Current Promising Biomarkers and Methods in the Diagnostics of Antiphospholipid Syndrome: A Review
Abstract
:1. Introduction
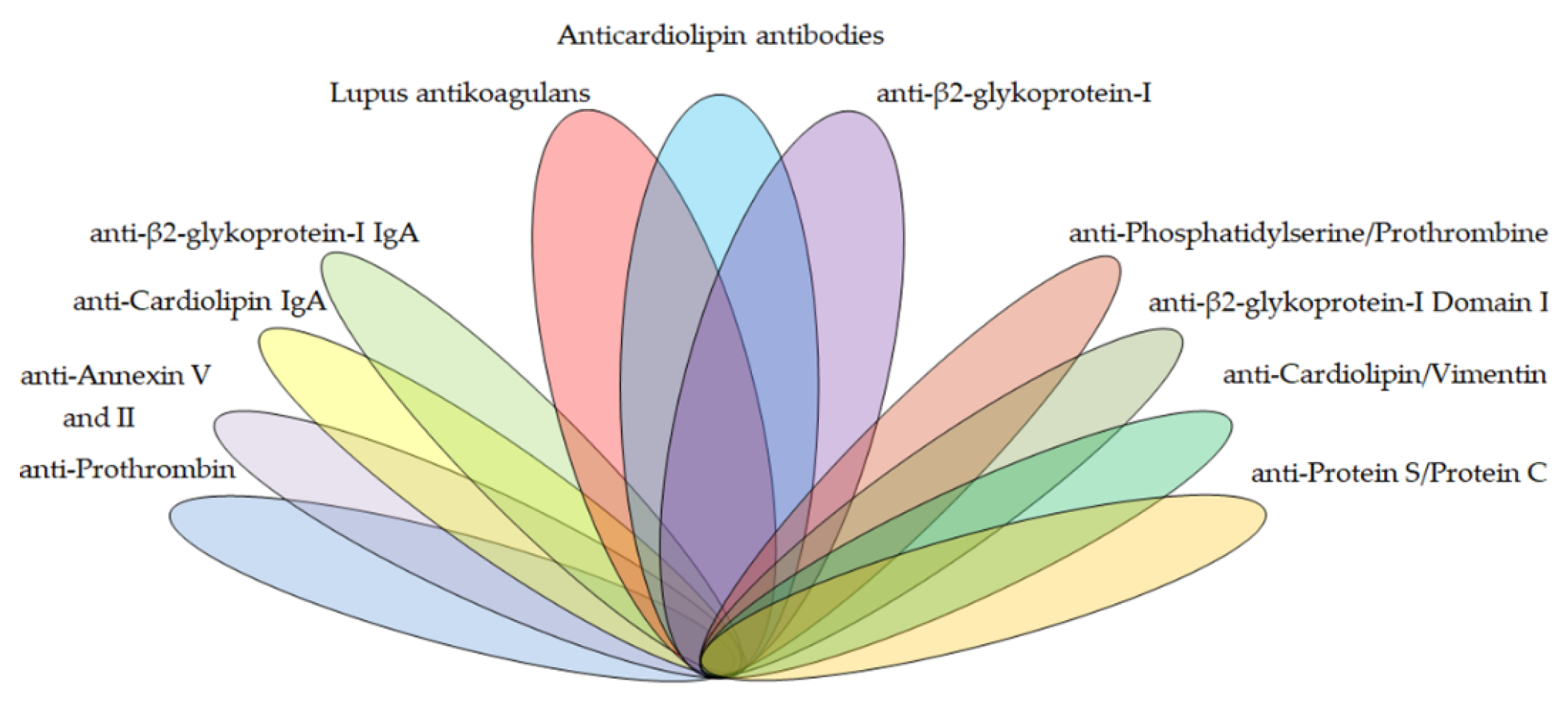
2. Antiphospholipid Antibodies
2.1. APS Criteria Antibodies
2.1.1. Lupus Anticoagulant
2.1.2. Anti-β2-Glycoprotein-I
2.1.3. Anti-Cardiolipin
2.2. APS Non-Criteria Antibodies
2.2.1. Anti-β2-Glycoprotein-I Domain I
2.2.2. Anti-β2-Glycoprotein-I IgA
2.2.3. Anti-Cardiolipin IgA
2.2.4. Anti-Prothrombin and Anti-Phosphatidylserine/Prothrombin Complex
2.2.5. Anti-Annexin V and Anti-Annexin II
2.2.6. Anti-Cardiolipin/Vimentin
2.2.7. Anti-Protein S/Protein C
2.2.8. Antibodies Against Phospholipid Antigens
3. Methods
3.1. Liquid-Phase Assay
Lupus Anticoagulant
3.2. Solid-Phase Assay
3.2.1. Enzyme-Linked Imunosorbent Assay
3.2.2. Fluorescence Enzyme Immunoassay
3.2.3. Chemiluminescence Immunoassay
3.2.4. Multiplex Flow Fluorescence Immunoassay
3.2.5. Multiline Dot Assay
3.2.6. Line Immunoassay
3.2.7. Thin-Layer Chromatography TLC
4. Conclusions
Seronegative APS
Author Contributions
Funding
Conflicts of Interest
References
- Hughes, G.R. Thrombosis, abortion, cerebral disease, and the lupus anticoagulant. Br. Med. J. 1983, 287, 1088–1089. [Google Scholar] [CrossRef] [Green Version]
- López-Pedrera, C.; Barbarroja, N.; Patiño-Trives, A.M.; Collantes, E.; Aguirre, M.A.; Perez-Sanchez, C. New Biomarkers for Atherothrombosis in Antiphospholipid Syndrome: Genomics and Epigenetics Approaches. Front. Immunol. 2019, 10, 764. [Google Scholar] [CrossRef] [PubMed]
- Radic, M.; Pattanaik, D. Cellular and Molecular Mechanisms of Anti-Phospholipid Syndrome. Front. Immunol. 2018, 9, 969. [Google Scholar] [CrossRef] [Green Version]
- Schreiber, K.; Sciascia, S.; de Groot, P.G.; Devreese, K.; Jacobsen, S.; Ruiz-Irastorza, G.; Salmon, J.E.; Shoenfeld, Y.; Shovman, O.; Hunt, B.J. Antiphospholipid syndrome. Nat. Rev. Dis. Primers 2018, 4, 17103. [Google Scholar] [CrossRef] [PubMed]
- Abeysekera, R.A.; Wazil, A.W.M.; Nanayakkara, N.; Ratnatunga, N.V.I.; Fernando, K.M.; Thinnarachchi, J. Primary antiphospholipid syndrome presenting as antiphospholipid syndrome nephropathy: A case report. J. Med. Case Rep. 2015, 9, 28. [Google Scholar] [CrossRef] [Green Version]
- Rand, J.H. The antiphospholipid syndrome. Hematol. Am. Soc. Hematol. Educ. Program 2007, 2007, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Meroni, P.L.; Toubi, E.; Shoenfeld, Y. Are Anti-Phospholipid Syndrome and Systemic Lupus Erythematosus Two Different Diseases? A 10-Year Late Remake. Isr. Med. Assoc. J. 2019, 21, 491–493. [Google Scholar]
- Cervera, R. Antiphospholipid syndrome. Thromb. Res. 2017, 151, S43–S47. [Google Scholar] [CrossRef]
- Cervera, R.; Serrano, R.; Pons-Estel, G.J.; Ceberio-Hualde, L.; Shoenfeld, Y.; de Ramón, E.; Buonaiuto, V.; Jacobsen, S.; Zeher, M.M.; Tarr, T.; et al. Morbidity and mortality in the antiphospholipid syndrome during a 10-year period: A multicentre pro-spective study of 1000 patients. Ann. Rheum. Dis. 2015, 74, 1011–1018. [Google Scholar] [CrossRef] [PubMed]
- Garcia, D.; Erkan, D. Diagnosis and Management of the Antiphospholipid Syndrome. N. Engl. J. Med. 2018, 378, 2010–2021. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Pintó, I.; Moitinho, M.; Santacreu, I.; Shoenfeld, Y.; Erkan, D.; Espinosa, G.; Cervera, R. Catastrophic antiphospholipid syndrome (CAPS): Descriptive analysis of 500 patients from the International CAPS Registry. Autoimmun. Rev. 2016, 15, 1120–1124. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Sun, D. Pregnancy outcomes in patients with primary antiphospholipid syndrome: A systematic review and meta-analysis. Medicine 2019, 98, e15733. [Google Scholar] [CrossRef] [PubMed]
- Whitaker, K.L. Antiphospholipid antibody syndrome: The difficulties of diagnosis. JAAPA 2017, 30, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Khamashta, M.A. Management of thrombosis and pregnancy loss in the antiphospholipid syndrome. Lupus 1998, 7, S162–S165. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Irastorza, G.; Crowther, M.; Branch, W.; Khamashta, M.A. Antiphospholipid syndrome. Lancet 2010, 376, 1498–1509. [Google Scholar] [CrossRef] [Green Version]
- Miyakis, S.; Lockshin, M.D.; Atsumi, T.; Branch, D.W.; Brey, R.L.; Cervera, R.; Derksen, R.H.W.M.; De Groot, P.G.; Koike, T.; Meroni, P.L.; et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J. Thromb. Haemost. 2006, 4, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Devreese, K.M.J.; Ortel, T.L.; Pengo, V.; De Laat, B.; Antibodies, T.S.O.L.A. Laboratory criteria for antiphospholipid syndrome: Communication from the SSC of the ISTH. J. Thromb. Haemost. 2018, 16, 809–813. [Google Scholar] [CrossRef] [Green Version]
- Pengo, V.; Banzato, A.; Bison, E.; Bracco, A.; Denas, G.; Ruffatti, A. What have we learned about antiphospholipid syndrome from patients and antiphospholipid carrier co-horts? Semin. Thromb. Hemost. 2012, 38, 322–327. [Google Scholar]
- Pengo, V.; Ruffatti, A.; Del Ross, T.; Tonello, M.; Cuffaro, S.; Hoxha, A.; Banzato, A.; Bison, E.; Denas, G.; Bracco, A.; et al. Confirmation of initial antiphospholipid antibody positivity depends on the antiphospholipid antibody profile. J. Thromb. Haemost. 2013, 11, 1527–1531. [Google Scholar] [CrossRef]
- Pengo, V.; Ruffatti, A.; Legnani, C.; Gresele, P.; Barcellona, D.; Erba, N.; Testa, S.; Marongiu, F.; Bison, E.; Denas, G.; et al. Clinical course of high-risk patients diagnosed with antiphospholipid syndrome. J. Thromb. Haemost. 2010, 8, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Pengo, V.; Denas, G. Diagnostics and treatment of thrombotic antiphospholipid syndrome (APS): A personal perspec-tive. Thromb. Res. 2018, 169, 35–40. [Google Scholar] [CrossRef]
- Tripodi, A. Laboratory Testing for Lupus Anticoagulants: A Review of Issues Affecting Results. Clin. Chem. 2007, 53, 1629–1635. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, S.; McCrae, K.R. Diagnosis and management of the antiphospholipid syndrome. Blood Rev. 2017, 31, 406–417. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Gu, J.; Wan, L.; Hu, Q.; Teng, J.; Liu, H.; Cheng, X.; Ye, J.; Su, Y.; Sun, Y.; et al. “Non-criteria” antiphospholipid antibodies add value to antiphospholipid syndrome diagnoses in a large Chinese cohort. Arthritis Res. 2020, 22, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, H.; Ahn, S.S.; Song, J.J.; Park, Y.; Song, J.; Lee, S.-W. Anti-phospholipid antibody syndrome occurrence in patients with persistent anti-phospholipid antibodies. Rheumatol. Int. 2019, 39, 1359–1367. [Google Scholar] [CrossRef] [PubMed]
- De Groot, P.G.; Meijers, J.C. β(2)-Glycoprotein I: Evolution, structure and function. J. Thromb. Haemost. 2011, 9, 1275–1284. [Google Scholar] [CrossRef]
- Misasi, R.; Capozzi, A.; Longo, A.; Recalchi, S.; Lococo, E.; Alessandri, C.; Conti, F.; Valesini, G.; Sorice, M. “New” antigenic targets and methodological approaches for refining laboratory diagnosis of antiphospho-lipid syndrome. J. Immunol. Res. 2015, 2015, 858542. [Google Scholar] [CrossRef] [Green Version]
- Chayoua, W.; Kelchtermans, H.; Moore, G.W.; Musiał, J.; Wahl, D.; De Laat, B.; Devreese, K.M.J. Identification of high thrombotic risk triple-positive antiphospholipid syndrome patients is dependent on anti-cardiolipin and anti-β2glycoprotein I antibody detection assays. J. Thromb. Haemost. 2018, 16, 2016–2023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Decavele, A.S.; Schouwers, S.; Devreese, K.M. Evaluation of three commercial ELISA kits for anticardiolipin and anti-β2-glycoprotein I antibodies in the laboratory diagnosis of the antiphospholipid syndrome. Int. J. Lab. Hematol. 2011, 33, 97–108. [Google Scholar] [CrossRef]
- Gebhart, J.; Posch, F.; Koder, S.; Quehenberger, P.; Perkmann, T.; Kuessel, L.; Ay, C.; Pabinger, I. High risk of adverse pregnancy outcomes in women with a persistent lupus anticoagulant. Blood Adv. 2019, 3, 769–776. [Google Scholar] [CrossRef] [Green Version]
- Pierangeli, S.S.; Favaloro, E.J.; Lakos, G.; Meroni, P.L.; Tincani, A.; Wong, R.C.; Harris, E.N. Standards and reference materials for the anticardiolipin and anti-β2glycoprotein I assays: A report of recommendations from the APL Task Force at the 13th International Congress on Antiphospholipid Antibodies. Clin. Chim. Acta 2012, 413, 358–360. [Google Scholar] [CrossRef]
- Willis, R.; Lakos, G.; Harris, E.N. Standardization of Antiphospholipid Antibody Testing—Historical Perspectives and Ongoing Initiatives. Semin. Thromb. Hemost. 2014, 40, 172–177. [Google Scholar] [CrossRef]
- Janek, D.; Slavik, L.; Ulehlova, J.; Krcova, V.; Hlusi, A.; Prochazkova, J. Validation of a New Panel of Automated Chemiluminescence Assays for Anticardiolipin Antibodies in the Screening for Antiphospholipid Syndrome. Clin. Lab. 2016, 62, 1309–1315. [Google Scholar] [CrossRef]
- Meroni, P.L.; Tincani, A.; Harris, E.N.; Valesini, G.; Hughes, G.R.; Balestrieri, G. The pathophysiology of anti-phospholipid antibodies. Clin. Exp. Rheumatol. 1989, 7, 81–84. [Google Scholar]
- Yin, D.; Chayoua, W.; Kelchtermans, H.; de Groot, P.G.; Moore, G.W.; Gris, J.C.; Zuily, S.; Musial, J.; de Laat, B.; Devreese, K.M.J. Detection of anti-domain I antibodies by chemiluminescence enables the identification of high-risk an-tiphospholipid syndrome patients: A multicenter multiplatform study. J. Thromb. Haemost. 2020, 18, 463–478. [Google Scholar] [CrossRef] [PubMed]
- Mahler, M.; Norman, G.L.; Meroni, P.L.; Khamashta, M. Autoantibodies to domain 1 of β2 glycoprotein 1: A promising candidate biomarker for risk manage-ment in antiphospholipid syndrome. Autoimmun. Rev. 2012, 12, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Radin, M.; Cecchi, I.; Roccatello, D.; Meroni, P.L.; Sciascia, S. Prevalence and Thrombotic Risk Assessment of Anti-β2 Glycoprotein I Domain I Antibodies: A Systematic Review. Semin. Thromb. Hemost. 2017, 44, 466–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tonello, M.; Mattia, E.; Del Ross, T.; Favaro, M.; Calligaro, A.; Hoxha, A.; Bison, E.; Pengo, V.; Ruffatti, A. Clinical value of anti-domain I-β2Glycoprotein 1 antibodies in antiphospholipid antibody carriers. A single centre, prospective observational follow-up study. Clin. Chim. Acta 2018, 485, 74–78. [Google Scholar] [CrossRef]
- Serrano, M.; Martinez-Flores, J.A.; Norman, G.L.; Naranjo, L.; Morales, J.M.; Serrano, A. The IgA Isotype of Anti-β2 Glycoprotein I Antibodies Recognizes Epitopes in Domains 3, 4, and 5 That Are Located in a Lateral Zone of the Molecule (L-Shaped). Front. Immunol. 2019, 10, 1031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slavik, L.; Janek, D.; Ulehlova, J.; Krcova, V.; Hlusi, A. Detection of Anti-Domain I β-2 Glycoprotein I Antibodies as New Potential Target in Antiphospholipid Syndrome Diagnosis. J. Hematol. Thrombo. Dis. 2017, 5, 276. [Google Scholar]
- Pérez, D.; Tincani, A.; Serrano, M.; Shoenfeld, Y.; Serrano, A. Antiphospholipid syndrome and IgA anti-β2-glycoprotein I antibodies: When Cinderella becomes a princess. Lupus 2017, 27, 177–178. [Google Scholar] [CrossRef] [PubMed]
- Morales, J.M.; Serrano, M.; Martinez-Flores, J.A.; Gainza, F.J.; Marcen, R.; Arias, M.; Escuin, F.; Pérez, D.; Andres, A.; Martínez, M.A.; et al. Pretransplant IgA-Anti-Beta 2 Glycoprotein I Antibodies as a Predictor of Early Graft Thrombosis after Renal Transplantation in the Clinical Practice: A Multicenter and Prospective Study. Front. Immunol. 2018, 9, 468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pericleous, C.; Ferreira, I.; Borghi, O.; Pregnolato, F.; McDonnell, T.; Garza-Garcia, A.; Driscoll, P.; Pierangeli, S.; Isenberg, D.; Ioannou, Y.; et al. Measuring IgA Anti-β2-Glycoprotein I and IgG/IgA Anti-Domain I Antibodies Adds Value to Current Serological Assays for the Antiphospholipid Syndrome. PLoS ONE 2016, 11, e0156407. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-García, R.; Serrano, M.; Martínez-Flores, J.Á.; Mora, S.; Morillas, L.; Martín-Mola, M.Á.; Morales, J.M.; Paz-Artal, E.; Serrano, A. Isolated IgA Anti-β2 Glycoprotein I Antibodies in Patients with Clinical Criteria for Antiphospholipid Syndrome. J. Immunol. Res. 2014, 2014, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Vlagea, A.; Pascual-Salcedo, D.; Doforno, R.Á.; Lavilla, P.; Diez, J.; Merlano, B.P.; Cuesta, M.V.; Gil, A. IgA anti-β2 glycoprotein I antibodies: Experience from a large center. Thromb. Res. 2018, 162, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Chayoua, W.; Yin, D.-M.; Kelchtermans, H.; Moore, G.W.; Gris, J.-C.; Musiał, J.; Zuily, S.; Cate, H.T.; De Laat, B.; Devreese, K.M.J. Is There an Additional Value in Detecting Anticardiolipin and Anti-β2 glycoprotein I IgA Antibodies in the Antiphospholipid Syndrome? Thromb. Haemost. 2020, 120, 1557–1568. [Google Scholar] [CrossRef]
- Devreese, K.M.J. Testing for antiphospholipid antibodies: Advances and best practices. Int. J. Lab. Hematol. 2020, 42, 49–58. [Google Scholar] [CrossRef]
- Bizzaro, N.; Ghirardello, A.; Zampieri, S.; Iaccarino, L.; Tozzoli, R.; Ruffatti, A.; Villalta, D.; Tonutti, E.; Doria, A. Anti-prothrombin antibodies predict thrombosis in patients with systemic lupus erythematosus: A 15-year longitudinal study. J. Thromb. Haemost. 2007, 5, 1158–1164. [Google Scholar] [CrossRef]
- Sciascia, S.; Sanna, G.; Murru, V.; Roccatello, D.; Khamashta, M.A.; Bertolaccini, M.L. Anti-prothrombin (aPT) and anti-phosphatidylserine/prothrombin (aPS/PT) antibodies and the risk of thrombosis in the antiphospholipid syndrome. Thromb. Haemost. 2014, 111, 354–364. [Google Scholar] [CrossRef] [PubMed]
- Cifu’, A.; Domenis, R.; Pistis, C.; Curcio, F.; Fabris, M. Anti-β2-glycoprotein I and anti-phosphatidylserine/prothrombin antibodies exert similar pro-thrombotic effects in peripheral blood monocytes and endothelial cells. Autoimmun. Highlights 2019, 10, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Sciascia, S.; Radin, M.; Sanna, G.; Cecchi, I.; Roccatello, D.; Bertolaccini, M.L. Clinical utility of the global anti-phospholipid syndrome score for risk stratification: A pooled analysis. Rheumatology 2018, 57, 661–665. [Google Scholar] [CrossRef] [Green Version]
- Conti, F.; Capozzi, A.; Truglia, S.; Lococo, E.; Longo, A.; Misasi, R.; Alessandri, C.; Valesini, G.; Sorice, M. The mosaic of “seronegative” antiphospholipid syndrome. J. Immunol. Res. 2014, 2014, 389601. [Google Scholar] [CrossRef] [Green Version]
- Ho, W.K.; Rigano, J. Prevalence of autoantibodies directed against prothrombin in unprovoked venous thromboembo-lism. J. Thromb. Thrombolysis 2020, 49, 446–450. [Google Scholar] [CrossRef]
- Shi, H.; Zheng, H.; Yin, Y.; Hu, Q.; Teng, J.; Sun, Y.; Liu, H.-L.; Cheng, X.; Ye, J.; Su, Y.; et al. Antiphosphatidylserine/prothrombin antibodies (aPS/PT) as potential diagnostic markers and risk predictors of venous thrombosis and obstetric complications in antiphospholipid syndrome. Clin. Chem. Lab. Med. 2017, 56, 614–624. [Google Scholar] [CrossRef] [Green Version]
- Rand, J.H. Antiphospholipid Antibody-mediated Disruption of the Annexin-V Antithrombotic Shield: A Thrombogenic Mechanism for the Antiphospholipid Syndrome. J. Autoimmun. 2000, 15, 107–111. [Google Scholar] [CrossRef]
- Bertolaccini, M.L.; Amengual, O.; Atsumi, T.; Binder, W.L.; de Laat, B.; Forastiero, R.; Kutteh, W.H.; Lambert, M.; Matsubayashi, H.; Murthy, V.; et al. ‘Non-criteria’ aPL tests: Report of a task force and preconference workshop at the 13th International Congress on Antiphospholipid Antibodies, Galveston, TX, USA, April 2010. Lupus 2011, 20, 191–205. [Google Scholar] [CrossRef] [PubMed]
- Cañas, F.; Simonin, L.; Couturaud, F.; Renaudineau, Y. Annexin A2 autoantibodies in thrombosis and autoimmune diseases. Thromb. Res. 2015, 135, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Ortona, E.; Capozzi, A.; Colasanti, T.; Conti, F.; Alessandri, C.; Longo, A.; Garofalo, T.; Margutti, P.; Misasi, R.; Khamashta, M.A.; et al. Vimentin/cardiolipin complex as a new antigenic target of the antiphospholipid syndrome. Blood 2010, 116, 2960–2967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arachchillage, D.R.J.; Efthymiou, M.; Mackie, I.J.; Lawrie, A.S.; Machin, S.J.; Cohen, H. Anti-protein C antibodies are associated with resistance to endogenous protein C activation and a severe thrombotic phenotype in antiphospholipid syndrome. J. Thromb. Haemost. 2014, 12, 1801–1809. [Google Scholar] [CrossRef] [PubMed]
- Matyas, G.R.; Alving, C.R. Antigen-specific enhancement of natural human IgG antibodies to phosphatidylcholine, phosphatidylglycerol, phosphatidylinositol-4-phosphate, cholesterol, and lipid A by a liposomal vaccine containing lipid A. Vaccine 2011, 29, 5137–5144. [Google Scholar] [CrossRef] [PubMed]
- Pignatelli, P.; Ettorre, E.; Menichelli, D.; Pani, A.; Violi, F.; Pastori, D. Seronegative antiphospholipid syndrome: Refining the value of “non-criteria” antibodies for di-agnosis and clinical management. Haematologica 2020, 105, 562–572. [Google Scholar] [CrossRef] [Green Version]
- Korematsu, S.; Yamada, H.; Miyahara, H.; Ihara, K. Increased levels of anti-phosphatidylcholine and anti-phosphatidylethanolamine antibodies in pediatric patients with cerebral infarction. Brain Dev. 2017, 39, 542–546. [Google Scholar] [CrossRef] [PubMed]
- Alessandri, C.; Bombardieri, M.; Di Prospero, L.; Conigliaro, P.; Conti, F.; Labbadia, G.; Misasi, R.; Sorice, M.; Valesini, G. Anti-lysobisphosphatidic acid antibodies in patients with antiphospholipid syndrome and systemic lupus erythematosus. Clin. Exp. Immunol. 2005, 140, 173–180. [Google Scholar] [CrossRef] [Green Version]
- Castanon, A.; Pierre, G.; Willis, R.; Harris, E.N.; Papalardo, E.; Romay-Penabad, Z.; Schleh, A.; Jajoria, P.; Smikle, M.; DeCeulaer, K.; et al. Performance Evaluation and Clinical Associations of Immunoassays That Detect Antibodies to Nega-tively Charged Phospholipids Other Than Cardiolipin. Am. J. Clin. Pathol. 2018, 149, 401–411. [Google Scholar] [CrossRef] [Green Version]
- Park, H.S.; Gu, J.Y.; Jung, H.S.; Kim, H.K. Thrombotic Risk of Non-Criteria Anti-Phospholipid Antibodies Measured by Line Immunoassay: Superi-ority of Anti-Phosphatidylserine and Anti-Phosphatidic Acid Antibodies. Clin. Lab. 2019, 65, 171207. [Google Scholar] [CrossRef] [PubMed]
- Pengo, V.; Tripodi, A.; Reber, G.; Rand, J.H.; Ortel, T.L.; Galli, M.; De Groot, P.G. Update of the guidelines for lupus anticoagulant detection. Subcommittee on Lupus Anticoagu-lant/Antiphospholipid Antibody of the Scientific and Standardisation Committee of the International Society on Throm-bosis and Haemostasis. J. Thromb. Haemost 2009, 7, 1737–1740. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, T.C.R.; Willis, R.; Pericleous, C.; Ripoll, V.M.; Giles, I.P.; Isenberg, D.A.; Brasier, A.R.; Gonzalez, E.B.; Papalardo, E.; Romay-Penabad, Z.; et al. PEGylated Domain I of Beta-2-Glycoprotein I Inhibits the Binding, Coagulopathic, and Thrombo-genic Properties of IgG from Patients with the Antiphospholipid Syndrome. Front. Immunol. 2018, 9, 2413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linnemann, B. Antiphospholipid syndrome—An update. Vasa 2018, 47, 451–464. [Google Scholar] [CrossRef] [PubMed]
- Devreese, K.M.J. How to Interpret Antiphospholipid Laboratory Tests. Curr. Rheumatol. Rep. 2020, 22, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Tripodi, A.; Chantarangkul, V.; Cini, M.; Devreese, K.; Dlott, J.S.; Giacomello, R.; Gray, E.; Legnani, C.; Martinuzzo, M.E.; Pradella, P.; et al. Variability of cut-off values for the detection of lupus anticoagulants: Results of an international multi-center multiplatform study. J. Thromb. Haemost. 2017, 15, 1180–1190. [Google Scholar] [CrossRef]
- Moore, G.W. Current Controversies in Lupus Anticoagulant Detection. Antibodies 2016, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- Cohen, H.; Mackie, I.J.; Devreese, K.M.J. Clinical and laboratory practice for lupus anticoagulant testing: An Interna-tional Society of Thrombosis and Haemostasis Scientific and Standardization Committee survey. J. Thromb. Haemost. 2019, 17, 1715–1732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pradella, P.; Azzarini, G.; Santarossa, L.; Caberlotto, L.; Bardin, C.; Poz, A.; D’Aurizio, F.; Giacomello, R. Cooperation experience in a multicentre study to define the upper limits in a normal population for the diagnostic assessment of the functional lupus anticoagulant assays. Clin. Chem. Lab. Med. 2013, 51, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Aydin, S. A short history, principles, and types of ELISA, and our laboratory experience with peptide/protein analyses using ELISA. Peptides 2015, 72, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Kohl, T.O.; Ascoli, C.A. Immunometric Double-Antibody Sandwich Enzyme-Linked Immunosorbent Assay. Cold Spring Harb. Protoc. 2017, 2017, 093724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kohl, T.O.; Ascoli, C.A. Indirect Immunometric ELISA. Cold Spring Harb. Protoc. 2017, 2017, 93708. [Google Scholar] [CrossRef] [Green Version]
- Tebo, A.E.; Jaskowski, T.D.; Phansalkar, A.R.; Litwin, C.M.; Branch, D.W.; Hill, H.R. Diagnostic Performance of Phospholipid-Specific Assays for the Evaluation of Antiphospholipid Syndrome. Am. J. Clin. Pathol. 2008, 129, 870–875. [Google Scholar] [CrossRef]
- Vanoverschelde, L.; Kelchtermans, H.; Musial, J.; de Laat, B.; Devreese, K.M.J. Influence of anticardiolipin and anti-β2 glycoprotein I antibody cutoff values on antiphospholip-id syndrome classification. Res. Pract. Thromb. Haemost. 2019, 3, 515–527. [Google Scholar] [CrossRef] [PubMed]
- Bor, M.V.; Jacobsen, I.S.; Gram, J.B.; Sidelmann, J.J. Revisiting the Phadia/EliA cut-off values for anticardiolipin and anti-β2-glycoprotein I antibodies: A sys-tematic evaluation according to the guidelines. Lupus 2018, 27, 1446–1454. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Hou, X.; Zhang, H.; Wang, T.; Cui, L. The Clinical Performance of a New Chemiluminescent Immunoassay in Measuring Anti-β2 Glycoprotein 1 and Anti-Cardiolipin Antibodies. Med. Sci. Monit. 2018, 24, 6816–6822. [Google Scholar] [CrossRef] [PubMed]
- Yin, D.; De Laat, B.; Devreese, K.M.; Kelchtermans, H. The clinical value of assays detecting antibodies against domain I of β2-glycoprotein I in the antiphospholipid syndrome. Autoimmun. Rev. 2018, 17, 1210–1218. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Oku, K.; Amengual, O.; Ohmura, K.; Fujieda, Y.; Kato, M.; Bohgaki, T.; Yasuda, S.; Atsumi, T. First-Line, Non-Criterial Antiphospholipid Antibody Testing for the Diagnosis of Antiphospholipid Syndrome in Clinical Practice: A Combination of Anti-β(2)-Glycoprotein I Domain I and An-ti-Phosphatidylserine/Prothrombin Complex Antibodies Tests. Arthritis Care Res. 2018, 70, 627–634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chayoua, W.; Kelchtermans, H.; Moore, G.W.; Gris, J.-C.; Musial, J.; Wahl, D.; Zuily, S.; Gianniello, F.; Fontana, P.; Remijn, J.; et al. Detection of Anti-Cardiolipin and Anti-β2glycoprotein I Antibodies Differs between Platforms without Influence on Association with Clinical Symptoms. Thromb. Haemost. 2019, 119, 797–806. [Google Scholar] [CrossRef] [PubMed]
- Salma, N.; Julie, L.; Boutahar, B.; Sylvie, L.N.; Eleonore, B.; Fabien, L.N.; Elisabeth, P.; Sandrine, J.J.; Francis, C.; Sophie, H.; et al. Thrombotic risk assessment and analytical performance of the chemiluminescent analyzer IDS-iSYS for the detection of anti-cardiolipin and anti-β2 glycoprotein I autoantibodies. Clin. Immunol. 2018, 194, 92–99. [Google Scholar] [CrossRef]
- Chayoua, W.; Kelchtermans, H.; Gris, J.C.; Moore, G.W.; Musiał, J.; Wahl, D.; de Groot, P.G.; de Laat, B.; Devreese, K.M.J. The (non-)sense of detecting anti-cardiolipin and anti-β2glycoprotein I IgM antibodies in the antiphos-pholipid syndrome. J. Thromb. Haemost. 2020, 18, 169–179. [Google Scholar] [CrossRef] [Green Version]
- Grossi, V.; Infantino, M.; Benucci, M.; Gobbi, F.L.; Bandinelli, F.; Damiani, A.; Bodio, C.; Borghi, M.O.; Mahler, M.; Aure, M.A.; et al. Two Novel Technologies for the Detection of Anti-cardiolipin and Anti β2–Glycoprotein Antibodies in the Real Life: Chemiluminescent in Comparison to the Addressable Laser Bead Immunoassays. Immunol. Investig. 2019, 49, 58–68. [Google Scholar] [CrossRef]
- Bevers, E.M.; Zwaal, R.F.; Willems, G.M. The effect of phospholipids on the formation of immune complexes between autoantibodies and β2-glycoprotein I or prothrombin. Clin. Immunol. 2004, 112, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Egerer, K.; Roggenbuck, D.; Buettner, T.; Lehmann, B.; Kohn, A.; Von Landenberg, P.; Hiemann, R.; Feist, E.; Burmester, G.-R.; Dorner, T. Single-step autoantibody profiling in antiphospholipid syndrome using a multi-line dot assay. Arthritis Res. Ther. 2011, 13, R118. [Google Scholar] [CrossRef] [Green Version]
- Thaler, M.A.; Bietenbeck, A.; Steigerwald, U.; Büttner, T.; Schierack, P.; Lindhoff-Last, E.; Roggenbuck, D.; Luppa, P.B. Evaluation of the sensitivity and specificity of a novel line immunoassay for the detection of criteria and non-criteria antiphospholipid antibodies in comparison to established ELISAs. PLoS ONE 2019, 14, e0220033. [Google Scholar] [CrossRef] [Green Version]
- Roggenbuck, D.; Borghi, M.O.; Somma, V.; Büttner, T.; Schierack, P.; Hanack, K.; Grossi, C.; Bodio, C.; Macor, P.; von Landenberg, P.; et al. Antiphospholipid antibodies detected by line immunoassay differentiate among patients with an-tiphospholipid syndrome, with infections and asymptomatic carriers. Arthritis Res. Ther. 2016, 18, 111. [Google Scholar] [CrossRef] [Green Version]
- Nalli, C.; Somma, V.; Andreoli, L.; Büttner, T.; Schierack, P.; Mahler, M.; Roggenbuck, D.; Tincani, A. Anti-phospholipid IgG antibodies detected by line immunoassay differentiate patients with an-ti-phospholipid syndrome and other autoimmune diseases. Auto Immun. Highlights 2018, 9, 6. [Google Scholar] [CrossRef] [Green Version]
- Conti, F.; Alessandri, C.; Sorice, M.; Capozzi, A.; Longo, A.; Garofalo, T.; Misasi, R.; Bompane, D.; Hughes, G.R.V.; Khamashta, M.A.; et al. Thin-layer chromatography immunostaining in detecting anti-phospholipid antibodies in seronegative anti-phospholipid syndrome. Clin. Exp. Immunol. 2011, 167, 429–437. [Google Scholar] [CrossRef]
- Conti, F.; Alessandri, C.; Spinelli, F.; Capozzi, A.; Martinelli, F.; Recalchi, S.; Misasi, R.; Valesini, G.; Sorice, M. TLC immunostaining for detection of “antiphospholipid” antibodies. Methods Mol. Biol. 2014, 1134, 95–101. [Google Scholar] [PubMed]
- Sorice, M.; Griggi, T.; Circella, A.; Garofalo, T.; D’Agostino, F.; Pittoni, V.; Pontieri, G.; Lenti, L.; Valesini, G. Detection of antiphospholipid antibodies by immunostaining on thin layer chromatography plates. J. Immunol. Methods 1994, 173, 49–54. [Google Scholar] [CrossRef]
- Albay, A.; Esen, B.A.; Pericleous, C.; Wincup, C.; Giles, I.; Rahman, A.; McDonnell, T. Domain I of β2GPI is capable of blocking serum IgA antiphospholipid antibodies binding in vitro: An effect enhanced by PEGylation. Lupus 2019, 28, 893–897. [Google Scholar] [CrossRef] [PubMed]
- Cervera, R.; Conti, F.; Doria, A.; Iaccarino, L.; Valesini, G. Does seronegative antiphospholipid syndrome really exist? Autoimmun. Rev. 2012, 11, 581–584. [Google Scholar] [CrossRef] [PubMed]
- Conti, F.; Andreoli, L.; Crisafulli, F.; Mancuso, S.; Truglia, S.; Tektonidou, M.G. Does seronegative obstetric APS exist? “pro” and “cons”. Autoimmun. Rev. 2019, 18, 102407. [Google Scholar] [CrossRef]
- Truglia, S.; Capozzi, A.; Mancuso, S.; Recalchi, S.; Spinelli, F.R.; Perricone, C.; De Carolis, C.; Manganelli, V.; Riitano, G.; Garofalo, T.; et al. A Monocentric Cohort of Obstetric Seronegative Anti-Phospholipid Syndrome. Front. Immunol. 2018, 9, 1678. [Google Scholar] [CrossRef] [PubMed]
- Salle, V. Seronegative antiphospholipid syndrome: Myth or reality? Rev. Med. Interne. 2020, 41, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Fabris, M.; Giacomello, R.; Poz, A.; Pantarotto, L.; Tanzi, N.; Curcio, F.; Tonutti, E. The introduction of anti-phosphatidylserine/prothrombin autoantibodies in the laboratory diagnostic process of anti-phospholipid antibody syndrome: 6 months of observation. Autoimmun. Highlights 2014, 5, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Meroni, P.L.; Chighizola, C.B.; Rovelli, F.; Gerosa, M. Antiphospholipid syndrome in 2014: More clinical manifestations, novel pathogenic players and emerging biomarkers. Arthritis Res. Ther. 2014, 16, 209. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Methods | Assay | Determination |
|---|---|---|
| Dilute Russell’s viper venom time (DRVVT) Activated partial thromboplastin time (aPTT) | Liquid-phase | Quantitative |
| Enzyme-linked immunosorbent assay (ELISA) | Solid-phase | Quantitative |
| Fluorescence enzyme immunoassay (EliA) | Quantitative | |
| Chemiluminescence immunoassay (CLIA) | Quantitative | |
| Multiplex flow fluorescence immunoassay (MFFIA) | Quantitative | |
| Multiline dot assay (MLDA) | Semi-quantitative | |
| Line immunoassay (LIA) | Qualitative | |
| Thin-layer chromatography (TLC) | Qualitative |
| Biomarkers | Methods | Reference |
|---|---|---|
| Lupus anticoagulant (LA) | DRVVT aPTT | Liu [24], Choi [25], Pengo [66], Linnemann [68] |
| Anti-β2-glycoprotein-I (anti-β2GPI) IgG, IgM | ELISA EliA CLIA MFFIA MLDA LIA | Miykis [16], Liu [24], Misasi [27], Serrano [39] Vanouverchelde [78], Bor [79], Chayoua [83] Janek [33], Chayoua [83], Salma [84] Chayoua [83], Chayoua [85], Grossi [86] Misasi [27], Bevers [87], Egerer [88] Park [65], Egerer [88], Thaler [89], Roggenbuck [90], Nalli [91] |
| Anti-cardiolipin (aCL) IgG, IgM | ELISA EliA CLIA MFFIA MLDA LIA | Miykis [16], Liu [24] Vanouverchelde [78], Bor [79], Chayoua [83] Janek [33], Chayoua [83], Salma [84] Chayoua [83], Chayoua [85], Grossi [86] [Misasi [27], Bevers [87], Egerer [88] Park [65], Egerer [88], Thaler [89], Roggenbuck [90], Nalli [91] |
| Anti-β2-glycoprotein-I domain I (anti-DI) | ELISA CLIA | Serrano [39] Slavik [40] |
| Anti-β2-glycoprotein-I IgA | ELISA EliA CLIA MFFIA | Ruiz-Garcia [44], Vlagea [45] Chayoua [46] Chayoua [46] Chayoua [46] |
| Anti-cardiolipin IgA | CLIA | Liu [24] |
| Anti-prothrombin (anti-PT) Anti-phosphatidylserine/prothrombin (anti-PS/PT) | ELISA | Liu [24], Shi [54] |
| Anti-annexin V Anti-annexin II | ELISA | Canas [57] |
| Anti-cardiolipin/vimentin (aCL/Vim) | ELISA | Ortona [58] |
| Anti-protein S/protein C (anti-PS/PC) | LIA | Arachchillage [59] |
| Anti-phosphatidic acid (anti-PA) Anti-phosphatidylserine (anti-PS) Anti-phosphatidyletanolamine (anti-PE) Anti-phosphatidylinositol (anti-PI) Anti-phosphatidylcholine (aPC) Anti-phosphatidylglycerol (aPG) Anti-lyso-bis-phosphatidic acid (anti-LBPA) Anti-mixture of phospholipids (APhL) | ELISA LIA | Castanon [64] Park [65] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bradacova, P.; Slavik, L.; Ulehlova, J.; Skoumalova, A.; Ullrychova, J.; Prochazkova, J.; Hlusi, A.; Manukyan, G.; Kriegova, E. Current Promising Biomarkers and Methods in the Diagnostics of Antiphospholipid Syndrome: A Review. Biomedicines 2021, 9, 166. https://doi.org/10.3390/biomedicines9020166
Bradacova P, Slavik L, Ulehlova J, Skoumalova A, Ullrychova J, Prochazkova J, Hlusi A, Manukyan G, Kriegova E. Current Promising Biomarkers and Methods in the Diagnostics of Antiphospholipid Syndrome: A Review. Biomedicines. 2021; 9(2):166. https://doi.org/10.3390/biomedicines9020166
Chicago/Turabian StyleBradacova, Pavla, Ludek Slavik, Jana Ulehlova, Adela Skoumalova, Jana Ullrychova, Jana Prochazkova, Antonin Hlusi, Gayane Manukyan, and Eva Kriegova. 2021. "Current Promising Biomarkers and Methods in the Diagnostics of Antiphospholipid Syndrome: A Review" Biomedicines 9, no. 2: 166. https://doi.org/10.3390/biomedicines9020166
APA StyleBradacova, P., Slavik, L., Ulehlova, J., Skoumalova, A., Ullrychova, J., Prochazkova, J., Hlusi, A., Manukyan, G., & Kriegova, E. (2021). Current Promising Biomarkers and Methods in the Diagnostics of Antiphospholipid Syndrome: A Review. Biomedicines, 9(2), 166. https://doi.org/10.3390/biomedicines9020166






