [CrossRef]
- Fritz, M.; El Rawas, R.; Klement, S.; Kummer, K.; Mayr, M.J.; Eggart, V.; Salti, A.; Bardo, M.T.; Saria, A.; Zernig, G.; et al. Differential effects of accumbens core vs. shell lesions in a rat concurrent conditioned place preference paradigm for cocaine vs. social interaction. PLoS ONE 2011, 6, e26761. [Google Scholar] [CrossRef]
- Lemos, C.; Salti, A.; Amaral, I.M.; Fontebasso, V.; Singewald, N.; Dechant, G.; Hofer, A.; El Rawas, R. Social interaction reward in rats has anti-stress effects. Addict. Biol. 2021, 26, e12878. [Google Scholar] [CrossRef]
- Amaral, I.M.; Lemos, C.; Cera, I.; Dechant, G.; Hofer, A.; El Rawas, R. Involvement of camp-dependent protein kinase in the nucleus accumbens in cocaine versus social interaction reward. Int. J. Mol. Sci. 2021, 22, 345. [Google Scholar] [CrossRef]
- Sampedro-Piquero, P.; Ávila-Gámiz, F.; Moreno Fernández, R.D.; Castilla-Ortega, E.; Santín, L.J. The presence of a social stimulus reduces cocaine-seeking in a place preference conditioning paradigm. J. Psychopharmacol. 2019, 33, 1501–1511. [Google Scholar] [CrossRef]
- Bregolin, T.; Pinheiro, B.S.; El Rawas, R.; Zernig, G. Preventive strength of dyadic social interaction against reacquisition/reexpression of cocaine conditioned place preference. Front. Behav. Neurosci. 2017, 11, 225. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro Do Couto, B.; Aguilar, M.A.; Lluch, J.; Rodríguez-Arias, M.; Miñarro, J. Social experiences affect reinstatement of cocaine-induced place preference in mice. Psychopharmacology 2009, 207, 485–498. [Google Scholar] [CrossRef]
- Kelley, A.E. Memory and addiction: Shared neural circuitry and molecular mechanisms. Neuron 2004, 44, 161–179. [Google Scholar] [CrossRef] [PubMed]
- Olsen, C.M. Natural rewards, neuroplasticity, and non-drug addictions. Neuropharmacology 2011, 61, 1109–1122. [Google Scholar] [CrossRef] [PubMed]
- Olsen, R.W. Extrasynaptic GABAA receptors in the nucleus accumbens are necessary for alcohol drinking. Proc. Natl. Acad. Sci. USA 2011, 108, 4699–4700. [Google Scholar] [CrossRef]
- Leggio, G.M.; Di Marco, R.; Gulisano, W.; D’Ascenzo, M.; Torrisi, S.A.; Geraci, F.; Lavanco, G.; Dahl, K.; Giurdanella, G.; Castorina, A.; et al. Dopaminergic-GABAergic interplay and alcohol binge drinking. Pharmacol. Res. 2019, 141, 384–391. [Google Scholar] [CrossRef]
- Teague, C.D.; Nestler, E.J. Key transcription factors mediating cocaine-induced plasticity in the nucleus accumbens. Mol. Psychiatry 2021. [Google Scholar] [CrossRef] [PubMed]
- Gerdjikov, T.V.; Giles, A.C.; Swain, S.N.; Beninger, R.J. Nucleus accumbens PKA inhibition blocks acquisition but enhances expression of amphetamine-produced conditioned activity in rats. Psychopharmacology 2007, 190, 65–72. [Google Scholar] [CrossRef]
- Self, D.W.; Genova, L.M.; Hope, B.T.; Barnhart, W.J.; Spencer, J.J.; Nestler, E.J. Involvement of cAMP-dependent protein kinase in the nucleus accumbens in cocaine self-administration and relapse of cocaine-seeking behavior. J. Neurosci. 1998, 18, 1848–1859. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, A.E.; Sadeghian, K.; Holahan, M.R.; Kelley, A.E. Appetitive instrumental learning is impaired by inhibition of cAMP-dependent protein kinase within the nucleus accumbens. Neurobiol. Learn. Mem. 2002, 77, 44–62. [Google Scholar] [CrossRef]
- Misra, K.; Pandey, S.C. The decreased cyclic-AMP dependent-protein kinase a function in the nucleus accumbens: A role in alcohol drinking but not in anxiety-like behaviors in rats. Neuropsychopharmacology 2006, 31, 1406–1419. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Caffino, L.; Cassina, C.; Giannotti, G.; Orrù, A.; Moro, F.; Di Clemente, A.; Racagni, G.; Fumagalli, F.; Cervo, L. Short-term abstinence from cocaine self-administration, but not passive cocaine infusion, elevates αcaMKII autophosphorylation in the rat nucleus accumbens and medial prefrontal cortex. Int. J. Neuropsychopharmacol. 2014, 17, 323–329. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ferrario, C.R.; Loweth, J.A.; Milovanovic, M.; Ford, K.A.; Galiñanes, G.L.; Heng, L.J.; Tseng, K.Y.; Wolf, M.E. Alterations in AMPA receptor subunits and TARPs in the rat nucleus accumbens related to the formation of Ca2+-permeable AMPA receptors during the incubation of cocaine craving. Neuropharmacology 2011, 61, 1141–1151. [Google Scholar] [CrossRef]
- Horan, B.; Gardner, E.L.; Ashby, C.R. Enhancement of conditioned place preference response to cocaine in rats following subchronic administration of 3,4-methylenedioxymethamphetamine (MDMA). Synapse 2000, 35, 160–162. [Google Scholar] [CrossRef]
- Kong, H.; Kuang, W.; Li, S.; Xu, M. Activation of dopamine D3 receptors inhibits reward-related learning induced by cocaine. Neuroscience 2011, 176, 152–161. [Google Scholar] [CrossRef][Green Version]
- Kummer, K.K.K.K.; Hofhansel, L.; Barwitz, C.M.C.M.; Schardl, A.; Prast, J.M.J.M.; Salti, A.; El Rawas, R.; Zernig, G. Differences in social interaction- vs. cocaine reward in mouse vs. rat. Front. Behav. Neurosci. 2014, 8, 363. [Google Scholar] [CrossRef]
- Di Chiara, G. Nucleus accumbens shell and core dopamine: Differential role in behavior and addiction. Behav. Brain Res. 2002, 137, 75–114. [Google Scholar] [CrossRef]
- Ikemoto, S.; Qin, M.; Liu, Z.H. The functional divide for primary reinforcement of D-amphetamine lies between the medial and lateral ventral striatum: Is the division of the accumbens core, shell, and olfactory tubercle valid? J. Neurosci. 2005, 25, 5061–5065. [Google Scholar] [CrossRef] [PubMed]
- Corbit, L.H.; Fischbach, S.C.; Janak, P.H. Nucleus accumbens core and shell are differentially involved in general and outcome-specific forms of Pavlovian-instrumental transfer with alcohol and sucrose rewards. Eur. J. Neurosci. 2016, 43, 1229–1236. [Google Scholar] [CrossRef] [PubMed]
- Floresco, S.B.; Montes, D.R.; Tse, M.M.T.; van Holstein, M. Differential contributions of nucleus accumbens subregions to cue-guided risk/reward decision making and implementation of conditional rules. J. Neurosci. 2018, 38, 1901–1914. [Google Scholar] [CrossRef]
- Kai, K.; Sabine, K.; Vincent, E.; Michael, J.M.; Alois, S.; Gerald, Z.; Kummer, K.; Klement, S.; Eggart, V.; Mayr, M.J.; et al. Conditioned place preference for social interaction in rats: Contribution of sensory components. Front. Behav. Neurosci. 2011, 5, 80. [Google Scholar] [CrossRef]
- Sakurai, S.; Yu, L.; Tan, S.E. Roles of hippocampal N-methyl-D-aspartate receptors and calcium/calmodulin-dependent protein kinase II in amphetamine-produced conditioned place preference in rats. Behav. Pharmacol. 2007, 18, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, X.-D.D.; Zhang, J.-J.J.; Yu, L.-C.C. Increases in αCaMKII phosphorylated on Thr286 in the nucleus accumbens shell but not the core during priming-induced reinstatement of morphine-seeking in rats. Neurosci. Lett. 2012, 526, 39–44. [Google Scholar] [CrossRef]
- El Rawas, R.; Klement, S.; Kummer, K.K.; Fritz, M.; Dechant, G.; Saria, A.; Zernig, G. Brain regions associated with the acquisition of conditioned place preference for cocaine vs. social interaction. Front. Behav. Neurosci. 2012, 6, 63. [Google Scholar] [CrossRef] [PubMed]
- Salti, A.; Kummer, K.K.K.K.; Sadangi, C.; Dechant, G.; Saria, A.; El Rawas, R. Social interaction reward decreases p38 activation in the nucleus accumbens shell of rats. Neuropharmacology 2015, 99, 510–516. [Google Scholar] [CrossRef][Green Version]
- El Rawas, R.; Klement, S.; Salti, A.; Fritz, M.; Dechant, G.; Saria, A.; Zernig, G. Preventive role of social interaction for cocaine conditioned place preference: Correlation with FosB/DeltaFosB and pCREB expression in rat mesocorticolimbic areas. Front. Behav. Neurosci. 2012, 6, 8. [Google Scholar] [CrossRef]
- Harda, Z.; Dzik, J.M.; Nalberczak-Skóra, M.; Meyza, K.; Łukasiewicz, K.; Łęski, S.; Radwanska, K. Autophosphorylation of αCaMKII affects social interactions in mice. Genes Brain Behav. 2018, 17, e12457. [Google Scholar] [CrossRef]
- Stephenson, J.R.; Wang, X.; Perfitt, T.L.; Parrish, W.P.; Shonesy, B.C.; Marks, C.R.; Mortlock, D.P.; Nakagawa, T.; Sutcliffe, J.S.; Colbran, R.J. A novel human CAMK2a mutation disrupts dendritic morphology and synaptic transmission, and causes ASD-related behaviors. J. Neurosci. 2017, 37, 2216–2233. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.H.; Samal, A.B.; Lee, M.; Vlach, J.; Novikov, N.; Niedziela-Majka, A.; Feng, J.Y.; Koltun, D.O.; Brendza, K.M.; Kwon, H.J.; et al. The KN-93 Molecule Inhibits Calcium/Calmodulin-Dependent Protein Kinase II (CaMKII) Activity by Binding to Ca2+/CaM. J. Mol. Biol. 2019, 431, 1440–1459. [Google Scholar] [CrossRef]
- Johnson, C.N.; Pattanayek, R.; Potet, F.; Rebbeck, R.T.; Blackwell, D.J.; Nikolaienko, R.; Sequeira, V.; Le Meur, R.; Radwański, P.B.; Davis, J.P.; et al. The CaMKII inhibitor KN93-calmodulin interaction and implications for calmodulin tuning of NaV1.5 and RyR2 function. Cell Calcium 2019, 82, 102063. [Google Scholar] [CrossRef] [PubMed]
- EL Rawas, R.; Amaral, I.M.; Hofer, A. Social interaction reward: A resilience approach to overcome vulnerability to drugs of abuse. Eur Neuropsychopharmacol. 2020, 37, 12–28. [Google Scholar] [CrossRef] [PubMed]
- El Rawas, R.; Amaral, I.M.; Hofer, A. Is p38 MAPK associated to drugs of abuse-induced abnormal behaviors? Int. J. Mol. Sci. 2020, 21, 4833. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amaral, I.M.; Scheffauer, L.; Langeder, A.B.; Hofer, A.; El Rawas, R. Rewarding Social Interaction in Rats Increases CaMKII in the Nucleus Accumbens. Biomedicines 2021, 9, 1886. https://doi.org/10.3390/biomedicines9121886
Amaral IM, Scheffauer L, Langeder AB, Hofer A, El Rawas R. Rewarding Social Interaction in Rats Increases CaMKII in the Nucleus Accumbens. Biomedicines. 2021; 9(12):1886. https://doi.org/10.3390/biomedicines9121886
Chicago/Turabian StyleAmaral, Inês M., Laura Scheffauer, Angelika B. Langeder, Alex Hofer, and Rana El Rawas. 2021. "Rewarding Social Interaction in Rats Increases CaMKII in the Nucleus Accumbens" Biomedicines 9, no. 12: 1886. https://doi.org/10.3390/biomedicines9121886
APA StyleAmaral, I. M., Scheffauer, L., Langeder, A. B., Hofer, A., & El Rawas, R. (2021). Rewarding Social Interaction in Rats Increases CaMKII in the Nucleus Accumbens. Biomedicines, 9(12), 1886. https://doi.org/10.3390/biomedicines9121886






