Controlled Synthesis of Up-Conversion NaYF4:Yb,Tm Nanoparticles for Drug Release under Near IR-Light Therapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Np-UCs
2.2. Synthesis of 7-(4-(tert-butoxycarbonyl)piperazin-1-yl)-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylic Acid (CF)
2.3. Tert-butyl 4-(1-cyclopropyl-3-((((diphenylmethylene)amino)oxy)carbonyl)-6-fluoro-4-oxo-1,4-dihydroquinolin-7-yl)piperazine-1-carboxylate (CF-OE)
2.4. Characterization Methods
2.5. Photophysical Measurements
2.6. Light-Induced Drug Delivered Experiments
3. Results and Discussion
3.1. Optimization of the Synthesis of NaYF4:Yb/Tm UC Materials
3.2. Light-Driven Drug Delivery
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tiwari, G.; Tiwari, R.; Sriwastawa, B.; Bhati, L.; Pandey, S.; Pandey, P.; Bannerjee, S.K. Drug Delivery Systems: An Updated Review. Int. J. Pharm. Investig. 2012, 2, 2–11. [Google Scholar] [CrossRef] [Green Version]
- Manzari, M.T.; Shamay, Y.; Kiguchi, H.; Rosen, N.; Scaltriti, M.; Heller, D.A. Targeted Drug Delivery Strategies for Precision Medicines. Nat. Rev. Mater. 2021, 6, 351–370. [Google Scholar] [CrossRef]
- He, H.; Lu, Y.; Qi, J.; Zhu, Q.; Chen, Z.; Wu, W. Adapting Liposomes for Oral Drug Delivery. Acta Pharm. Sin. B 2019, 9, 36–48. [Google Scholar] [CrossRef]
- Antimisiaris, S.G.; Marazioti, A.; Kannavou, M.; Natsaridis, E.; Gkartziou, F.; Kogkos, G.; Mourtas, S. Overcoming Barriers by Local Drug Delivery with Liposomes. Adv. Drug Deliv. Rev. 2021, 174, 53–86. [Google Scholar] [CrossRef] [PubMed]
- Nuin, E.; Gómez-Mendoza, M.; Andreu, I.; Marin, M.L.; Miranda, M.A. New Photoactive Compounds to Probe Cholic Acid and Cholesterol inside Mixed Micelles. Org. Lett. 2013, 15, 298–301. [Google Scholar] [CrossRef]
- Nuin, E.; Gomez-Mendoza, M.; Marin, M.L.; Andreu, I.; Miranda, M.A. Influence of Drug Encapsulation within Mixed Micelles on the Excited State Dynamics and Accessibility to Ionic Quenchers. J. Phys. Chem. B 2013, 117, 9327–9332. [Google Scholar] [CrossRef]
- Rodriguez-Muñiz, G.M.; Gomez-Mendoza, M.; Nuin, E.; Andreu, I.; Marin, M.L.; Miranda, M.A. “Snorkelling” vs. “Diving” in Mixed Micelles Probed by Means of a Molecular Bathymeter. Org. Biomol. Chem. 2017, 15, 10281–10288. [Google Scholar] [CrossRef] [PubMed]
- Bartelds, R.; Nematollahi, M.H.; Pols, T.; Stuart, M.C.A.; Pardakhty, A.; Asadikaram, G.; Poolman, B. Niosomes, an Alternative for Liposomal Delivery. PLoS ONE 2018, 13, e0194179. [Google Scholar] [CrossRef] [Green Version]
- Gomez-Mendoza, M.; Nuin, E.; Andreu, I.; Marin, M.L.; Miranda, M.A. Photophysical Probes to Assess the Potential of Cholic Acid Aggregates as Drug Carriers. J. Phys. Chem. B 2012, 116, 10213–10218. [Google Scholar] [CrossRef]
- Gomez-Mendoza, M.; Marin, M.L.; Miranda, M.A. Photoactive Bile Salts with Critical Micellar Concentration in the Micromolar Range. Phys. Chem. Chem. Phys. 2016, 18, 12976–12982. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Mendoza, M.; Marin, M.L.; Miranda, M.A. Dansyl Derivatives of Cholic Acid as Tools to Build Speciation Diagrams for Sodium Cholate Aggregation. J. Phys. Chem. Lett. 2011, 2, 782–785. [Google Scholar] [CrossRef]
- Gomez-Mendoza, M.; Marin, M.L.; Miranda, M.A. Dansyl-Labeled Cholic Acid as a Tool to Build Speciation Diagrams for the Aggregation of Bile Acids. J. Phys. Chem. B 2012, 116, 14776–14780. [Google Scholar] [CrossRef] [PubMed]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; del Pilar Rodriguez-Torres, M.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano Based Drug Delivery Systems: Recent Developments and Future Prospects. J. Nanobiotechnology 2018, 16, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering Precision Nanoparticles for Drug Delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Del Gaudio, C.; Crognale, V.; Serino, G.; Galloni, P.; Audenino, A.; Ribatti, D.; Morbiducci, U. Natural Polymeric Microspheres for Modulated Drug Delivery. Mater. Sci. Eng. C 2017, 75, 408–417. [Google Scholar] [CrossRef]
- Chandan, R.; Banerjee, R. Pro-Apoptotic Liposomes-Nanobubble Conjugate Synergistic with Paclitaxel: A Platform for Ultrasound Responsive Image-Guided Drug Delivery. Sci. Rep. 2018, 8, 2624. [Google Scholar] [CrossRef]
- Sherje, A.P.; Jadhav, M.; Dravyakar, B.R.; Kadam, D. Dendrimers: A Versatile Nanocarrier for Drug Delivery and Targeting. Int. J. Pharm. 2018, 548, 707–720. [Google Scholar] [CrossRef]
- Patel, V.; Rajani, C.; Paul, D.; Borisa, P.; Rajpoot, K.; Youngren-Ortiz, S.R.; Tekade, R.K. Chapter 8—Dendrimers as Novel Drug-Delivery System and Its Applications. In Advances in Pharmaceutical Product Development and Research; Tekade, R.K., Ed.; Elsevier Inc.: Amsterdam, The Netherlands; Academic Press: Cambridge, MA, USA, 2020; pp. 333–392. ISBN 978-0-12-814487-9. [Google Scholar] [CrossRef]
- Silindir Gunay, M.; Ozer, Y.; Chalon, S. Drug Delivery Systems for Imaging and Therapy of Parkinson’s Disease. Curr. Neuropharmacol. 2015, 14, 376–391. [Google Scholar] [CrossRef]
- Zhai, B.; Zeng, Y.; Zeng, Z.; Zhang, N.; Li, C.; Zeng, Y.; You, Y.; Wang, S.; Chen, X.; Sui, X.; et al. Drug Delivery Systems for Elemene, Its Main Active Ingredient β-Elemene, and Its Derivatives in Cancer Therapy. Int. J. Nanomed. 2018, 13, 6279–6296. [Google Scholar] [CrossRef] [Green Version]
- Porter, C.J.H.; Trevaskis, N.L.; Charman, W.N. Lipids and Lipid-Based Formulations: Optimizing the Oral Delivery of Lipophilic Drugs. Nat. Rev. Drug Discov. 2007, 6, 231–248. [Google Scholar] [CrossRef]
- Hammad, M.A.; Müller, B.W. Increasing Drug Solubility by Means of Bile Salt–Phosphatidylcholine-Based Mixed Micelles. Eur. J. Pharm. Biopharm. 1998, 46, 361–367. [Google Scholar] [CrossRef]
- Gao, W.; Chan, J.M.; Farokhzad, O.C. PH-Responsive Nanoparticles for Drug Delivery. Mol. Pharm. 2010, 7, 1913–1920. [Google Scholar] [CrossRef]
- Ding, J.; Sun, Y.; Li, J.; Wang, H.; Mao, S. Enhanced Blood–Brain Barrier Transport of Vinpocetine by Oral Delivery of Mixed Micelles in Combination with a Message Guider. J. Drug Target. 2017, 25, 532–540. [Google Scholar] [CrossRef]
- Lasic, D.D. Mixed Micelles in Drug Delivery. Nature 1992, 355, 279–280. [Google Scholar] [CrossRef]
- Cheng, L.; Kamkaew, A.; Sun, H.; Jiang, D.; Valdovinos, H.F.; Gong, H.; England, C.G.; Goel, S.; Barnhart, T.E.; Cai, W. Dual-Modality Positron Emission Tomography/Optical Image-Guided Photodynamic Cancer Therapy with Chlorin E6-Containing Nanomicelles. ACS Nano 2016, 10, 7721–7730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and Computational Approaches to Estimate Solubility and Permeability in Drug Discovery and Development Settings. Adv. Drug Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Dikmen, G.; Genç, L.; Guney Eskiler, G. Advantage and Disadvantage in Drug Delivery Systems. J. Mater. Sci. Eng. 2011, 5, 468–472. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Chen, B.; Xu, S.; Li, X.; Zhang, J.; Sun, J.; Zheng, H.; Tong, L.; Sui, G.; Zhong, H.; et al. Dually Functioned Core-Shell NaYF4:Er3+/Yb3+@NaYF4:Tm3+/Yb3+ Nanoparticles as Nano-Calorifiers and Nano-Thermometers for Advanced Photothermal Therapy. Sci. Rep. 2017, 7, 11849. [Google Scholar] [CrossRef]
- Dai, Y.; Ma, P.; Cheng, Z.; Kang, X.; Zhang, X.; Hou, Z.; Li, C.; Yang, D.; Zhai, X.; Lin, J. Up-Conversion Cell Imaging and PH-Induced Thermally Controlled Drug Release from NaYF4:Yb3+/Er3+@Hydrogel Core–Shell Hybrid Microspheres. ACS Nano 2012, 6, 3327–3338. [Google Scholar] [CrossRef]
- Hou, Z.; Li, C.; Ma, P.; Cheng, Z.; Li, X.; Zhang, X.; Dai, Y.; Yang, D.; Lian, H.; Lin, J. Up-Conversion Luminescent and Porous NaYF4:Yb3+, Er3+@SiO2 Nanocomposite Fibers for Anti-Cancer Drug Delivery and Cell Imaging. Adv. Funct. Mater. 2012, 22, 2713–2722. [Google Scholar] [CrossRef]
- Wang, C.; Cheng, L.; Liu, Z. Drug Delivery with Upconversion Nanoparticles for Multi-Functional Targeted Cancer Cell Imaging and Therapy. Biomaterials 2011, 32, 1110–1120. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, C.; Liu, H.; Li, Y.; Xu, Z.; Li, L.; Whittaker, A. Controllable Synthesis of Up-Conversion Nanoparticles UCNPs@MIL-PEG for PH-Responsive Drug Delivery and Potential Up-Conversion Luminescence/Magnetic Resonance Dual-Mode Imaging. J. Alloys Compd. 2018, 749, 939–947. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, C.; Xu, C.; Lin, C.; Sun, K.; Wang, J.; Chen, X.; Li, L.; Whittaker, A.K.; Xu, H.-B. Controlled Synthesis of Up-Conversion Luminescent Gd/Tm-MOFs for PH-Responsive Drug Delivery and UCL/MRI Dual-Modal Imaging. Dalton Trans. 2018, 47, 11253–11263. [Google Scholar] [CrossRef]
- Lim, S. Phototherapy and the Benefits of LEDs. J. Soc. Inf. Disp. 2011, 19, 882–887. [Google Scholar] [CrossRef]
- Liu, Y.; Tu, D.; Zhu, H.; Chen, X. Lanthanide-Doped Luminescent Nanoprobes: Controlled Synthesis, Optical Spectroscopy, and Bioapplications. Chem. Soc. Rev. 2013, 42, 6924–6958. [Google Scholar] [CrossRef]
- Gray, V.; Dzebo, D.; Abrahamsson, M.; Albinsson, B.; Moth-Poulsen, K. Triplet–Triplet Annihilation Photon-Upconversion: Towards Solar Energy Applications. Phys. Chem. Chem. Phys. 2014, 16, 10345–10352. [Google Scholar] [CrossRef]
- Kerzig, C.; Wenger, O.S. Sensitized Triplet–Triplet Annihilation Upconversion in Water and Its Application to Photochemical Transformations. Chem. Sci. 2018, 9, 6670–6678. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Castellano, F.N.; Schmidt, T.W.; Hanson, K. On the Quantum Yield of Photon Upconversion via Triplet–Triplet Annihilation. ACS Energy Lett. 2020, 5, 2322–2326. [Google Scholar] [CrossRef]
- Li, Z.; Wang, L.; Wang, Z.; Liu, X.; Xiong, Y. Modification of NaYF4:Yb,Er@SiO2 Nanoparticles with Gold Nanocrystals for Tunable Green-to-Red Upconversion Emissions. J. Phys. Chem. C 2011, 115, 3291–3296. [Google Scholar] [CrossRef]
- Wang, L.; Qin, W.; Liu, Z.; Zhao, D.; Qin, G.; Di, W.; He, C. Improved 800 Nm Emission of Tm3+ Sensitized by Yb3+ and Ho3+ in β-NaYF4 Nanocrystals under 980 Nm Excitation. Opt. Express 2012, 20, 7602–7607. [Google Scholar] [CrossRef]
- Homann, C.; Krukewitt, L.; Frenzel, F.; Grauel, B.; Würth, C.; Resch-Genger, U.; Haase, M. NaYF4:Yb,Er/NaYF4 Core/Shell Nanocrystals with High Upconversion Luminescence Quantum Yield. Angew. Chem. Int. Ed. 2018, 57, 8765–8769. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Tian, G.; Jian, S.; Gu, Z.; Zhou, L.; Yan, L.; Jin, S.; Yin, W.; Zhao, Y. TWEEN Coated NaYF4:Yb,Er/NaYF4 Core/Shell Upconversion Nanoparticles for Bioimaging and Drug Delivery. RSC Adv. 2012, 2, 7037–7041. [Google Scholar] [CrossRef]
- Tian, G.; Gu, Z.; Zhou, L.; Yin, W.; Liu, X.; Yan, L.; Jin, S.; Ren, W.; Xing, G.; Li, S.; et al. Mn2+ Dopant-Controlled Synthesis of NaYF4:Yb/Er Upconversion Nanoparticles for in Vivo Imaging and Drug Delivery. Adv. Mater. 2012, 24, 1226–1231. [Google Scholar] [CrossRef]
- Tu, D.; Liu, Y.; Zhu, H.; Li, R.; Liu, L.; Chen, X. Breakdown of Crystallographic Site Symmetry in Lanthanide-Doped NaYF4 Crystals. Angew. Chem. Int. Ed. 2013, 52, 1128–1133. [Google Scholar] [CrossRef]
- Shan, S.-N.; Wang, X.-Y.; Jia, N.-Q. Synthesis of NaYF4:Yb3+, Er3+ Upconversion Nanoparticles in Normal Microemulsions. Nanoscale Res. Lett. 2011, 6, 539. [Google Scholar] [CrossRef] [Green Version]
- D’Vries, R.F.; de la Peña-O’Shea, V.A.; Snejko, N.; Iglesias, M.; Gutiérrez-Puebla, E.; Monge, M.A. Insight into the Correlation between Net Topology and Ligand Coordination Mode in New Lanthanide MOFs Heterogeneous Catalysts: A Theoretical and Experimental Approach. Cryst. Growth Des. 2012, 12, 5535–5545. [Google Scholar] [CrossRef]
- D’Vries, R.F.; de la Peña-O’Shea, V.A.; Snejko, N.; Iglesias, M.; Gutiérrez-Puebla, E.; Monge, M.A. H3O2 Bridging Ligand in a Metal–Organic Framework. Insight into the Aqua-Hydroxo↔Hydroxyl Equilibrium: A Combined Experimental and Theoretical Study. J. Am. Chem. Soc. 2013, 135, 5782–5792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quesada, M.; Monrabal, M.; Aromí, G.; de la Peña-O’Shea, V.A.; Gich, M.; Molins, E.; Roubeau, O.; Teat, S.J.; MacLean, E.J.; Gamez, P.; et al. Spin transition in a triazine-based Fe(II) complex: Variable-temperature structural, thermal, magnetic and spectroscopic studies. J. Mater. Chem. 2006, 16, 2669–2676. [Google Scholar] [CrossRef]
- Jain, D.; Banerjee, R. Comparison of Ciprofloxacin Hydrochloride-Loaded Protein, Lipid, and Chitosan Nanoparticles for Drug Delivery. J. Biomed. Mater. Res. Part B Appl. Biomater. 2008, 86B, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Kloskowski, T.; Gurtowska, N.; Bajek, A.; Drewa, T. Ciprofloxacin as a Prophylactic Agent against Prostate Cancer: A “Two Hit” Hypothesis. Med. Hypotheses 2012, 78, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Kloskowski, T.; Gurtowska, N.; Olkowska, J.; Nowak Marcin, J.; Adamowicz, J.; Tworkiewicz, J.; Dębski, R.; Grzanka, A.; Drewa, T. Ciprofloxacin Is a Potential Topoisomerase II Inhibitor for the Treatment of NSCLC. Int. J. Oncol. 2012, 41, 1943–1949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geller, L.T.; Barzily-Rokni, M.; Danino, T.; Jonas, O.H.; Shental, N.; Nejman, D.; Gavert, N.; Zwang, Y.; Cooper, Z.A.; Shee, K.; et al. Potential Role of Intratumor Bacteria in Mediating Tumor Resistance to the Chemotherapeutic Drug Gemcitabine. Science 2017, 357, 1156–1160. [Google Scholar] [CrossRef] [Green Version]
- Straussman, R.; Morikawa, T.; Shee, K.; Barzily-Rokni, M.; Qian, Z.R.; Du, J.; Davis, A.; Mongare, M.M.; Gould, J.; Frederick, D.T.; et al. Tumour Micro-Environment Elicits Innate Resistance to RAF Inhibitors through HGF Secretion. Nature 2012, 487, 500–504. [Google Scholar] [CrossRef] [Green Version]
- Ji, C.; Miller, P.A.; Miller, M.J. Syntheses and Antibacterial Activity of N-Acylated Ciprofloxacin Derivatives Based on the Trimethyl Lock. ACS Med. Chem. Lett. 2015, 6, 707–710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.; Yu, Y.; Liebeskind, L.S. N-Substituted Imines by the Copper-Catalyzed N-Imination of Boronic Acids and Organostannanes with O-Acyl Ketoximes. Org. Lett. 2007, 9, 1947–1950. [Google Scholar] [CrossRef]
- Kannan, P.; Rahim, F.A.; Teng, X.; Chen, R.; Sun, H.; Huang, L.; Kim, D.-H. Enhanced Emission of NaYF4:Yb,Er/Tm Nanoparticles by Selective Growth of Au and Ag Nanoshells. RSC Adv. 2013, 3, 7718–7721. [Google Scholar] [CrossRef]
- Zeng, S.; Ren, G.; Xu, C.; Yang, Q. High Uniformity and Monodispersity of Sodium Rare-Earth Fluoride Nanocrystals: Controllable Synthesis, Shape Evolution and Optical Properties. Cryst. Eng. Comm. 2011, 13, 1384–1390. [Google Scholar] [CrossRef]
- Li, C.; Quan, Z.; Yang, J.; Yang, P.; Lin, J. Highly Uniform and Monodisperse Beta-NaYF(4):Ln(3+) (Ln = Eu, Tb, Yb/Er, and Yb/Tm) Hexagonal Microprism Crystals: Hydrothermal Synthesis and Luminescent Properties. Inorg. Chem. 2007, 46, 6329–6337. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, H.; Li, X.; Chen, D. Monodisperse, Size-Tunable and Highly Efficient β-NaYF4:Yb,Er(Tm) up-Conversion Luminescent Nanospheres: Controllable Synthesis and Their Surface Modifications. J. Mater. Chem. 2009, 19, 3546–3553. [Google Scholar] [CrossRef]
- Li, C.; Yang, J.; Quan, Z.; Yang, P.; Kong, D.; Lin, J. Different Microstructures of β-NaYF4 Fabricated by Hydrothermal Process: Effects of PH Values and Fluoride Sources. Chem. Mater. 2007, 19, 4933–4942. [Google Scholar] [CrossRef]
- Lin, M.; Zhao, Y.; Liu, M.; Qiu, M.; Dong, Y.; Duan, Z.; Li, Y.H.; Pingguan-Murphy, B.; Lu, T.J.; Xu, F. Synthesis of Upconversion NaYF4:Yb3+,Er3+ Particles with Enhanced Luminescent Intensity through Control of Morphology and Phase. J. Mater. Chem. C 2014, 2, 3671–3676. [Google Scholar] [CrossRef]
- Lutz, T.; Veissier, L.; Thiel, C.; Cone, R.; Barclay, P.; Tittel, W. Modification of Phonon Processes in Nano-Structured Rare-Earth-Ion-Doped Crystals. Phys. Rev. A 2015, 94, 013801. [Google Scholar] [CrossRef] [Green Version]
- Harder, S.; Fuhr, U.; Beermann, D.; Staib, A.H. Ciprofloxacin absorption in different regions onf the human gastrointestinal tract. Investigations with the hf-capsule. Br. J. Clin. Pharmacol. 1990, 30, 35–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Staib, A.H.; Beermann, D.; Harder, S.; Fuhr, U.; Liermann, D. Absorption differences of ciprofloxacin along the human gastrointestinal tract determined using a remote-control drug delivery device (HF-capsule). Am. J. Med. 1989, 87, 566–569. [Google Scholar]
- Yang, R.; Fu, Y.; Li, L.-D.; Liu, J.-M. Medium Effects on Fluorescence of Ciprofloxacin Hydrochloride. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2003, 59, 2723–2732. [Google Scholar] [CrossRef]
- Halubek-Gluchowska, K.; Szymański, D.; Tran, T.N.; Ferrari, M.; Lukowiak, A. Upconversion Luminescence of Silica–Calcia Nanoparticles Co-Doped with Tm3+ and Yb3+ Ions. Materials 2021, 14, 937. [Google Scholar] [CrossRef]

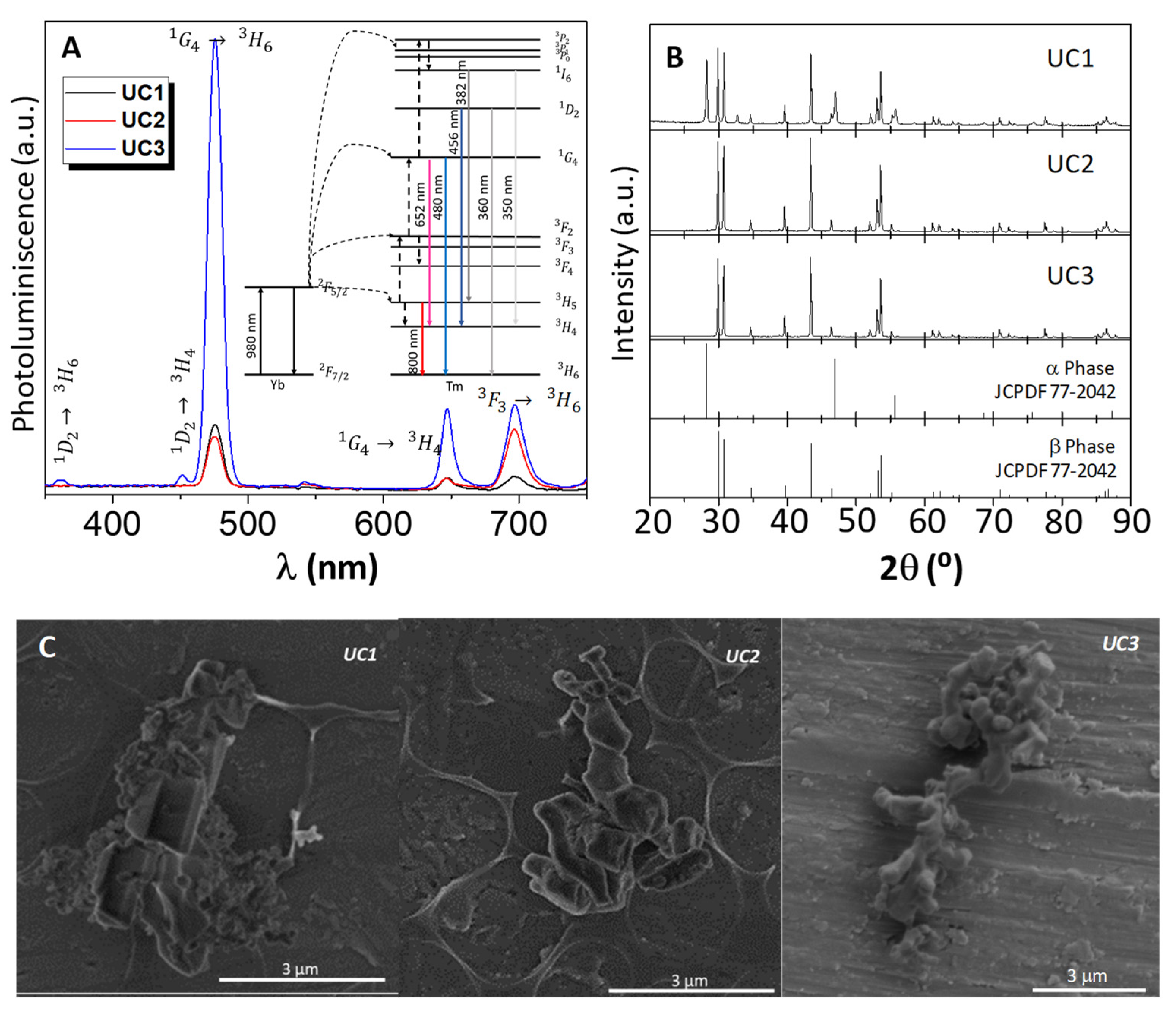




| UCi(i) | pH | Time (h) | Thermal Treatment (400 °C) | Proportion of β (%) |
|---|---|---|---|---|
| 1 | 5 | 12 | YES | 56.9 |
| 2 | 5 | 24 | YES | 100 |
| 3 | 5 | 24 | NO | 100 |
| 4 | 6 | 24 | NO | 100 |
| 5 | 7 | 24 | NO | 100 |
| 6 | 8 | 24 | NO | 100 |
| 7 | 10 | 24 | NO | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moyano Rodríguez, E.; Gomez-Mendoza, M.; Pérez-Ruiz, R.; Peñín, B.; Sampedro, D.; Caamaño, A.; de la Peña O’Shea, V.A. Controlled Synthesis of Up-Conversion NaYF4:Yb,Tm Nanoparticles for Drug Release under Near IR-Light Therapy. Biomedicines 2021, 9, 1953. https://doi.org/10.3390/biomedicines9121953
Moyano Rodríguez E, Gomez-Mendoza M, Pérez-Ruiz R, Peñín B, Sampedro D, Caamaño A, de la Peña O’Shea VA. Controlled Synthesis of Up-Conversion NaYF4:Yb,Tm Nanoparticles for Drug Release under Near IR-Light Therapy. Biomedicines. 2021; 9(12):1953. https://doi.org/10.3390/biomedicines9121953
Chicago/Turabian StyleMoyano Rodríguez, Edelweiss, Miguel Gomez-Mendoza, Raúl Pérez-Ruiz, Beatriz Peñín, Diego Sampedro, Antonio Caamaño, and Víctor A. de la Peña O’Shea. 2021. "Controlled Synthesis of Up-Conversion NaYF4:Yb,Tm Nanoparticles for Drug Release under Near IR-Light Therapy" Biomedicines 9, no. 12: 1953. https://doi.org/10.3390/biomedicines9121953







