Dopamine in Health and Disease: Much More Than a Neurotransmitter
Abstract
:1. Introduction
2. Dopamine, Dopamine Receptors and Catechol-Related Enzymes
3. Features of DA Receptors That Are Important in Dopaminergic Transmission in Both Health and Disease
4. Dopamine, L-DOPA and Parkinson’s Disease
5. Dopamine Derivatives in Neurological and Neuropsychiatric Diseases
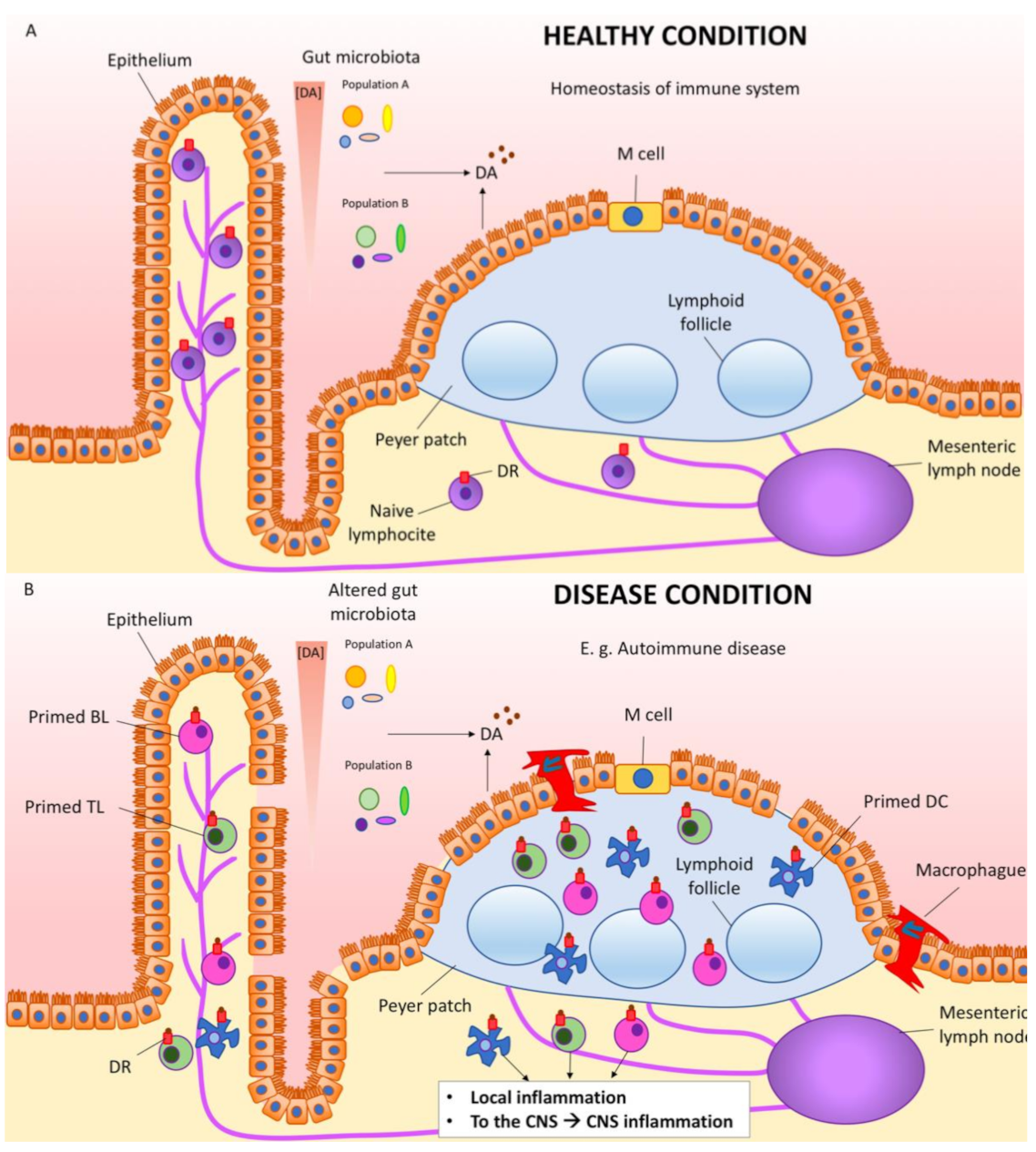
6. Dopamine in the Gastrointestinal Tract
7. Dopamine, Immune Cells, Inflammation, Autoimmunity and Parkinson’s Disease
8. Future Perspectives of DA as Neurotransmitter
9. Future Perspectives of DA as Regulatory Molecule
Author Contributions
Funding
Conflicts of Interest
References
- Raab, W.; Gigee, W. Concentration and Distribution of “Encephalin” in the Brain of Humans, and Animals. Proc. Soc. Exp. Biol. Med. 1951, 76, 97–100. [Google Scholar] [CrossRef] [PubMed]
- Vogt, M. The concentration of sympathin in different parts of the central nervous system under normal conditions and after the administration of drugs. J. Physiol. 1954, 123, 451–481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montagu, K.A. Catechol compounds in rat tissues and in brains of different animals. Nature 1957, 180, 244–245. [Google Scholar] [CrossRef]
- Weil-Malherbe, H.; Bone, A.D. Effect of reserpine on the intracellular distribution of catecholamines in the brain stem of the rabbit. Nature 1958, 181, 1474–1475. [Google Scholar] [CrossRef]
- Holtz, P.; Balzer, H.; Westermann, E.; Wezler, E. Beeinflussung der Evipannarkose durch Reserpin, Iproniazid und biogene Amine. Naunyn-Schmiedeberg’s Arch. Exp. Pathol. Pharmakol. 1957, 231, 333–348. [Google Scholar] [CrossRef]
- Hornykiewicz, O. The action of dopamine on the arterial blood pressure of the guinea-pig. Br. J. Pharmacol. Chemother. 1958, 13, 91–94. [Google Scholar] [CrossRef] [Green Version]
- Holzer, G.; Hornykiewicz, O. Über den Dopamin-(Hydroxytyramin-)Stoffwechsel im Gehirn der Ratte. Naunyn-Schmiedeberg’s Arch. Exp. Pathol. Pharmakol. 1959, 237, 27–33. [Google Scholar]
- Hornykiewicz, O. The discovery of dopamine deficiency in the parkinsonian brain. J. Neural Transm. Suppl. 2006, 70, 9–15. [Google Scholar]
- Hassler, R. Zur Pathologie der Paralysis agitans und des postenzephalitischen Parkinsonismus. J. Psychol. Neurol. 1938, 48, 387–476. [Google Scholar]
- Hornykiewicz, O. Die topische Lokalization und des Verhalten von Noradrenalin und Dopamin (3-Hydroxytyramin) in der Substantia nigra der normalen und Parkinson Kranken Menschen. Wien. Klin. Wochschr. 1963, 75, 309–312. [Google Scholar]
- Rico, A.J.; Dopeso-Reyes, I.G.; Martínez-Pinilla, E.; Sucunza, D.; Pignataro, D.; Roda, E.; Marín-Ramos, D.; Labandeira-García, J.L.; George, S.R.; Franco, R.; et al. Neurochemical evidence supporting dopamine D1–D2 receptor heteromers in the striatum of the long-tailed macaque: Changes following dopaminergic manipulation. Brain Struct. Funct. 2016, 222, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lévesque, M.; Parent, A. The striatofugal fiber system in primates: A reevaluation of its organization based on single-axon tracing studies. Proc. Natl. Acad. Sci. USA 2005, 102, 11888–11893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agnati, L.F.; Santarossa, L.; Genedani, S.; Canela, E.I.; Leo, G.; Franco, R.; Woods, A.; Lluis, C.; Ferré, S.; Fuxe, K. On the nested hierarchical organization of CNS: Basic characteristics of neuronal molecular networks. Lect. Notes Comput. Sci. 2004, 3146, 24–54. [Google Scholar]
- Fuxe, K.; Borroto-Escuela, D.O. Volume transmission and receptor-receptor interactions in heteroreceptor complexes: Understanding the role of new concepts for brain communication. Neural Regen. Res. 2016, 11, 1220–1223. [Google Scholar] [CrossRef] [PubMed]
- Fuxe, K.; Agnati, L.F.; Marcoli, M.; Borroto-Escuela, D.O. Volume Transmission in Central Dopamine and Noradrenaline Neurons and Its Astroglial Targets. Neurochem. Res. 2015, 40, 2600–2614. [Google Scholar] [CrossRef]
- Zoli, M.; Torri, C.; Ferrari, R.; Jansson, A.; Zini, I.; Fuxe, K.; Agnati, L.F. The emergence of the volume transmission concept. In Brain Research Reviews; Elsevier: Amsterdam, The Netherlands, 1998; Volume 26, pp. 136–147. [Google Scholar]
- Alexander, S.P.; Christopoulos, A.; Davenport, A.P.; Kelly, E.; Mathie, A.; Peters, J.A.; Veale, E.L.; Armstrong, J.F.; Faccenda, E.; Harding, S.D.; et al. The concise guide to pharmacology 2019/20: G protein-coupled receptors. Br. J. Pharmacol. 2019, 176, S21–S141. [Google Scholar] [CrossRef] [Green Version]
- Heber, D.; Carpenter, C.L. Addictive genes and the relationship to obesity and inflammation. Mol. Neurobiol. 2011, 44, 160–165. [Google Scholar] [CrossRef] [Green Version]
- Kranzler, H.R.; Edenberg, H.J. Pharmacogenetics of Alcohol and Alcohol Dependence Treatment. Curr. Pharm. Des. 2010, 16, 2141–2148. [Google Scholar] [CrossRef]
- Le Foll, B.; Gallo, A.; Le Strat, Y.; Lu, L.; Gorwood, P. Genetics of dopamine receptors and drug addiction: A comprehensive review. Behav. Pharmacol. 2009, 20, 1–17. [Google Scholar] [CrossRef]
- Smith, L.; Watson, M.; Gates, S.; Ball, D.; Foxcroft, D. Meta-analysis of the association of the Taq1A polymorphism with the risk of alcohol dependency: A HuGE gene-disease association review. Am. J. Epidemiol. 2008, 167, 125–138. [Google Scholar] [CrossRef]
- Tyndale, R.F. Genetics of alcohol and tobacco use in humans. Ann. Med. 2003, 35, 94–121. [Google Scholar] [CrossRef]
- Wong, A.H.C.; Buckle, C.E.; Van Tol, H.H.M. Polymorphisms in dopamine receptors: What do they tell us? Eur. J. Pharmacol. 2000, 410, 183–203. [Google Scholar] [CrossRef]
- Comings, D.E.; Blum, K. Reward deficiency syndrome: Genetic aspects of behavioral disorders. Prog. Brain Res. 2000, 126, 325–341. [Google Scholar]
- Botticelli, L.; Di Bonaventura, E.M.; Del Bello, F.; Giorgioni, G.; Piergentili, A.; Romano, A.; Quaglia, W.; Cifani, C.; Di Bonaventura, M.V.M. Underlying susceptibility to eating disorders and drug abuse: Genetic and pharmacological aspects of dopamine D4 receptors. Nutrients 2020, 12, 2288. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.P.; So, C.H.; Rashid, A.J.; Varghese, G.; Cheng, R.; Lança, A.J.; O’Dowd, B.F.; George, S.R. Dopamine D1 and D2 receptor Co-activation generates a novel phospholipase C-mediated calcium signal. J. Biol. Chem. 2004, 279, 35671–35678. [Google Scholar] [CrossRef] [Green Version]
- Rashid, A.J.; So, C.H.; Kong, M.M.C.; Furtak, T.; El-Ghundi, M.; Cheng, R.; O’Dowd, B.F.; George, S.R. D1-D2 dopamine receptor heterooligomers with unique pharmacology are coupled to rapid activation of Gq/11 in the striatum. Proc. Natl. Acad. Sci. USA 2007, 104, 654–659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasbi, A.; Fan, T.; Alijaniaram, M.; Nguyen, T.; Perreault, M.L.; O’Dowd, B.F.; George, S.R. Calcium signaling cascade links dopamine D1-D2 receptor heteromer to striatal BDNF production and neuronal growth. Proc. Natl. Acad. Sci. USA 2009, 106, 21377–21382. [Google Scholar] [CrossRef] [Green Version]
- Hasbi, A.; Perreault, M.L.; Shen, M.Y.F.; Fan, T.; Nguyen, T.; Alijaniaram, M.; Banasikowski, T.J.; Grace, A.A.; O’Dowd, B.F.; Fletcher, P.J.; et al. Activation of dopamine D1-D2 receptor complex attenuates cocaine reward and reinstatement of cocaine-seeking through inhibition of DARPP-32, ERK, and ΔFosB. Front. Pharmacol. 2018, 8, 924. [Google Scholar] [CrossRef]
- Perreault, M.L.; Hasbi, A.; Shen, M.Y.F.; Fan, T.; Navarro, G.; Fletcher, P.J.; Franco, R.; Lanciego, J.L.; George, S.R. Disruption of a dopamine receptor complex amplifies the actions of cocaine. Eur. Neuropsychopharmacol. 2016, 26, 1366–1377. [Google Scholar] [CrossRef]
- Hasbi, A.; Nguyen, T.; Rahal, H.; Manduca, J.D.; Miksys, S.; Tyndale, R.F.; Madras, B.K.; Perreault, M.L.; George, S.R. Sex difference in dopamine D1-D2 receptor complex expression and signaling affects depression- and anxiety-like behaviors. Biol. Sex Differ. 2020, 11, 8. [Google Scholar] [CrossRef] [Green Version]
- Ferré, S.; Baler, R.; Bouvier, M.; Caron, M.G.; Devi, L.A.; Durroux, T.; Fuxe, K.; George, S.R.; Javitch, J.A.; Lohse, M.J.; et al. Building a new conceptual framework for receptor heteromers. Nat. Chem. Biol. 2009, 5, 131–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franco, N.; Franco, R. Understanding the added value of g-protein-coupled receptor heteromers. Scientifica 2014, 2014, 362937. [Google Scholar] [CrossRef] [PubMed]
- Franco, R.; Casadó, V.; Cortés, A.; Ferrada, C.; Mallol, J.; Woods, A.; Lluis, C.; Canela, E.I.; Ferré, S. Basic concepts in G-protein-coupled receptor homo- and heterodimerization. Sci. World J. 2007, 7, 48–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gines, S.; Hillion, J.; Torvinen, M.; Le Crom, S.; Casado, V.; Canela, E.I.; Rondin, S.; Lew, J.Y.; Watson, S.; Zoli, M.; et al. Dopamine D1 and adenosine A1 receptors form functionally interacting heteromeric complexes. Proc. Natl. Acad. Sci. USA 2000, 97, 8606–8611. [Google Scholar] [CrossRef] [Green Version]
- Hillion, J.; Canals, M.; Torvinen, M.; Casado, V.; Scott, R.; Terasmaa, A.; Hansson, A.; Watson, S.; Olah, M.E.; Mallol, J.; et al. Coaggregation, cointernalization, and codesensitization of adenosine A2A receptors and dopamine D2 receptors. J. Biol. Chem. 2002, 277, 18091–18097. [Google Scholar] [CrossRef] [Green Version]
- Fuxe, K.; Ferré, S.; Canals, M.; Torvinen, M.; Terasmaa, A.; Marcellino, D.; Goldberg, S.R.; Staines, W.; Jacobsen, K.X.; Lluis, C.; et al. Adenosine A2A and Dopamine D2 Heteromeric Receptor Complexes and Their Function. J. Mol. Neurosci. 2005, 26, 209–220. [Google Scholar] [CrossRef]
- Franco, R.; Lluis, C.; Canela, E.I.; Mallol, J.; Agnati, L.; Casadó, V.; Ciruela, F.; Ferré, S.; Fuxe, K. Receptor-receptor interactions involving adenosine A1 or dopamine D1 receptors and accessory proteins. J. Neural Transm. (Vienna) 2007, 114, 93–104. [Google Scholar] [CrossRef]
- Navarro, G.; Borroto-Escuela, D.O.D.O.; Fuxe, K.; Franco, R. Purinergic signaling in Parkinson’s disease. Relevance for treatment. Neuropharmacology 2015, 104, 161–168. [Google Scholar] [CrossRef]
- Fuxe, K.; Agnati, L.F.F.; Jacobsen, K.; Hillion, J.; Canals, M.; Torvinen, M.; Tinner-Staines, B.; Staines, W.; Rosin, D.; Terasmaa, A.; et al. Receptor heteromerization in adenosine A2A receptor signaling: Relevance for striatal function and Parkinson’s disease. Neurology 2003, 61, S19–S23. [Google Scholar] [CrossRef]
- Marcellino, D.; Ferré, S.; Casadó, V.; Cortés, A.; Le Foll, B.; Mazzola, C.; Drago, F.; Saur, O.; Stark, H.; Soriano, A.; et al. Identification of dopamine D1-D3 receptor heteromers: Indications for a role of synergistic D1-D3 receptor interactions in the striatum. J. Biol. Chem. 2008, 283, 26016–26025. [Google Scholar] [CrossRef] [Green Version]
- Fiorentini, C.; Busi, C.; Gorruso, E.; Gotti, C.; Spano, P.F.; Missale, C. Reciprocal regulation of dopamine D1 and D3 receptor function and trafficking by heterodimerization. Mol. Pharmacol. 2008, 74, 59–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scarselli, M.; Novi, F.; Schallmach, E.; Lin, R.; Baragli, A.; Colzi, A.; Griffon, N.; Corsini, G.U.; Sokoloff, P.; Levenson, R.; et al. D2/D3 Dopamine Receptor Heterodimers Exhibit Unique Functional Properties. J. Biol. Chem. 2001, 276, 30308–30314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borroto-Escuela, D.O.; Van Craenenbroeck, K.; Romero-Fernandez, W.; Guidolin, D.; Woods, A.S.; Rivera, A.; Haegeman, G.; Agnati, L.F.; Tarakanov, A.O.; Fuxe, K. Dopamine D2 and D4 receptor heteromerization and its allosteric receptor–receptor interactions. Biochem. Biophys. Res. Commun. 2011, 404, 928–934. [Google Scholar] [CrossRef] [PubMed]
- Ferrada, C.; Moreno, E.; Casadó, V.; Bongers, G.; Cortés, A.; Mallol, J.; Canela, E.I.; Leurs, R.; Ferré, S.; Lluís, C.; et al. Marked changes in signal transduction upon heteromerization of dopamine D1 and histamine H3 receptors. Br. J. Pharmacol. 2009, 157, 64–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrada, C.; Ferré, S.; Casadó, V.; Cortés, A.; Justinova, Z.; Barnes, C.; Canela, E.I.; Goldberg, S.R.; Leurs, R.; Lluis, C.; et al. Interactions between histamine H3 and dopamine D2 receptors and the implications for striatal function. Neuropharmacology 2008, 55, 190–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González, S.; Moreno-Delgado, D.; Moreno, E.; Pérez-Capote, K.; Franco, R.; Mallol, J.; Cortés, A.; Casadó, V.; Lluís, C.; Ortiz, J.; et al. Circadian-related heteromerization of adrenergic and dopamine D 4 receptors modulates melatonin synthesis and release in the pineal gland. PLoS Biol. 2012, 10, e1001347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borroto-Escuela, D.O.; Brito, I.; Romero-Fernandez, W.; Di Palma, M.; Oflijan, J.; Skieterska, K.; Duchou, J.; Van Craenenbroeck, K.; Suárez-Boomgaard, D.; Rivera, A.; et al. The G protein-coupled receptor heterodimer network (GPCR-HetNet) and its hub components. Int. J. Mol. Sci. 2014, 15, 8570–8590. [Google Scholar] [CrossRef]
- Grandy, D.K.; Marchionni, M.A.; Makam, H.; Stofko, R.E.; Alfano, M.; Frothingham, L.; Fischer, J.B.; Burke-Howie, K.J.; Bunzow, J.R.; Server, A.C.; et al. Cloning of the cDNA and gene for a human D2 dopamine receptor. Proc. Natl. Acad. Sci. USA 1989, 86, 9762–9766. [Google Scholar] [CrossRef] [Green Version]
- Timmerman, W.; Dubocovich, M.L.; Westerink, B.H.C.; De Vries, J.B.; Tepper, P.G.; Horn, A.S. The enantiomers of the dopamine agonist N-0437: In vivo and in vitro effects on the release of striatal dopamine. Eur. J. Pharmacol. 1989, 166, 1–11. [Google Scholar] [CrossRef]
- Akaoka, H.; Charléty, P.; Saunier, C.F.; Buda, M.; Chouvet, G. Inhibition of nigral dopamine neurons by systemic and local apomorphine: Possible contribution of dendritic autoreceptors. Neuroscience 1992, 49, 879–891. [Google Scholar] [CrossRef]
- Lichter, J.B.; Barr, C.L.; Kennedy, J.L.; Van Tol, H.H. m.; Kidd, K.K.; Livak, K.J. A hypervariable segment in the human dopamine receptor D4 (DRD4) gene. Hum. Mol. Genet. 1993, 2, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Asghari, V.; Sanyal, S.; Buchwaldt, S.; Paterson, A.; Jovanovic, V.; Van Tol, H.H.M. Modulation of Intracellular Cyclic AMP Levels by Different Human Dopamine D4 Receptor Variants. J. Neurochem. 1995, 65, 1157–1165. [Google Scholar] [CrossRef] [PubMed]
- Berry, D.; Deater-Deckard, K.; McCartney, K.; Wang, Z.; Petrill, S.A. Gene-environment interaction between dopamine receptor D4 7-repeat polymorphism and early maternal sensitivity predicts inattention trajectories across middle childhood. Dev. Psychopathol. 2013, 25, 291–306. [Google Scholar] [CrossRef] [PubMed]
- Navarro, G.; Cordomí, A.; Zelman-Femiak, M.; Brugarolas, M.; Moreno, E.; Aguinaga, D.; Perez-Benito, L.; Cortés, A.; Casadó, V.; Mallol, J.; et al. Quaternary structure of a G-protein-coupled receptor heterotetramer in complex with Gi and Gs. BMC Biol. 2016, 14, 26. [Google Scholar] [CrossRef] [Green Version]
- Navarro, G.; Cordomí, A.; Brugarolas, M.; Moreno, E.; Aguinaga, D.; Pérez-Benito, L.; Ferre, S.; Cortés, A.; Casadó, V.; Mallol, J.; et al. Cross-communication between Gi and Gs in a G-protein-coupled receptor heterotetramer guided by a receptor C-terminal domain. BMC Biol. 2018, 16, 1–15. [Google Scholar] [CrossRef]
- Cordomí, A.; Navarro, G.; Aymerich, M.S.; Franco, R. Structures for G-Protein-Coupled Receptor Tetramers in Complex with G Proteins. Trends Biochem. Sci. 2015, 40, 548–551. [Google Scholar] [CrossRef] [Green Version]
- González, S.; Rangel-Barajas, C.; Peper, M.; Lorenzo, R.; Moreno, E.; Ciruela, F.; Borycz, J.; Ortiz, J.; Lluís, C.; Franco, R.; et al. Dopamine D4 receptor, but not the ADHD-associated D4.7 variant, forms functional heteromers with the dopamine D2S receptor in the brain. Mol. Psychiatry 2012, 17, 650–662. [Google Scholar] [CrossRef] [Green Version]
- Clow, A.; Jenner, P.; Theodorou, A.; Marsden, C.D. Changes in cerebral dopamine metabolism and receptors during one-year neuroleptic administration and subsequent withdrawal: Relevance to brain biochemistry in schizophrenia. Adv. Biochem. Psychopharmacol. 1980, 24, 53–55. [Google Scholar]
- Langer, D.H.; Brown, G.L.; Docherty, J.P. Dopamine receptor supersensitivity and schizophrenia: A review. Schizophr. Bull. 1981, 7, 208–224. [Google Scholar] [CrossRef] [Green Version]
- Fuxe, K.; Borroto-Escuela, D.; Fisone, G.; Agnati, L.F.; Tanganelli, S. Understanding the role of heteroreceptor complexes in the central nervous system. Curr. Protein Pept. Sci. 2014, 15, 647–654. [Google Scholar] [CrossRef]
- Blum, K.; Braverman, E.R.; Holder, J.M.; Lubar, J.F.; Monastra, V.I.; Miller, D.; Lubar, J.O.; Chen, T.J.H.; Comings, D.E. The reward deficiency syndrome: A biogenetic model for the diagnosis and treatment of impulsive, addictive and compulsive behaviors. J. Psychoact. Drugs 2000, 32, 1–112. [Google Scholar] [CrossRef] [PubMed]
- Grinchii, D.; Dremencov, E. Mechanism of action of atypical antipsychotic drugs in mood disorders. Int. J. Mol. Sci. 2020, 21, 9532. [Google Scholar] [CrossRef] [PubMed]
- Cossu, G.; Rinaldi, R.; Colosimo, C. The rise and fall of impulse control behavior disorders. Park. Relat. Disord. 2018, 46, S24–S29. [Google Scholar] [CrossRef] [PubMed]
- Birkmayer, W.; Hornykiewicz, O. Additional experimental studies on L-DOPA in Parkinson’s syndrome and reserpine parkinsonism. Arch. Psychiatr. Nervenkr. 1964, 206, 367–381. [Google Scholar] [CrossRef] [PubMed]
- Olanow, C.W.; Agid, Y.; Mizuno, Y.; Albanese, A.; Bonuccelli, U.; Bonucelli, U.; Damier, P.; De Yebenes, J.; Gershanik, O.; Guttman, M.; et al. Levodopa in the treatment of Parkinson’s disease: Current controversies. Mov. Disord. 2004, 19, 997–1005. [Google Scholar] [CrossRef] [PubMed]
- Marín-Valencia, I.; Serrano, M.; Ormazabal, A.; Pérez-Dueñas, B.; García-Cazorla, A.; Campistol, J.; Artuch, R. Biochemical diagnosis of dopaminergic disturbances in paediatric patients: Analysis of cerebrospinal fluid homovanillic acid and other biogenic amines. Clin. Biochem. 2008, 41, 1306–1315. [Google Scholar] [CrossRef]
- Molero-Luis, M.; Serrano, M.; Ormazábal, A.; Pérez-Dueñas, B.; García-Cazorla, À.; Pons, R.; Artuch, R. Homovanillic acid in cerebrospinal fluid of 1388 children with neurological disorders. Dev. Med. Child Neurol. 2013, 55, 559–566. [Google Scholar] [CrossRef]
- Hyland, K. Clinical utility of monoamine neurotransmitter metabolite analysis in cerebrospinal fluid. Clin. Chem. 2008, 54, 633–641. [Google Scholar] [CrossRef] [Green Version]
- Muñoz, P.; Huenchuguala, S.; Paris, I.; Segura-Aguilar, J. Dopamine Oxidation and Autophagy. Parkinsons. Dis. 2012, 2012, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, R.; Wang, G. Impact of Dopamine Oxidation on Dopaminergic Neurodegeneration. ACS Chem. Neurosci. 2019, 10, 945–953. [Google Scholar] [CrossRef] [PubMed]
- Finberg, J.P.M. Inhibitors of MAO-B and COMT: Their effects on brain dopamine levels and uses in Parkinson’s disease. J. Neural Transm. 2019, 126, 433–448. [Google Scholar] [CrossRef] [PubMed]
- Kupsch, A.; Sautter, J.; Götz, M.E.; Breithaupt, W.; Schwarz, J.; Youdim, M.B.H.; Riederer, P.; Gerlach, M.; Oertel, W.H. Monoamine oxidase-inhibition and MPTP-induced neurotoxicity in the non-human primate: Comparison of rasagiline (TVP 1012) with selegiline. J. Neurol. 2001, 248, 985–1009. [Google Scholar] [CrossRef] [PubMed]
- Goudreau, J.L.; Maraganore, D.M.; Farrer, M.J.; Lesnick, T.G.; Singleton, A.B.; Bower, J.H.; Hardy, J.A.; Rocca, W.A. Case-control study of dopamine transporter-1, monoamine oxidase-B, and catechol-O-methyl transferase polymorphisms in Parkinson’s disease. Mov. Disord. 2002, 17, 1305–1311. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Lopez, E.; Vrana, K.E. Dopamine beta-hydroxylase and its genetic variants in human health and disease. J. Neurochem. 2020, 152, 157–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oleskin, A.V.; Shenderov, B.A. Probiotics and Psychobiotics: The Role of Microbial Neurochemicals. Probiotics Antimicrob. Proteins 2019, 11, 1071–1085. [Google Scholar] [CrossRef]
- Oleskin, A.V.; Shenderov, B.A.; Rogovsky, V.S. Role of Neurochemicals in the Interaction between the Microbiota and the Immune and the Nervous System of the Host Organism. Probiotics Antimicrob. Proteins 2017, 9, 215–234. [Google Scholar] [CrossRef]
- Oleskin, A.V.; El’-Registan, G.I.; Shenderov, B.A. Role of neuromediators in the functioning of the human microbiota: “Business talks” among microorganisms and the microbiota-host dialogue. Microbiology 2016, 85, 1–22. [Google Scholar] [CrossRef]
- Lyte, M. Microbial endocrinology: An ongoing personal journey. In Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2016; Volume 874, pp. 1–24. [Google Scholar]
- González-Arancibia, C.; Urrutia-Piñones, J.; Illanes-González, J.; Martinez-Pinto, J.; Sotomayor-Zárate, R.; Julio-Pieper, M.; Bravo, J.A. Do your gut microbes affect your brain dopamine? Psychopharmacology 2019, 236, 1611–1622. [Google Scholar] [CrossRef]
- Collins, G.G.; West, G.B. The release of 3H-dopamine from the isolated rabbit ileum. Br. J. Pharmacol. 1968, 34, 514–522. [Google Scholar] [CrossRef] [Green Version]
- Penttilä, A. Effect of Incubation in Krebs-Ringer solution or humid air on the amine content, fluorescence and staining characteristics of the duodenal enterochromaffin and dopamine cells. Virchows Arch. B 1968, 1, 269–282. [Google Scholar]
- Falck, B. Observations on the possibilities of the cellular localization of monoamines by a fluorescence method. Acta Physiol. Scand. 1962, 56, 1–25. [Google Scholar]
- Strandwitz, P. Neurotransmitter modulation by the gut microbiota. Brain Res. 2018, 1693, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Penttilä, A.; Ahonen, A. Binding of 1-3,4-dihydroxyphenylalanine and dopamine in cytoplasmic granules of paneth cells. Experientia 1969, 25, 70–72. [Google Scholar] [CrossRef]
- Vaishnava, S.; Behrendt, C.L.; Ismail, A.S.; Eckmann, L.; Hooper, L.V. Paneth cells directly sense gut commensals and maintain homeostasis at the intestinal host-microbial interface. Proc. Natl. Acad. Sci. USA 2008, 105, 20858–20863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liévin-Le Moal, V.; Servin, A.L. The front line of enteric host defense against unwelcome intrusion of harmful microorganisms: Mucins, antimicrobial peptides, and Microbiota. Clin. Microbiol. Rev. 2006, 19, 315–337. [Google Scholar] [CrossRef] [Green Version]
- Pelaseyed, T.; Bergström, J.H.; Gustafsson, J.K.; Ermund, A.; Birchenough, G.M.H.; Schütte, A.; van der Post, S.; Svensson, F.; Rodríguez-Piñeiro, A.M.; Nyström, E.E.L.; et al. The mucus and mucins of the goblet cells and enterocytes provide the first defense line of the gastrointestinal tract and interact with the immune system. Immunol. Rev. 2014, 260, 8–20. [Google Scholar] [CrossRef] [Green Version]
- Salzman, N.H.; Underwood, M.A.; Bevins, C.L. Paneth cells, defensins, and the commensal microbiota: A hypothesis on intimate interplay at the intestinal mucosa. Semin. Immunol. 2007, 19, 70–83. [Google Scholar] [CrossRef]
- Yang, E.; Shen, J. The roles and functions of Paneth cells in Crohn’s disease: A critical review. Cell Prolif. 2020, e12958. [Google Scholar] [CrossRef]
- Magro, F.; Cunha, E.; Araujo, F.; Meireles, E.; Pereira, P.; Dinis-Ribeiro, M.; Veloso, F.T.; Medeiros, R.; Soares-Da-Silva, P. Dopamine D2 receptor polymorphisms in inflammatory bowel disease and the refractory response to treatment. Dig. Dis. Sci. 2006, 51, 2039–2044. [Google Scholar] [CrossRef]
- Pacheco, R.; Gallart, T.; Lluis, C.; Franco, R. Role of glutamate on T-cell mediated immunity. J. Neuroimmunol. 2007, 185, 9–19. [Google Scholar] [CrossRef]
- Papa, I.; Saliba, D.; Ponzoni, M.; Bustamante, S.; Canete, P.F.; Gonzalez-Figueroa, P.; McNamara, H.A.; Valvo, S.; Grimbaldeston, M.; Sweet, R.A.; et al. TFH-derived dopamine accelerates productive synapses in germinal centres. Nature 2017, 547, 318–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagai, Y.; Ueno, S.; Saeki, Y.; Soga, F.; Hirano, M.; Yanagihara, T. Decrease of the D3 dopamine receptor mRNA expression in lymphocytes from patients with Parkinson’s disease. Neurology 1996, 46, 791–795. [Google Scholar] [CrossRef] [PubMed]
- Elgueta, D.; Aymerich, M.S.; Contreras, F.; Montoya, A.; Celorrio, M.; Rojo-Bustamante, E.; Riquelme, E.; Gonzalez, H.; Vasquez, M.; Franco, R.; et al. Pharmacologic antagonism of dopamine receptor D3 attenuates neurodegeneration and motor impairment in a mouse model of Parkinson’s disease. Neuropharmacology 2017, 113, 110–123. [Google Scholar] [CrossRef] [PubMed]
- González, H.; Contreras, F.; Prado, C.; Elgueta, D.; Franz, D.; Bernales, S.; Pacheco, R. Dopamine receptor D3 expressed on CD4+ T cells favors neurodegeneration of dopaminergic neurons during Parkinson’s disease. J. Immunol. 2013, 190, 5048–5056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campos-Acuña, J.; Elgueta, D.; Pacheco, R. T-cell-driven inflammation as a mediator of the gut-brain axis involved in Parkinson’s disease. Front. Immunol. 2019, 10, 239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parashar, A.; Udayabanu, M. Gut microbiota: Implications in Parkinson’s disease. Park. Relat. Disord. 2017, 38, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharyya, D.; Bhunia, A. Gut-Brain Axis in Parkinson’s disease Etiology: The Role of Lipopolysaccharide. Chem. Phys. Lipids 2020, 235, 105029. [Google Scholar] [CrossRef]
- Mulak, A.; Bonaz, B. Brain-gut-microbiota axis in Parkinson’s disease. World J. Gastroenterol. 2015, 21, 10609–10620. [Google Scholar] [CrossRef]
- Moraga-Amaro, R.; Gonzalez, H.; Pacheco, R.; Stehberg, J. Dopamine receptor D3 deficiency results in chronic depression and anxiety. Behav. Brain Res. 2014, 274, 186–193. [Google Scholar] [CrossRef]
- Franz, D.; Contreras, F.; González, H.; Prado, C.; Elgueta, D.; Figueroa, C.; Pacheco, R. Dopamine receptors D3 and D5 regulate CD4+T-cell activation and differentiation by modulating ERK activation and cAMP production. J. Neuroimmunol. 2015, 284, 18–29. [Google Scholar] [CrossRef]
- Contreras, F.; Prado, C.; González, H.; Franz, D.; Osorio-Barrios, F.; Osorio, F.; Ugalde, V.; Lopez, E.; Elgueta, D.; Figueroa, A.; et al. Dopamine Receptor D3 Signaling on CD4 + T Cells Favors Th1- and Th17-Mediated Immunity. J. Immunol. 2016, 196, 4143–4149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prado, C.; Gaiazzi, M.; González, H.; Ugalde, V.; Figueroa, A.; Osorio-Barrios, F.J.; López, E.; Lladser, A.; Rasini, E.; Marino, F.; et al. Dopaminergic stimulation of myeloid antigen-presenting cells attenuates signal transducer and activator of transcription 3-activation favouring the development of experimental autoimmune encephalomyelitis. Front. Immunol. 2018, 9, 571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pacheco, R.; Contreras, F.; Zouali, M. The dopaminergic system in autoimmune diseases. Front. Immunol. 2014, 5, 117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pacheco, R.; Prado, C.E.; Barrientos, M.J.; Bernales, S. Role of dopamine in the physiology of T-cells and dendritic cells. J. Neuroimmunol. 2009, 216, 8–19. [Google Scholar] [CrossRef]
- Figueroa, C.; Gálvez-Cancino, F.; Oyarce, C.; Contreras, F.; Prado, C.; Valeria, C.; Cruz, S.; Lladser, A.; Pacheco, R. Inhibition of dopamine receptor D3 signaling in dendritic cells increases antigen cross-presentation to CD8+ T-cells favoring anti-tumor immunity. J. Neuroimmunol. 2017, 303, 99–107. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franco, R.; Reyes-Resina, I.; Navarro, G. Dopamine in Health and Disease: Much More Than a Neurotransmitter. Biomedicines 2021, 9, 109. https://doi.org/10.3390/biomedicines9020109
Franco R, Reyes-Resina I, Navarro G. Dopamine in Health and Disease: Much More Than a Neurotransmitter. Biomedicines. 2021; 9(2):109. https://doi.org/10.3390/biomedicines9020109
Chicago/Turabian StyleFranco, Rafael, Irene Reyes-Resina, and Gemma Navarro. 2021. "Dopamine in Health and Disease: Much More Than a Neurotransmitter" Biomedicines 9, no. 2: 109. https://doi.org/10.3390/biomedicines9020109
APA StyleFranco, R., Reyes-Resina, I., & Navarro, G. (2021). Dopamine in Health and Disease: Much More Than a Neurotransmitter. Biomedicines, 9(2), 109. https://doi.org/10.3390/biomedicines9020109





