Angiotensin System Modulations in Spontaneously Hypertensive Rats and Consequences on Erythrocyte Properties; Action of MLN-4760 and Zofenopril
Abstract
1. Introduction
2. Materials and Methods
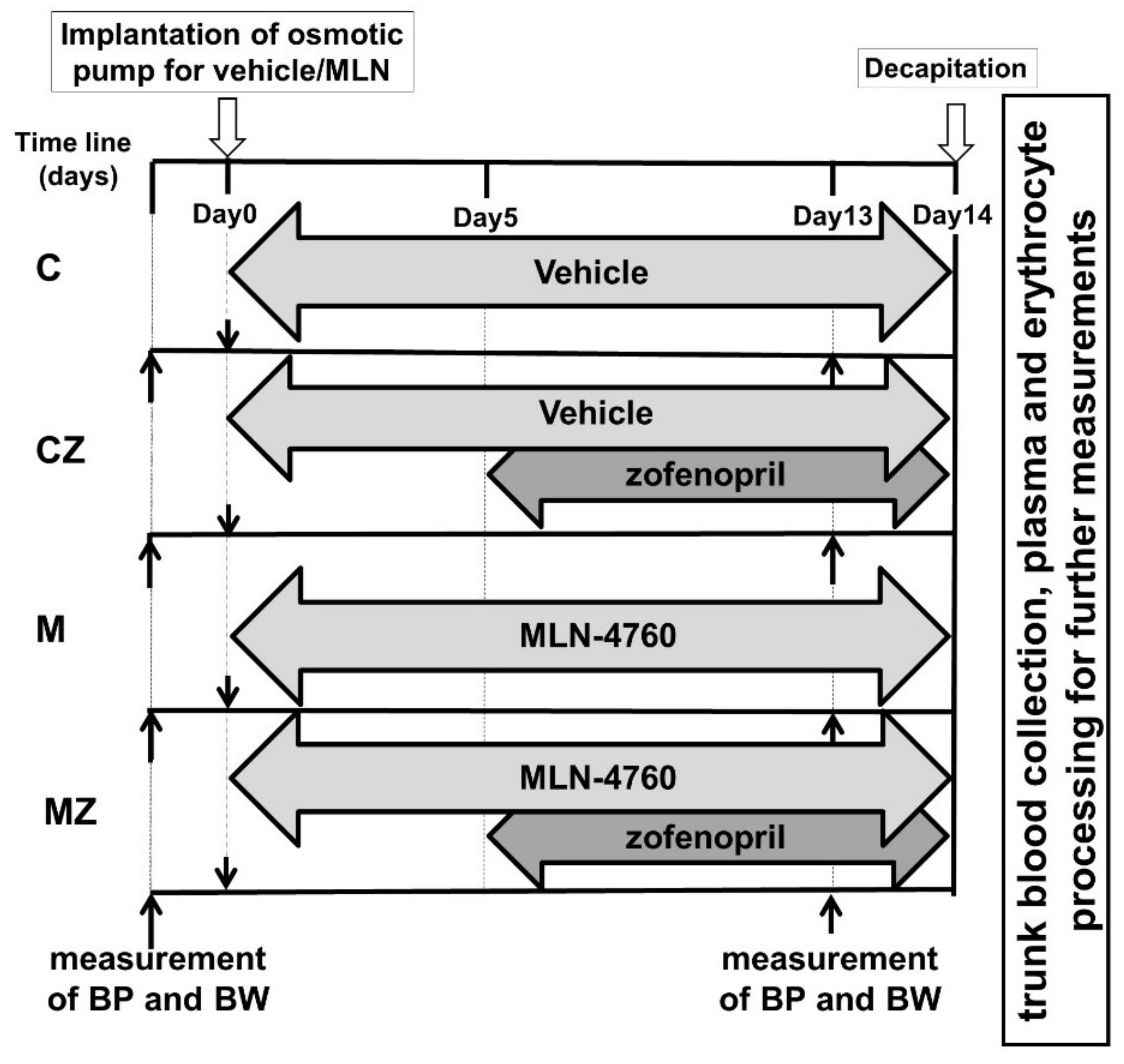
2.1. Experimental Model
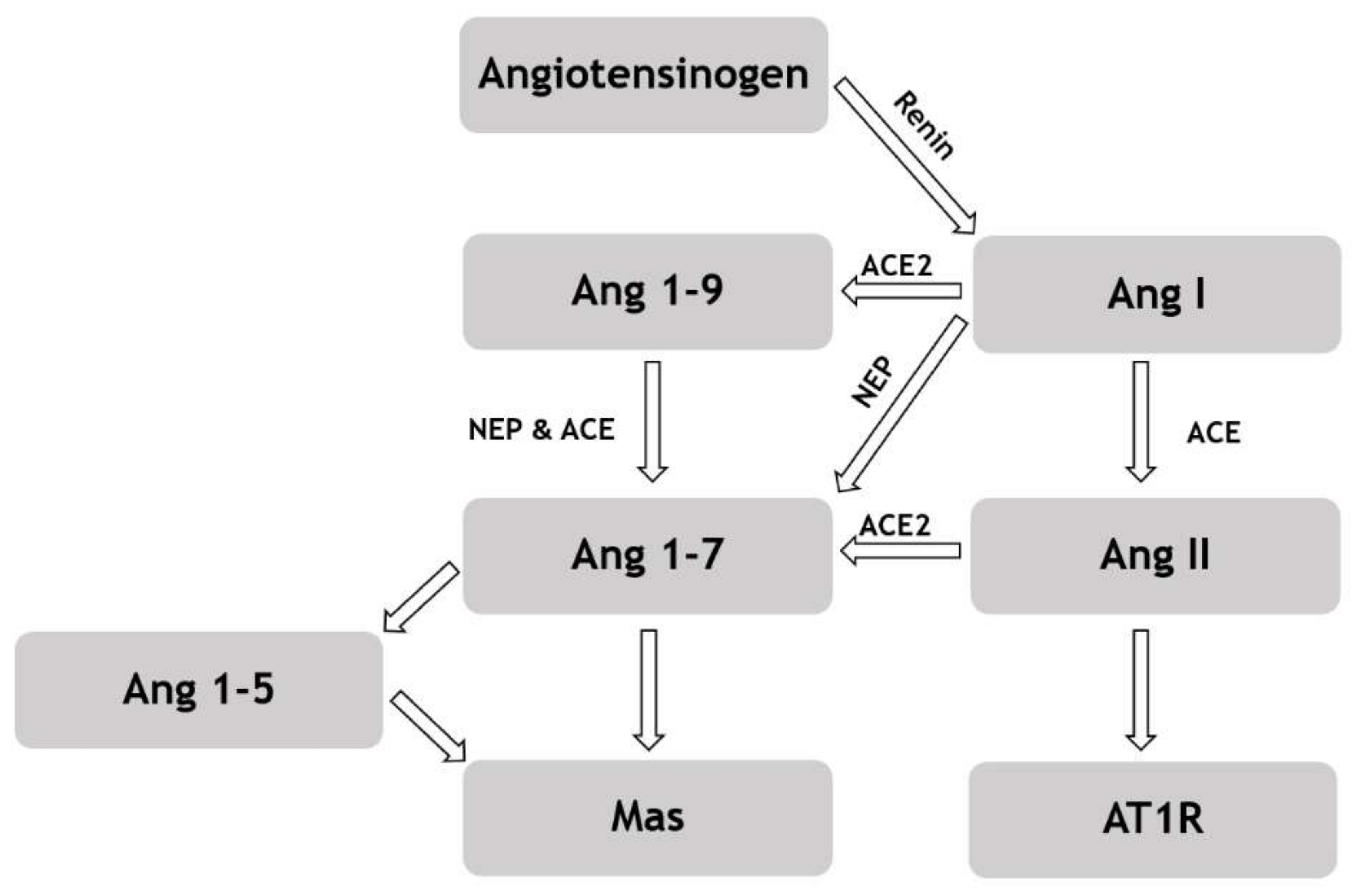
2.2. RAS Peptide Concentrations
2.3. Parameters of Antioxidant Status and Oxidative Stress in Blood Plasma and Hemolyzed RBCs
2.4. Measurement of Plasma H2S Concentration
2.5. Matrix Metalloproteinase 9 Activities
2.6. Blood Processing and RBC Isolation
2.7. RBC Deformability
2.8. RBC Nitric Oxide Production
2.9. RBC Free Radical Measurement
2.10. Na,K-ATPase Kinetics
2.11. Determination of RBC Osmotic Resistance
2.12. Statistical Analyses
3. Results
3.1. Basic Biometric Parameters
3.2. Angiotensin Peptide Concentration in Blood Plasma
3.3. Parameters of Antioxidant Status and Oxidative Stress in Blood Plasma
3.4. Parameters of Antioxidant Status in Hemolyzed RBCs
3.5. Plasma H2S Concentration
3.6. Activity of MMP-9 in Plasma
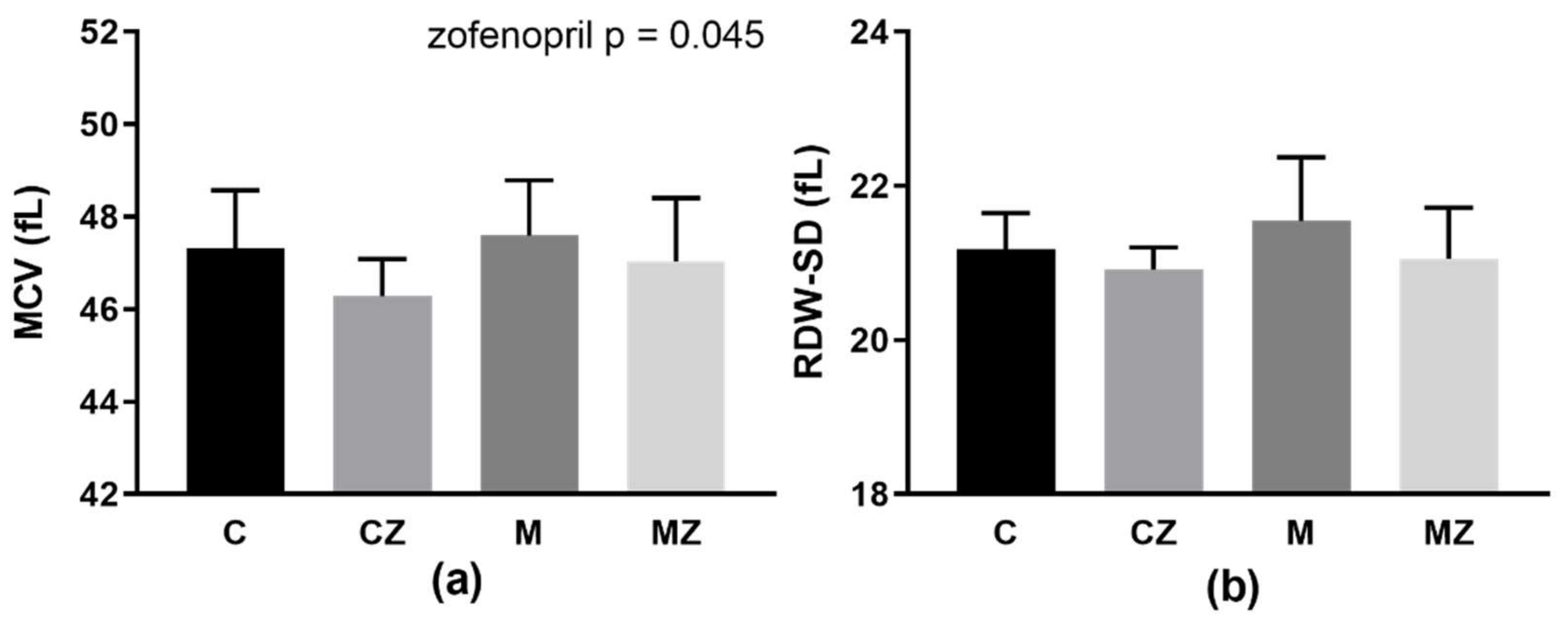
3.7. Erythrocyte Parameters MCV and RDW-SD
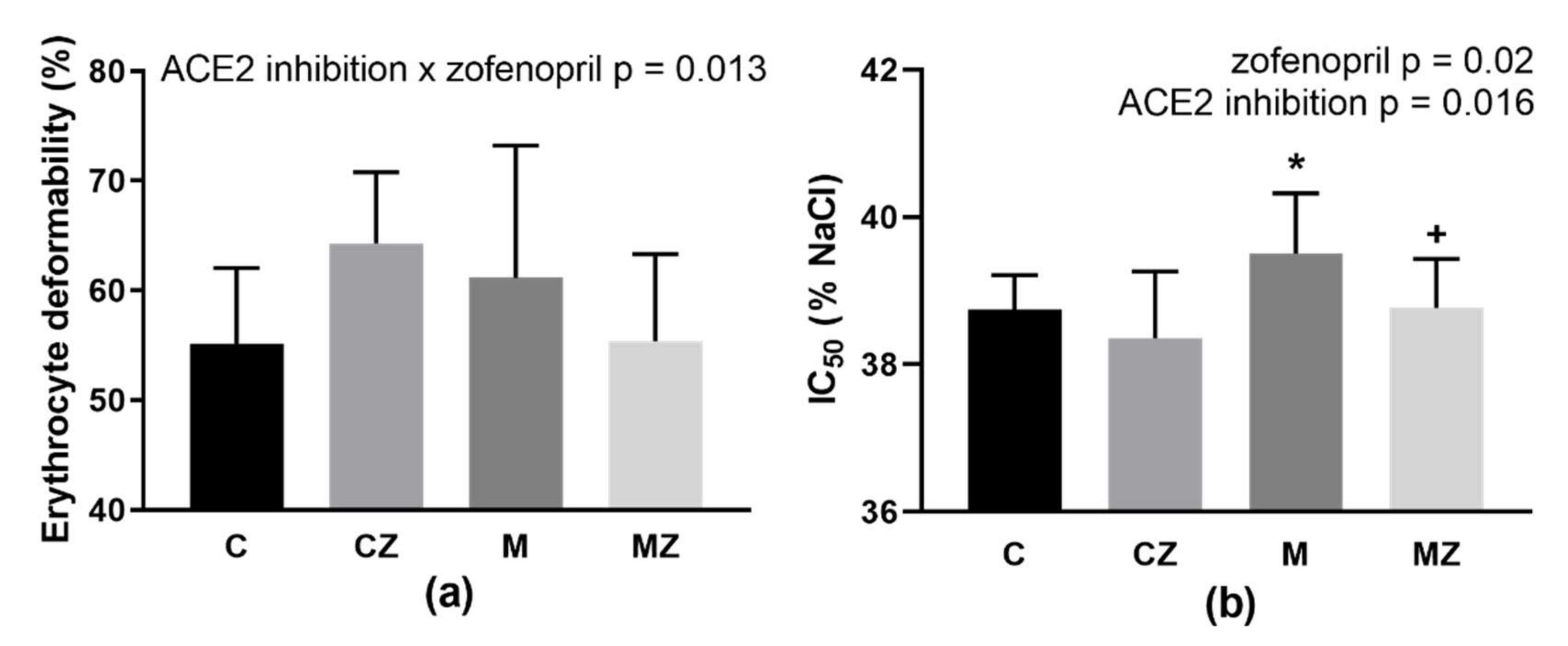
3.8. RBC Deformability and Osmotic Resistance
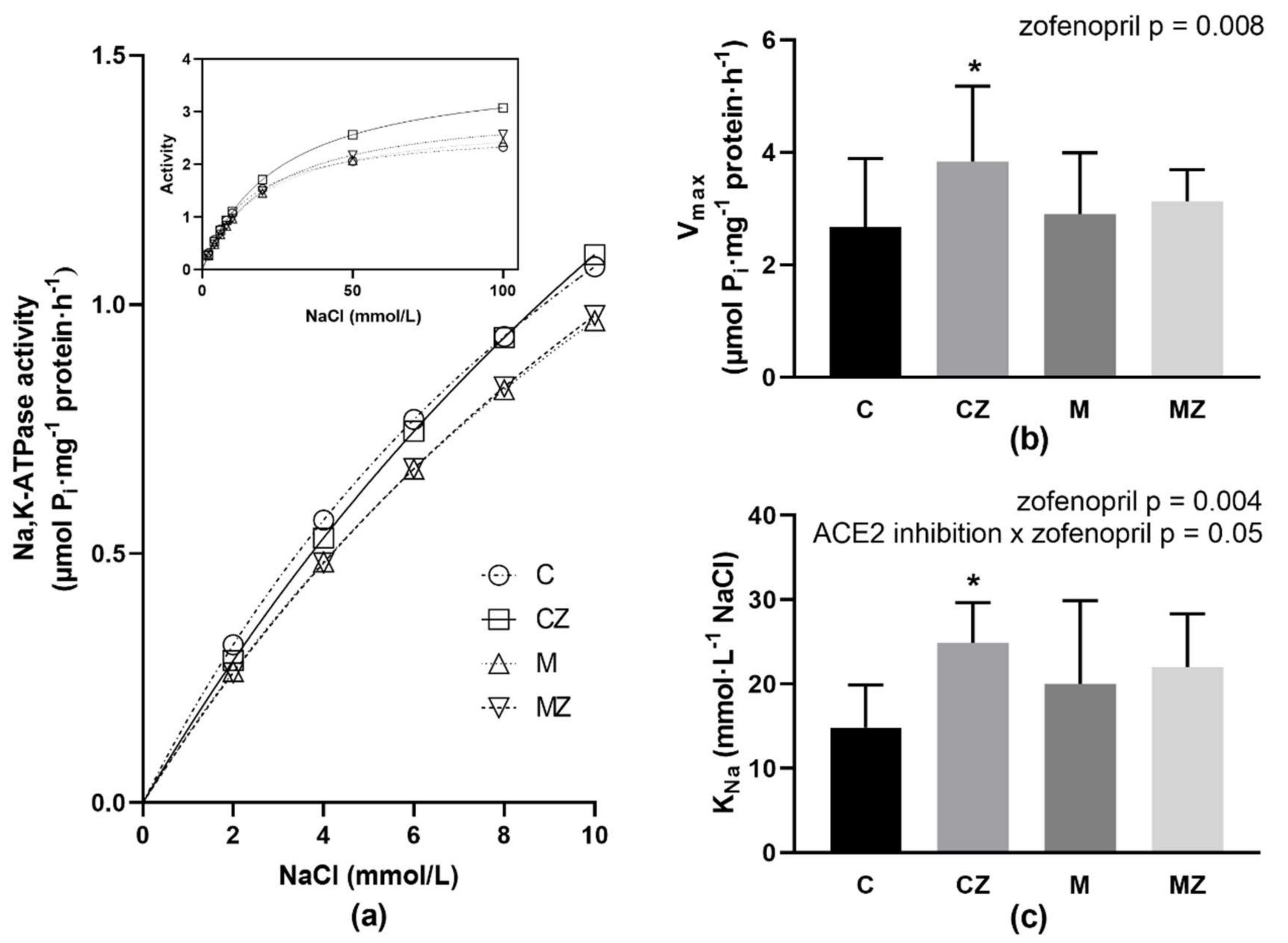
3.9. Na,K-ATPase Kinetics
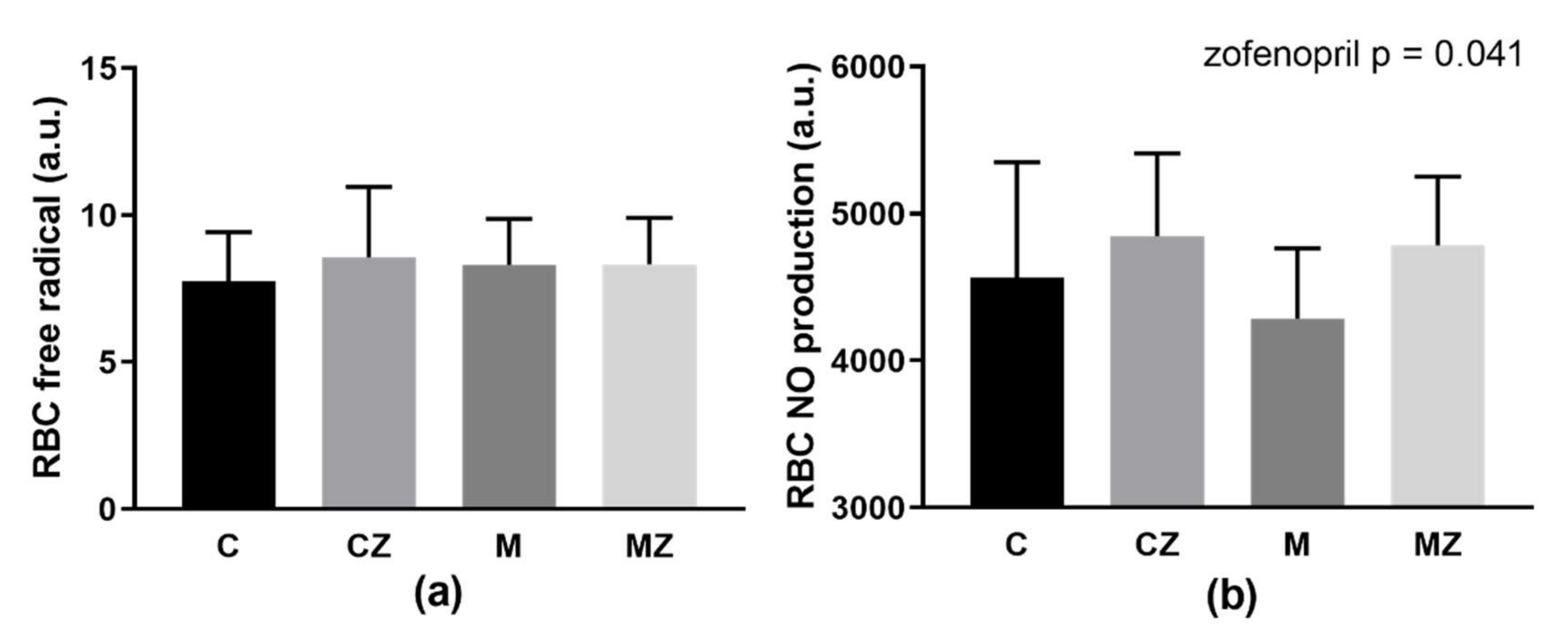
3.10. RBC Free Radical and NO Production
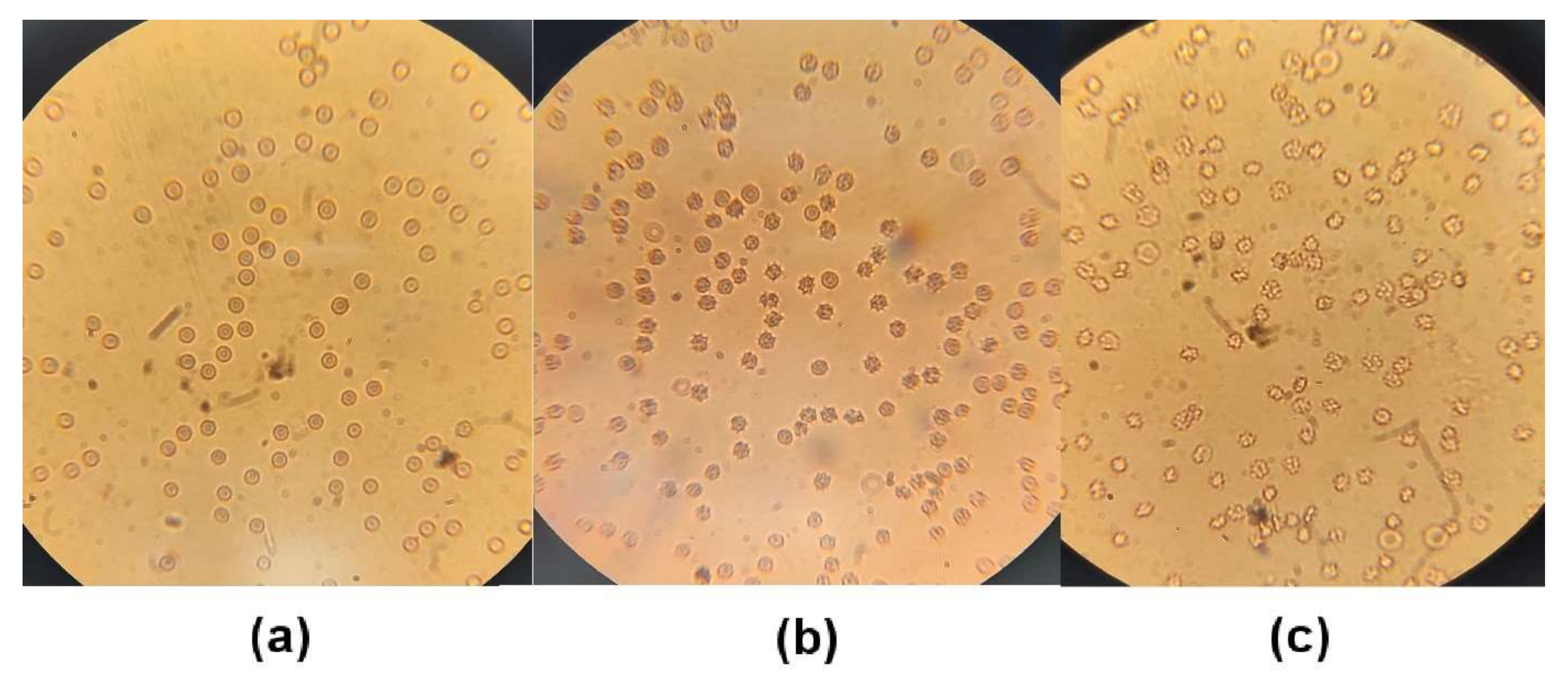
3.11. Erythrocyte Morphology
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berzuini, A.; Bianco, C.; Paccapelo, C.; Bertolini, F.; Gregato, G.; Cattaneo, A.; Erba, E.; Bandera, A.; Gori, A.; Lamorte, G.; et al. Red Cell–Bound Antibodies and Transfusion Requirements in Hospitalized Patients with COVID-19. Blood 2020, 136, 766–768. [Google Scholar] [CrossRef]
- Khakwani, M.; Horgan, C.; Ewing, J. COVID-19-associated Oxidative Damage to Red Blood Cells. Br. J. Haematol. 2021, 193, 481. [Google Scholar] [CrossRef] [PubMed]
- Thomas, T.; Stefanoni, D.; Dzieciatkowska, M.; Issaian, A.; Nemkov, T.; Hill, R.C.; Francis, R.O.; Hudson, K.E.; Buehler, P.W.; Zimring, J.C.; et al. Evidence of Structural Protein Damage and Membrane Lipid Remodeling in Red Blood Cells from COVID-19 Patients. J. Proteome Res. 2020, 19, 4455–4469. [Google Scholar] [CrossRef]
- Chien, S. Determinants of Blood Viscosity and Red Cell Deformability. Scand. J. Clin. Lab. Investig. 1981, 41, 7–12. [Google Scholar] [CrossRef]
- Renoux, C.; Fort, R.; Nader, E.; Boisson, C.; Joly, P.; Stauffer, E.; Robert, M.; Girard, S.; Cibiel, A.; Gauthier, A.; et al. Impact of COVID-19 on Red Blood Cell Rheology. Br. J. Haematol. 2021, 192, e108–e111. [Google Scholar] [CrossRef]
- Farber, P.L. Can Erythrocytes Behavior in Microcirculation Help the Understanding the Physiopathology and Improve Prevention and Treatment for Covid-19? Clin. Hemorheol. Microcirc. 2021, 78, 41–47. [Google Scholar] [CrossRef]
- Radosinska, J.; Vrbjar, N. The Role of Red Blood Cell Deformability and Na,K-ATPase Function in Selected Risk Factors of Cardiovascular Diseases in Humans: Focus on Hypertension, Diabetes Mellitus and Hypercholesterolemia. Physiol. Res. 2016, 65, S43–S54. [Google Scholar] [CrossRef]
- Harrison, S.L.; Buckley, B.J.R.; Rivera-Caravaca, J.M.; Zhang, J.; Lip, G.Y.H. Cardiovascular Risk Factors, Cardiovascular Disease, and COVID-19: An Umbrella Review of Systematic Reviews. Eur. Hear. J.-Qual. Care Clin. Outcomes 2021, 7, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Tadic, M.; Cuspidi, C.; Grassi, G.; Mancia, G. COVID-19 and Arterial Hypertension: Hypothesis or Evidence? J. Clin. Hypertens. 2020, 22, 1120–1126. [Google Scholar] [CrossRef]
- Radosinska, J.; Barancik, M.; Vrbjar, N. Heart Failure and Role of Circulating MMP-2 and MMP-9. Panminerva Med. 2017, 59, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Onal, I.K.; Altun, B.; Onal, E.D.; Kırkpantur, A.; Oz, S.G.; Turgan, C. Serum Levels of MMP-9 and TIMP-1 in Primary Hypertension and Effect of Antihypertensive Treatment. Eur. J. Intern. Med. 2009, 20, 369–372. [Google Scholar] [CrossRef]
- Schieffer, B.; Bünte, C.; Witte, J.; Hoeper, K.; Böger, R.H.; Schwedhelm, E.; Drexler, H. Comparative Effects of AT1-Antagonism and Angiotensin-Converting Enzyme Inhibition on Markers of Inflammation and Platelet Aggregation in Patients with Coronary Artery Disease. J. Am. Coll. Cardiol. 2004, 44, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Abers, M.S.; Delmonte, O.M.; Ricotta, E.E.; Fintzi, J.; Fink, D.L.; de Jesus, A.A.A.; Zarember, K.A.; Alehashemi, S.; Oikonomou, V.; Desai, J.V.; et al. An Immune-Based Biomarker Signature Is Associated with Mortality in COVID-19 Patients. JCI Insight 2021, 6, e144455. [Google Scholar] [CrossRef]
- Clerkin, K.J.; Fried, J.A.; Raikhelkar, J.; Sayer, G.; Griffin, J.M.; Masoumi, A.; Jain, S.S.; Burkhoff, D.; Kumaraiah, D.; Rabbani, L.R.; et al. COVID-19 and Cardiovascular Disease. Circulation 2020, 141, 1648–1655. [Google Scholar] [CrossRef] [PubMed]
- Aleksova, A.; Gagno, G.; Sinagra, G.; Beltrami, A.P.; Janjusevic, M.; Ippolito, G.; Zumla, A.; Fluca, A.L.; Ferro, F. Effects of SARS-CoV-2 on Cardiovascular System: The Dual Role of Angiotensin-Converting Enzyme 2 (ACE2) as the Virus Receptor and Homeostasis Regulator-Review. Int. J. Mol. Sci. 2021, 22, 4526. [Google Scholar] [CrossRef] [PubMed]
- Borghi, C.; Omboni, S. Angiotensin-Converting Enzyme Inhibition: Beyond Blood Pressure Control—The Role of Zofenopril. Adv. Ther. 2020, 37, 4068–4085. [Google Scholar] [CrossRef]
- Donnarumma, E.; Ali, M.J.; Rushing, A.M.; Scarborough, A.L.; Bradley, J.M.; Organ, C.L.; Islam, K.N.; Polhemus, D.J.; Evangelista, S.; Cirino, G.; et al. Zofenopril Protects Against Myocardial Ischemia–Reperfusion Injury by Increasing Nitric Oxide and Hydrogen Sulfide Bioavailability. J. Am. Hear. Assoc. 2016, 5, e003531. [Google Scholar] [CrossRef]
- Vitvitsky, V.; Yadav, P.K.; Kurthen, A.; Banerjee, R. Sulfide Oxidation by a Noncanonical Pathway in Red Blood Cells Generates Thiosulfate and Polysulfides. J. Biol. Chem. 2015, 290, 8310–8320. [Google Scholar] [CrossRef]
- Fadyukova, O.E.; Koshelev, V.B. Effect of Hydrogen Sulfide on Deformability of Rat Erythrocytes. Bull. Exp. Biol. Med. 2020, 169, 725–728. [Google Scholar] [CrossRef] [PubMed]
- Zicha, J.; Kunes, J. Ontogenetic Aspects of Hypertension Development: Analysis in the Rat. Physiol. Rev. 1999, 79, 1227–1282. [Google Scholar] [CrossRef] [PubMed]
- Púzserová, A.; Ilovska, V.; Balis, P.; Slezak, P.; Bernatova, I. Age-Related Alterations in Endothelial Function of Femoral Artery in Young SHR and WKY Rats. BioMed Res. Int. 2014, 2014, 1–12. [Google Scholar] [CrossRef]
- Smith, T.L.; Hutchins, P.M. Central Hemodynamics in the Developmental Stage of Spontaneous Hypertension in the Unanesthetized Rat. Hypertension 1979, 1, 508–517. [Google Scholar] [CrossRef] [PubMed]
- Ülker, S.; Mcmaster, D.; Mckeown, P.P.; Bayraktutan, U. Impaired Activities of Antioxidant Enzymes Elicit Endothelial Dysfunction in Spontaneous Hypertensive Rats despite Enhanced Vascular Nitric Oxide Generation. Cardiovasc. Res. 2003, 59, 488–500. [Google Scholar] [CrossRef]
- Kodavanti, U.P.; Schladweiler, M.C.; Ledbetter, A.D.; Watkinson, W.P.; Campen, M.J.; Winsett, D.W.; Richards, J.R.; Crissman, K.M.; Hatch, G.E.; Costa, D.L. The Spontaneously Hypertensive Rat as a Model of Human Cardiovascular Disease: Evidence of Exacerbated Cardiopulmonary Injury and Oxidative Stress from Inhaled Emission Particulate Matter. Toxicol. Appl. Pharmacol. 2000, 164, 250–263. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Poglitsch, M.; McWhinney, B.C.; Ungerer, J.P.J.; Ahmed, A.H.; Gordon, R.D.; Wolley, M.; Stowasser, M. Measurement of Equilibrium Angiotensin II in the Diagnosis of Primary Aldosteronism. Clin. Chem. 2020, 66, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Kollarova, M.; Puzserova, A.; Balis, P.; Radosinska, D.; Tothova, L.; Bartekova, M.; Barancik, M.; Radosinska, J. Age-and Phenotype-Dependent Changes in Circulating MMP-2 and MMP-9 Activities in Normotensive and Hypertensive Rats. Int. J. Mol. Sci. 2020, 21, 7286. [Google Scholar] [CrossRef]
- Dayar, E.; Kara, E.; Hocaoglu, N.; Bozkurt, O.; Gidener, S.; Durmus, N.; Yetik-Anacak, G. Do Penile Haemodynamics Change in the Presence of Hydrogen Sulphide (H2S) Donor in Metabolic Syndrome-Induced Erectile Dysfunction? Andrologia 2017, 50, e12885. [Google Scholar] [CrossRef]
- Shen, X.; Pattillo, C.B.; Pardue, S.; Bir, S.C.; Wang, R.; Kevil, C.G. Measurement of Plasma Hydrogen Sulfide in Vivo and in Vitro. Free. Radic. Biol. Med. 2011, 50, 1021–1031. [Google Scholar] [CrossRef]
- Jasenovec, T.; Radosinska, D.; Kollarova, M.; Balis, P.; Ferenczyova, K.; Kalocayova, B.; Bartekova, M.; Tothova, L.; Radosinska, J. Beneficial Effect of Quercetin on Erythrocyte Properties in Type 2 Diabetic Rats. Molecules 2021, 26, 4868. [Google Scholar] [CrossRef]
- Bhuyan, A.A.M.; Bissinger, R.; Cao, H.; Lang, F. Triggering of Suicidal Erythrocyte Death by Exemestane. Cell. Physiol. Biochem. 2017, 42, 1–12. [Google Scholar] [CrossRef]
- Radosinska, J.; Mezesova, L.; Okruhlicova, L.; Frimmel, K.; Breierova, E.; Bartekova, M.; Vrbjar, N. Effect of Yeast Biomass with High Content of Carotenoids on Erythrocyte Deformability, NO Production and Na,K-ATPase Activity in Healthy and LPS Treated Rats. Clin. Hemorheol. Microcirc. 2016, 64, 125–134. [Google Scholar] [CrossRef]
- Taussky, H.H.; Shorr, E.; Kurzmann, G. A Microcolorimetric Method for the Determination of Inorganic Phosphorus. J. Biol. Chem. 1953, 202, 675–685. [Google Scholar]
- Radosinska, J.; Jasenovec, T.; Radosinska, D.; Balis, P.; Puzserova, A.; Skratek, M.; Manka, J.; Bernatova, I. Ultra-Small Superparamagnetic Iron-Oxide Nanoparticles Exert Different Effects on Erythrocytes in Normotensive and Hypertensive Rats. Biomedicines 2021, 9, 377. [Google Scholar] [CrossRef]
- Zhou, X.; Shang, D.; Zhang, T.; Li, L.; Zhou, T.; Lu, W. Modeling of angiotensin II–angiotensin-(1-7) counterbalance in disease progression in spontaneously hypertensive rats treated with/without perindopril. Pharmacol. Res. 2012, 66, 177–184. [Google Scholar] [CrossRef]
- Jiang, F.; Yang, J.; Zhang, Y.; Dong, M.; Wang, S.; Zhang, Q.; Liu, F.F.; Zhang, K.; Zhang, C. Angiotensin-converting enzyme 2 and angiotensin 1–7: Novel therapeutic targets. Nat. Rev. Cardiol. 2014, 11, 413–426. [Google Scholar] [CrossRef] [PubMed]
- Cooper, S.L.; Boyle, E.; Jefferson, S.R.; Heslop, C.R.A.; Mohan, P.; Mohanraj, G.G.J.; Sidow, H.A.; Tan, R.C.P.; Hill, S.J.; Woolard, J. Role of the Renin–Angiotensin–Aldosterone and Kinin–Kallikrein Systems in the Cardiovascular Complications of COVID-19 and Long COVID. Int. J. Mol. Sci. 2021, 22, 8255. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.D.; Karnik, S.S. Angiotensin Receptors: Structure, Function, Signaling and Clinical Applications. J. Cell Signal. 2016, 1, 111. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Roso, M.; Montero, M.; Carrón, R.; Sevilla, M. Cardiovascular Changes in Spontaneously Hypertensive Rats Are Improved by Chronic Treatment with Zofenopril: Effects of Chronic Treatment with Zofenopril in SHR. Br. J. Pharmacol. 2009, 158, 1911–1921. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wen, H.; Gwathmey, J.K.; Xie, L.-H. Oxidative Stress-Mediated Effects of Angiotensin II in the Cardiovascular System. World J. Hypertens. 2012, 2, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Pernow, J.; Mahdi, A.; Yang, J.; Zhou, Z. Red Blood Cell Dysfunction: A New Player in Cardiovascular Disease. Cardiovasc. Res. 2019, 115, 1596–1605. [Google Scholar] [CrossRef]
- Mahdi, A.; Cortese-Krott, M.M.; Kelm, M.; Li, N.; Pernow, J. Novel Perspectives on Redox Signaling in Red Blood Cells and Platelets in Cardiovascular Disease. Free. Radic. Biol. Med. 2021, 168, 95–109. [Google Scholar] [CrossRef] [PubMed]
- Suvorava, T.; Cortese-Krott, M.M. Exercise-Induced Cardioprotection via ENOS: A Putative Role of Red Blood Cell Signaling. Curr. Med. Chem. 2018, 25, 4457–4474. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.C.; Nichols, W.W.; Mehta, J.L. Cardioprotective Effects of Red Blood Cells on Ischemia and Reperfusion Injury in Isolated Rat Heart: Release of Nitric Oxide as a Potential Mechanism. J. Cardiovasc. Pharmacol. Ther. 1996, 1, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, V.; Diederich, L.; Keller, T.C.S.; Kramer, C.M.; Lückstädt, W.; Panknin, C.; Suvorava, T.; Isakson, B.E.; Kelm, M.; Cortese-Krott, M.M. Red Blood Cell Function and Dysfunction: Redox Regulation, Nitric Oxide Metabolism, Anemia. Antioxidants Redox Signal. 2017, 26, 718–742. [Google Scholar] [CrossRef]
- Revin, V.V.; Ushakova, A.A.; Gromova, N.V.; Balykova, L.A.; Revina, E.S.; Stolyarova, V.V.; Stolbova, T.A.; Solomadin, I.N.; Tychkov, A.Y.; Revina, N.V.; et al. Study of Erythrocyte Indices, Erythrocyte Morphometric Indicators, and Oxygen-Binding Properties of Hemoglobin Hematoporphyrin Patients with Cardiovascular Diseases. Adv. Hematol. 2017, 2017, 1–9. [Google Scholar] [CrossRef]
- Borghi, C.; Bacchelli, S.; Degli Esposti, D.; Ambrosioni, E. A Review of the Angiotensin-Converting Enzyme Inhibitor, Zofenopril, in the Treatment of Cardiovascular Diseases. Expert Opin. Pharmacother. 2004, 5, 1965–1977. [Google Scholar] [CrossRef]
- May, J.E.; Marques, M.B.; Reddy, V.V.B.; Gangaraju, R. Three Neglected Numbers in the CBC: The RDW, MPV, and NRBC Count. Clevel. Clin. J. Med. 2019, 86, 167–172. [Google Scholar] [CrossRef]
- Fava, C.; Cattazzo, F.; Hu, Z.-D.; Lippi, G.; Montagnana, M. The Role of Red Blood Cell Distribution Width (RDW) in Cardiovascular Risk Assessment: Useful or Hype? Ann. Transl. Med. 2019, 7, 581. [Google Scholar] [CrossRef] [PubMed]
- Cortese-Krott, M.M.; Kelm, M. Endothelial Nitric Oxide Synthase in Red Blood Cells: Key to a New Erythrocrine Function? Redox Biol. 2014, 2, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Tomaiuolo, G. Biomechanical Properties of Red Blood Cells in Health and Disease towards Microfluidics. Biomicrofluidics 2014, 8, 051501. [Google Scholar] [CrossRef]
- Pretorius, E.; Olumuyiwa-Akeredolu, O.-O.O.; Mbotwe, S.; Bester, J. Erythrocytes and Their Role as Health Indicator: Using Structure in a Patient-Orientated Precision Medicine Approach. Blood Rev. 2016, 30, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Van Putten, L.M.; Croon, F. The Life Span of Red Cells in the Rat and the Mouse as Determined by Labeling with DFP32 in Vivo. Blood 1958, 13, 789–794. [Google Scholar] [CrossRef] [PubMed]
| C | CZ | M | MZ | Zof. | ACE2i | Inter. | |
|---|---|---|---|---|---|---|---|
| Δ BP (mmHg) | 9.28 ± 15.25 | −5.36 ± 14.72 † | 11.56 ± 18.66 | 8.83 ± 16.48 | x | x | |
| Δ Body weight (g) | 17.65 ± 5.77 | 9.15 ± 8.67 †† | 24.85 ± 6.17 †† | 14.5 ± 8.95 *** | xxxx | xxx | |
| HW/Tibia (mg/mm) | 33 ± 3 | 31 ± 1 | 34 ± 2 | 29 ± 1 **** | xxxx | ||
| LW/Tibia (mg/mm) | 344 ± 29 | 321 ± 16 | 353 ± 29 | 327 ± 17 * | xx | ||
| KW/Tibia (mg/mm) | 64 ± 3 | 66 ± 2 † | 65 ± 4 | 63 ± 3 | |||
| Hematocrit (%) | 50.04 ± 3.03 | 52.46 ± 0.95 | 51.25 ± 1.48 | 50.17 ± 2.06 | x |
| C | CZ | M | MZ | Zof. | ACE2i | Inter. | |
|---|---|---|---|---|---|---|---|
| Ang I (1-10) (pmol/L) | 94.5 ± 15.8 | 372 ± 135.7 †††† | 105.9 ± 23.7 | 484.6 ± 112.9 **** | xxxx | ||
| Ang II (1-8) (pmol/L) | 161.1 ± 29.3 | 225.2 ± 33.9 †† | 186.4 ± 32.2 | 259.2 ± 32.2 *** | xxxx | x | |
| Ang 1-7 (pmol/L) | 6.6 ± 2.4 | 16.4 ± 7.0 †† | 6.4 ± 2.2 | 19.5 ± 6.3 **** | xxxx | ||
| Ang 1-5 (pmol/L) | 20.3 ± 3.1 | 38.6 ± 9.7 †††† | 20.9 ± 5.1 | 46.3 ± 6.9 **** | xxxx | ||
| PRA (pmol/L) | 255.6 ± 41.6 | 597.2 ± 167.1 †††† | 292.4 ± 53.1 | 743.8 ± 104 **** | xxxx | x | |
| ACE | 1.7 ± 0.2 | 0.7 ± 0.1 †††† | 1.8 ± 0.2 | 0.6 ± 0.2 **** | xxxx | ||
| ALT | 0.096 ± 0.01 | 0.084 ± 0.005 † | 0.084 ± 0.01 † | 0.081 ± 0.01 | x | x |
| C | CZ | M | MZ | Zof. | ACE2i | Inter. | |
|---|---|---|---|---|---|---|---|
| GSH/GSSG | 7.05 (5.66; 12.41) | 8.74 (7.39; 15.17) | 23.94 † (10.47; 35.46) | 5.81 ** (5.14; 16.06) | xx | ||
| FRAP (μmol/L) | 520 ± 100.7 | 480 ± 38.8 | 465 ± 58.1 | 445 ± 99.9 | |||
| TAC (μmol/L) | 1.83 ± 0.207 | 1.86 ± 0.077 | 1.71 ± 0.194 | 2.02 ± 0.381 * | |||
| AGEs (g/L) | 0.96 ± 0.099 | 1.00 ± 0.090 | 0.87 ± 0.125 | 0.95 ± 0.077 | x | ||
| FRUC (mmol/L) | 1.34 ± 0.119 | 1.29 ± 0.127 | 1.29 ± 0.117 | 1.23 ± 0.064 | |||
| AOPP (μmol/L) | 195 ± 63.6 | 183 ± 54.9 | 170 ± 57.6 | 161 ± 48.4 | |||
| TBARS (μmol/L) | 410 ± 129.0 | 448 ± 86.2 | 350 ± 82.7 | 477 ± 147.3 | x |
| C | CZ | M | MZ | Zof. | ACE2i | Inter. | |
|---|---|---|---|---|---|---|---|
| GSH/GSSG | 0.37 ± 0.222 | 0.16 ± 0.099 † | 0.22 ± 0.1 † | 0.15 ± 0.051 | xx | ||
| FRAP (mmol/L) | 13 ± 1.94 | 12.8 ± 1.36 | 15.4 ± 2.26 † | 10.8 ± 1.40 *** | xxx | xxx |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jasenovec, T.; Radosinska, D.; Kollarova, M.; Balis, P.; Dayar, E.; Bernatova, I.; Zorad, S.; Vrbjar, N.; Cacanyova, S.; Radosinska, J. Angiotensin System Modulations in Spontaneously Hypertensive Rats and Consequences on Erythrocyte Properties; Action of MLN-4760 and Zofenopril. Biomedicines 2021, 9, 1902. https://doi.org/10.3390/biomedicines9121902
Jasenovec T, Radosinska D, Kollarova M, Balis P, Dayar E, Bernatova I, Zorad S, Vrbjar N, Cacanyova S, Radosinska J. Angiotensin System Modulations in Spontaneously Hypertensive Rats and Consequences on Erythrocyte Properties; Action of MLN-4760 and Zofenopril. Biomedicines. 2021; 9(12):1902. https://doi.org/10.3390/biomedicines9121902
Chicago/Turabian StyleJasenovec, Tomas, Dominika Radosinska, Marta Kollarova, Peter Balis, Ezgi Dayar, Iveta Bernatova, Stefan Zorad, Norbert Vrbjar, Sona Cacanyova, and Jana Radosinska. 2021. "Angiotensin System Modulations in Spontaneously Hypertensive Rats and Consequences on Erythrocyte Properties; Action of MLN-4760 and Zofenopril" Biomedicines 9, no. 12: 1902. https://doi.org/10.3390/biomedicines9121902
APA StyleJasenovec, T., Radosinska, D., Kollarova, M., Balis, P., Dayar, E., Bernatova, I., Zorad, S., Vrbjar, N., Cacanyova, S., & Radosinska, J. (2021). Angiotensin System Modulations in Spontaneously Hypertensive Rats and Consequences on Erythrocyte Properties; Action of MLN-4760 and Zofenopril. Biomedicines, 9(12), 1902. https://doi.org/10.3390/biomedicines9121902






