Lower Functional and Proportional Characteristics of Cord Blood Treg of Male Newborns Compared with Female Newborns
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects and Sample Collection
2.2. Flow Cytometry
2.3. Magnetic Bead-Based Cell Isolation
2.4. Genomic DNA and Total RNA Isolation from Treg
2.5. Real-Time qPCR Gene Expression Analysis
2.6. Bisulphite Conversion of Treg DNA
2.7. High-Resolution Melting Analysis of TSDR Demethylation
2.8. Cell Culture
2.9. ELISA
2.10. Statistics
3. Results
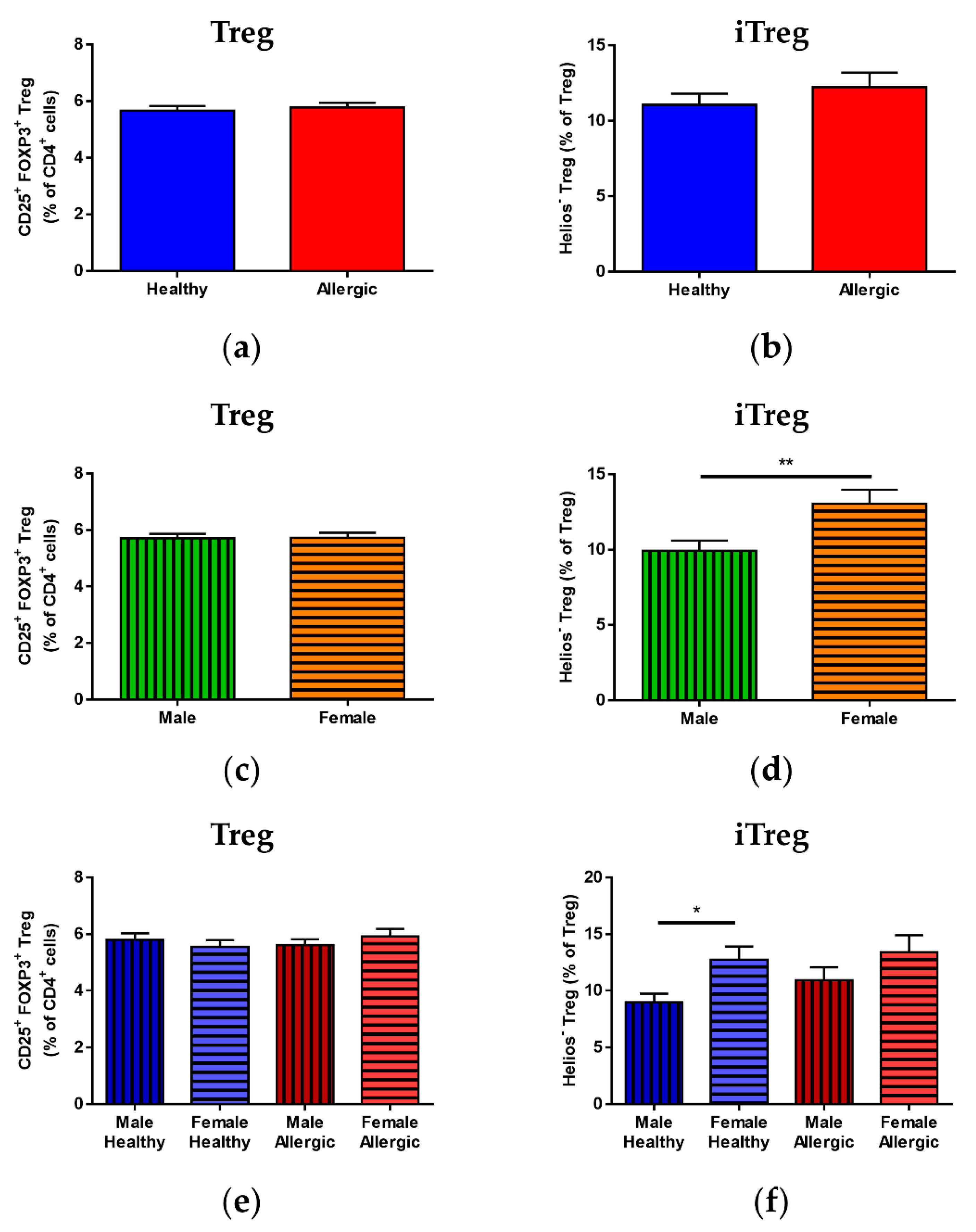
3.1. Treg Population Ratios in Cord Blood
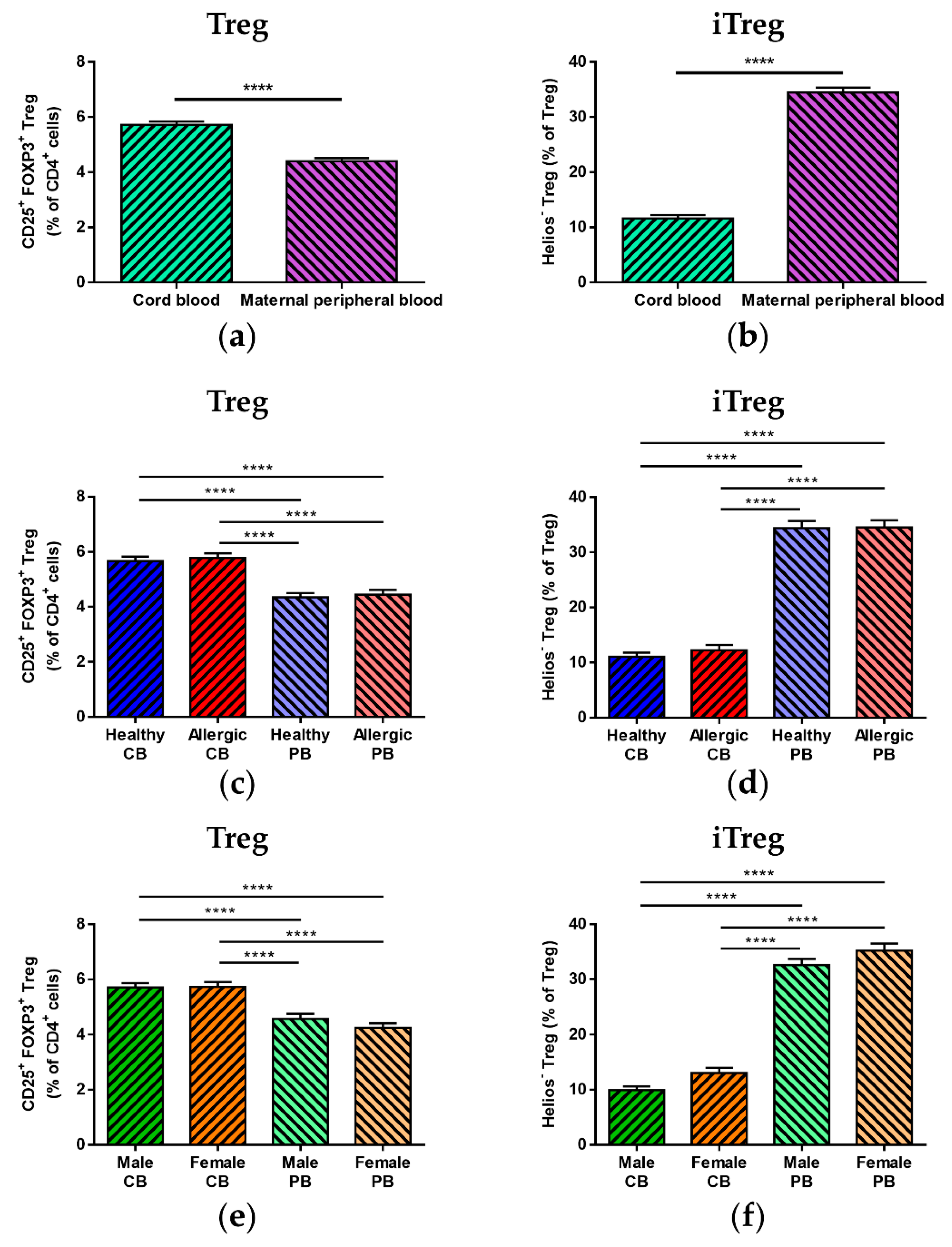
3.2. Treg Population Ratio Comparison between Cord Blood and Maternal Peripheral Blood
3.3. Median of Fluorescence Intensity of FOXP3 and Helios
3.4. Correlation of Immune Parameters
3.5. Correlation of Immune Parameters in Cord Blood
3.6. Correlation of Immune Parameters between Cord Blood and Maternal Blood
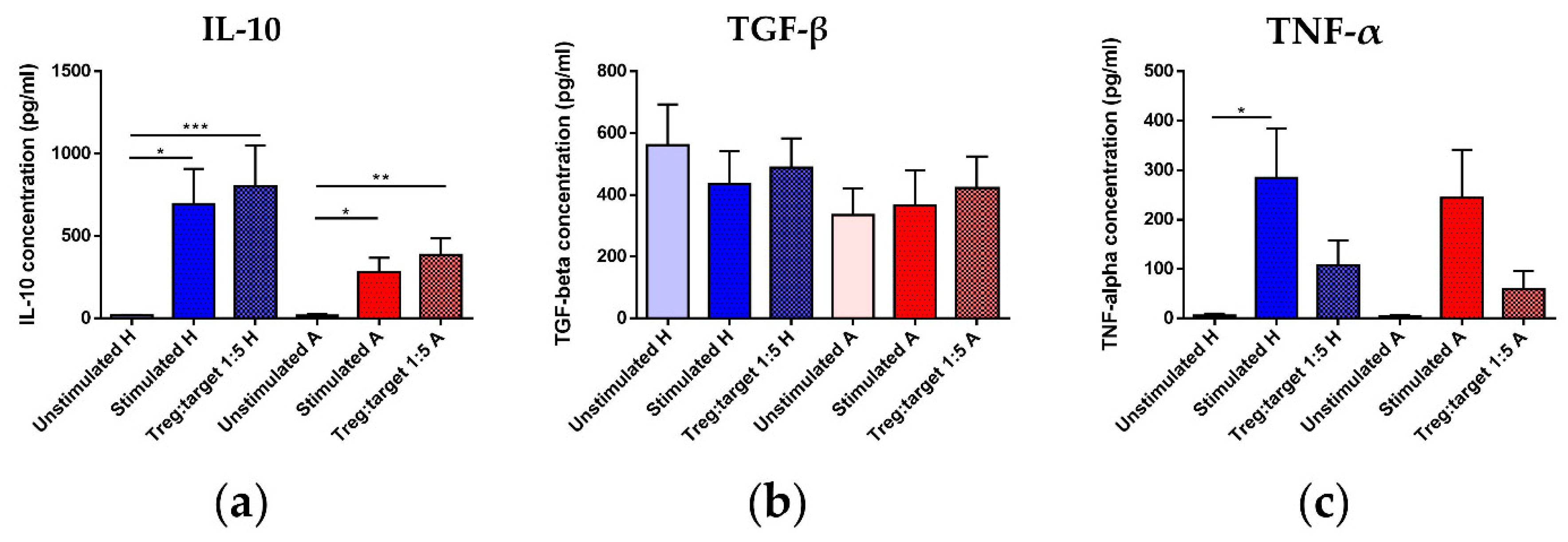
3.7. Treg Functional Parameters: Coculture Assay and Gene Expression Analysis
3.8. Epigenetic Analysis of TSDR Demethylation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sykes, L.; MacIntyre, D.A.; Yap, X.J.; Teoh, T.G.; Bennett, P.R. The Th1:th2 dichotomy of pregnancy and preterm labour. Mediat. Inflamm. 2012, 2012, 967629. [Google Scholar] [CrossRef] [PubMed]
- McCoy, K.D.; Köller, Y. New developments providing mechanistic insight into the impact of the microbiota on allergic disease. Clin. Immunol. 2015, 159, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Jutel, M.; Akdis, C.A. T-cell Subset Regulation in Atopy. Curr. Allergy Asthma Rep. 2011, 11, 139–145. [Google Scholar] [CrossRef]
- Bacchetta, R.; Passerini, L.; Gambineri, E.; Dai, M.; Allan, S.E.; Perroni, L.; Dagna-Bricarelli, F.; Sartirana, C.; Matthes-Martin, S.; Lawitschka, A.; et al. Defective regulatory and effector T cell functions in patients with FOXP3 mutations. J. Clin. Investig. 2006, 116, 1713–1722. [Google Scholar] [CrossRef]
- Alroqi, F.J.; Chatila, T.A. T Regulatory Cell Biology in Health and Disease. Curr. Allergy Asthma Rep. 2016, 16. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Truong, N.; Grossman, W.J.; Haribhai, D.; Williams, C.B.; Wang, J.; Martín, M.G.; Chatila, T.A. Allergic dysregulation and hyperimmunoglobulinemia E in Foxp3 mutant mice. J. Allergy Clin. Immunol. 2005, 116, 1106–1115. [Google Scholar] [CrossRef]
- Steinborn, A.; Engst, M.; Haensch, G.M.; Mahnke, K.; Schmitt, E.; Meuer, S.; Sohn, C. Small for gestational age (SGA) neonates show reduced suppressive activity of their regulatory T cells. Clin. Immunol. 2010, 134, 188–197. [Google Scholar] [CrossRef]
- Shevach, E.M. Mechanisms of Foxp3+ T Regulatory Cell-Mediated Suppression. Immunity 2009, 30, 636–645. [Google Scholar] [CrossRef]
- Ohkura, N.; Hamaguchi, M.; Morikawa, H.; Sugimura, K.; Tanaka, A.; Ito, Y.; Osaki, M.; Tanaka, Y.; Yamashita, R.; Nakano, N.; et al. T Cell Receptor Stimulation-Induced Epigenetic Changes and Foxp3 Expression Are Independent and Complementary Events Required for Treg Cell Development. Immunity 2012, 37, 785–799. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, H.; Sakaguchi, S. Genetic and epigenetic basis of Treg cell development and function: From a FoxP3-centered view to an epigenome-defined view of natural Treg cells. Immunol. Rev. 2014, 259, 192–205. [Google Scholar] [CrossRef]
- Baron, U.; Floess, S.; Wieczorek, G.; Baumann, K.; Grützkau, A.; Dong, J.; Thiel, A.; Boeld, T.J.; Hoffmann, P.; Edinger, M.; et al. DNA demethylation in the human FOXP3 locus discriminates regulatory T cells from activated FOXP3+ conventional T cells. Eur. J. Immunol. 2007, 37, 2378–2389. [Google Scholar] [CrossRef] [PubMed]
- Barzaghi, F.; Passerini, L.; Gambineri, E.; Ciullini Mannurita, S.; Cornu, T.; Kang, E.S.; Choe, Y.H.; Cancrini, C.; Corrente, S.; Ciccocioppo, R.; et al. Demethylation analysis of the FOXP3 locus shows quantitative defects of regulatory T cells in IPEX-like syndrome. J. Autoimmun. 2012, 38, 49–58. [Google Scholar] [CrossRef]
- Ohkura, N.; Yasumizu, Y.; Kitagawa, Y.; Tanaka, A.; Nakamura, Y.; Motooka, D.; Nakamura, S.; Okada, Y.; Sakaguchi, S. Regulatory T Cell-Specific Epigenomic Region Variants Are a Key Determinant of Susceptibility to Common Autoimmune Diseases. Immunity 2020, 52, 1119–1132.e4. [Google Scholar] [CrossRef]
- Swamy, R.S.; Reshamwala, N.; Hunter, T.; Vissamsetti, S.; Santos, C.B.; Baroody, F.M.; Hwang, P.H.; Hoyte, E.G.; Garcia, M.A.; Nadeau, K.C. Epigenetic modifications and improved regulatory T-cell function in subjects undergoing dual sublingual immunotherapy. J. Allergy Clin. Immunol. 2012, 130, 215–224.e7. [Google Scholar] [CrossRef] [PubMed]
- Syed, A.; Garcia, M.A.; Lyu, S.-C.; Bucayu, R.; Kohli, A.; Ishida, S.; Berglund, J.P.; Tsai, M.; Maecker, H.; O’Riordan, G.; et al. Peanut oral immunotherapy results in increased antigen-induced regulatory T-cell function and hypomethylation of forkhead box protein 3 (FOXP3). J. Allergy Clin. Immunol. 2014, 133, 500–510. [Google Scholar] [CrossRef]
- Paparo, L.; Nocerino, R.; Cosenza, L.; Aitoro, R.; D’Argenio, V.; Del Monaco, V.; Di Scala, C.; Amoroso, A.; Di Costanzo, M.; Salvatore, F.; et al. Epigenetic features of FoxP3 in children with cow’s milk allergy. Clin. Epigenetics 2016, 8. [Google Scholar] [CrossRef]
- Shevach, E.M.; Thornton, A.M. tTregs, pTregs, and iTregs: Similarities and differences. Immunol. Rev. 2014, 259, 88–102. [Google Scholar] [CrossRef]
- Chen, W.; Jin, W.; Hardegen, N.; Lei, K.-J.; Li, L.; Marinos, N.; McGrady, G.; Wahl, S.M. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J. Exp. Med. 2003, 198, 1875–1886. [Google Scholar] [CrossRef] [PubMed]
- Haribhai, D.; Williams, J.B.; Jia, S.; Nickerson, D.; Schmitt, E.G.; Edwards, B.; Ziegelbauer, J.; Yassai, M.; Li, S.-H.; Relland, L.M.; et al. A Requisite Role for Induced Regulatory T Cells in Tolerance Based on Expanding Antigen Receptor Diversity. Immunity 2011, 35, 109–122. [Google Scholar] [CrossRef]
- Thornton, A.M.; Korty, P.E.; Tran, D.Q.; Wohlfert, E.A.; Murray, P.E.; Belkaid, Y.; Shevach, E.M. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J. Immunol. 2010, 184, 3433–3441. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, S.; Vignali, D.A.A.; Rudensky, A.Y.; Niec, R.E.; Waldmann, H. The plasticity and stability of regulatory T cells. Nat. Rev. Immunol. 2013, 13, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Diller, M.L.; Kudchadkar, R.R.; Delman, K.A.; Lawson, D.H.; Ford, M.L. Balancing Inflammation: The Link between Th17 and Regulatory T Cells. Mediat. Inflamm. 2016, 2016, 6309219. [Google Scholar] [CrossRef]
- Lal, G.; Bromberg, J.S. Epigenetic mechanisms of regulation of Foxp3 expression. Blood 2009, 114, 3727–3735. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Chen, M.; Liu, Y.; Guo, Z.; He, X.; Brand, D.; Zheng, S.G. Advances in distinguishing natural from induced Foxp3(+) regulatory T cells. Int. J. Clin. Exp. Pathol. 2013, 6, 116–123. [Google Scholar] [PubMed]
- Chen, Q.; Kim, Y.C.; Laurence, A.; Punkosdy, G.A.; Shevach, E.M. IL-2 controls the stability of Foxp3 expression in TGF-beta-induced Foxp3+ T cells in vivo. J. Immunol. 2011, 186, 6329–6337. [Google Scholar] [CrossRef]
- Yue, X.; Trifari, S.; Äijö, T.; Tsagaratou, A.; Pastor, W.A.; Zepeda-Martínez, J.A.; Lio, C.-W.J.; Li, X.; Huang, Y.; Vijayanand, P.; et al. Control of Foxp3 stability through modulation of TET activity. J. Exp. Med. 2016, 213, 377–397. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Park, K.; Kim, J.; Min, H.; Seong, R.H. Foxp3 expression in induced regulatory T cells is stabilized by C/EBP in inflammatory environments. EMBO Rep. 2018, e45995. [Google Scholar] [CrossRef]
- Mikami, N.; Kawakami, R.; Chen, K.Y.; Sugimoto, A.; Ohkura, N.; Sakaguchi, S. Epigenetic conversion of conventional T cells into regulatory T cells by CD28 signal deprivation. Proc. Natl. Acad. Sci. USA 2020, 117, 12258–12268. [Google Scholar] [CrossRef]
- Prokešová, L.; Novotná, O.; Janatková, I.; Zanvít, P.; Žižka, J.; Lodinová-Žádníková, R.; Kocourková, I.; Šterzl, I. IgE against food and respiratory allergens in healthy and allergic mothers and their children. Folia Microbiol. (Praha) 2008, 53, 67–72. [Google Scholar] [CrossRef]
- Peters, J.L.; Cohen, S.; Staudenmayer, J.; Hosen, J.; Platts-Mills, T.A.E.; Wright, R.J. Prenatal negative life events increases cord blood IgE: Interactions with dust mite allergen and maternal atopy. Allergy 2012, 67, 545–551. [Google Scholar] [CrossRef]
- Hrdý, J.; Zanvít, P.; Novotná, O.; Kocourková, I.; Žižka, J.; Prokešová, L. Cytokine expression in cord blood cells of children of healthy and allergic mothers. Folia Microbiol. 2010, 55, 515–519. [Google Scholar] [CrossRef]
- Chung, E.K.; Miller, R.L.; Wilson, M.T.; McGeady, S.J.; Culhane, J.F. Antenatal risk factors, cytokines and the development of atopic disease in early childhood. Arch. Dis. Child. Fetal Neonatal Ed. 2007, 92, F68–F73. [Google Scholar] [CrossRef] [PubMed]
- Belderbos, M.E.; Knol, E.F.; Houben, M.L.; Bleek, G.M.; Wilbrink, B.; Kimpen, J.L.L.; Rovers, M.; Bont, L. Low neonatal Toll-like receptor 4-mediated interleukin-10 production is associated with subsequent atopic dermatitis. Clin. Exp. Allergy 2012, 42, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Hrdý, J.; Vlasáková, K.; Černý, V.; Súkeníková, L.; Novotná, O.; Petrásková, P.; Boráková, K.; Lodinová-Žádníková, R.; Kolářová, L.; Prokešová, L. Decreased allergy incidence in children supplemented with E. coli O83:K24:H31 and its possible modes of action. Eur. J. Immunol. 2018, 48, 2015–2030. [Google Scholar] [CrossRef]
- Bullens, D.M.A.; Kasran, A.; Dilissen, E.; Ceuppens, J.L. Neonatal IL-10 production and risk of allergy development. Clin. Exp. Allergy 2012, 42, 483–484. [Google Scholar] [CrossRef]
- Rindsjö, E.; Joerink, M.; Johansson, C.; Bremme, K.; Malmström, V.; Scheynius, A. Maternal allergic disease does not affect the phenotype of T and B cells or the immune response to allergens in neonates: No effect of maternal allergy on neonatal lymphocytes. Allergy 2009, 65, 822–830. [Google Scholar] [CrossRef]
- Schaub, B.; Liu, J.; Höppler, S.; Schleich, I.; Huehn, J.; Olek, S.; Wieczorek, G.; Illi, S.; von Mutius, E. Maternal farm exposure modulates neonatal immune mechanisms through regulatory T cells. J. Allergy Clin. Immunol. 2009, 123, 774–782.e5. [Google Scholar] [CrossRef] [PubMed]
- Prescott, S.L.; King, B.; Strong, T.L.; Holt, P.G. The value of perinatal immune responses in predicting allergic disease at 6 years of age. Allergy 2003, 58, 1187–1194. [Google Scholar] [CrossRef] [PubMed]
- Björkander, S.; Hallberg, J.; Persson, J.-O.; Lilja, G.; Nilsson, C.; Sverremark-Ekström, E. The allergic phenotype during the first 10 years of life in a prospective cohort. Immun. Inflamm. Dis. 2019, 7, 170–182. [Google Scholar] [CrossRef]
- Hrdý, J.; Novotná, O.; Kocourková, I.; Prokešová, L. Gene expression of subunits of the IL-12 family cytokines in moDCs derived in vitro from the cord blood of children of healthy and allergic mothers. Folia Biol. (Praha) 2014, 60, 74–82. [Google Scholar]
- Wieczorek, G.; Asemissen, A.; Model, F.; Turbachova, I.; Floess, S.; Liebenberg, V.; Baron, U.; Stauch, D.; Kotsch, K.; Pratschke, J.; et al. Quantitative DNA methylation analysis of FOXP3 as a new method for counting regulatory T cells in peripheral blood and solid tissue. Cancer Res. 2009, 69, 599–608. [Google Scholar] [CrossRef]
- Černý, V.; Hrdý, J.; Novotná, O.; Petrásková, P.; Boráková, K.; Kolářová, L.; Prokešová, L. Distinct characteristics of Tregs of newborns of healthy and allergic mothers. PLoS ONE 2018, 13, e0207998. [Google Scholar] [CrossRef]
- Lodinová-Žádníková, R.; Prokešová, L.; Kocourková, I.; Hrdý, J.; Žižka, J. Prevention of allergy in infants of allergic mothers by probiotic Escherichia coli. Int. Arch. Allergy Immunol. 2010, 153, 201–206. [Google Scholar] [CrossRef]
- Stein, M.M.; Hrusch, C.L.; Gozdz, J.; Igartua, C.; Pivniouk, V.; Murray, S.E.; Ledford, J.G.; Marques dos Santos, M.; Anderson, R.L.; Metwali, N.; et al. Innate Immunity and Asthma Risk in Amish and Hutterite Farm Children. N. Engl. J. Med. 2016, 375, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Hinz, D.; Bauer, M.; Röder, S.; Olek, S.; Huehn, J.; Sack, U.; Borte, M.; Simon, J.C.; Lehmann, I.; Herberth, G.; et al. Cord blood Tregs with stable FOXP3 expression are influenced by prenatal environment and associated with atopic dermatitis at the age of one year. Allergy 2012, 67, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.A.; Holloway, J.A.; Warner, J.O. Does atopic disease start in foetal life? Allergy 2000, 55, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Hrdý, J.; Kocourková, I.; Prokešová, L. Impaired function of regulatory T cells in cord blood of children of allergic mothers: Tregs in cord blood and allergy risk. Clin. Exp. Immunol. 2012, 170, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Bili, H.; Fleva, A.; Pados, G.; Argyriou, T.; Tsolakidis, D.; Pavlitou, A.; Tarlatzis, B.C. Regulatory Τ-cell Differentiation Between Maternal and Cord Blood Samples in Pregnancies with Spontaneous Vaginal Delivery and with Elective Cesarian Section: REGULATORY AND γ/δ T-CELLS IN NORMAL PREGNANCY. Am. J. Reprod. Immunol. 2011, 65, 173–179. [Google Scholar] [CrossRef]
- Yildiran, A.; Yurdakul, E.; Guloglu, D.; Dogu, F.; Arsan, S.; Arikan, M.; Cengiz, L.; Tezcan, S.; İkinciogullari, A. The Effect of Mode of Delivery on T Regulatory (Treg) Cells of Cord Blood. Indian J. Pediatr. 2011, 78, 1234–1238. [Google Scholar] [CrossRef]
- Słabuszewska-Jóźwiak, A.; Szymański, J.K.; Ciebiera, M.; Sarecka-Hujar, B.; Jakiel, G. Pediatrics Consequences of Caesarean Section-A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2020, 17, 31. [Google Scholar] [CrossRef]
- Kristensen, K.; Henriksen, L. Cesarean section and disease associated with immune function. J. Allergy Clin. Immunol. 2016, 137, 587–590. [Google Scholar] [CrossRef] [PubMed]
- Bager, P.; Wohlfahrt, J.; Westergaard, T. Caesarean delivery and risk of atopy and allergic disesase: Meta-analyses. Clin. Exp. Allergy 2008, 38, 634–642. [Google Scholar] [CrossRef] [PubMed]
- McLoughlin, R.M.; Calatroni, A.; Visness, C.M.; Wallace, P.K.; Cruikshank, W.W.; Tuzova, M.; Ly, N.P.; Ruiz-Perez, B.; Kattan, M.; Bloomberg, G.R.; et al. Longitudinal relationship of early life immunomodulatory T cell phenotype and function to development of allergic sensitization in an urban cohort. Clin. Exp. Allergy 2012, 42, 392–404. [Google Scholar] [CrossRef]
- Strömbeck, A.; Rabe, H.; Lundell, A.-C.; Andersson, K.; Johansen, S.; Adlerberth, I.; Wold, A.E.; Hesselmar, B.; Rudin, A. High proportions of FOXP3+ CD25high T cells in neonates are positively associated with allergic sensitization later in childhood. Clin. Exp. Allergy 2014, 44, 940–952. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.-S.; Gao, R.; Yan, B.; Ren, J.; Wu, F.; Chen, P.; Zhang, J.; Wang, L.-F.; Xiao, Y.-M.; Liu, J. Maternal allergic disease history affects childhood allergy development through impairment of neonatal regulatory T-cells. Respir. Res. 2016, 17, 114. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Lou, H.; Wang, C.; Lou, W.; Wang, Y.; Zheng, T.; Zhang, L. T cell subsets in cord blood are influenced by maternal allergy and associated with atopic dermatitis. Pediatric Allergy Immunol. 2013, 24, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Law, J.P.; Hirschkorn, D.F.; Owen, R.E.; Biswas, H.H.; Norris, P.J.; Lanteri, M.C. The importance of Foxp3 antibody and fixation/permeabilization buffer combinations in identifying CD4+CD25+Foxp3+ regulatory T cells. Cytom. A 2009, 75, 1040–1050. [Google Scholar] [CrossRef]
- Presicce, P.; Moreno-Fernandez, M.E.; Lages, C.S.; Orsborn, K.I.; Chougnet, C.A. Association of two clones allows for optimal detection of human FOXP3. Cytom. A 2010, 77, 571–579. [Google Scholar] [CrossRef]
- Lima, J.; Martins, C.; Nunes, G.; Sousa, M.-J.; Branco, J.C.; Borrego, L.-M. Regulatory T Cells Show Dynamic Behavior During Late Pregnancy, Delivery, and the Postpartum Period. Reprod. Sci. 2017, 24, 1025–1032. [Google Scholar] [CrossRef]
- Jutel, M.; Akdis, M.; Budak, F.; Aebischer-Casaulta, C.; Wrzyszcz, M.; Blaser, K.; Akdis, C.A. IL-10 and TGF-beta cooperate in the regulatory T cell response to mucosal allergens in normal immunity and specific immunotherapy. Eur. J. Immunol. 2003, 33, 1205–1214. [Google Scholar] [CrossRef]
- Fuseini, H.; Newcomb, D.C. Mechanisms Driving Gender Differences in Asthma. Curr. Allergy Asthma Rep. 2017, 17, 19. [Google Scholar] [CrossRef] [PubMed]
- Zein, J.G.; Erzurum, S.C. Asthma is Different in Women. Curr. Allergy Asthma Rep. 2015, 15, 28. [Google Scholar] [CrossRef] [PubMed]
- Vink, N.M.; Postma, D.S.; Schouten, J.P.; Rosmalen, J.G.M.; Boezen, H.M. Gender differences in asthma development and remission during transition through puberty: The TRacking Adolescents’ Individual Lives Survey (TRAILS) study. J. Allergy Clin. Immunol. 2010, 126, 498–504.e6. [Google Scholar] [CrossRef]
- Almqvist, C.; Worm, M.; Leynaert, B. Impact of gender on asthma in childhood and adolescence: A GA2 LEN review. Allergy 2007, 63, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Kurukulaaratchy, R.J.; Karmaus, W.; Raza, A.; Matthews, S.; Roberts, G.; Arshad, S.H. The influence of gender and atopy on the natural history of rhinitis in the first 18 years of life: Rhinitis trends through childhood and adolescence. Clin. Exp. Allergy 2011, 41, 851–859. [Google Scholar] [CrossRef]
- Pinart, M.; Keller, T.; Reich, A.; Fröhlich, M.; Cabieses, B.; Hohmann, C.; Postma, D.S.; Bousquet, J.; Antó, J.M.; Keil, T. Sex-Related Allergic Rhinitis Prevalence Switch from Childhood to Adulthood: A Systematic Review and Meta-Analysis. Int. Arch. Allergy Immunol. 2017, 172, 224–235. [Google Scholar] [CrossRef]
- Loh, W.; Tang, M.L.K. The Epidemiology of Food Allergy in the Global Context. Int. J. Environ. Res. Public Health 2018, 15, 43. [Google Scholar] [CrossRef] [PubMed]
- Kelly, C.; Gangur, V. Sex Disparity in Food Allergy: Evidence from the PubMed Database. J. Allergy 2009, 2009, 1–7. [Google Scholar] [CrossRef]
- Karpa, K.D.; Paul, I.M.; Leckie, J.A.; Shung, S.; Carkaci-Salli, N.; Vrana, K.E.; Mauger, D.; Fausnight, T.; Poger, J. A retrospective chart review to identify perinatal factors associated with food allergies. Nutr. J. 2012, 11, 87. [Google Scholar] [CrossRef] [PubMed]
- Peters, R.L.; Allen, K.J.; Dharmage, S.C.; Lodge, C.J.; Koplin, J.J.; Ponsonby, A.-L.; Wake, M.; Lowe, A.J.; Tang, M.L.K.; Matheson, M.C.; et al. Differential factors associated with challenge-proven food allergy phenotypes in a population cohort of infants: A latent class analysis. Clin. Exp. Allergy 2015, 45, 953–963. [Google Scholar] [CrossRef]
- Kim, H.-B.; Ahn, K.M.; Kim, K.W.; Shin, Y.H.; Yu, J.; Seo, J.-H.; Kim, H.Y.; Kwon, J.-W.; Kim, B.-J.; Kwon, J.-Y.; et al. Cord Blood Cellular Proliferative Response as a Predictive Factor for Atopic Dermatitis at 12 Months. J. Korean Med. Sci. 2012, 27, 1320. [Google Scholar] [CrossRef]
- Lee, S.; Hess, E.P.; Lohse, C.; Gilani, W.; Chamberlain, A.M.; Campbell, R.L. Trends, characteristics, and incidence of anaphylaxis in 2001-2010: A population-based study. J. Allergy Clin. Immunol. 2017, 139, 182–188.e2. [Google Scholar] [CrossRef] [PubMed]
- Akinbami, L.J.; Simon, A.E.; Schoendorf, K.C. Trends in allergy prevalence among children aged 0-17 years by asthma status, United States, 2001–2013. J. Asthma 2016, 53, 356–362. [Google Scholar] [CrossRef]
- Salo, P.M.; Arbes, S.J.; Jaramillo, R.; Calatroni, A.; Weir, C.H.; Sever, M.L.; Hoppin, J.A.; Rose, K.M.; Liu, A.H.; Gergen, P.J.; et al. Prevalence of allergic sensitization in the United States: Results from the National Health and Nutrition Examination Survey (NHANES) 2005-2006. J. Allergy Clin. Immunol. 2014, 134, 350–359. [Google Scholar] [CrossRef]
- Georgiev, P.; Charbonnier, L.-M.; Chatila, T.A. Regulatory T Cells: The Many Faces of Foxp3. J. Clin. Immunol. 2019, 39, 623–640. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Chi, H. Metabolic Control of Treg Cell Stability, Plasticity, and Tissue-Specific Heterogeneity. Front. Immunol. 2019, 10, 2716. [Google Scholar] [CrossRef]
- Hori, S. Lineage stability and phenotypic plasticity of Foxp3+ regulatory T cells. Immunol. Rev. 2014, 259, 159–172. [Google Scholar] [CrossRef]
- Xiong, H.; Zhou, C.; Qi, G. Proportional changes of CD4+CD25+Foxp3+ Regulatory T cells in maternal peripheral blood during pregnancy and labor at term and preterm. CIM 2010, 33, 422. [Google Scholar] [CrossRef]
- Gomez-Lopez, N.; StLouis, D.; Lehr, M.A.; Sanchez-Rodriguez, E.N.; Arenas-Hernandez, M. Immune cells in term and preterm labor. Cell Mol. Immunol. 2014, 11, 571–581. [Google Scholar] [CrossRef]
- Keelan, J.A. Intrauterine inflammatory activation, functional progesterone withdrawal, and the timing of term and preterm birth. J. Reprod. Immunol. 2018, 125, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Mold, J.E.; Michaëlsson, J.; Burt, T.D.; Muench, M.O.; Beckerman, K.P.; Busch, M.P.; Lee, T.-H.; Nixon, D.F.; McCune, J.M. Maternal alloantigens promote the development of tolerogenic fetal regulatory T cells in utero. Science 2008, 322, 1562–1565. [Google Scholar] [CrossRef] [PubMed]
- Santner-Nanan, B.; Straubinger, K.; Hsu, P.; Parnell, G.; Tang, B.; Xu, B.; Makris, A.; Hennessy, A.; Peek, M.J.; Busch, D.H.; et al. Fetal-Maternal Alignment of Regulatory T Cells Correlates with IL-10 and Bcl-2 Upregulation in Pregnancy. J. Immunol. 2013, 191, 145–153. [Google Scholar] [CrossRef]
- Kmieciak, M.; Gowda, M.; Graham, L.; Godder, K.; Bear, H.D.; Marincola, F.M.; Manjili, M.H. Human T cells express CD25 and Foxp3 upon activation and exhibit effector/memory phenotypes without any regulatory/suppressor function. J. Transl. Med. 2009, 7, 89. [Google Scholar] [CrossRef]
- Allan, S.E.; Crome, S.Q.; Crellin, N.K.; Passerini, L.; Steiner, T.S.; Bacchetta, R.; Roncarolo, M.G.; Levings, M.K. Activation-induced FOXP3 in human T effector cells does not suppress proliferation or cytokine production. Int. Immunol. 2007, 19, 345–354. [Google Scholar] [CrossRef]
- Iizuka-Koga, M.; Nakatsukasa, H.; Ito, M.; Akanuma, T.; Lu, Q.; Yoshimura, A. Induction and maintenance of regulatory T cells by transcription factors and epigenetic modifications. J. Autoimmun. 2017, 83, 113–121. [Google Scholar] [CrossRef]
- Kitagawa, Y.; Ohkura, N.; Sakaguchi, S. Epigenetic control of thymic Treg-cell development. Eur. J. Immunol. 2015, 45, 11–16. [Google Scholar] [CrossRef]
- Singh, K.; Hjort, M.; Thorvaldson, L.; Sandler, S. Concomitant analysis of Helios and Neuropilin-1 as a marker to detect thymic derived regulatory T cells in naïve mice. Sci. Rep. 2015, 5, 7767. [Google Scholar] [CrossRef] [PubMed]
- Akimova, T.; Beier, U.H.; Wang, L.; Levine, M.H.; Hancock, W.W. Helios expression is a marker of T cell activation and proliferation. PLoS ONE 2011, 6, e24226. [Google Scholar] [CrossRef]
- Szurek, E.; Cebula, A.; Wojciech, L.; Pietrzak, M.; Rempala, G.; Kisielow, P.; Ignatowicz, L. Differences in Expression Level of Helios and Neuropilin-1 Do Not Distinguish Thymus-Derived from Extrathymically-Induced CD4+Foxp3+ Regulatory T Cells. PLoS ONE 2015, 10, e0141161. [Google Scholar] [CrossRef] [PubMed]
- Thornton, A.M.; Shevach, E.M. Helios: Still behind the clouds. Immunology 2019, 158, 161–170. [Google Scholar] [CrossRef]
- Lord, J.; Chen, J.; Thirlby, R.C.; Sherwood, A.M.; Carlson, C.S. T-cell receptor sequencing reveals the clonal diversity and overlap of colonic effector and FOXP3+ T cells in ulcerative colitis. Inflamm. Bowel Dis. 2015, 21, 19–30. [Google Scholar] [CrossRef]
- Thornton, A.M.; Lu, J.; Korty, P.E.; Kim, Y.C.; Martens, C.; Sun, P.D.; Shevach, E.M. Helios+ and Helios− Treg subpopulations are phenotypically and functionally distinct and express dissimilar TCR repertoires. Eur. J. Immunol. 2019, 49, 398–412. [Google Scholar] [CrossRef] [PubMed]
- Akdis, M.; Burgler, S.; Crameri, R.; Eiwegger, T.; Fujita, H.; Gomez, E.; Klunker, S.; Meyer, N.; O’Mahony, L.; Palomares, O.; et al. Interleukins, from 1 to 37, and interferon-γ: Receptors, functions, and roles in diseases. J. Allergy Clin. Immunol. 2011, 127, 701–721.e1–70. [Google Scholar] [CrossRef]
- Fife, B.T.; Bluestone, J.A. Control of peripheral T-cell tolerance and autoimmunity via the CTLA-4 and PD-1 pathways. Immunol. Rev. 2008, 224, 166–182. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, N.; Nakajima, H. Coinhibitory molecules in autoimmune diseases. Clin. Dev. Immunol. 2012, 2012, 269756. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Verma, A.K.; Das, M.; Dwivedi, P.D. A molecular insight of CTLA-4 in food allergy. Immunol. Lett. 2013, 149, 101–109. [Google Scholar] [CrossRef]
- Nocentini, G.; Riccardi, C. GITR: A modulator of immune response and inflammation. Adv. Exp. Med. Biol. 2009, 647, 156–173. [Google Scholar] [CrossRef]
- Huang, C.-T.; Workman, C.J.; Flies, D.; Pan, X.; Marson, A.L.; Zhou, G.; Hipkiss, E.L.; Ravi, S.; Kowalski, J.; Levitsky, H.I.; et al. Role of LAG-3 in regulatory T cells. Immunity 2004, 21, 503–513. [Google Scholar] [CrossRef]
- Liang, B.; Workman, C.; Lee, J.; Chew, C.; Dale, B.M.; Colonna, L.; Flores, M.; Li, N.; Schweighoffer, E.; Greenberg, S.; et al. Regulatory T cells inhibit dendritic cells by lymphocyte activation gene-3 engagement of MHC class II. J. Immunol. 2008, 180, 5916–5926. [Google Scholar] [CrossRef] [PubMed]
- Rosser, E.C.; Mauri, C. Regulatory B cells: Origin, phenotype, and function. Immunity 2015, 42, 607–612. [Google Scholar] [CrossRef]
- Palomares, O.; Akdis, M.; Martín-Fontecha, M.; Akdis, C.A. Mechanisms of immune regulation in allergic diseases: The role of regulatory T and B cells. Immunol. Rev. 2017, 278, 219–236. [Google Scholar] [CrossRef]
- Wang, S.; Xia, P.; Chen, Y.; Qu, Y.; Xiong, Z.; Ye, B.; Du, Y.; Tian, Y.; Yin, Z.; Xu, Z.; et al. Regulatory Innate Lymphoid Cells Control Innate Intestinal Inflammation. Cell 2017, 171, 201–216.e18. [Google Scholar] [CrossRef]
- Saradna, A.; Do, D.C.; Kumar, S.; Fu, Q.-L.; Gao, P. Macrophage polarization and allergic asthma. Transl. Res. 2018, 191, 1–14. [Google Scholar] [CrossRef]
- Palomares, O.; Martín-Fontecha, M.; Lauener, R.; Traidl-Hoffmann, C.; Cavkaytar, O.; Akdis, M.; Akdis, C.A. Regulatory T cells and immune regulation of allergic diseases: Roles of IL-10 and TGF-β. Genes Immun. 2014, 15, 511–520. [Google Scholar] [CrossRef]
- Akdis, C.A.; Akdis, M. Mechanisms of allergen-specific immunotherapy. J. Allergy Clin. Immunol. 2011, 127, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Akdis, C.A.; Akdis, M. Mechanisms of allergen-specific immunotherapy and immune tolerance to allergens. World Allergy Organ J. 2015, 8, 17. [Google Scholar] [CrossRef] [PubMed]
- Palomares, O.; Rückert, B.; Jartti, T.; Kücüksezer, U.C.; Puhakka, T.; Gomez, E.; Fahrner, H.B.; Speiser, A.; Jung, A.; Kwok, W.W.; et al. Induction and maintenance of allergen-specific FOXP3+ Treg cells in human tonsils as potential first-line organs of oral tolerance. J. Allergy Clin. Immunol. 2012, 129, 510–520.e1–9. [Google Scholar] [CrossRef]
- Branchett, W.J.; Lloyd, C.M. Regulatory cytokine function in the respiratory tract. Mucosal Immunol 2019, 12, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Ruan, G.; Wen, X.; Yuan, Z. Correlation between miR-223 and IL-35 and their regulatory effect in children with allergic rhinitis. Clin. Immunol. 2020, 214, 108383. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Liu, X.; Wei, Z.; Wang, X.; Xu, D.; Dai, S.; Li, Y.; Gao, M.; Ji, C.; Guo, C.; et al. The expression of a novel anti-inflammatory cytokine IL-35 and its possible significance in childhood asthma. Immunol. Lett. 2014, 162, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Layhadi, J.A.; Eguiluz-Gracia, I.; Shamji, M.H. Role of IL-35 in sublingual allergen immunotherapy. Curr. Opin. Allergy Clin. Immunol. 2019, 19, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Overacre, A.E.; Vignali, D.A. Treg stability: To be or not to be. Curr. Opin. Immunol. 2016, 39, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Barbi, J.; Pardoll, D.; Pan, F. Treg functional stability and its responsiveness to the microenvironment. Immunol. Rev. 2014, 259, 115–139. [Google Scholar] [CrossRef] [PubMed]
- Korn, T.; Muschaweckh, A. Stability and Maintenance of Foxp3+ Treg Cells in Non-lymphoid Microenvironments. Front. Immunol. 2019, 10, 2634. [Google Scholar] [CrossRef]
- Herberth, G.; Hinz, D.; Röder, S.; Schlink, U.; Sack, U.; Diez, U.; Borte, M.; Lehmann, I. Maternal immune status in pregnancy is related to offspring’s immune responses and atopy risk: Immune status in pregnancy and offspring’s immune responses. Allergy 2011, 66, 1065–1074. [Google Scholar] [CrossRef]
- Hinz, D.; Simon, J.C.; Maier-Simon, C.; Milkova, L.; Röder, S.; Sack, U.; Borte, M.; Lehmann, I.; Herberth, G. Reduced maternal regulatory T cell numbers and increased T helper type 2 cytokine production are associated with elevated levels of immunoglobulin E in cord blood. Clin. Exp. Allergy 2010, 40, 419–426. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Černý, V.; Novotná, O.; Petrásková, P.; Hudcová, K.; Boráková, K.; Prokešová, L.; Kolářová, L.; Hrdý, J. Lower Functional and Proportional Characteristics of Cord Blood Treg of Male Newborns Compared with Female Newborns. Biomedicines 2021, 9, 170. https://doi.org/10.3390/biomedicines9020170
Černý V, Novotná O, Petrásková P, Hudcová K, Boráková K, Prokešová L, Kolářová L, Hrdý J. Lower Functional and Proportional Characteristics of Cord Blood Treg of Male Newborns Compared with Female Newborns. Biomedicines. 2021; 9(2):170. https://doi.org/10.3390/biomedicines9020170
Chicago/Turabian StyleČerný, Viktor, Olga Novotná, Petra Petrásková, Kateřina Hudcová, Kristýna Boráková, Ludmila Prokešová, Libuše Kolářová, and Jiří Hrdý. 2021. "Lower Functional and Proportional Characteristics of Cord Blood Treg of Male Newborns Compared with Female Newborns" Biomedicines 9, no. 2: 170. https://doi.org/10.3390/biomedicines9020170
APA StyleČerný, V., Novotná, O., Petrásková, P., Hudcová, K., Boráková, K., Prokešová, L., Kolářová, L., & Hrdý, J. (2021). Lower Functional and Proportional Characteristics of Cord Blood Treg of Male Newborns Compared with Female Newborns. Biomedicines, 9(2), 170. https://doi.org/10.3390/biomedicines9020170





