Figure 1.
Study design. Traumatic brain injury (TBI) was induced by lateral fluid-percussion injury (FPI). Lesion endophenotype (focal inflammatory [TBIFI] vs. cavity-forming [TBICF]) was assessed with T2-weighted magnetic resonance imaging (MRI) at 6 weeks after TBI. At 8 weeks post-TBI, rats received a single injection of lipopolysaccharide (LPS; 5 mg/kg, i.p.) or vehicle. Epidural skull electrodes (ei) were implanted at 14 weeks following TBI. Thereafter, 4-week-long video-electroencephalogram (vEEG) monitoring was performed starting at 16 weeks after TBI to detect spontaneous seizures. The pentylenetetrazole (PTZ) seizure-susceptibility test was performed under vEEG at 23 weeks post-TBI (i.e., 15 weeks after LPS injection). Finally, rats were perfused for histology at 2 h after PTZ administration (~6 months post-TBI). Sham-operated controls underwent all of the same procedures except the induction of TBI.
Figure 1.
Study design. Traumatic brain injury (TBI) was induced by lateral fluid-percussion injury (FPI). Lesion endophenotype (focal inflammatory [TBIFI] vs. cavity-forming [TBICF]) was assessed with T2-weighted magnetic resonance imaging (MRI) at 6 weeks after TBI. At 8 weeks post-TBI, rats received a single injection of lipopolysaccharide (LPS; 5 mg/kg, i.p.) or vehicle. Epidural skull electrodes (ei) were implanted at 14 weeks following TBI. Thereafter, 4-week-long video-electroencephalogram (vEEG) monitoring was performed starting at 16 weeks after TBI to detect spontaneous seizures. The pentylenetetrazole (PTZ) seizure-susceptibility test was performed under vEEG at 23 weeks post-TBI (i.e., 15 weeks after LPS injection). Finally, rats were perfused for histology at 2 h after PTZ administration (~6 months post-TBI). Sham-operated controls underwent all of the same procedures except the induction of TBI.
Figure 2.
Cortical lesion endophenotypes. Representative unfolded MRI and histologic cortical maps of 2 rats with lateral fluid-percussion-induced traumatic brain injury (TBI), showing the extent and location of the cortical lesion in the focal-inflammatory (rat #17 from the TBI
FI-LPS group; left panels) and cavity-forming (rat #36 from the TBI
CF-LPS group; right panels) endophenotypes. The horizontal lines indicate the 2 rostrocaudal levels, from which the immunostained sections were sampled for analysis of c-Fos expression. (
A) Four representative coronal T
2-weighted MRI slices (a
1 most rostral, a
4 most caudal) used for the unfolding of the lesion in MRI images at 6 weeks post-TBI. White arrows indicate the lesion. (
B) Four thionin-stained sections (23 weeks post-TBI) corresponding to levels of MRI slices in panel (
A). Dashed 1 mm wide squares in b
1 (rostral) and b
4 (caudal), extending throughout layers I-VI indicate the areas used for the quantitative analysis of c-Fos-immunoreactivity (ir) in the medial (m) and lateral (l) perilesional cortex. (
C) Unfolded MRI (blue) and histologic (pink) cortical lesion overlaid on the unfolded template prepared as previously described [
26]. Abbreviations: CF, cavity-forming; FI, focal inflammatory; ir, immunoreactivity; L, lateral; LPS, lipopolysaccharide; M, medial; MRI, magnetic resonance imaging; TBI, traumatic brain injury. Scale bar = 1 mm.
Figure 2.
Cortical lesion endophenotypes. Representative unfolded MRI and histologic cortical maps of 2 rats with lateral fluid-percussion-induced traumatic brain injury (TBI), showing the extent and location of the cortical lesion in the focal-inflammatory (rat #17 from the TBI
FI-LPS group; left panels) and cavity-forming (rat #36 from the TBI
CF-LPS group; right panels) endophenotypes. The horizontal lines indicate the 2 rostrocaudal levels, from which the immunostained sections were sampled for analysis of c-Fos expression. (
A) Four representative coronal T
2-weighted MRI slices (a
1 most rostral, a
4 most caudal) used for the unfolding of the lesion in MRI images at 6 weeks post-TBI. White arrows indicate the lesion. (
B) Four thionin-stained sections (23 weeks post-TBI) corresponding to levels of MRI slices in panel (
A). Dashed 1 mm wide squares in b
1 (rostral) and b
4 (caudal), extending throughout layers I-VI indicate the areas used for the quantitative analysis of c-Fos-immunoreactivity (ir) in the medial (m) and lateral (l) perilesional cortex. (
C) Unfolded MRI (blue) and histologic (pink) cortical lesion overlaid on the unfolded template prepared as previously described [
26]. Abbreviations: CF, cavity-forming; FI, focal inflammatory; ir, immunoreactivity; L, lateral; LPS, lipopolysaccharide; M, medial; MRI, magnetic resonance imaging; TBI, traumatic brain injury. Scale bar = 1 mm.
![Biomedicines 09 01946 g002 Biomedicines 09 01946 g002]()
Figure 3.
Video-electroencephalogram (vEEG) analysis. (
A) A spontaneous seizure in a rat (#22) from the TBI
FI-Veh group that occurred during the transition from stage III sleep to rapid eye movement sleep (REM). Black arrows indicate the beginning and end of the seizure. Asterisks refer to arousals. The duration of the electrographic seizure was 117 s, and the behavioral Racine score was 3 [
31]. The X-Y scale in the right lower corner indicates the voltage and duration or electrographic patterns and applies to panels A-C. Stars indicate muscle artifacts. (
B) An example of the epileptiform discharge (ED) lasting 1.2 s in a rat (#39) from the TBI
FI-LPS group. Note the peak in relative power at 5.5 Hz. (
C) An example of a spike-and-wave discharge (SWD) in a rat (#40) in the TBI
FI-Veh group. Note the peak in relative power at 8 Hz and subsequent harmonics. SWDs were also found in the sham-operated group and were considered to be age-related oscillations in Sprague–Dawley rats]. (
D) Green circle indicates the craniotomy. Positions of the 4 epidural recording electrodes (Ø 1 mm, blue circles), a reference electrode (black), and ground electrode (orange). Abbreviations: cF, contralateral frontal; cP, contralateral posterior; ED, epileptiform discharge; FI, focal inflammatory; iF, ipsilateral frontal; iP, ipsilateral posterior; LPS, lipopolysaccharide; REM, rapid eye movement sleep; SWD, spike-and-wave discharge; TBI, traumatic brain injury; Veh, vehicle.
Figure 3.
Video-electroencephalogram (vEEG) analysis. (
A) A spontaneous seizure in a rat (#22) from the TBI
FI-Veh group that occurred during the transition from stage III sleep to rapid eye movement sleep (REM). Black arrows indicate the beginning and end of the seizure. Asterisks refer to arousals. The duration of the electrographic seizure was 117 s, and the behavioral Racine score was 3 [
31]. The X-Y scale in the right lower corner indicates the voltage and duration or electrographic patterns and applies to panels A-C. Stars indicate muscle artifacts. (
B) An example of the epileptiform discharge (ED) lasting 1.2 s in a rat (#39) from the TBI
FI-LPS group. Note the peak in relative power at 5.5 Hz. (
C) An example of a spike-and-wave discharge (SWD) in a rat (#40) in the TBI
FI-Veh group. Note the peak in relative power at 8 Hz and subsequent harmonics. SWDs were also found in the sham-operated group and were considered to be age-related oscillations in Sprague–Dawley rats]. (
D) Green circle indicates the craniotomy. Positions of the 4 epidural recording electrodes (Ø 1 mm, blue circles), a reference electrode (black), and ground electrode (orange). Abbreviations: cF, contralateral frontal; cP, contralateral posterior; ED, epileptiform discharge; FI, focal inflammatory; iF, ipsilateral frontal; iP, ipsilateral posterior; LPS, lipopolysaccharide; REM, rapid eye movement sleep; SWD, spike-and-wave discharge; TBI, traumatic brain injury; Veh, vehicle.
![Biomedicines 09 01946 g003 Biomedicines 09 01946 g003]()
Figure 4.
Effect of lesion endophenotype on PTZ seizure susceptibility test. (A) Occurrence of PTZ-induced seizures was increased by the “second hit” in the TBIFI endophenotype (TBIFI-LPS 100% vs. TBIFI-Veh 43%). (B) Latency to the first electrographic seizure was not affected by the endophenotype of the cortical lesion. Please note that only a subpopulation of animals developed PTZ-induced seizures (e.g., only 1 rat showed a seizure after PTZ administration in the sham-operated group). (C) TBI-LPS rats showed a longer cumulative seizure duration (163 ± 90 s, range 77–338 s, median 168 s) compared with TBI-Veh rats (35 ± 19 s, range 10–52 s, median 46 s). (D) Further analysis revealed that the difference resulted from the prolonged cumulative seizure duration in the TBIFI endophenotype. Data are presented as mean ± standard error of the mean (SEM) (panels (B,C)). Statistical significances: # p < 0.05 (χ2-test); * p < 0.05, ** p < 0.01 (Mann–Whitney U test). Abbreviations: CF, cavity-forming; FI, focal inflammatory; LPS, lipopolysaccharide; PTZ, pentylenetetrazole; TBI, traumatic brain injury; Veh, vehicle.
Figure 4.
Effect of lesion endophenotype on PTZ seizure susceptibility test. (A) Occurrence of PTZ-induced seizures was increased by the “second hit” in the TBIFI endophenotype (TBIFI-LPS 100% vs. TBIFI-Veh 43%). (B) Latency to the first electrographic seizure was not affected by the endophenotype of the cortical lesion. Please note that only a subpopulation of animals developed PTZ-induced seizures (e.g., only 1 rat showed a seizure after PTZ administration in the sham-operated group). (C) TBI-LPS rats showed a longer cumulative seizure duration (163 ± 90 s, range 77–338 s, median 168 s) compared with TBI-Veh rats (35 ± 19 s, range 10–52 s, median 46 s). (D) Further analysis revealed that the difference resulted from the prolonged cumulative seizure duration in the TBIFI endophenotype. Data are presented as mean ± standard error of the mean (SEM) (panels (B,C)). Statistical significances: # p < 0.05 (χ2-test); * p < 0.05, ** p < 0.01 (Mann–Whitney U test). Abbreviations: CF, cavity-forming; FI, focal inflammatory; LPS, lipopolysaccharide; PTZ, pentylenetetrazole; TBI, traumatic brain injury; Veh, vehicle.
![Biomedicines 09 01946 g004 Biomedicines 09 01946 g004]()
Figure 5.
c-Fos expression in the rostral and caudal perilesional cortex. (A) Rostral perilesional cortex. In the Sham-Veh group, the density of c-Fos expression was comparable between the ipsilateral and contralateral perilesional cortex rostrally. In the TBI-Veh group, c-Fos expression was increased ipsilaterally compared with the Sham-Veh group. In the TBI-LPS group, the density of c-Fos expression was higher both ipsilaterally and contralaterally than in the Sham-Veh group. Contralateral labeling was also higher than that in the TBI-Veh group. Inter-hemispheric analysis showed higher c-Fos labeling ipsilaterally than contralaterally in both the TBI-Veh and TBI-LPS groups. (B) Caudal perilesional cortex. In the Sham-Veh group, the density of c-Fos expression was comparable between the ipsilateral and contralateral perilesional cortex caudally. The c-Fos labeling density was higher ipsilaterally in the TBI-Veh group than in the Sham-Veh group. In the TBI-LPS group, c-Fos expression was increased ipsilaterally compared with the Sham-Veh and TBI-Veh groups. Interhemispheric analysis revealed more c-Fos activation ipsilaterally than contralaterally in both the TBI-Veh and TBI-LPS groups. Comparison of c-Fos expression between the rostral and caudal perilesional cortex showed that c-Fos expression in the ipsilateral perilesional cortex was higher rostrally than caudally in the TBI-Veh group (p < 0.05, Wilcoxon). In the TBI-LPS group, rostral c-Fos expression was increased bilaterally compared with the caudal c-Fos expression (p < 0.05, Wilcoxon). Abbreviations: CF, cavity-forming; contra, contralateral; FI, focal inflammatory; ipsi, ipsilateral; LPS, lipopolysaccharide; TBI, traumatic brain injury; Veh, vehicle. Statistical significances: # p < 0.05, ## p < 0.01, ### p < 0.001 (Wilcoxon); * p < 0.05, ** p < 0.01, *** p < 0.001 (Mann–Whitney U test).
Figure 5.
c-Fos expression in the rostral and caudal perilesional cortex. (A) Rostral perilesional cortex. In the Sham-Veh group, the density of c-Fos expression was comparable between the ipsilateral and contralateral perilesional cortex rostrally. In the TBI-Veh group, c-Fos expression was increased ipsilaterally compared with the Sham-Veh group. In the TBI-LPS group, the density of c-Fos expression was higher both ipsilaterally and contralaterally than in the Sham-Veh group. Contralateral labeling was also higher than that in the TBI-Veh group. Inter-hemispheric analysis showed higher c-Fos labeling ipsilaterally than contralaterally in both the TBI-Veh and TBI-LPS groups. (B) Caudal perilesional cortex. In the Sham-Veh group, the density of c-Fos expression was comparable between the ipsilateral and contralateral perilesional cortex caudally. The c-Fos labeling density was higher ipsilaterally in the TBI-Veh group than in the Sham-Veh group. In the TBI-LPS group, c-Fos expression was increased ipsilaterally compared with the Sham-Veh and TBI-Veh groups. Interhemispheric analysis revealed more c-Fos activation ipsilaterally than contralaterally in both the TBI-Veh and TBI-LPS groups. Comparison of c-Fos expression between the rostral and caudal perilesional cortex showed that c-Fos expression in the ipsilateral perilesional cortex was higher rostrally than caudally in the TBI-Veh group (p < 0.05, Wilcoxon). In the TBI-LPS group, rostral c-Fos expression was increased bilaterally compared with the caudal c-Fos expression (p < 0.05, Wilcoxon). Abbreviations: CF, cavity-forming; contra, contralateral; FI, focal inflammatory; ipsi, ipsilateral; LPS, lipopolysaccharide; TBI, traumatic brain injury; Veh, vehicle. Statistical significances: # p < 0.05, ## p < 0.01, ### p < 0.001 (Wilcoxon); * p < 0.05, ** p < 0.01, *** p < 0.001 (Mann–Whitney U test).
![Biomedicines 09 01946 g005 Biomedicines 09 01946 g005]()
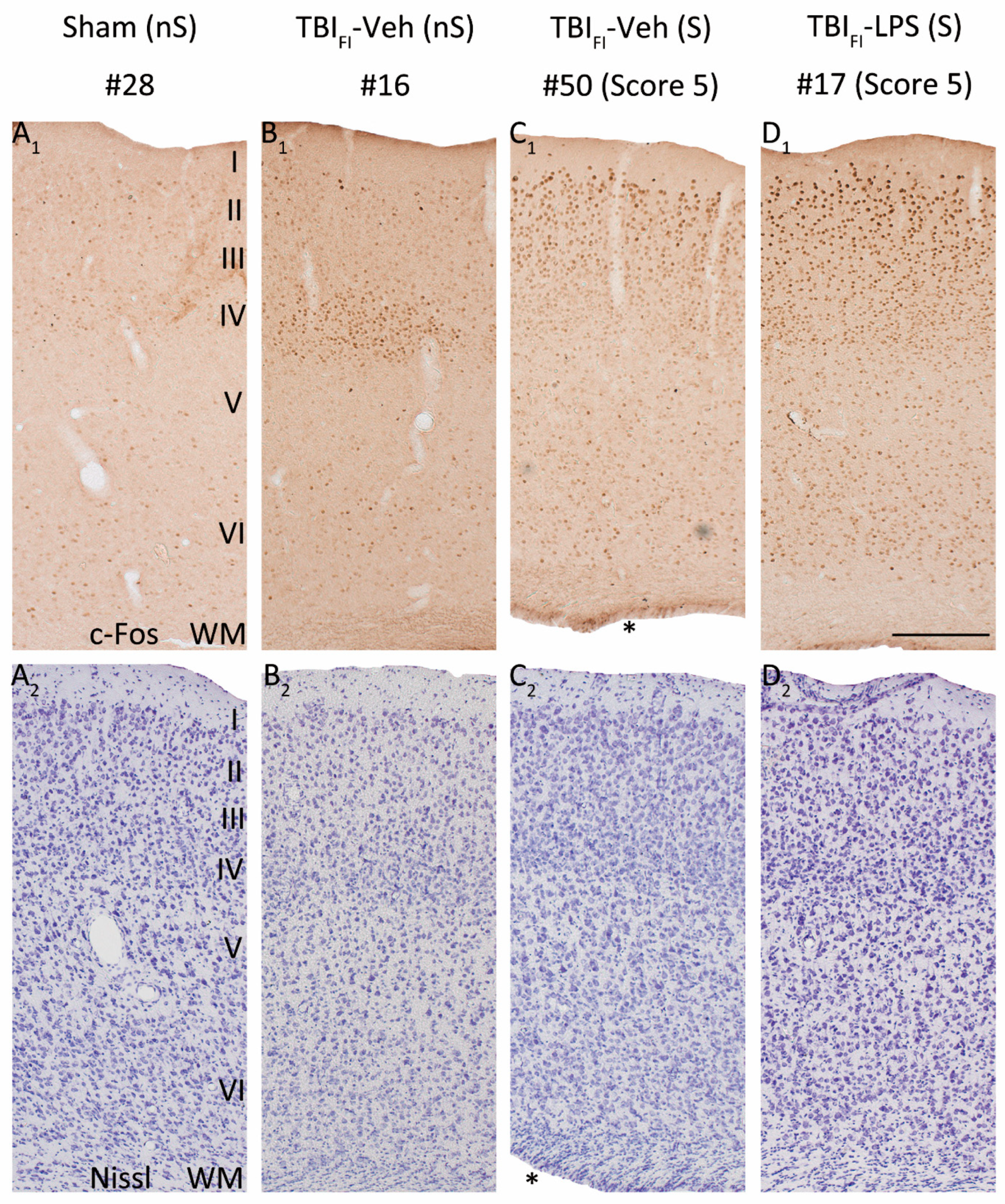
Figure 6.
LPS enhances the seizure-induced c-Fos expression and changes its pattern in the rostromedial perilesional cortex. Representative high-magnification photomicrographs showing c-Fos immunolabeling (
A1–
D1) and thionin staining (
A2–
D2) in the rostromedial perilesional cortex (somatosensory cortex, see
Figure 3C) of a vehicle-treated sham-operated rat and a vehicle or LPS-treated injured rat with a TBI
FI endophenotype at 2 h after PTZ injection (15 weeks after LPS injection and 23 weeks post-TBI). (
A1,
A2) A rat from the Sham-Veh group without PTZ-induced seizure (#28). Note the very low c-Fos expression level throughout the cortical layers. (
B1,
B2) A rat from the TBI
FI-Veh group without a PTZ-induced seizure (#16). Note the intense c-Fos labeling in layer IV apparently reporting on the TBI-induced chronic excitability. (
C1,
C2) A rat from the TBI
FI-Veh group with a PTZ-induced seizure (#50, Racine score 5 seizure). Note the intense c-Fos labeling in layers II-III. (
D1,
D2) A rat from the TBI
FI-LPS group with a PTZ-induced seizure (#17, Racine score 5 seizure) with intense c-Fos labeling in layers II-III and scattered immunopositive cells in deeper layers. Note that the occurrence of PTZ-induced seizures changed the overall pattern of perilesional c-Fos expression in TBI animals (with or without LPS). In addition to labeling in layer IV, layers I-III were activated (e.g., (
D1) vs. (
B1)). A rat in the TBI
FI-Veh group with PTZ-induced seizure (
C1) showed higher c-Fos activation than a rat without a seizure ((
C1) vs. (
B1)), particularly in the supragranular layers. An LPS-treated rat with a TBI
FI endophenotype and a PTZ-induced seizure had robustly enhanced c-Fos expression in all cortical layers (
D1) compared with a vehicle-treated seizing TBI rat (
C1). Abbreviations: FI, focal inflammatory; FPI, fluid-percussion injury; LPS, lipopolysaccharide; nS, no seizure; PTZ, pentylenetetrazole; S, seizure; TBI, traumatic brain injury; Veh, vehicle; WM, white matter. Scale bars = 200 μm. * Enlarged ipsilateral lateral ventricle.
Figure 6.
LPS enhances the seizure-induced c-Fos expression and changes its pattern in the rostromedial perilesional cortex. Representative high-magnification photomicrographs showing c-Fos immunolabeling (
A1–
D1) and thionin staining (
A2–
D2) in the rostromedial perilesional cortex (somatosensory cortex, see
Figure 3C) of a vehicle-treated sham-operated rat and a vehicle or LPS-treated injured rat with a TBI
FI endophenotype at 2 h after PTZ injection (15 weeks after LPS injection and 23 weeks post-TBI). (
A1,
A2) A rat from the Sham-Veh group without PTZ-induced seizure (#28). Note the very low c-Fos expression level throughout the cortical layers. (
B1,
B2) A rat from the TBI
FI-Veh group without a PTZ-induced seizure (#16). Note the intense c-Fos labeling in layer IV apparently reporting on the TBI-induced chronic excitability. (
C1,
C2) A rat from the TBI
FI-Veh group with a PTZ-induced seizure (#50, Racine score 5 seizure). Note the intense c-Fos labeling in layers II-III. (
D1,
D2) A rat from the TBI
FI-LPS group with a PTZ-induced seizure (#17, Racine score 5 seizure) with intense c-Fos labeling in layers II-III and scattered immunopositive cells in deeper layers. Note that the occurrence of PTZ-induced seizures changed the overall pattern of perilesional c-Fos expression in TBI animals (with or without LPS). In addition to labeling in layer IV, layers I-III were activated (e.g., (
D1) vs. (
B1)). A rat in the TBI
FI-Veh group with PTZ-induced seizure (
C1) showed higher c-Fos activation than a rat without a seizure ((
C1) vs. (
B1)), particularly in the supragranular layers. An LPS-treated rat with a TBI
FI endophenotype and a PTZ-induced seizure had robustly enhanced c-Fos expression in all cortical layers (
D1) compared with a vehicle-treated seizing TBI rat (
C1). Abbreviations: FI, focal inflammatory; FPI, fluid-percussion injury; LPS, lipopolysaccharide; nS, no seizure; PTZ, pentylenetetrazole; S, seizure; TBI, traumatic brain injury; Veh, vehicle; WM, white matter. Scale bars = 200 μm. * Enlarged ipsilateral lateral ventricle.
![Biomedicines 09 01946 g006 Biomedicines 09 01946 g006]()
Figure 7.
LPS enhances the seizure-induced c-Fos expression and changes its pattern in the caudomedial perilesional cortex. Representative high magnification photomicrographs showing c-Fos immunochemistry (A1–D1) and Nissl staining (A2–D2) in the caudomedial perilesional cortex in rats from the Sham-Veh and TBIFI endophenotype groups at 2 h after PTZ injection (23 weeks post-FPI and 15 weeks after LPS injection). (A1,A2) Example of a rat from the Sham-Veh group without PTZ-induced seizure (#28). (B1,B2) Example of a rat from the TBIFI-Veh group without a PTZ-induced seizure (#16). (C1,C2) Example of a rat from TBIFI-Veh group with a PTZ-induced seizure (#50, seizure Racine score 5). (D1,D2) Example of a rat from TBIFI-LPS group with a PTZ-induced seizure (#17, seizure Racine score 5). Note that in rats without a PTZ-induced seizure, TBI increased PTZ-induced c-Fos expression ((B1) vs. (A1)). In the TBIFI-Veh group, a rat with a PTZ-induced seizure (C1) exhibited higher c-Fos activation compared to a rat without a seizure (B1), particularly in the supragranular layers. In the TBIFI endophenotype with PTZ-induced seizure, LPS treatment at a chronic time-point after TBI further enhanced c-Fos expression (D1) compared with vehicle treatment (C1) in all layers. Abbreviations: FI, focal inflammatory; FPI, fluid-percussion injury; LPS, lipopolysaccharide; nS, no seizure; PTZ, pentylenetetrazole; S, seizure; TBI, traumatic brain injury; Veh, vehicle; WM, white matter. Scale bars = 200 μm.
Figure 7.
LPS enhances the seizure-induced c-Fos expression and changes its pattern in the caudomedial perilesional cortex. Representative high magnification photomicrographs showing c-Fos immunochemistry (A1–D1) and Nissl staining (A2–D2) in the caudomedial perilesional cortex in rats from the Sham-Veh and TBIFI endophenotype groups at 2 h after PTZ injection (23 weeks post-FPI and 15 weeks after LPS injection). (A1,A2) Example of a rat from the Sham-Veh group without PTZ-induced seizure (#28). (B1,B2) Example of a rat from the TBIFI-Veh group without a PTZ-induced seizure (#16). (C1,C2) Example of a rat from TBIFI-Veh group with a PTZ-induced seizure (#50, seizure Racine score 5). (D1,D2) Example of a rat from TBIFI-LPS group with a PTZ-induced seizure (#17, seizure Racine score 5). Note that in rats without a PTZ-induced seizure, TBI increased PTZ-induced c-Fos expression ((B1) vs. (A1)). In the TBIFI-Veh group, a rat with a PTZ-induced seizure (C1) exhibited higher c-Fos activation compared to a rat without a seizure (B1), particularly in the supragranular layers. In the TBIFI endophenotype with PTZ-induced seizure, LPS treatment at a chronic time-point after TBI further enhanced c-Fos expression (D1) compared with vehicle treatment (C1) in all layers. Abbreviations: FI, focal inflammatory; FPI, fluid-percussion injury; LPS, lipopolysaccharide; nS, no seizure; PTZ, pentylenetetrazole; S, seizure; TBI, traumatic brain injury; Veh, vehicle; WM, white matter. Scale bars = 200 μm.
![Biomedicines 09 01946 g007 Biomedicines 09 01946 g007]()
Figure 8.
Pattern of c-Fos expression in the rostromedial perilesional cortex. Representative high magnification photomicrographs showing c-Fos immunochemistry (A1–D1) and Nissl staining (A2–D2) in the rostromedial perilesional cortex in rats from the Sham-Veh group and TBICF endophenotype at 2 h after PTZ injection (23 weeks post-FPI and 15 weeks after LPS injection). (A1,A2) Example of a rat from the Sham-Veh group without a PTZ-induced seizure (#28). (B1,B2) Example of a rat from the TBICF-Veh group without a PTZ-induced seizure (#33). (C1,C2) Example of a rat from the TBICF-Veh group with a PTZ-induced seizure (#48, seizure Racine score 0, only electroencephalographic seizure). (D1,D2) Example of a rat from the TBICF-LPS group with a PTZ-induced seizure (#45, seizure Racine score 4). Note that in rats without a PTZ-induced seizure, TBI increased PTZ-induced c-Fos expression, particularly in layer IV ((B1) vs. (A1)). In the TBICF-Veh group, a rat with a PTZ-induced seizure (C1) revealed higher c-Fos activation compared to a rat without a seizure (B1), particularly in the supragranular layers. In the TBICF endophenotype with a PTZ-induced seizure, LPS treatment at a chronic time-point after TBI further enhanced c-Fos expression (D1) compared with vehicle treatment (C1) in all layers. Abbreviations: CF, cavity-forming; FPI, fluid-percussion injury; LPS, lipopolysaccharide; nS, no seizure; PTZ, pentylenetetrazole; S, seizure; TBI, traumatic brain injury; Veh, vehicle; WM, white matter. Scale bars = 200 μm. * Enlarged ipsilateral lateral ventricle.
Figure 8.
Pattern of c-Fos expression in the rostromedial perilesional cortex. Representative high magnification photomicrographs showing c-Fos immunochemistry (A1–D1) and Nissl staining (A2–D2) in the rostromedial perilesional cortex in rats from the Sham-Veh group and TBICF endophenotype at 2 h after PTZ injection (23 weeks post-FPI and 15 weeks after LPS injection). (A1,A2) Example of a rat from the Sham-Veh group without a PTZ-induced seizure (#28). (B1,B2) Example of a rat from the TBICF-Veh group without a PTZ-induced seizure (#33). (C1,C2) Example of a rat from the TBICF-Veh group with a PTZ-induced seizure (#48, seizure Racine score 0, only electroencephalographic seizure). (D1,D2) Example of a rat from the TBICF-LPS group with a PTZ-induced seizure (#45, seizure Racine score 4). Note that in rats without a PTZ-induced seizure, TBI increased PTZ-induced c-Fos expression, particularly in layer IV ((B1) vs. (A1)). In the TBICF-Veh group, a rat with a PTZ-induced seizure (C1) revealed higher c-Fos activation compared to a rat without a seizure (B1), particularly in the supragranular layers. In the TBICF endophenotype with a PTZ-induced seizure, LPS treatment at a chronic time-point after TBI further enhanced c-Fos expression (D1) compared with vehicle treatment (C1) in all layers. Abbreviations: CF, cavity-forming; FPI, fluid-percussion injury; LPS, lipopolysaccharide; nS, no seizure; PTZ, pentylenetetrazole; S, seizure; TBI, traumatic brain injury; Veh, vehicle; WM, white matter. Scale bars = 200 μm. * Enlarged ipsilateral lateral ventricle.
![Biomedicines 09 01946 g008 Biomedicines 09 01946 g008]()
Figure 9.
Pattern of c-Fos expression in the caudomedial perilesional cortex. Representative high magnification photomicrographs showing c-Fos immunochemistry (A1–D1) and Nissl staining (A2–D2) in the caudomedial perilesional cortex in rats from Sham-Veh group and TBICF endophenotype at 2 h after PTZ injection (23 weeks post-FPI and 15 weeks after LPS injection). (A1,A2) Example of a rat from the Sham-Veh group without a PTZ-induced seizure (#28). (B1,B2) Example of a rat from the TBICF-Veh group without a PTZ-induced seizure (#33). (C1,C2) Example of a rat from the TBICF-Veh group with a PTZ-induced seizure (#48, seizure Racine score 0, only electroencephalographic seizure). (D1,D2) Example of a rat from the TBICF-LPS group with a PTZ-induced seizure (#45, seizure Racine score 4). Note that in rats without a PTZ-induced seizure, TBI increased PTZ-induced c-Fos expression ((B1) vs. (A1)). In the TBICF-Veh group, no difference was detected between rats with a PTZ-induced seizure (C1) and those without a seizure (B1). In the TBICF endophenotype with a PTZ-induced seizure, LPS treatment at a chronic time-point after TBI further enhanced c-Fos expression (D1) compared with vehicle treatment (C1) in all layers. Abbreviations: CF, cavity-forming; FPI, fluid-percussion injury; LPS, lipopolysaccharide; nS, no seizure; PTZ, pentylenetetrazole; S, seizure; TBI, traumatic brain injury; Veh, vehicle; WM, white matter. Scale bars = 200 μm. * Enlarged ipsilateral lateral ventricle.
Figure 9.
Pattern of c-Fos expression in the caudomedial perilesional cortex. Representative high magnification photomicrographs showing c-Fos immunochemistry (A1–D1) and Nissl staining (A2–D2) in the caudomedial perilesional cortex in rats from Sham-Veh group and TBICF endophenotype at 2 h after PTZ injection (23 weeks post-FPI and 15 weeks after LPS injection). (A1,A2) Example of a rat from the Sham-Veh group without a PTZ-induced seizure (#28). (B1,B2) Example of a rat from the TBICF-Veh group without a PTZ-induced seizure (#33). (C1,C2) Example of a rat from the TBICF-Veh group with a PTZ-induced seizure (#48, seizure Racine score 0, only electroencephalographic seizure). (D1,D2) Example of a rat from the TBICF-LPS group with a PTZ-induced seizure (#45, seizure Racine score 4). Note that in rats without a PTZ-induced seizure, TBI increased PTZ-induced c-Fos expression ((B1) vs. (A1)). In the TBICF-Veh group, no difference was detected between rats with a PTZ-induced seizure (C1) and those without a seizure (B1). In the TBICF endophenotype with a PTZ-induced seizure, LPS treatment at a chronic time-point after TBI further enhanced c-Fos expression (D1) compared with vehicle treatment (C1) in all layers. Abbreviations: CF, cavity-forming; FPI, fluid-percussion injury; LPS, lipopolysaccharide; nS, no seizure; PTZ, pentylenetetrazole; S, seizure; TBI, traumatic brain injury; Veh, vehicle; WM, white matter. Scale bars = 200 μm. * Enlarged ipsilateral lateral ventricle.
![Biomedicines 09 01946 g009 Biomedicines 09 01946 g009]()
Figure 10.
c-Fos expression and seizure susceptibility. Correlations between the density of rostral perilesional c-Fos expression and seizure susceptibility (maximal behavioral seizure score, cumulative seizure duration) in the PTZ-test. (A1) Ipsilaterally, the higher the density of c-Fos expression, the higher the maximal behavioral seizure score in the TBI-Veh group (r = 0.949, p < 0.05). (A2) Contralaterally, the higher the c-Fos expression, the higher the maximal behavioral score in the TBI-LPS group (r = 0.764, p < 0.05). No correlations between the density of c-Fos expression and the cumulative seizure duration were detected (B1) ipsilaterally or (B2) contralaterally in the TBI-Veh or TBI-LPS groups. Abbreviations: LPS, lipopolysaccharide; ns, non-significant; r, correlation coefficient; TBI, traumatic brain injury; Veh, vehicle. Statistical significances: * p < 0.05 (r, Spearman’s rho correlations).
Figure 10.
c-Fos expression and seizure susceptibility. Correlations between the density of rostral perilesional c-Fos expression and seizure susceptibility (maximal behavioral seizure score, cumulative seizure duration) in the PTZ-test. (A1) Ipsilaterally, the higher the density of c-Fos expression, the higher the maximal behavioral seizure score in the TBI-Veh group (r = 0.949, p < 0.05). (A2) Contralaterally, the higher the c-Fos expression, the higher the maximal behavioral score in the TBI-LPS group (r = 0.764, p < 0.05). No correlations between the density of c-Fos expression and the cumulative seizure duration were detected (B1) ipsilaterally or (B2) contralaterally in the TBI-Veh or TBI-LPS groups. Abbreviations: LPS, lipopolysaccharide; ns, non-significant; r, correlation coefficient; TBI, traumatic brain injury; Veh, vehicle. Statistical significances: * p < 0.05 (r, Spearman’s rho correlations).
Figure 11.
c-Fos expression in the dentate gyrus (DG). (A) Percentage of animals with increased c-Fos expression in the dentate gyrus was higher in the TBI-LPS than in the TBI-Veh and Sham-Veh groups. (B) All rats in the TBIFI-LPS group more commonly showed increased c-Fos activation in the dentate gyrus than rats in the TBIFI-Veh and Sham-Veh groups. Abbreviations: CF, cavity-forming; DG, dentate gyrus; FI, focal inflammatory; LPS, lipopolysaccharide; TBI, traumatic brain injury; Veh, vehicle. Statistical significances: * p < 0.05, ** p < 0.01 (χ2-test).
Figure 11.
c-Fos expression in the dentate gyrus (DG). (A) Percentage of animals with increased c-Fos expression in the dentate gyrus was higher in the TBI-LPS than in the TBI-Veh and Sham-Veh groups. (B) All rats in the TBIFI-LPS group more commonly showed increased c-Fos activation in the dentate gyrus than rats in the TBIFI-Veh and Sham-Veh groups. Abbreviations: CF, cavity-forming; DG, dentate gyrus; FI, focal inflammatory; LPS, lipopolysaccharide; TBI, traumatic brain injury; Veh, vehicle. Statistical significances: * p < 0.05, ** p < 0.01 (χ2-test).
Figure 12.
Pattern of c-Fos expression in the dentate gyrus. Representative photomicrographs showing mild granule cell damage and dispersion in the dentate gyrus (A–D) and calcifications in the ipsilateral thalamus (E,F) in a rat (#38, Racine score 5) in the TBIFI-Veh group with an induced seizure at 2 h after PTZ injection. A c-Fos immunostained (A) and a Nissl-stained section (C). A higher-magnification photomicrographs of c-Fos immunohistochemistry (B) and Nissl staining (D) taken from the region indicated with a dashed box in panel (A,C), respectively. Note the granule cell loss indicated by a closed arrow with iron deposits (dark dots) and dispersed granule cells in the molecular layer. (E) Thalamic calcification (dashed box). (F) A higher magnification photomicrograph was taken from the region indicated with a dashed box in panel (E). Abbreviations: FI, focal inflammatory; FPI, fluid-percussion injury; PTZ, pentylenetetrazole; TBI, traumatic brain injury; Veh, vehicle. Scale bar = 200 μm (panel (A,C,E)); 50 μm (panel (B,D,F)).
Figure 12.
Pattern of c-Fos expression in the dentate gyrus. Representative photomicrographs showing mild granule cell damage and dispersion in the dentate gyrus (A–D) and calcifications in the ipsilateral thalamus (E,F) in a rat (#38, Racine score 5) in the TBIFI-Veh group with an induced seizure at 2 h after PTZ injection. A c-Fos immunostained (A) and a Nissl-stained section (C). A higher-magnification photomicrographs of c-Fos immunohistochemistry (B) and Nissl staining (D) taken from the region indicated with a dashed box in panel (A,C), respectively. Note the granule cell loss indicated by a closed arrow with iron deposits (dark dots) and dispersed granule cells in the molecular layer. (E) Thalamic calcification (dashed box). (F) A higher magnification photomicrograph was taken from the region indicated with a dashed box in panel (E). Abbreviations: FI, focal inflammatory; FPI, fluid-percussion injury; PTZ, pentylenetetrazole; TBI, traumatic brain injury; Veh, vehicle. Scale bar = 200 μm (panel (A,C,E)); 50 μm (panel (B,D,F)).
![Biomedicines 09 01946 g012 Biomedicines 09 01946 g012]()
Table 1.
Electroencephalograhic (EEG) events in the pentylenetetrazol (PTZ) seizure susceptibility test at 23 weeks after traumatic brain injury (TBI) and 15 weeks after lipopolysaccharide (LPS) administration in the whole animal group.
Table 1.
Electroencephalograhic (EEG) events in the pentylenetetrazol (PTZ) seizure susceptibility test at 23 weeks after traumatic brain injury (TBI) and 15 weeks after lipopolysaccharide (LPS) administration in the whole animal group.
| Parameter | Sham
(1/5) | TBI + Veh
(5/11) | TBI + LPS
(8/10) |
|---|
| latency to the first spike (s) | 287 ± 230 | 730 ± 765 | 374 ± 401 |
| latency to the first ED (s) | 288 ± 229 | 775 ± 802 | 400 ± 399 |
| occurrence of PTZ-induced seizures | 20% | 46% | 80% |
| latency to the first electrographic seizure (s) | 1 628 | 604 ± 345 | 331 ± 258 |
| mean seizure duration per rat (s) | 24 | 35 ± 19 | 114 ± 53 * |
| mean cumulative seizure duration per rat (s) | 24 | 35 ± 19 | 163 ± 90 ** |
| mean behavioral seizure score per rat | 3 | 3.2 ± 2.0 | 4.0 ± 1.0 |