Laparoscopic Peritoneal Wash Cytology-Derived Primary Human Mesothelial Cells for In Vitro Cell Culture and Simulation of Human Peritoneum
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation of PWC-Derived Primary Mesothelial Cells
2.2. Isolation of Primary Human Peritoneal Fibroblasts
2.3. Primary Human Peritoneal Tissue Samples
2.4. HaCat (Primary Human Keratinocyte Cells) Culture, BJ Fibroblasts (Primary Human Fibroblasts)
2.5. Immunofluorescence
2.6. Flow Cytometry
2.7. Raman Analysis
2.8. Image and Data Analysis
3. Results
3.1. Isolation Methodology and Cell Culture of PWC-Derived Mesothelial Cells
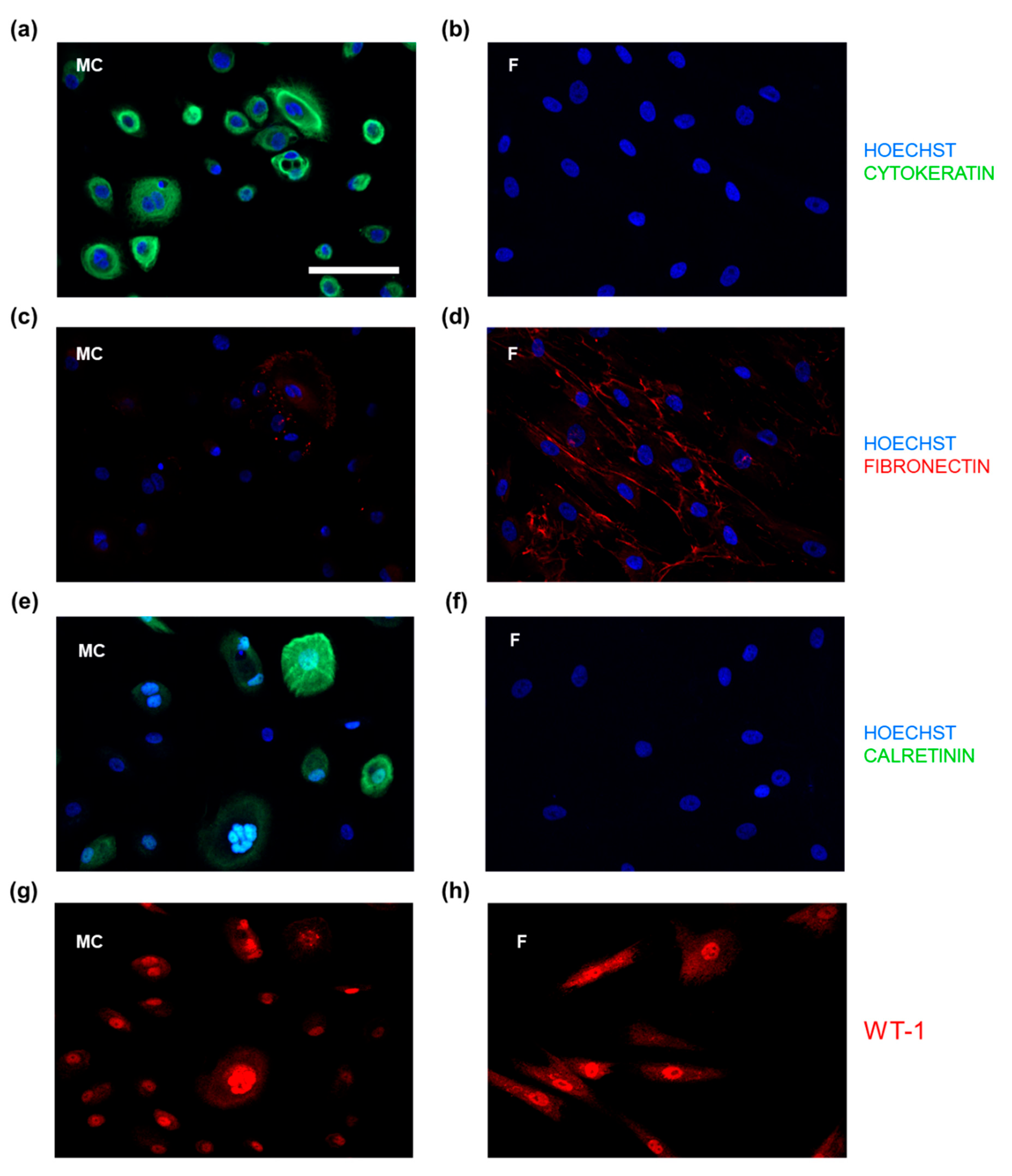
3.2. IF-Microscopic Characterization of PWC-Derived Mesothelial Cells and Suitable Molecular Markers to Distinguish from Peritoneal Fibroblasts
3.3. PWC-Derived Primary Mesothelial Cells Revealed above-Average Purity
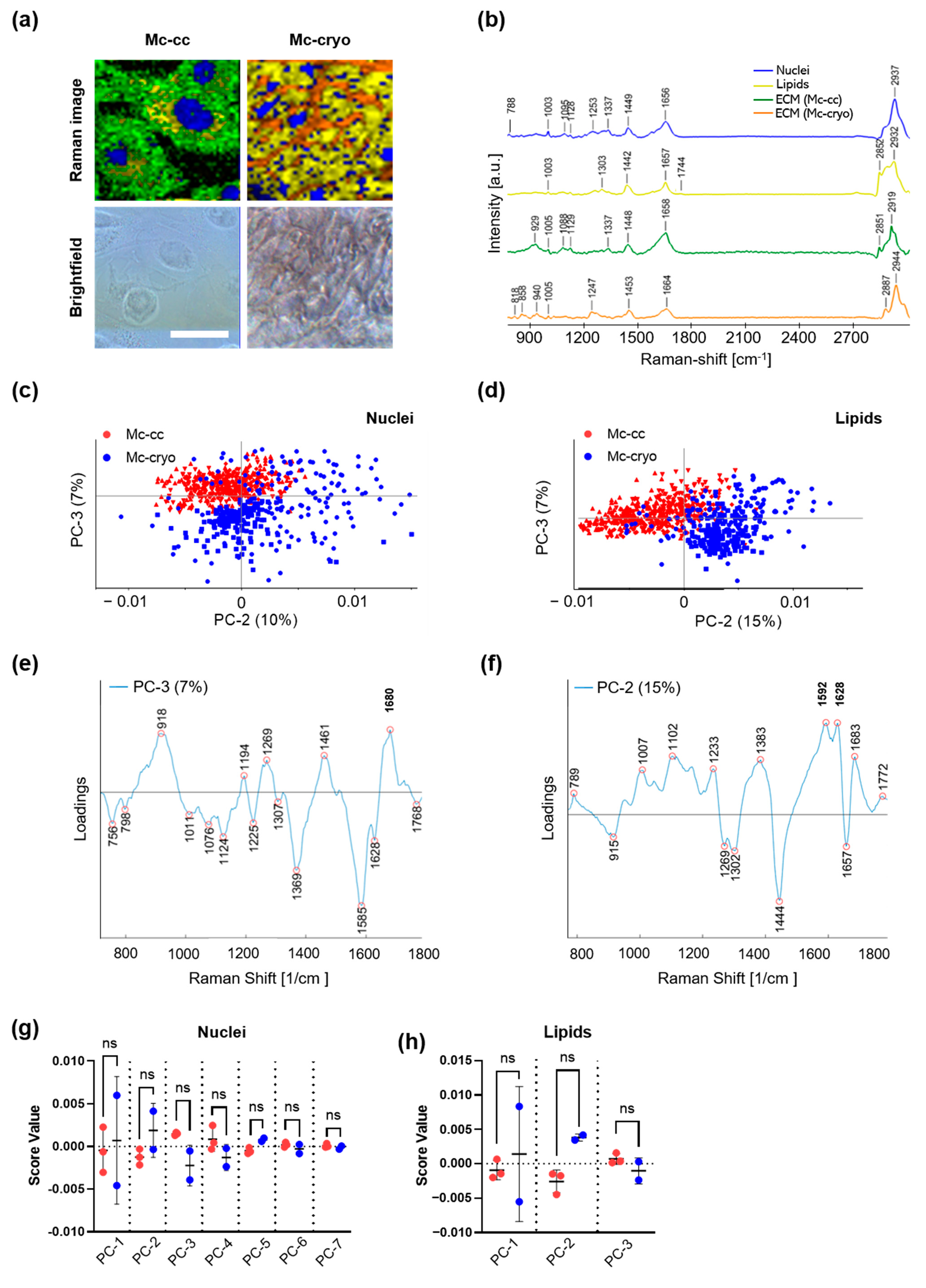
3.4. Characterization of the Molecular Structure and Composition of PWC-Derived Primary Mesothelial Cells Cryo-Preserved Peritoneal Tissue Samples Using Raman Imaging
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Herrick, S.E.; Mutsaers, S.E. Mesothelial progenitor cells and their potential in tissue engineering. Int. J. Biochem. Cell Biol. 2004, 36, 621–642. [Google Scholar] [CrossRef] [PubMed]
- Mutsaers, S.E.; Prêle, C.M.-A.; Pengelly, S.; Herrick, S.E. Mesothelial cells and peritoneal homeostasis. Fertil. Steril. 2016, 106, 1018–1024. [Google Scholar] [CrossRef]
- Mutsaers, S.E. The mesothelial cell. Int. J. Biochem. Cell Biol. 2004, 36, 9–16. [Google Scholar] [CrossRef]
- Capobianco, A.; Cottone, L.; Monno, A.; Manfredi, A.A.; Rovere-Querini, P. The peritoneum: Healing, immunity, and diseases. J. Pathol. 2017, 243, 137–147. [Google Scholar] [CrossRef]
- Whitaker, D.; Papadimitriou, J. Mesothelial healing: Morphological and kinetic investigations. J. Pathol. 1985, 145, 159–175. [Google Scholar] [CrossRef]
- Dauleh, S.; Santeramo, I.; Fielding, C.; Ward, K.; Herrmann, A.; Murray, P.; Wilm, B. Characterisation of Cultured Mesothelial Cells Derived from the Murine Adult Omentum. PLoS ONE 2016, 11, e0158997. [Google Scholar] [CrossRef]
- Fischer, A.; Koopmans, T.; Ramesh, P.; Christ, S.; Strunz, M.; Wannemacher, J.; Aichler, M.; Feuchtinger, A.; Walch, A.; Ansari, M.; et al. Post-surgical adhesions are triggered by calcium-dependent membrane bridges between mesothelial surfaces. Nat. Commun. 2020, 11, 3068. [Google Scholar] [CrossRef]
- Lee, H.B.; Ha, H. Mechanisms of epithelial-mesenchymal transition of peritoneal mesothelial cells during peritoneal dialysis. J. Korean Med. Sci. 2007, 22, 943–945. [Google Scholar] [CrossRef]
- van Baal, J.O.; Van de Vijver, K.K.; Nieuwland, R.; van Noorden, C.J.; van Driel, W.J.; Sturk, A.; Kenter, G.G.; Rikkert, L.G.; Lok, C.A. The histophysiology and pathophysiology of the peritoneum. Tissue Cell 2017, 49, 95–105. [Google Scholar] [CrossRef] [PubMed]
- van Hinsbergh, V.W.; Kooistra, T.; Scheffer, M.A.; Hajo van Bockel, J.; van Muijen, G.N. Characterization and fibrinolytic properties of human omental tissue mesothelial cells. Comparison with endothelial cells. Blood 1990, 75, 1490–1497. [Google Scholar] [CrossRef]
- Stylianou, E.; Jenner, L.A.; Davies, M.; Coles, G.A.; Williams, J.D. Isolation, culture and characterization of human peritoneal mesothelial cells. Kidney Int. 1990, 37, 1563–1570. [Google Scholar] [CrossRef]
- Yung, S.; Li, F.K.; Chan, T.M. Peritoneal Mesothelial Cell Culture and Biology. Perit. Dial. Int. 2006, 26, 162–193. [Google Scholar] [CrossRef]
- Pronk, A.; Leguit, P.; Hoynck van Papendrecht, A.A.G.M.; Hagelen, E.; van Vroonhoven, T.J.M.V.; Verbrugh, H.A. A cobblestone cell isolated from the human omentum: The mesothelial cell; isolation, identification, and growth characteristics. In Vitro Cell. Dev. Biol. Anim. 1993, 29, 127–134. [Google Scholar] [CrossRef]
- Yamamoto, T.; Izumotani, T.; Otoshi, T.; Kim, M. Morphological studies of mesothelial cells in CAPD effluent and their clinical significance. Am. J. Kidney Dis. 1998, 32, 946–952. [Google Scholar] [CrossRef]
- Chan, T.M.; Leung, J.K.H.; Sun, Y.; Lai, K.N.; Tsang, R.C.W.; Yung, S. Different effects of amino acid-based and glucose-based dialysate from peritoneal dialysis patients on mesothelial cell ultrastructure and function. Nephrol. Dial. Transplant. 2003, 18, 1086–1094. [Google Scholar] [CrossRef]
- Takashima, A. Establishment of Fibroblast Cultures. Curr. Protoc. Cell Biol. 1998, 2.1.1–2.1.12. [Google Scholar] [CrossRef]
- Wenzel, T.; Carvajal Berrio, D.A.; Daum, R.; Reisenauer, C.; Weltmann, K.-D.; Wallwiener, D.; Brucker, S.Y.; Schenke-Layland, K.; Brauchle, E.-M.; Weiss, M. Molecular Effects and Tissue Penetration Depth of Physical Plasma in Human Mucosa Analyzed by Contact- and Marker-Independent Raman Microspectroscopy. ACS Appl. Mater. Interfaces 2019, 11, 42885–42895. [Google Scholar] [CrossRef]
- Wenzel, T.; Carvajal Berrio, D.A.; Reisenauer, C.; Layland, S.; Koch, A.; Wallwiener, D.; Brucker, S.Y.; Schenke-Layland, K.; Brauchle, E.M.; Weiss, M. Trans-Mucosal Efficacy of Non-Thermal Plasma Treatment on Cervical Cancer Tissue and Human Cervix Uteri by a Next Generation Electrosurgical Argon Plasma Device. Cancers 2020, 12, 267. [Google Scholar] [CrossRef]
- Notingher, I.; Green, C.; Dyer, C.; Perkins, E.; Hopkins, N.; Lindsay, C.; Hench, L.L. Discrimination between ricin and sulphur mustard toxicity in vitro using Raman spectroscopy. J. R. Soc. Interface 2004, 1, 79–90. [Google Scholar] [CrossRef]
- Malini, R.; Venkatakrishna, K.; Kurien, J.; Pai, K.M.; Rao, L.; Kartha, V.B.; Krishna, C.M. Discrimination of normal, inflammatory, premalignant, and malignant oral tissue: A Raman spectroscopy study. Biopolymers 2006, 81, 179–193. [Google Scholar] [CrossRef]
- Hanlon, E.B.; Manoharan, R.; Koo, T.W.; Shafer, K.E.; Motz, J.T.; Fitzmaurice, M.; Kramer, J.R.; Itzkan, I.; Dasari, R.R.; Feld, M.S. Prospects for in vivo Raman spectroscopy. Phys. Med. Biol. 2000, 45, R1–R59. [Google Scholar] [CrossRef]
- Dukor, R.K. Vibrational Spectroscopy in the Detection of Cancer. In Handbook of Vibrational Spectroscopy; John Wiley & Sons: Hoboken, NJ, USA, 2001. [Google Scholar] [CrossRef]
- Kline, N.J.; Treado, P.J. Raman Chemical Imaging of Breast Tissue. J. Raman Spectrosc. 1997, 28, 119–124. [Google Scholar] [CrossRef]
- Koljenović, S.; Schut, T.B.; Vincent, A.; Kros, J.M.; Puppels, G.J. Detection of Meningioma in Dura Mater by Raman Spectroscopy. Anal. Chem. 2005, 77, 7958–7965. [Google Scholar] [CrossRef]
- Lau, D.P.; Huang, Z.; Lui, H.; Man, C.S.; Berean, K.; Morrison, M.D.; Zeng, H. Raman spectroscopy for optical diagnosis in normal and cancerous tissue of the nasopharynx-preliminary findings. Lasers Surg. Med. 2003, 32, 210–214. [Google Scholar] [CrossRef]
- Jyothi Lakshmi, R.; Kartha, V.B.; Murali Krishna, C.; JG, R.S.; Ullas, G.; Uma Devi, P. Tissue Raman spectroscopy for the study of radiation damage: Brain irradiation of mice. Radiat. Res. 2002, 157, 175–182. [Google Scholar] [CrossRef]
- Frank, C.J.; McCreery, R.L.; Redd, D.C. Raman spectroscopy of normal and diseased human breast tissues. Anal. Chem. 1995, 67, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.T.; Liu, M.T.; Liu, H.N.; Lin, S.Y. Micro-Raman spectroscopy used to identify and grade human skin pilomatrixoma. Microsc. Res. Tech. 2005, 68, 75–79. [Google Scholar] [CrossRef]
- Shetty, G.; Kendall, C.; Shepherd, N.; Stone, N.; Barr, H. Raman spectroscopy: Elucidation of biochemical changes in carcinogenesis of oesophagus. Br. J. Cancer 2006, 94, 1460–1464. [Google Scholar] [CrossRef] [PubMed]
- Viehoever, A.R.; Anderson, D.; Jansen, D.; Mahadevan-Jansen, A. Organotypic raft cultures as an effective in vitro tool for understanding Raman spectral analysis of tissue. Photochem. Photobiol. 2003, 78, 517–524. [Google Scholar] [CrossRef]
- Stone, N.; Kendall, C.; Smith, J.; Crow, P.; Barr, H. Raman spectroscopy for identification of epithelial cancers. Faraday Discuss. 2004, 126, 141–157. [Google Scholar] [CrossRef]
- Huang, Z.; McWilliams, A.; Lui, H.; McLean, D.I.; Lam, S.; Zeng, H. Near-infrared Raman spectroscopy for optical diagnosis of lung cancer. Int. J. Cancer 2003, 107, 1047–1052. [Google Scholar] [CrossRef]
- Naumann, D. Infrared and NIR Raman Spectroscopy in Medical Microbiology. In Proceedings of the BiOS ’98 International Biomedical Optics Symposium, San Jose, CA, USA, 27 January 1998; SPIE: London, UK, 1998; Volume 3257. [Google Scholar]
- Schulz, H.; Baranska, M. Identification and quantification of valuable plant substances by IR and Raman spectroscopy. Vib. Spectrosc. 2007, 43, 13–25. [Google Scholar] [CrossRef]
- Liu, F.Y.; Duan, S.B.; Long, Z.G. Culture and characterization of human peritoneal mesothelial cells. Hunan Yi Ke Da Xue Xue Bao 2001, 26, 321–324. [Google Scholar] [PubMed]
- Fang, C.C.; Yen, C.J.; Chen, Y.M.; Shyu, R.S.; Tsai, T.J.; Lee, P.H.; Hsieh, B.S. Pentoxifylline inhibits human peritoneal mesothelial cell growth and collagen synthesis: Effects on TGF-beta. Kidney Int. 2000, 57, 2626–2633. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.-C.; Huang, J.-W.; Shyu, R.-S.; Yen, C.-J.; Shiao, C.-H.; Chiang, C.-K.; Hu, R.-H.; Tsai, T.-J. Fibrin-Induced Epithelial-to-Mesenchymal Transition of Peritoneal Mesothelial Cells as a Mechanism of Peritoneal Fibrosis: Effects of Pentoxifylline. PLoS ONE 2012, 7, e44765. [Google Scholar] [CrossRef] [PubMed]
- Chung-Welch, N.; Patton, W.F.; Shepro, D.; Cambria, R.P. Human omental microvascular endothelial and mesothelial cells: Characterization of two distinct mesodermally derived epithelial cells. Microvasc. Res. 1997, 54, 108–120. [Google Scholar] [CrossRef]
- Rayner, S.G.; Zheng, Y. Engineered Microvessels for the Study of Human Disease. J. Biomech. Eng. 2016, 138, 1108011–11080111. [Google Scholar] [CrossRef]
- Fang, C.C.; Yen, C.J.; Chen, Y.M.; Chu, T.S.; Lin, M.T.; Yang, J.Y.; Tsai, T.J. Diltiazem suppresses collagen synthesis and IL-1beta-induced TGF-beta1 production on human peritoneal mesothelial cells. Nephrol. Dial. Transplant. 2006, 21, 1340–1347. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yáñez-Mó, M.; Lara-Pezzi, E.; Selgas, R.; Ramírez-Huesca, M.; Domínguez-Jiménez, C.; Jiménez-Heffernan, J.A.; Aguilera, A.; Sánchez-Tomero, J.A.; Bajo, M.A.; Álvarez, V.; et al. Peritoneal Dialysis and Epithelial-to-Mesenchymal Transition of Mesothelial Cells. N. Engl. J. Med. 2003, 348, 403–413. [Google Scholar] [CrossRef]
- Makin, C.A.; Bobrow, L.G.; Bodmer, W.F. Monoclonal antibody to cytokeratin for use in routine histopathology. J. Clin. Pathol. 1984, 37, 975. [Google Scholar] [CrossRef]
- Von Koskull, H.; Virtanen, I. Induction of cytokeratin expression in human mesenchymal cells. J. Cell. Physiol. 1987, 133, 321–329. [Google Scholar] [CrossRef]
- Moll, R.; Franke, W.W.; Schiller, D.L.; Geiger, B.; Krepler, R. The catalog of human cytokeratins: Patterns of expression in normal epithelia, tumors and cultured cells. Cell 1982, 31, 11–24. [Google Scholar] [CrossRef]
- Yang, A.H.; Chen, J.Y.; Lin, J.K. Myofibroblastic conversion of mesothelial cells. Kidney Int. 2003, 63, 1530–1539. [Google Scholar] [CrossRef]
- Lugli, A.; Forster, Y.; Haas, P.; Nocito, A.; Bucher, C.; Bissig, H.; Mirlacher, M.; Storz, M.; Mihatsch, M.J.; Sauter, G. Calretinin expression in human normal and neoplastic tissues: A tissue microarray analysis on 5233 tissue samples. Hum. Pathol. 2003, 34, 994–1000. [Google Scholar] [CrossRef]
- Betjes, M.G.; Bos, H.J.; Krediet, R.T.; Arisz, L. The mesothelial cells in CAPD effluent and their relation to peritonitis incidence. Perit. Dial. Int. 1991, 11, 22–26. [Google Scholar] [CrossRef]
- Harvey, W.; Amlot, P.L. Collagen production by human mesothelial cells in vitro. J. Pathol. 1983, 139, 337–347. [Google Scholar] [CrossRef]
- Fetsch, P.A.; Simsir, A.; Abati, A. Comparison of antibodies to HBME-1 and calretinin for the detection of mesothelial cells in effusion cytology. Diagn. Cytopathol. 2001, 25, 158–161. [Google Scholar] [CrossRef] [PubMed]
- Czernobilsky, B.; Moll, R.; Levy, R.; Franke, W.W. Co-expression of Cytokeratin and Vimentin Filaments in Mesothelial, Granulosa and Rete Ovarii Cells of the Human Ovary. Eur. J. Cell Biol. 1985, 37, 175–190. [Google Scholar]
- Marzi, J.; Brauchle, E.M.; Schenke-Layland, K.; Rolle, M.W. Non-invasive functional molecular phenotyping of human smooth muscle cells utilized in cardiovascular tissue engineering. Acta Biomater. 2019, 89, 193–205. [Google Scholar] [CrossRef]
- Brauchle, E.; Schenke-Layland, K. Raman spectroscopy in biomedicine—Non-invasive in vitro analysis of cells and extracellular matrix components in tissues. Biotechnol. J. 2013, 8, 288–297. [Google Scholar] [CrossRef]
- Parlatan, U.; Inanc, M.T.; Ozgor, B.Y.; Oral, E.; Bastu, E.; Unlu, M.B.; Basar, G. Raman spectroscopy as a non-invasive diagnostic technique for endometriosis. Sci. Rep. 2019, 9, 19795. [Google Scholar] [CrossRef]
- Gaifulina, R.; Maher, A.T.; Kendall, C.; Nelson, J.; Rodriguez-Justo, M.; Lau, K.; Thomas, G.M. Label-free Raman spectroscopic imaging to extract morphological and chemical information from a formalin-fixed, paraffin-embedded rat colon tissue section. Int. J. Exp. Pathol. 2016, 97, 337–350. [Google Scholar] [CrossRef]
- Frtús, A.; Smolková, B.; Uzhytchak, M.; Lunova, M.; Jirsa, M.; Hof, M.; Jurkiewicz, P.; Lozinsky, V.I.; Wolfová, L.; Petrenko, Y.; et al. Hepatic Tumor Cell Morphology Plasticity under Physical Constraints in 3D Cultures Driven by YAP–mTOR Axis. Pharmaceuticals 2020, 13, 430. [Google Scholar] [CrossRef]


| Primary Antibody | IgG Source | Dilution | Application | Company |
|---|---|---|---|---|
| Cytokeratin–broad spectrum | Mouse (IgG1) | 1:100 | IF | Zytomed, Berlin, Germany |
| Fibronectin | Rabbit | 1:100 | IF | Abcam, Berlin, Germany |
| Calretinin | Mouse (IgG2b) | 1:100 | IF | Santa Cruz, Dallas, USA |
| Wilms’ tumor protein | Rabbit | 1:100 | IF | Santa Cruz, Dallas, USA |
| MC | F | |
|---|---|---|
| No. of patients, n (%) | 8 (100) | 3 (100) |
| Median age, years (range) | 35.8 (22–55) | 28.7 (25–31) |
| Gravidity, n (range) | 1.5 (0–5) | 1.0 (1) |
| Parity, n (range) | 0.5 (0–2) | 1.0 (1) |
| Ovarian function, n (%) | ||
| premenopausal | 6 (75.0) | 3 (100) |
| perimenopausal | 1 (12.5) | - |
| Cause for surgery | ||
| Hypermenorrhea/Hysterectomy | 2 (25.0) | - |
| Diagnostic/Pain | 2 (25.0) | - |
| Diagnostic/Childwish | 1 (12.5) | - |
| Cysts | 3 (37.5) | - |
| Cesarean section | - | 3 (100) |
| Intraoperative findings | ||
| Adhesions | 5 (62.5) | - |
| Endometriosis | 1 (12.5) | - |
| none | 2 (25.0) | - |
| Peaks (cm−1) | Assignment | Reference |
|---|---|---|
| 756 | Tryptophan | [31] |
| 788 | O-P-O stretching | [19] |
| 818 | C-C stretching in collagen | [27] |
| 918 | Proline | [28] |
| 929 | C-C stretching in amino acids such as proline and valine | [25] |
| 1005 | Phenylalanine | [19] |
| 1011 | Carbohydrates | [22] |
| 1076 | C-C in lipids | [20] |
| 1095 | DNA backbone | [20] |
| 1124 | Lipid backbone | [28] |
| 1247 | Amide III in collagen | [27] |
| 1269 | C-C vibration | [30] |
| 1302 | CH2 wagging | [32] |
| 1369 | Lipids | [31] |
| 1442 | C-C in fatty acids | [22] |
| 1444 | CH2 scissoring | [29] |
| 1453 | CH3 bending and CH2 scissoring in collagen | [25] |
| 1592 | C=C | [33] |
| 1628 | C=C | [34] |
| 1657 | C=C stretching | [21,22] |
| 1658 | Amide I | [26] |
| 1680 | Amide I | [29] |
| 1774 | Carbonyl feature in fatty acids | [23] |
| 2852 | CH2 symmetric stretch of lipids | [24] |
| 2912 | CH stretches of proteins | [24] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holl, M.; Becker, L.; Keller, A.-L.; Feuerer, N.; Marzi, J.; Carvajal Berrio, D.A.; Jakubowski, P.; Neis, F.; Pauluschke-Fröhlich, J.; Brucker, S.Y.; et al. Laparoscopic Peritoneal Wash Cytology-Derived Primary Human Mesothelial Cells for In Vitro Cell Culture and Simulation of Human Peritoneum. Biomedicines 2021, 9, 176. https://doi.org/10.3390/biomedicines9020176
Holl M, Becker L, Keller A-L, Feuerer N, Marzi J, Carvajal Berrio DA, Jakubowski P, Neis F, Pauluschke-Fröhlich J, Brucker SY, et al. Laparoscopic Peritoneal Wash Cytology-Derived Primary Human Mesothelial Cells for In Vitro Cell Culture and Simulation of Human Peritoneum. Biomedicines. 2021; 9(2):176. https://doi.org/10.3390/biomedicines9020176
Chicago/Turabian StyleHoll, Myriam, Lucas Becker, Anna-Lena Keller, Nora Feuerer, Julia Marzi, Daniel A. Carvajal Berrio, Peter Jakubowski, Felix Neis, Jan Pauluschke-Fröhlich, Sara Y. Brucker, and et al. 2021. "Laparoscopic Peritoneal Wash Cytology-Derived Primary Human Mesothelial Cells for In Vitro Cell Culture and Simulation of Human Peritoneum" Biomedicines 9, no. 2: 176. https://doi.org/10.3390/biomedicines9020176
APA StyleHoll, M., Becker, L., Keller, A.-L., Feuerer, N., Marzi, J., Carvajal Berrio, D. A., Jakubowski, P., Neis, F., Pauluschke-Fröhlich, J., Brucker, S. Y., Schenke-Layland, K., Krämer, B., & Weiss, M. (2021). Laparoscopic Peritoneal Wash Cytology-Derived Primary Human Mesothelial Cells for In Vitro Cell Culture and Simulation of Human Peritoneum. Biomedicines, 9(2), 176. https://doi.org/10.3390/biomedicines9020176








