Monoassociation of Preterm Germ-Free Piglets with Bifidobacterium animalis Subsp. lactis BB-12 and Its Impact on Infection with Salmonella Typhimurium
Abstract
:1. Introduction
2. Material and Methods
2.1. Bacterial Cultures and Inocula
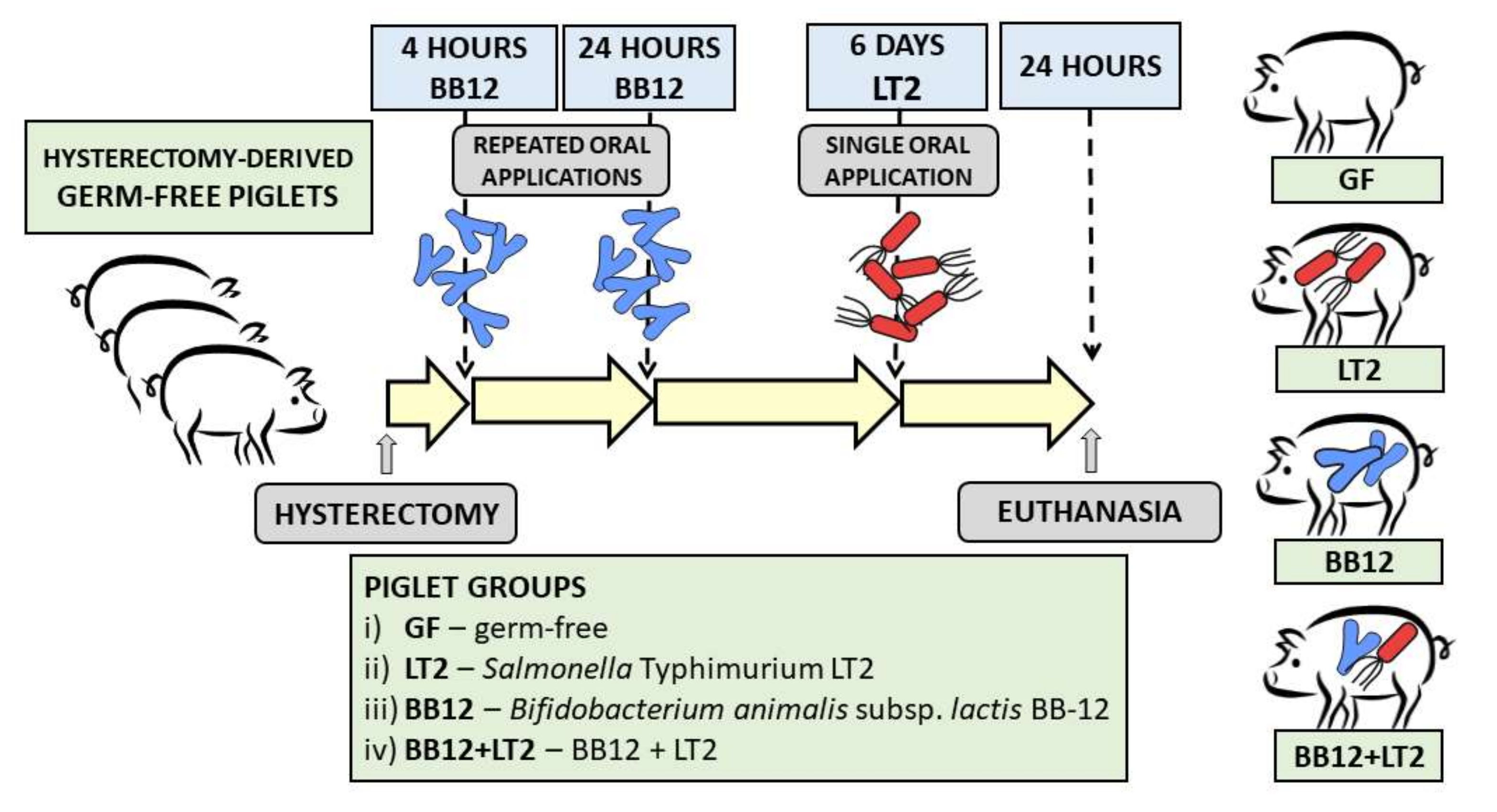
2.2. Preterm Gnotobiotic Piglets
2.3. Clinical Signs of Salmonellosis
2.4. Bacterial Counts in the Intestine and Translocation to Organs
2.5. Ileal Morphometry and Histopathological Evaluation
2.6. Intestinal Lavage and Blood Plasma
2.7. Total RNA Isolation and Reverse Transcription
2.8. LNA Probe-Based Real-Time PCR
2.9. Luminex xMAP Technology
2.10. Statistical Analysis
3. Results
3.1. Clinical Signs of Salmonellosis
3.2. Colonization of the Intestine with Bifidobacterium animalis Subsp. lactis BB-12 and Its Translocation
3.3. Colonization of the Intestine with Salmonella Typhimurium LT2 and Its Translocation
3.4. Histopathological Evaluation of the Ileum
3.5. Changes in Claudin-1 and Occludin mRNA Expression in the Ileum and Colon
3.6. Intestinal Inflammatory Cytokine Concentrations
3.7. Plasma Inflammatory Cytokine Concentrations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Donovan, S.M. Evolution of the gut microbiome in infancy within an ecological context. Curr. Opin. Clin. Nutr. Metab. Care 2020, 23, 223–227. [Google Scholar] [CrossRef]
- Rutayisire, E.; Huang, K.; Liu, Y.; Tao, F. The mode of delivery affects the diversity and colonization pattern of the gut microbiota during the first year of infants’ life: A systematic review. BMC Gastroenterol. 2016, 16, 86. [Google Scholar] [CrossRef] [Green Version]
- Davis, E.C.; Dinsmoor, A.M.; Wang, M.; Donovan, S.M. Microbiome composition in pediatric populations from birth to adolescence: Impact of diet and prebiotic and probiotic interventions. Dig. Dis. Sci. 2020, 65, 706–722. [Google Scholar] [CrossRef] [Green Version]
- Milani, C.; Duranti, S.; Bottacini, F.; Casey, E.; Turroni, F.; Mahony, J.; Belzer, C.; Delgado, P.S.; Arboleya, M.S.; Mancabelli, L.; et al. The first microbial colonizers of the human gut: Composition, activities, and health implications of the infant gut microbiota. Microbiol. Mol. Biol. Rev. 2017, 81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagpal, R.; Tsuji, H.; Takahashi, T.; Nomoto, K.; Kawashima, K.; Nagata, S.; Yamashiro, Y. Gut dysbiosis following C-section instigates higher colonisation of toxigenic Clostridium perfringens in infants. Benef. Microbes. 2017, 8, 353–365. [Google Scholar] [CrossRef] [PubMed]
- Osuchowski, M.F.; Ayala, A.; Bahrami, S.; Bauer, M.; Boros, M.; Cavaillon, J.M.; Chaudry, I.H.; Coopersmith, C.M.; Deutschman, C.; Drechsler, S.; et al. Minimum quality threshold in pre-clinical sepsis studies (MQTiPSS): An international expert consensus initiative for improvement of animal modeling in sepsis. Infection 2018, 46, 687–691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tlaskalova-Hogenova, H.; Stepankova, R.; Kozakova, H.; Hudcovic, T.; Vannucci, L.; Tuckova, L.; Rossmann, P.; Hrncir, T.; Kverka, M.; Zakostelska, Z.; et al. The role of gut microbiota (commensal bacteria) and the mucosal barrier in the pathogenesis of inflammatory and autoimmune diseases and cancer: Contribution of germ-free and gnotobiotic animal models of human diseases. Cell. Mol. Immunol. 2011, 8, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Burrin, D.; Sangild, P.T.; Stoll, B.; Thymann, T.; Buddington, R.; Marini, J.; Olutoye, O.; Shulman, R.J. Translational advances in pediatric Nutrition and gastroenterology: New insights from pig models. Annu. Rev. Anim. Biosci. 2020, 8, 321–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meurens, F.; Summerfield, A.; Nauwynck, H.; Saif, L.; Gerdts, V. The pig: A model for human infectious diseases. Trends Microbiol. 2012, 20, 50–57. [Google Scholar] [CrossRef]
- Waterhouse, A.; Leslie, D.C.; Bolgen, D.E.; Lightbown, S.; Dimitrakakis, N.; Cartwright, M.J.; Seiler, B.; Lightbown, K.; Smith, K.; Lombardo, P.; et al. Modified clinical monitoring assesment criteria for multi-organ failure during bacteremia and sepsis progression in a pig model. Advan Crit. Care Med. 2018, 1, 002. [Google Scholar]
- Xiao, L.; Estelle, J.; Kiilerich, P.; Ramayo-Caldas, Y.; Xia, Z.; Feng, Q.; Liang, S.; Pedersen, A.O.; Kjeldsen, N.J.; Liu, C.; et al. A reference gene catalogue of the pig gut microbiome. Nat. Microbiol. 2016, 1, 16161. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, L.; Feng, Q.; Liang, S.; Sonne, S.B.; Xia, Z.; Qiu, X.; Li, X.; Long, H.; Zhang, J.; Zhang, D.; et al. A catalog of the mouse gut metagenome. Nat. Biotechnol. 2015, 33, 1103–1108. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Hua, X.; Yang, Q.; Ding, D.; Che, C.; Cui, L.; Jia, W.; Bucheli, P.; Zhao, L. Inter-species transplantation of gut microbiota from human to pigs. ISME J. 2007, 1, 156–162. [Google Scholar] [CrossRef]
- Wang, M.; Donovan, S.M. Human microbiota-associated swine: Current progress and future opportunities. ILAR J. 2015, 56, 63–73. [Google Scholar] [CrossRef]
- Fischer, D.D.; Kandasamy, S.; Paim, F.C.; Langel, S.N.; Alhamo, M.A.; Shao, L.; Chepngeno, J.; Miyazaki, A.; Huang, H.C.; Kumar, A.; et al. Protein malnutrition alters tryptophan and angiotensin-converting enzyme 2 homeostasis and adaptive immune responses in human rotavirus-infected gnotobiotic pigs with human infant fecal microbiota transplant. Clin. Vaccine Immunol. 2017, 24. [Google Scholar] [CrossRef] [Green Version]
- Kverka, M.; Tlaskalova-Hogenova, H. Intestinal Microbiota: Facts and fiction. Dig. Dis. 2017, 35, 139–147. [Google Scholar] [CrossRef]
- Schroeder, B.O.; Backhed, F. Signals from the gut microbiota to distant organs in physiology and disease. Nat. Med. 2016, 22, 1079–1089. [Google Scholar] [CrossRef]
- Roberts, R.M.; Green, J.A.; Schulz, L.C. The evolution of the placenta. Reproduction 2016, 152, R179–R189. [Google Scholar] [CrossRef] [Green Version]
- Salmon, H.; Berri, M.; Gerdts, V.; Meurens, F. Humoral and cellular factors of maternal immunity in swine. Dev. Comp. Immunol. 2009, 33, 384–393. [Google Scholar] [CrossRef]
- Splichalova, A.; Slavikova, V.; Splichalova, Z.; Splichal, I. Preterm life in sterile conditions: A study on preterm, germ-free piglets. Front. Immunol. 2018, 9, 220. [Google Scholar] [CrossRef] [Green Version]
- Trebichavsky, I.; Rada, V.; Splichalova, A.; Splichal, I. Cross-talk of human gut with bifidobacteria. Nutr. Rev. 2009, 67, 77–82. [Google Scholar] [CrossRef]
- Turroni, F.; Peano, C.; Pass, D.A.; Foroni, E.; Severgnini, M.; Claesson, M.J.; Kerr, C.; Hourihane, J.; Murray, D.; Fuligni, F.; et al. Diversity of bifidobacteria within the infant gut microbiota. PLoS ONE 2012, 7, e36957. [Google Scholar] [CrossRef] [Green Version]
- Fushinobu, S. Unique sugar metabolic pathways of bifidobacteria. Biosci. Biotechnol. Biochem. 2010, 74, 2374–2384. [Google Scholar] [CrossRef]
- Turroni, F.; Milani, C.; Duranti, S.; Ferrario, C.; Lugli, G.A.; Mancabelli, L.; van, S.D.; Ventura, M. Bifidobacteria and the infant gut: An example of co-evolution and natural selection. Cell. Mol. Life Sci. 2018, 75, 103–118. [Google Scholar] [CrossRef]
- Bottacini, F.; van, S.D.; Ventura, M. Omics of bifidobacteria: Research and insights into their health-promoting activities. Biochem. J. 2017, 474, 4137–4152. [Google Scholar] [CrossRef] [PubMed]
- Abdulkadir, B.; Nelson, A.; Skeath, T.; Marrs, E.C.; Perry, J.D.; Cummings, S.P.; Embleton, N.D.; Berrington, J.E.; Stewart, C.J. Routine use of probiotics in preterm infants: Longitudinal impact on the microbiome and metabolome. Neonatology 2016, 109, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Bertelli, C.; Pillonel, T.; Torregrossa, A.; Prod’hom, G.; Fischer, C.J.; Greub, G.; Giannoni, E. Bifidobacterium longum bacteremia in preterm infants receiving probiotics. Clin. Infect. Dis. 2015, 60, 924–927. [Google Scholar] [CrossRef] [Green Version]
- Zbinden, A.; Zbinden, R.; Berger, C.; Arlettaz, R. Case series of Bifidobacterium longum bacteremia in three preterm infants on probiotic therapy. Neonatology 2015, 107, 56–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ducarmon, Q.R.; Zwittink, R.D.; Hornung, B.V.H.; van, S.W.; Young, V.B.; Kuijper, E.J. Gut icrobiota and colonization resistance against bacterial enteric infection. Microbiol. Mol. Biol. Rev. 2019, 83. [Google Scholar] [CrossRef] [PubMed]
- Hurley, D.; McCusker, M.P.; Fanning, S.; Martins, M. Salmonella-host interactions—modulation of the host innate immune system. Front. Immunol. 2014, 5, 481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Kingsley, R.A.; Santos, R.L.; Andrews-Polymenis, H.; Raffatellu, M.; Figueiredo, J.; Nunes, J.; Tsolis, R.M.; Adams, L.G.; Baumler, A.J. Molecular pathogenesis of Salmonella enterica serotype Typhimurium-induced diarrhea. Infect. Immun. 2003, 71, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaiser, P.; Hardt, W.D. Salmonella typhimurium diarrhea: Switching the mucosal epithelium from homeostasis to defense. Curr. Opin. Immunol. 2011, 23, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Campos, J.; Mourao, J.; Peixe, L.; Antunes, P. Non-typhoidal Salmonella in the pig production chain: A comprehensive analysis of Its impact on human health. Pathogens 2019, 8, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barthel, M.; Hapfelmeier, S.; Quintanilla-Martinez, L.; Kremer, M.; Rohde, M.; Hogardt, M.; Pfeffer, K.; Russmann, H.; Hardt, W.D. Pretreatment of mice with streptomycin provides a Salmonella enterica serovar Typhimurium colitis model that allows analysis of both pathogen and host. Infect. Immun. 2003, 71, 2839–2858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, S.C.; Best, E.; Nourse, C. Non-typhoidal Salmonella infections in children: Review of literature and recommendations for management. J. Paediatr. Child. Health 2017, 53, 936–941. [Google Scholar] [CrossRef]
- Czaplewski, L.; Bax, R.; Clokie, M.; Dawson, M.; Fairhead, H.; Fischetti, V.A.; Foster, S.; Gilmore, B.F.; Hancock, R.E.; Harper, D.; et al. Alternatives to antibiotics-a pipeline portfolio review. Lancet Infect. Dis. 2016, 16, 239–251. [Google Scholar] [CrossRef] [Green Version]
- Jackson, N.; Czaplewski, L.; Piddock, L.J.V. Discovery and development of new antibacterial drugs: Learning from experience? J. Antimicrob. Chemother. 2018, 73, 1452–1459. [Google Scholar] [CrossRef] [Green Version]
- Stavropoulou, E.; Bezirtzoglou, E. Probiotics in medicine: A long debate. Front. Immunol. 2020, 11, 2192. [Google Scholar] [CrossRef]
- Splichalova, A.; Pechar, R.; Killer, J.; Splichalova, Z.; Neuzil Bunesova, V.; Vlkova, E.; Subrtova Salmonova, H.; Splichal, I. Colonization of germ-free piglets with mucinolytic and non-mucinolytic Bifidobacterium boum strains isolated from the intestine of wild boar and their interference with Salmonella Typhimurium. Microorganisms 2020, 8, 2002. [Google Scholar] [CrossRef]
- Splichalova, A.; Jenistova, V.; Splichalova, Z.; Splichal, I. Colonization of preterm gnotobiotic piglets with probiotic Lactobacillus rhamnosus GG and its interference with Salmonella Typhimurium. Clin. Exp. Immunol. 2019, 195, 381–394. [Google Scholar] [CrossRef]
- Splichal, I.; Donovan, S.M.; Splichalova, Z.; Neuzil Bunesova, V.; Vlkova, E.; Jenistova, V.; Killer, J.; Svejstil, R.; Skrivanova, E.; Splichalova, A. Colonization of germ-free piglets with commensal Lactobacillus amylovorus, Lactobacillus mucosae, and probiotic E. coli Nissle 1917 and their interference with Salmonella Typhimurium. Microorganisms 2019, 7, 273. [Google Scholar] [CrossRef] [Green Version]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Fanaroff, A.A.; Stoll, B.J.; Wright, L.L.; Carlo, W.A.; Ehrenkranz, R.A.; Stark, A.R.; Bauer, C.R.; Donovan, E.F.; Korones, S.B.; Laptook, A.R.; et al. Trends in neonatal morbidity and mortality for very low birthweight infants. Am. J. Obstet. Gynecol. 2007, 196, 147–148. [Google Scholar] [CrossRef] [PubMed]
- Stoll, B.J.; Hansen, N.I.; Bell, E.F.; Walsh, M.C.; Carlo, W.A.; Shankaran, S.; Laptook, A.R.; Sanchez, P.J.; Van Meurs, K.P.; Wyckoff, M.; et al. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993–2012. JAMA 2015, 314, 1039–1051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sangild, P.T.; Thymann, T.; Schmidt, M.; Stoll, B.; Burrin, D.G.; Buddington, R.K. Invited review: The preterm pig as a model in pediatric gastroenterology. J. Anim. Sci. 2013, 91, 4713–4729. [Google Scholar] [CrossRef] [Green Version]
- Bunesova, V.; Vlkova, E.; Rada, V.; Killer, J.; Musilova, S. Bifidobacteria from the gastrointestinal tract of animals: Differences and similarities. Benef. Microbes 2014, 5, 377–388. [Google Scholar] [CrossRef]
- Nagpal, R.; Kurakawa, T.; Tsuji, H.; Takahashi, T.; Kawashima, K.; Nagata, S.; Nomoto, K.; Yamashiro, Y. Evolution of gut Bifidobacterium population in healthy Japanese infants over the first three years of life: A quantitative assessment. Sci. Rep. 2017, 7, 10097. [Google Scholar] [CrossRef] [PubMed]
- Lamendella, R.; Santo Domingo, J.W.; Kelty, C.; Oerther, D.B. Bifidobacteria in feces and environmental waters. Appl. Environ. Microbiol. 2008, 74, 575–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Killer, J.; Mrazek, J.; Bunesova, V.; Havlik, J.; Koppova, I.; Benada, O.; Rada, V.; Kopecny, J.; Vlkova, E. Pseudoscardovia suis gen. nov., sp. nov., a new member of the family Bifidobacteriaceae isolated from the digestive tract of wild pigs (Sus scrofa). Syst. Appl. Microbiol. 2013, 36, 11–16. [Google Scholar] [CrossRef]
- Vlasova, A.N.; Chattha, K.S.; Kandasamy, S.; Liu, Z.; Esseili, M.; Shao, L.; Rajashekara, G.; Saif, L.J. Lactobacilli and bifidobacteria promote immune homeostasis by modulating innate immune responses to human rotavirus in neonatal gnotobiotic pigs. PLoS ONE 2013, 8, e76962. [Google Scholar] [CrossRef]
- Splichalova, A.; Trebichavsky, I.; Rada, V.; Vlkova, E.; Sonnenborn, U.; Splichal, I. Interference of Bifidobacterium choerinum or Escherichia coli Nissle 1917 with Salmonella Typhimurium in gnotobiotic piglets correlates with cytokine patterns in blood and intestine. Clin. Exp. Immunol. 2011, 163, 242–249. [Google Scholar] [CrossRef] [PubMed]
- van den Akker, C.H.P.; van Goudoever, J.B.; Shamir, R.; Domellof, M.; Embleton, N.D.; Hojsak, I.; Lapillonne, A.; Mihatsch, W.A.; Berni, C.R.; Bronsky, J.; et al. Probiotics and preterm infants: A position paper by the European society for paediatric gastroenterology hepatology and nutrition committee on nutrition and the European society for paediatric gastroenterology hepatology and nutrition working group for probiotics and prebiotics. J. Pediatr. Gastroenterol. Nutr. 2020, 70, 664–680. [Google Scholar] [CrossRef] [PubMed]
- van den Akker, C.H.P.; van Goudoever, J.B.; Szajewska, H.; Embleton, N.D.; Hojsak, I.; Reid, D.; Shamir, R. Probiotics for preterm infants: A strain-specific systematic review and network meta-analysis. J. Pediatr. Gastroenterol. Nutr. 2018, 67, 103–122. [Google Scholar] [CrossRef]
- Bunesova, V.; Killer, J.; Javurkova, B.; Vlkova, E.; Tejnecky, V.; Musilova, S.; Rada, V. Diversity of the subspecies Bifidobacterium animalis subsp. lactis. Anaerobe 2017, 44, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Dronkers, T.M.G.; Ouwehand, A.C.; Rijkers, G.T. Global analysis of clinical trials with probiotics. Heliyon 2020, 6, e04467. [Google Scholar] [CrossRef]
- Trebichavsky, I.; Splichal, I.; Rada, V.; Splichalova, A. Modulation of natural immunity in the gut by Escherichia coli strain Nissle 1917. Nutr. Rev. 2010, 68, 459–464. [Google Scholar] [CrossRef]
- Clarke, R.C.; Gyles, C.L. Virulence of wild and mutant strains of Salmonella typhimurium in ligated intestinal segments of calves, pigs, and rabbits. Am. J. Vet. Res. 1987, 48, 504–510. [Google Scholar]
- Splichal, I.; Rychlik, I.; Splichalova, I.; Karasova, D.; Splichalova, A. Toll-Like receptor 4 signaling in the ileum and colon of gnotobiotic piglets infected with Salmonella Typhimurium or its isogenic Δrfa mutants. Toxins 2020, 12, 545. [Google Scholar] [CrossRef]
- Fukuda, S.; Toh, H.; Hase, K.; Oshima, K.; Nakanishi, Y.; Yoshimura, K.; Tobe, T.; Clarke, J.M.; Topping, D.L.; Suzuki, T.; et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature 2011, 469, 543–547. [Google Scholar] [CrossRef]
- Behnsen, J.; Perez-Lopez, A.; Nuccio, S.P.; Raffatellu, M. Exploiting host immunity: The Salmonella paradigm. Trends Immunol. 2015, 36, 112–120. [Google Scholar] [CrossRef] [Green Version]
- Arguello, H.; Estelle, J.; Zaldivar-Lopez, S.; Jimenez-Marin, A.; Carvajal, A.; Lopez-Bascon, M.A.; Crispie, F.; O’Sullivan, O.; Cotter, P.D.; Priego-Capote, F.; et al. Early Salmonella Typhimurium infection in pigs disrupts microbiome composition and functionality principally at the ileum mucosa. Sci. Rep. 2018, 8, 7788. [Google Scholar] [CrossRef] [Green Version]
- Nagpal, R.; Yadav, H. Bacterial translocation from the gut to the distant organs: An overview. Ann. Nutr. Metab. 2017, 71 (Suppl. 1), 11–16. [Google Scholar] [CrossRef]
- Goldstein, G.P.; Sylvester, K.G. Biomarker discovery and utility in necrotizing enterocolitis. Clin. Perinatol. 2019, 46, 1–17. [Google Scholar] [CrossRef]
- Zhang, K.; Griffiths, G.; Repnik, U.; Hornef, M. Seeing is understanding: Salmonella’s way to penetrate the intestinal epithelium. Int. J. Med. Microbiol. 2018, 308, 97–106. [Google Scholar] [CrossRef]
- Santos, R.L.; Tsolis, R.M.; Baumler, A.J.; Adams, L.G. Pathogenesis of Salmonella-induced enteritis. Braz. J. Med. Biol. Res. 2003, 36, 3–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pammi, M.; Cope, J.; Tarr, P.I.; Warner, B.B.; Morrow, A.L.; Mai, V.; Gregory, K.E.; Kroll, J.S.; McMurtry, V.; Ferris, M.J.; et al. Intestinal dysbiosis in preterm infants preceding necrotizing enterocolitis: A systematic review and meta-analysis. Microbiome 2017, 5, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dilli, D.; Aydin, B.; Fettah, N.D.; Ozyazici, E.; Beken, S.; Zenciroglu, A.; Okumus, N.; Ozyurt, B.M.; Ipek, M.S.; Akdag, A.; et al. The propre-save study: Effects of probiotics and prebiotics alone or combined on necrotizing enterocolitis in very low birth weight infants. J. Pediatr. 2015, 166, 545–551. [Google Scholar] [CrossRef] [PubMed]
- LaRock, D.L.; Chaudhary, A.; Miller, S.I. Salmonellae interactions with host processes. Nat. Rev. Microbiol. 2015, 13, 191–205. [Google Scholar] [CrossRef] [PubMed]
- Perez-Lopez, A.; Behnsen, J.; Nuccio, S.P.; Raffatellu, M. Mucosal immunity to pathogenic intestinal bacteria. Nat. Rev. Immunol. 2016, 16, 135–148. [Google Scholar] [CrossRef]
- Splichalova, A.; Splichalova, Z.; Karasova, D.; Rychlik, I.; Trevisi, P.; Sinkora, M.; Splichal, I. Impact of the Lipopolysaccharide chemotype of Salmonella enterica serovar Typhimurium on virulence in gnotobiotic piglets. Toxins 2019, 11, 534. [Google Scholar] [CrossRef] [Green Version]
- Kohler, H.; Sakaguchi, T.; Hurley, B.P.; Kase, B.A.; Reinecker, H.C.; McCormick, B.A. Salmonella enterica serovar Typhimurium regulates intercellular junction proteins and facilitates transepithelial neutrophil and bacterial passage. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 293, G178–G187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foster, N.; Lovell, M.A.; Marston, K.L.; Hulme, S.D.; Frost, A.J.; Bland, P.; Barrow, P.A. Rapid protection of gnotobiotic pigs against experimental salmonellosis following induction of polymorphonuclear leukocytes by avirulent Salmonella enterica. Infect. Immun. 2003, 71, 2182–2191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Splichal, I.; Trebichavsky, I.; Splichalova, A.; Barrow, P.A. Protection of gnotobiotic pigs against Salmonella enterica serotype Typhimurium by rough mutant of the same serotype is accompanied by the change of local and systemic cytokine response. Vet. Immunol. Immunopathol. 2005, 103, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, V.K.; Hodges, K.; Hecht, G. Enteric infection meets intestinal function: How bacterial pathogens cause diarrhoea. Nat. Rev. Microbiol. 2009, 7, 110–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gunzel, D.; Yu, A.S. Claudins and the modulation of tight junction permeability. Physiol. Rev. 2013, 93, 525–569. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Xia, B.; He, T.; Li, D.; Su, J.H.; Guo, L.; Wang, J.F.; Zhu, Y.H. Oral administration of a select mixture of Lactobacillus and Bacillus alleviates inflammation and maintains mucosal barrier integrity in the ileum of pigs challenged with Salmonella Infantis. Microorganisms 2019, 7, 135. [Google Scholar] [CrossRef] [Green Version]
- Al-Sadi, R.; Khatib, K.; Guo, S.; Ye, D.; Youssef, M.; Ma, T. Occludin regulates macromolecule flux across the intestinal epithelial tight junction barrier. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 300, G1054–G1064. [Google Scholar] [CrossRef] [Green Version]
- Edelblum, K.L.; Shen, L.; Weber, C.R.; Marchiando, A.M.; Clay, B.S.; Wang, Y.; Prinz, I.; Malissen, B.; Sperling, A.I.; Turner, J.R. Dynamic migration of gammadelta intraepithelial lymphocytes requires occludin. Proc. Natl. Acad. Sci. USA 2012, 109, 7097–7102. [Google Scholar] [CrossRef] [Green Version]
- Dalton, J.E.; Cruickshank, S.M.; Egan, C.E.; Mears, R.; Newton, D.J.; Andrew, E.M.; Lawrence, B.; Howell, G.; Else, K.J.; Gubbels, M.J.; et al. Intraepithelial gammadelta+ lymphocytes maintain the integrity of intestinal epithelial tight junctions in response to infection. Gastroenterology 2006, 131, 818–829. [Google Scholar] [CrossRef]
- Ziesmann, M.T.; Marshall, J.C. Multiple organ dysfunction: The defining syndrome of sepsis. Surg. Infect. 2018, 19, 184–190. [Google Scholar] [CrossRef]
- Delanghe, J.R.; Speeckaert, M.M. Translational research and biomarkers in neonatal sepsis. Clin. Chim. Acta 2015, 451, 46–64. [Google Scholar] [CrossRef]
- Collins, A.; Weitkamp, J.H.; Wynn, J.L. Why are preterm newborns at increased risk of infection? Arch. Dis. Child. Fetal Neonatal Ed. 2018, 103, F391–F394. [Google Scholar] [CrossRef]
- Sampah, M.E.S.; Hackam, D.J. Dysregulated mucosal immunity and associated pathogeneses in preterm neonates. Front. Immunol. 2020, 11, 899. [Google Scholar] [CrossRef]
- Baggiolini, M.; Clark-Lewis, I. Interleukin-8, a chemotactic and inflammatory cytokine. FEBS Lett. 1992, 307, 97–101. [Google Scholar] [CrossRef] [Green Version]
- Pfeffer, K. Biological functions of tumor necrosis factor cytokines and their receptors. Cytokine Growth Factor Rev. 2003, 14, 185–191. [Google Scholar] [CrossRef]
- Saraiva, M.; Vieira, P.; O’Garra, A. Biology and therapeutic potential of interleukin-10. J. Exp. Med. 2020, 217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Splichal, I.; Splichalova, A. Experimental enteric bacterial infections in pigs. J. Infect. Dis 2018, 218, 504–505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hibbert, J.E.; Currie, A.; Strunk, T. Sepsis-induced immunosuppression in neonates. Front Pediatr 2018, 6, 357. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, H.; Zhou, J.; Zhong, Y.; Ali, M.M.; McGuire, F.; Nagarkatti, P.S.; Nagarkatti, M. Role of cytokines as a double-edged sword in sepsis. In Vivo 2013, 27, 669–684. [Google Scholar]
- Gogos, C.A.; Drosou, E.; Bassaris, H.P.; Skoutelis, A. Pro- versus anti-inflammatory cytokine profile in patients with severe sepsis: A marker for prognosis and future therapeutic options. J. Infect. Dis. 2000, 181, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Splichalova, A.; Splichal, I. Local and systemic occurrences of HMGB1 in gnotobiotic piglets infected with E. coli O55 are related to bacterial translocation and inflammatory cytokines. Cytokine 2012, 60, 597–600. [Google Scholar] [CrossRef] [PubMed]






| Gene | 5′-Forward Primer-3′ | 5′-Reverse Primer-3′ | #LNA Probe |
|---|---|---|---|
| BACT 1 | TCCCTGGAGAAGAGCTACGA | AAGAGCGCCTCTGGACAC | 9 |
| CYPA 2 | CCTGAAGCATACGGGTCCT | AAAGACCACATGTTTGCCATC | 48 |
| CLD-1 3 | CACCACTTTGCAAGCAACC | TGGCCACAAAGATGGCTATT | 3 |
| OCLN 4 | AAAGAGCTCTCTCGACTGGATAAA | AGCAGCAGCCATGTACTCTTC | 42 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Splichalova, A.; Donovan, S.M.; Tlaskalova-Hogenova, H.; Stranak, Z.; Splichalova, Z.; Splichal, I. Monoassociation of Preterm Germ-Free Piglets with Bifidobacterium animalis Subsp. lactis BB-12 and Its Impact on Infection with Salmonella Typhimurium. Biomedicines 2021, 9, 183. https://doi.org/10.3390/biomedicines9020183
Splichalova A, Donovan SM, Tlaskalova-Hogenova H, Stranak Z, Splichalova Z, Splichal I. Monoassociation of Preterm Germ-Free Piglets with Bifidobacterium animalis Subsp. lactis BB-12 and Its Impact on Infection with Salmonella Typhimurium. Biomedicines. 2021; 9(2):183. https://doi.org/10.3390/biomedicines9020183
Chicago/Turabian StyleSplichalova, Alla, Sharon M. Donovan, Helena Tlaskalova-Hogenova, Zbynek Stranak, Zdislava Splichalova, and Igor Splichal. 2021. "Monoassociation of Preterm Germ-Free Piglets with Bifidobacterium animalis Subsp. lactis BB-12 and Its Impact on Infection with Salmonella Typhimurium" Biomedicines 9, no. 2: 183. https://doi.org/10.3390/biomedicines9020183
APA StyleSplichalova, A., Donovan, S. M., Tlaskalova-Hogenova, H., Stranak, Z., Splichalova, Z., & Splichal, I. (2021). Monoassociation of Preterm Germ-Free Piglets with Bifidobacterium animalis Subsp. lactis BB-12 and Its Impact on Infection with Salmonella Typhimurium. Biomedicines, 9(2), 183. https://doi.org/10.3390/biomedicines9020183









