The Antibacterial Activity of Human Amniotic Membrane against Multidrug-Resistant Bacteria Associated with Urinary Tract Infections: New Insights from Normal and Cancerous Urothelial Models
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganisms
2.2. Biomimetic In Vitro Models of Normal and Cancerous Urinary Bladder Urothelium
2.3. The hAM Preparation
2.4. Antibacterial Susceptibility Testing on Agar Plates Using the hAM Homogenate and Various Antibiotic Discs
2.5. Analysis of the Antibacterial Activity of hAM Homogenate on Biomimetic In Vitro Models of Normal and Cancerous Urothelia Infected with MRSA
2.6. Cell Viability Assay
2.7. Scanning and Transmission Electron Microscopy
2.8. Statistical Analysis
3. Results
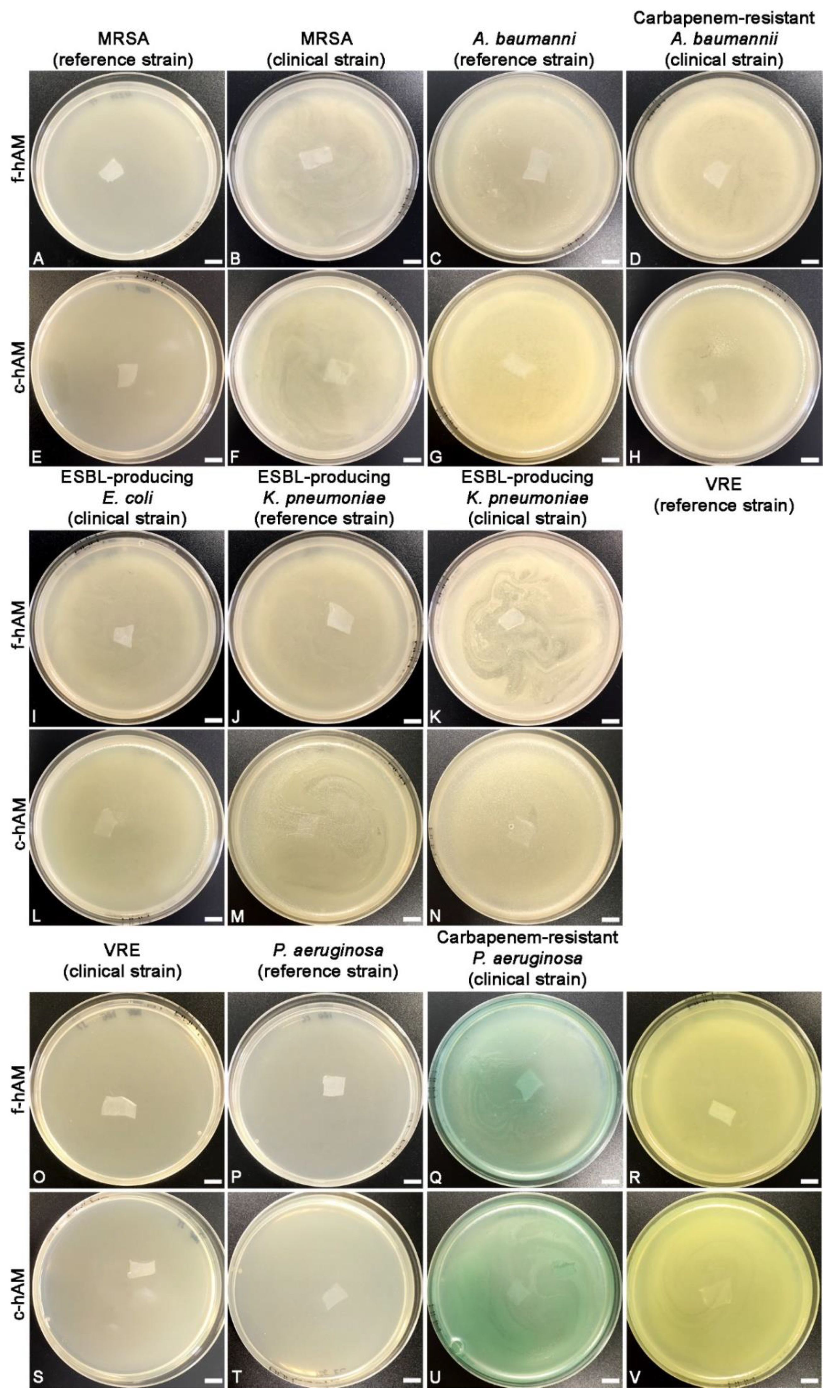
3.1. The hAM Patches Have No Antibacterial Activity against Selected Multidrug-Resistant Bacteria
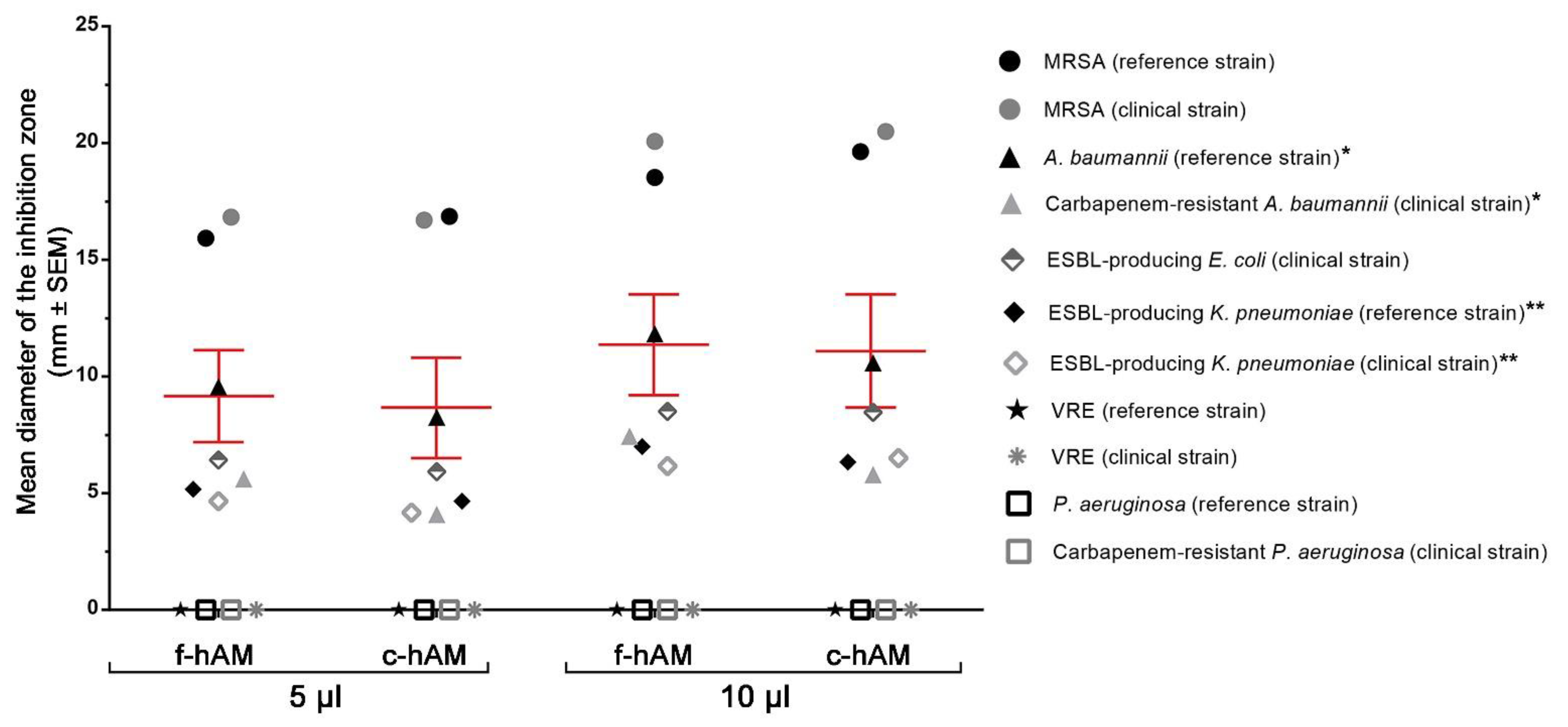
3.2. The hAM Homogenate Has Antibacterial Activity against Selected Multi-Drug Resistant Bacteria
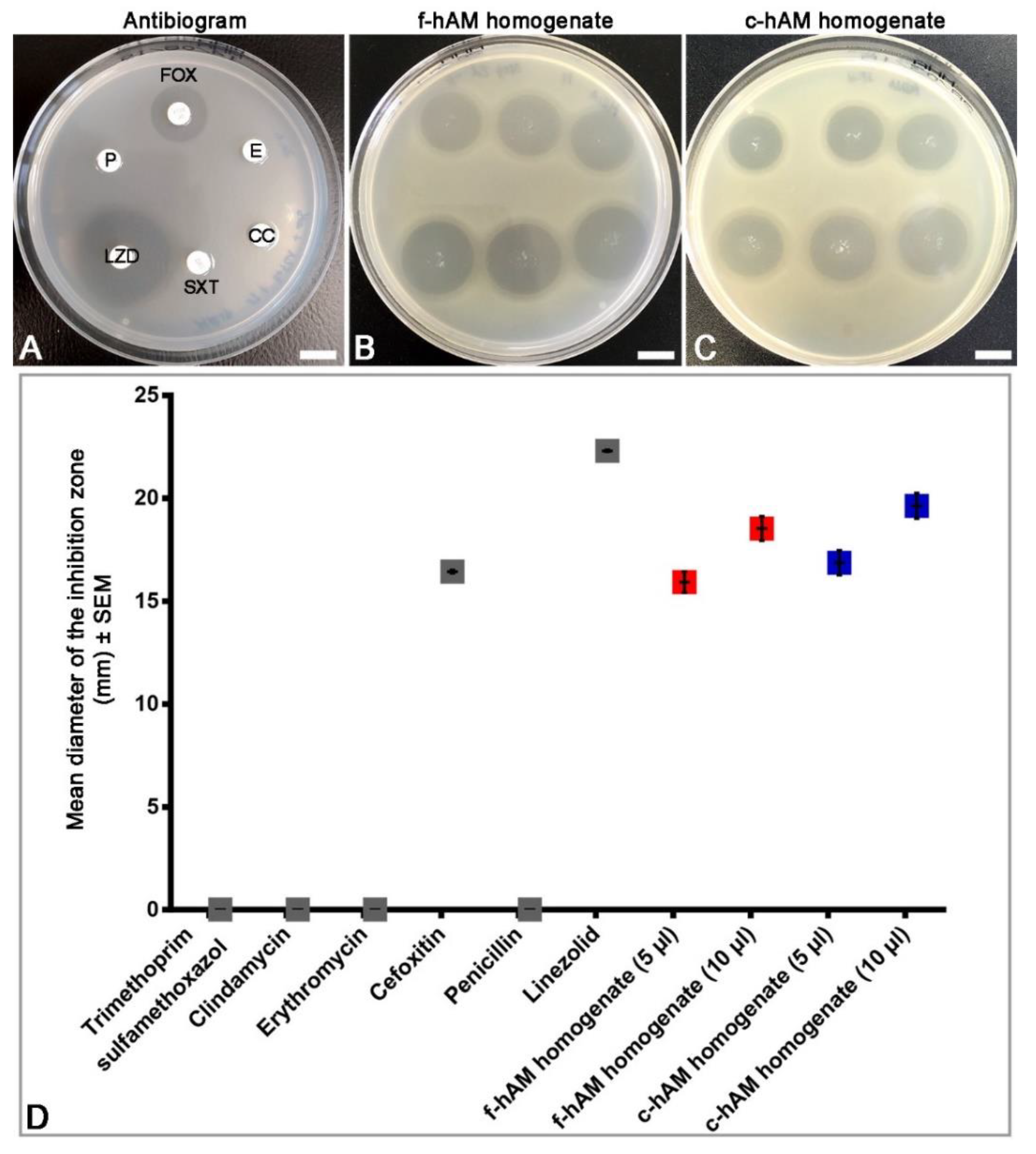
3.3. Comparison of the Antibacterial Activity of hAM Homogenates and Selected Antibiotics against MRSA
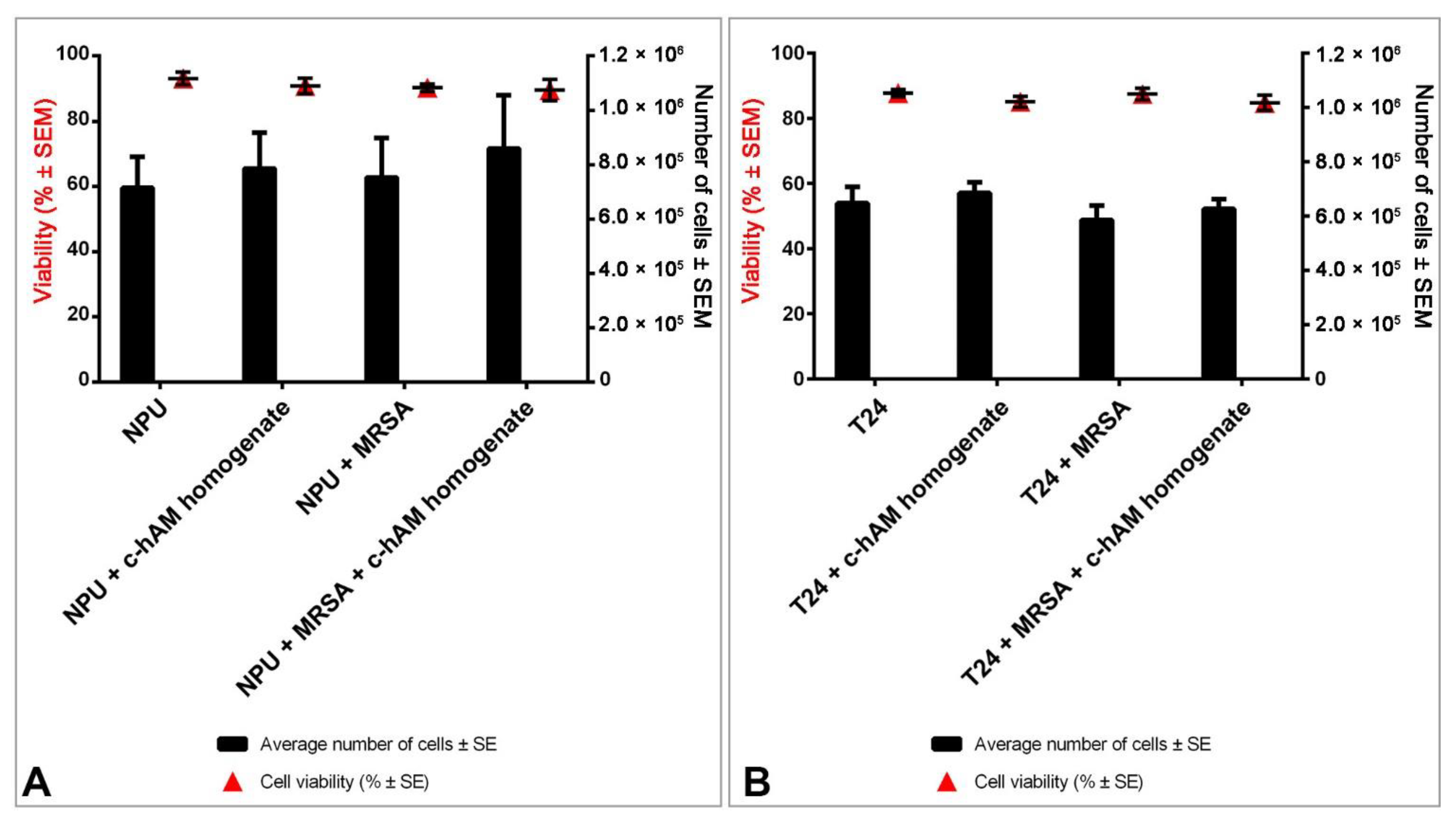
3.4. The c-hAM Homogenate Decreases the Number of Bacteria in Biomimetic In Vitro Models of the Normal and Cancerous Urothelium
3.5. A Short-Term Incubation in c-hAM Homogenate Does Not Affect the Cell Viability or Ultrastructure of Biomimetic In Vitro Models of the Normal and Cancerous Urothelium
4. Discussion
4.1. The hAM Patches Have No Antibacterial Activity against Multidrug-Resistant Bacteria
4.2. The hAM Homogenates Have Antibacterial Activity against Several Multidrug-Resistant Bacteria
4.3. The hAM Homogenate Demonstrated Antibacterial Activity against MRSA-Infected Biomimetic Models of the Normal and Cancerous Urothelium
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Waller, T.A.; Pantin, S.A.L.; Yenior, A.L.; Pujalte, G.G.A. Urinary Tract Infection Antibiotic Resistance in the United States. Prim. Care Clin. Off. Pract. 2018, 45, 455–466. [Google Scholar] [CrossRef]
- Morris, S.; Cerceo, E. Trends, Epidemiology, and Management of Multi-Drug Resistant Gram-Negative Bacterial Infections in the Hospitalized Setting. Antibiotics 2020, 9, 196. [Google Scholar] [CrossRef] [Green Version]
- Medina, M.; Castillo-Pino, E. An introduction to the epidemiology and burden of urinary tract infections. Ther. Adv. Urol. 2019, 11. [Google Scholar] [CrossRef] [Green Version]
- Ny, S.; Edquist, P.; Dumpis, U.; Gröndahl-Yli-Hannuksela, K.; Hermes, J.; Kling, A.M.; Klingeberg, A.; Kozlov, R.; Källman, O.; Lis, D.O.; et al. Antimicrobial resistance of Escherichia coli isolates from outpatient urinary tract infections in women in six European countries including Russia. J. Glob. Antimicrob. Resist. 2019, 17, 25–34. [Google Scholar] [CrossRef] [PubMed]
- van Driel, A.A.; Notermans, D.W.; Meima, A.; Mulder, M.; Donker, G.A.; Stobberingh, E.E.; Verbon, A. Antibiotic resistance of Escherichia coli isolated from uncomplicated UTI in general practice patients over a 10-year period. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 2151–2158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flores-Mireles, A.L.; Walker, J.N.; Caparon, M.; Hultgren, S.J. Urinary tract infections: Epidemiology, mechanisms of infection and treatment options. Nat. Rev. Microbiol. 2015, 13, 269–284. [Google Scholar] [CrossRef] [PubMed]
- Mazzariol, A.; Bazaj, A.; Cornaglia, G. Multi-drug-resistant Gram-negative bacteria causing urinary tract infections: A review. J. Chemother. 2017, 29, 2–9. [Google Scholar] [CrossRef] [Green Version]
- Ramírez-Castillo, F.Y.; Moreno-Flores, A.C.; Avelar-González, F.J.; Márquez-Díaz, F.; Harel, J.; Guerrero-Barrera, A.L. An evaluation of multidrug-resistant Escherichia coli isolates in urinary tract infections from Aguascalientes, Mexico: Cross-sectional study. Ann. Clin. Microbiol. Antimicrob. 2018, 17, 34. [Google Scholar] [CrossRef]
- van Driel, A. Antibiotic resistance of uropathogenic Escherichia coli and ESBL prevalence in general practice patients over 10 years. Br. J. Gen. Pr. 2020, 70. [Google Scholar] [CrossRef] [PubMed]
- Jean, S.S.; Coombs, G.; Ling, T.; Balaji, V.; Rodrigues, C.; Mikamo, H.; Kim, M.J.; Rajasekaram, D.G.; Mendoza, M.; Tan, T.Y.; et al. Epidemiology and antimicrobial susceptibility profiles of pathogens causing urinary tract infections in the Asia-Pacific region: Results from the Study for Monitoring Antimicrobial Resistance Trends (SMART), 2010–2013. Int. J. Antimicrob. Agents 2016, 47, 328–334. [Google Scholar] [CrossRef]
- Choe, H.S.; Lee, S.J.; Cho, Y.H.; Çek, M.; Tandoğdu, Z.; Wagenlehner, F.; Bjerklund-Johansen, T.E.; Naber, K. Aspects of urinary tract infections and antimicrobial resistance in hospitalized urology patients in Asia: 10-Year results of the Global Prevalence Study of Infections in Urology (GPIU). J. Infect. Chemother. 2018, 24, 278–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paul, R. State of the Globe: Rising Antimicrobial Resistance of Pathogens in Urinary Tract Infection. J. Glob. Infect. Dis. 2018, 10, 117–118. [Google Scholar] [CrossRef]
- Munita, J.M.; Arias, C.A. Mechanisms of Antibiotic Resistance. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef] [Green Version]
- Ramirez, M.S.; Tolmasky, M.E. Aminoglycoside modifying enzymes. Drug Resist. Updates 2010, 13, 151–171. [Google Scholar] [CrossRef] [Green Version]
- Wilson, D.N. Ribosome-targeting antibiotics and mechanisms of bacterial resistance. Nat. Rev. Microbiol. 2014, 12, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Bush, K. The ABCD’s of β-lactamase nomenclature. J. Infect. Chemother. 2013, 19, 549–559. [Google Scholar] [CrossRef]
- Nordmann, P.; Poirel, L.; Walsh, T.R.; Livermore, D.M. The emerging NDM carbapenemases. Trends Microbiol. 2011, 19, 588–595. [Google Scholar] [CrossRef]
- Pagès, J.M.; James, C.E.; Winterhalter, M. The porin and the permeating antibiotic: A selective diffusion barrier in Gram-negative bacteria. Nat. Rev. Microbiol. 2008, 6, 893–903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hancock, R.E.; Brinkman, F.S. Function of Pseudomonas porins in uptake and efflux. Annu. Rev. Microbiol. 2002, 56, 17–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poole, K. Efflux-mediated antimicrobial resistance. J. Antimicrob. Chemother. 2005, 56, 20–51. [Google Scholar] [CrossRef] [Green Version]
- Piddock, L.J. Clinically relevant chromosomally encoded multidrug resistance efflux pumps in bacteria. Clin. Microbiol. Rev. 2006, 19, 382–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dönhöfer, A.; Franckenberg, S.; Wickles, S.; Berninghausen, O.; Beckmann, R.; Wilson, D.N. Structural basis for TetM-mediated tetracycline resistance. Proc. Natl. Acad. Sci. USA 2012, 109, 16900–16905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aldred, K.J.; Kerns, R.J.; Osheroff, N. Mechanism of quinolone action and resistance. Biochemistry 2014, 53, 1565–1574. [Google Scholar] [CrossRef] [PubMed]
- Floss, H.G.; Yu, T.W. Rifamycin-mode of action, resistance, and biosynthesis. Chem. Rev. 2005, 105, 621–632. [Google Scholar] [CrossRef]
- Mendes, R.E.; Deshpande, L.M.; Jones, R.N. Linezolid update: Stable in vitro activity following more than a decade of clinical use and summary of associated resistance mechanisms. Drug Resist. Updat. 2014, 17, 1–12. [Google Scholar] [CrossRef]
- Roberts, M.C. Update on macrolide-lincosamide-streptogramin, ketolide, and oxazolidinone resistance genes. FEMS Microbiol. Lett. 2008, 282, 147–159. [Google Scholar] [CrossRef] [Green Version]
- Chambers, H.F.; Deleo, F.R. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat. Rev. Microbiol. 2009, 7, 629–641. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.R.; Munita, J.M.; Arias, C.A. Mechanisms of antibiotic resistance in enterococci. Expert Rev. Anti Infect. Ther. 2014, 12, 1221–1236. [Google Scholar] [CrossRef]
- Miller, W.R.; Murray, B.E.; Rice, L.B.; Arias, C.A. Vancomycin-Resistant Enterococci: Therapeutic Challenges in the 21st Century. Infect. Dis. Clin. N. Am. 2016, 30, 415–439. [Google Scholar] [CrossRef] [PubMed]
- Putty, S.; Vemula, H.; Bobba, S.; Gutheil, W.G. A liquid chromatography-tandem mass spectrometry assay for d-Ala-d-Lac: A key intermediate for vancomycin resistance in vancomycin-resistant enterococci. Anal. Biochem. 2013, 442, 166–171. [Google Scholar] [CrossRef]
- Vemula, H.; Ayon, N.J.; Gutheil, W.G. Cytoplasmic peptidoglycan intermediate levels in Staphylococcus aureus. Biochimie 2016, 121, 72–78. [Google Scholar] [CrossRef]
- Vemula, H.; Ayon, N.J.; Burton, A.; Gutheil, W.G. Antibiotic Effects on Methicillin-Resistant Staphylococcus aureus Cytoplasmic Peptidoglycan Intermediate Levels and Evidence for Potential Metabolite Level Regulatory Loops. Antimicrob. Agents Chemother. 2017, 61. [Google Scholar] [CrossRef] [Green Version]
- Reygaert, W.C. An overview of the antimicrobial resistance mechanisms of bacteria. AIMS Microbiol. 2018, 4, 482–501. [Google Scholar] [CrossRef]
- Bayer, A.S.; Schneider, T.; Sahl, H.G. Mechanisms of daptomycin resistance in Staphylococcus aureus: Role of the cell membrane and cell wall. Ann. N. Y. Acad. Sci. 2013, 1277, 139–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dadgostar, P. Antimicrobial Resistance: Implications and Costs. Infect. Drug Resist. 2019, 12, 3903–3910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Brien, V.P.; Hannan, T.J.; Nielsen, H.V.; Hultgren, S.J. Drug and Vaccine Development for the Treatment and Prevention of Urinary Tract Infections. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.; Wei, H.; Zhao, Y.; Shang, L.; Di, L.; Lyu, C.; Liu, J. Analysis of multidrug-resistant bacteria in 3223 patients with hospital-acquired infections (HAI) from a tertiary general hospital in China. Bosn. J. Basic Med. Sci. 2019, 19, 86–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Looney, A.T.; Redmond, E.J.; Davey, N.M.; Daly, P.J.; Troy, C.; Carey, B.F.; Cullen, I.M. Methicillin-resistant Staphylococcus aureus as a uropathogen in an Irish setting. Medicine 2017, 96, e4635. [Google Scholar] [CrossRef]
- Lunacek, A.; Koenig, U.; Mrstik, C.; Radmayr, C.; Horninger, W.; Plas, E. Unexpected Multidrug Resistance of Methicillin-Resistant Staphylococcus aureus in Urine Samples: A Single-Center Study. Korean J. Urol. 2014, 55, 349–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Araki, M.; Kariyama, R.; Monden, K.; Tsugawa, M.; Kumon, H. Molecular epidemiological studies of Staphylococcus aureus in urinary tract infection. J. Infect. Chemother. 2002, 8, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Picozzi, S.C.; Casellato, S.; Rossini, M.; Paola, G.; Tejada, M.; Costa, E.; Carmignani, L. Extended-spectrum β-lactamase-positive Escherichia coli causing complicated upper urinary tract infection: Urologist should act in time. Urol. Ann. 2014, 6, 107–112. [Google Scholar] [CrossRef]
- DeBusscher, J.; Zhang, L.; Buxton, M.; Foxman, B.; Barbosa-Cesnik, C. Persistent extended-spectrum β-lactamase urinary tract infection. Emerg. Infect. Dis. 2009, 15, 1862–1864. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, K.P.; Ranjan, N. Complicated urinary tract infection caused by extended spectrum β-lactamase-producing Escherichia coli. Urol. Ann. 2014, 6, 112–113. [Google Scholar] [PubMed]
- Caneiras, C.; Lito, L.; Melo-Cristino, J.; Duarte, A. Community- and Hospital-Acquired Klebsiella pneumoniae Urinary Tract Infections in Portugal: Virulence and Antibiotic Resistance. Microorganisms 2019, 7, 138. [Google Scholar] [CrossRef] [Green Version]
- Qureshi, Z.A.; Syed, A.; Clarke, L.G.; Doi, Y.; Shields, R.K. Epidemiology and clinical outcomes of patients with carbapenem-resistant Klebsiella pneumoniae bacteriuria. Antimicrob. Agents Chemother. 2014, 58, 3100–3104. [Google Scholar] [CrossRef] [Green Version]
- Jiménez-Guerra, G.; Heras-Cañas, V.; Gutiérrez-Soto, M.; Aznarte-Padial, M.D.P.; Expósito-Ruiz, M.; Navarro-Marí, J.M.; Gutiérrez-Fernández, J. Urinary tract infection by Acinetobacter baumannii and Pseudomonas aeruginosa: Evolution of antimicrobial resistance and therapeutic alternatives. J. Med. Microbiol. 2018. [Google Scholar] [CrossRef]
- Di Venanzio, G.; Flores-Mireles, A.L.; Calix, J.J.; Haurat, M.F.; Scott, N.E.; Palmer, L.D.; Potter, R.F.; Hibbing, M.E.; Friedman, L.; Wang, B.; et al. Urinary tract colonization is enhanced by a plasmid that regulates uropathogenic Acinetobacter baumannii chromosomal genes. Nat. Commun. 2019, 10, 2763. [Google Scholar] [CrossRef] [PubMed]
- Moubareck, C.A.; Halat, D.H. Insights into Acinetobacter baumannii: A Review of Microbiological, Virulence, and Resistance Traits in a Threatening Nosocomial Pathogen. Antibiotics 2020, 9, 119. [Google Scholar] [CrossRef] [Green Version]
- Heintz, B.H.; Halilovic, J.; Christensen, C.L. Vancomycin-resistant enterococcal urinary tract infections. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2010, 30, 1136–1149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Driscoll, T.; Crank, C.W. Vancomycin-resistant enterococcal infections: Epidemiology, clinical manifestations, and optimal management. Infect. Drug Resist. 2015, 8, 217–230. [Google Scholar] [CrossRef] [Green Version]
- Toner, L.; Papa, N.; Aliyu, S.H.; Dev, H.; Lawrentschuk, N.; Al-Hayek, S. Vancomycin resistant enterococci in urine cultures: Antibiotic susceptibility trends over a decade at a tertiary hospital in the United Kingdom. Investig. Clin. Urol. 2016, 57, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Lamas Ferreiro, J.L.; Álvarez Otero, J.; González González, L.; Novoa Lamazares, L.; Arca Blanco, A.; Bermúdez Sanjurjo, J.R.; Rodríguez Conde, I.; Fernández Soneira, M.; de la Fuente Aguado, J. Pseudomonas aeruginosa urinary tract infections in hospitalized patients: Mortality and prognostic factors. PLoS ONE 2017, 12, e0178178. [Google Scholar] [CrossRef] [Green Version]
- Gomila, A.; Carratalà, J.; Eliakim-Raz, N.; Shaw, E.; Wiegand, I.; Vallejo-Torres, L.; Gorostiza, A.; Vigo, J.M.; Morris, S.; Stoddart, M.; et al. Risk factors and prognosis of complicated urinary tract infections caused by Pseudomonas aeruginosa in hospitalized patients: A retrospective multicenter cohort study. Infect. Drug Resist. 2018, 11, 2571–2581. [Google Scholar] [CrossRef] [Green Version]
- Rocha, S.C.M.; Baptista, C.J.M. Biochemical Properties of Amniotic Membrane. In Amniotic Membrane; Mamede, A.C., Botelho, M.F., Eds.; Springer: Dordrecht, The Netherlands, 2015; pp. 19–40. [Google Scholar]
- Silini, A.R.; Cargnoni, A.; Magatti, M.; Pianta, S.; Parolini, O. The Long Path of Human Placenta, and Its Derivatives, in Regenerative Medicine. Front. Bioeng. Biotechnol. 2015, 3, 162. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Weidinger, A.; Hofer, M.; Steinborn, R.; Lindenmair, A.; Hennerbichler-Lugscheider, S.; Eibl, J.; Redl, H.; Kozlov, A.V.; Wolbank, S. Different metabolic activity in placental and reflected regions of the human amniotic membrane. Placenta 2015, 36, 1329–1332. [Google Scholar] [CrossRef] [PubMed]
- Parolini, O.; Soncini, M.; Evangelista, M.; Schmidt, D. Amniotic membrane and amniotic fluid-derived cells: Potential tools for regenerative medicine? Regen. Med. 2009, 4, 275–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koizumi, N.J.; Inatomi, T.J.; Sotozono, C.J.; Fullwood, N.J.; Quantock, A.J.; Kinoshita, S. Growth factor mRNA and protein in preserved human amniotic membrane. Curr. Eye Res. 2000, 20, 173–177. [Google Scholar] [CrossRef]
- Jin, E.; Kim, T.H.; Han, S.; Kim, S.W. Amniotic epithelial cells promote wound healing in mice through high epithelialization and engraftment. J. Tissue Eng. Regen. Med. 2015, 10, 613–622. [Google Scholar] [CrossRef]
- Gicquel, J.J.; Dua, H.S.; Brodie, A.; Mohammed, I.; Suleman, H.; Lazutina, E.; James, D.K.; Hopkinson, A. Epidermal growth factor variations in amniotic membrane used for ex vivo tissue constructs. Tissue Eng. Part A 2009, 15, 1919–1927. [Google Scholar] [CrossRef]
- Koh, J.W.; Shin, Y.J.; Oh, J.Y.; Kim, M.K.; Ko, J.H.; Hwang, J.M.; Wee, W.R.; Lee, J.H. The expression of TIMPs in cryo-preserved and freeze-dried amniotic membrane. Curr. Eye Res. 2007, 32, 611–616. [Google Scholar] [CrossRef]
- Riau, A.K.; Beuerman, R.W.; Lim, L.S.; Mehta, J.S. Preservation, sterilization and de-epithelialization of human amniotic membrane for use in ocular surface reconstruction. Biomaterials 2010, 31, 216–225. [Google Scholar] [CrossRef]
- SantAnna, L.B.; Hage, R.; Cardoso, M.A.; Arisawa, E.A.; Cruz, M.M.; Parolini, O.; Cargnoni, A.; Sant’Anna, N. Antifibrotic Effects of Human Amniotic Membrane Transplantation in Established Biliary Fibrosis Induced in Rats. Cell Transpl. 2016, 25, 2245–2257. [Google Scholar] [CrossRef] [PubMed]
- Tseng, S.C.; Li, D.Q.; Ma, X. Suppression of transforming growth factor-β isoforms, TGF-β receptor type II, and myofibroblast differentiation in cultured human corneal and limbal fibroblasts by amniotic membrane matrix. J. Cell Physiol. 1999, 179, 325–335. [Google Scholar] [CrossRef]
- Magatti, M.; De Munari, S.; Vertua, E.; Gibelli, L.; Wengler, G.S.; Parolini, O. Human amnion mesenchyme harbors cells with allogeneic T-cell suppression and stimulation capabilities. Stem Cells 2008, 26, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Magatti, M.; Caruso, M.; De Munari, S.; Vertua, E.; De, D.; Manuelpillai, U.; Parolini, O. Human Amniotic Membrane-Derived Mesenchymal and Epithelial Cells Exert Different Effects on Monocyte-Derived Dendritic Cell Differentiation and Function. Cell Transplant. 2015, 24, 1733–1752. [Google Scholar] [CrossRef]
- Magatti, M.; Vertua, E.; De Munari, S.; Caro, M.; Caruso, M.; Silini, A.; Delgado, M.; Parolini, O. Human amnion favours tissue repair by inducing the M1-to-M2 switch and enhancing M2 macrophage features. J. Tissue Eng. Regen. Med. 2016. [Google Scholar] [CrossRef] [PubMed]
- Magatti, M.; Vertua, E.; Cargnoni, A.; Silini, A.; Parolini, O. The Immunomodulatory Properties of Amniotic Cells: The Two Sides of the Coin. Cell Transplant. 2018, 27, 31–44. [Google Scholar] [CrossRef] [Green Version]
- Banas, R.A.; Trumpower, C.; Bentlejewski, C.; Marshall, V.; Sing, G.; Zeevi, A. Immunogenicity and immunomodulatory effects of amnion-derived multipotent progenitor cells. Hum. Immunol. 2008, 69, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Insausti, C.L.; Blanquer, M.; Garcia-Hernandez, A.M.; Castellanos, G.; Moraleda, J.M. Amniotic membrane-derived stem cells: Immunomodulatory properties and potential clinical application. Stem Cells Cloning Adv. Appl. 2014, 7, 53–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Niederkorn, J.Y.; Neelam, S.; Mayhew, E.; Word, R.A.; McCulley, J.P.; Alizadeh, H. Immunosuppressive factors secreted by human amniotic epithelial cells. Investig. Ophthalmol. Vis. Sci. 2005, 46, 900–907. [Google Scholar] [CrossRef] [Green Version]
- Magatti, M.; De Munari, S.; Vertua, E.; Parolini, O. Amniotic membrane-derived cells inhibit proliferation of cancer cell lines by inducing cell cycle arrest. J. Cell. Mol. Med. 2012, 16, 2208–2218. [Google Scholar] [CrossRef] [PubMed]
- Niknejad, H.; Yazdanpanah, G.; Mirmasoumi, M.; Abolghasemi, H.; Peirovi, H.; Ahmadiani, A. Inhibition of HSP90 could be possible mechanism for anti-cancer property of amniotic membrane. Med. Hypotheses 2013, 81, 862–865. [Google Scholar] [CrossRef]
- Niknejad, H.; Khayat-Khoei, M.; Peirovi, H.; Abolghasemi, H. Human amniotic epithelial cells induce apoptosis of cancer cells: A new anti-tumor therapeutic strategy. Cytotherapy 2014, 16, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Niknejad, H.; Yazdanpanah, G.; Ahmadiani, A. Induction of apoptosis, stimulation of cell-cycle arrest and inhibition of angiogenesis make human amnion-derived cells promising sources for cell therapy of cancer. Cell Tissue Res. 2016, 363, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Bu, S.; Zhang, Q.; Wang, Q.; Lai, D. Human amniotic epithelial cells inhibit growth of epithelial ovarian cancer cells via TGF-β1-mediated cell cycle arrest. Int. J. Oncol. 2017, 51, 1405–1414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riedel, R.; Pérez-Pérez, A.; Carmona-Fernández, A.; Jaime, M.; Casale, R.; Dueñas, J.L.; Guadix, P.; Sánchez-Margalet, V.; Varone, C.L.; Maymó, J.L. Human amniotic membrane conditioned medium inhibits proliferation and modulates related microRNAs expression in hepatocarcinoma cells. Sci. Rep. 2019, 9, 14193. [Google Scholar] [CrossRef] [PubMed]
- Mamede, A.C.; Laranjo, M.; Carvalho, M.J.; Abrantes, A.M.; Pires, A.S.; Brito, A.F.; Moura, P.; Maia, C.J.; Botelho, M.F. Effect of amniotic membrane proteins in human cancer cell lines: An exploratory study. J. Membr. Biol. 2014, 247, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Mamede, A.C.; Guerra, S.; Laranjo, M.; Carvalho, M.J.; Oliveira, R.C.; Goncalves, A.C.; Alves, R.; Castro, L.P.; Sarmento-Ribeiro, A.B.; Moura, P.; et al. Selective cytotoxicity and cell death induced by human amniotic membrane in hepatocellular carcinoma. Med. Oncol. 2015, 32, 257. [Google Scholar] [CrossRef] [PubMed]
- Mamede, A.C.; Guerra, S.; Laranjo, M.; Santos, K.; Carvalho, M.J.; Carvalheiro, T.; Moura, P.; Paiva, A.; Abrantes, A.M.; Maia, C.J.; et al. Oxidative Stress, DNA, Cell Cycle/Cell Cycle Associated Proteins and Multidrug Resistance Proteins: Targets of Human Amniotic Membrane in Hepatocellular Carcinoma. Pathol. Oncol. Res. 2016, 22, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Ramuta, T.Z.; Jerman, U.D.; Tratnjek, L.; Janev, A.; Magatti, M.; Vertua, E.; Signoroni, P.B.; Silini, A.R.; Parolini, O.; Kreft, M.E. The Cells and Extracellular Matrix of Human Amniotic Membrane Hinder the Growth and Invasive Potential of Bladder Urothelial Cancer Cells. Front. Bioeng. Biotechnol. 2020, 8, 1–18. [Google Scholar] [CrossRef]
- Ramuta, T.Z.; Starčič Erjavec, M.; Kreft, M.E. Amniotic Membrane Preparation Crucially Affects Its Broad-Spectrum Activity Against Uropathogenic Bacteria. Front. Microbiol. 2020, 11, 469. [Google Scholar] [CrossRef] [Green Version]
- Šket, T.; Ramuta, T.; Starčič Erjavec, M.; Kreft, M.E. Different Effects Of Amniotic Membrane Homogenate On The Growth Of Uropathogenic Escherichia coli, Staphylococcus aureus and Serratia marcescens. Infect. Drug Resist. 2019, 12, 3365–3375. [Google Scholar] [CrossRef] [Green Version]
- Yadav, M.K.; Go, Y.Y.; Kim, S.H.; Chae, S.W.; Song, J.J. Antimicrobial and Antibiofilm Effects of Human Amniotic/Chorionic Membrane Extract on Streptococcus pneumoniae. Front. Microbiol. 2017, 8, 1948. [Google Scholar] [CrossRef]
- Mao, Y.; Hoffman, T.; Singh-Varma, A.; Duan-Arnold, Y.; Moorman, M.; Danilkovitch, A.; Kohn, J. Antimicrobial Peptides Secreted From Human Cryopreserved Viable Amniotic Membrane Contribute to its Antibacterial Activity. Sci. Rep. 2017, 7, 13722. [Google Scholar] [CrossRef] [PubMed]
- Kjaergaard, N.; Hein, M.; Hyttel, L.; Helmig, R.B.; Schonheyder, H.C.; Uldbjerg, N.; Madsen, H. Antibacterial properties of human amnion and chorion in vitro. Eur. J. Obstet. Gynecol. Reprod. Biol. 2001, 94, 224–229. [Google Scholar] [CrossRef] [Green Version]
- Tehrani, F.A.; Modaresifar, K.; Azizian, S.; Niknejad, H. Induction of antimicrobial peptides secretion by IL-1β enhances human amniotic membrane for regenerative medicine. Sci. Rep. 2017, 7, 17022. [Google Scholar] [CrossRef] [Green Version]
- Višnjar, T.; Kocbek, P.; Kreft, M.E. Hyperplasia as a mechanism for rapid resealing urothelial injuries and maintaining high transepithelial resistance. Histochem. Cell Biol. 2012, 137, 177–186. [Google Scholar] [CrossRef]
- Višnjar, T.; Kreft, M.E. The complete functional recovery of chitosan-treated biomimetic hyperplastic and normoplastic urothelial models. Histochem. Cell Biol. 2015, 143, 95–107. [Google Scholar] [CrossRef]
- Višnjar, T.; Jerman, U.D.; Veranič, P.; Kreft, M.E. Chitosan hydrochloride has no detrimental effect on bladder urothelial cancer cells. Toxicol. Vitr. 2017, 44, 403–413. [Google Scholar] [CrossRef]
- Lojk, J.; Bregar, V.B.; Strojan, K.; Hudoklin, S.; Veranič, P.; Pavlin, M.; Kreft, M.E. Increased endocytosis of magnetic nanoparticles into cancerous urothelial cells versus normal urothelial cells. Histochem. Cell Biol. 2017. [Google Scholar] [CrossRef]
- Jerman, U.D.; Veranic, P.; Kreft, M.E. Amniotic membrane scaffolds enable the development of tissue-engineered urothelium with molecular and ultrastructural properties comparable to that of native urothelium. Tissue Eng. Part C Methods 2014, 20, 317–327. [Google Scholar] [CrossRef] [Green Version]
- Tenney, J.; Hudson, N.; Alnifaidy, H.; Li, J.T.C.; Fung, K.H. Risk factors for aquiring multidrug-resistant organisms in urinary tract infections: A systematic literature review. Saudi Pharm. J. 2018, 26, 678–684. [Google Scholar] [CrossRef]
- Milovanovic, T.; Dumic, I.; Veličkovic, J.; Lalosevic, M.S.; Nikolic, V.; Palibrk, I. Epidemiology and risk factors for multi-drug resistant hospital-acquired urinary tract infection in patients with liver cirrhosis: Single center experience in Serbia. BMC Infect. Dis. 2019, 19, 141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foxman, B. Urinary tract infection syndromes: Occurrence, recurrence, bacteriology, risk factors, and disease burden. Infect. Dis. Clin. N. Am. 2014, 28, 1–13. [Google Scholar] [CrossRef]
- Talmi, Y.P.; Sigler, L.; Inge, E.; Finkelstein, Y.; Zohar, Y. Antibacterial properties of human amniotic membranes. Placenta 1991, 12, 285–288. [Google Scholar] [PubMed]
- Mao, Y.; Hoffman, T.; Johnson, A.; Duan-Arnold, Y.; Danilkovitch, A.; Kohn, J. Human cryopreserved viable amniotic membrane inhibits the growth of bacteria associated with chronic wounds. J. Diabet. Foot Complicat. 2016, 8, 23–30. [Google Scholar]
- Tehrani, F.A.; Ahmadiani, A.; Niknejad, H. The effects of preservation procedures on antibacterial property of amniotic membrane. Cryobiology 2013, 67, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Zare Bidaki, M.; Lessani, T.; Khazaie, Z. Evaluation of anti-bacterial effects of chorionic membranes in vitro. J. Birjand Univ. Med Sci. 2012, 19, 140–147. [Google Scholar]
- Wang, X.; Xie, J.; Tan, L.; Huo, J.; Xie, H. Epithelium of human fresh amniotic membrane has antimicrobial effects in vitro. Afr. J. Microbiol. Res. 2012, 6, 4533–4537. [Google Scholar]
- Mao, Y.; Singh-Varma, A.; Hoffman, T.; Dhall, S.; Danilkovitch, A.; Kohn, J. The Effect of Cryopreserved Human Placental Tissues on Biofilm Formation of Wound-Associated Pathogens. J. Funct. Biomater. 2018, 9, 3. [Google Scholar] [CrossRef] [Green Version]
- King, A.E.; Paltoo, A.; Kelly, R.W.; Sallenave, J.M.; Bocking, A.D.; Challis, J.R. Expression of natural antimicrobials by human placenta and fetal membranes. Placenta 2007, 28, 161–169. [Google Scholar] [CrossRef]
- Klotman, M.E.; Chang, T.L. Defensins in innate antiviral immunity. Nat. Rev. Immunol. 2006, 6, 447–456. [Google Scholar] [CrossRef]
- Svinarich, D.M.; Gomez, R.; Romero, R. Detection of human defensins in the placenta. Am. J. Reprod. Immunol. 1997, 38, 252–255. [Google Scholar] [CrossRef]
- Buhimschi, I.A.; Jabr, M.; Buhimschi, C.S.; Petkova, A.P.; Weiner, C.P.; Saed, G.M. The novel antimicrobial peptide β3-defensin is produced by the amnion: A possible role of the fetal membranes in innate immunity of the amniotic cavity. Am. J. Obstet. Gynecol. 2004, 191, 1678–1687. [Google Scholar] [CrossRef]
- Denison, F.C.; Kelly, R.W.; Calder, A.A.; Riley, S.C. Secretory leukocyte protease inhibitor concentration increases in amniotic fluid with the onset of labour in women: Characterization of sites of release within the uterus. J. Endocrinol. 1999, 161, 299–306. [Google Scholar] [CrossRef] [Green Version]
- Zaga-Clavellina, V.; Ruiz, M.; Flores-Espinosa, P.; Vega-Sanchez, R.; Flores-Pliego, A.; Estrada-Gutierrez, G.; Sosa-Gonzalez, I.; Morales-Méndez, I.; Osorio-Caballero, M. Tissue-specific human β-defensins (HBD)-1, HBD-2 and HBD-3 secretion profile from human amniochorionic membranes stimulated with Candida albicans in a two-compartment tissue culture system. Reprod. Biol. Endocrinol. 2012, 10, 70. [Google Scholar] [CrossRef] [Green Version]
- Parthasarathy, M.; Sasikala, R.P.G.; Raja, J. Antimicrobial Activity of Human Amniotic and Chorionic Membranes. J. Acad. Ind. Res. 2014, 2, 545–547. [Google Scholar]
- Voidazan, S.; Albu, S.; Toth, R.; Grigorescu, B.; Rachita, A.; Moldovan, I. Healthcare Associated Infections—A New Pathology in Medical Practice? Int. J. Environ. Res. Public Health 2020, 17, 760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pana, Z.D.; Zaoutis, T. Treatment of extended-spectrum β-lactamase-producing Enterobacteriaceae (ESBLs) infections: What have we learned until now? F1000Research 2018, 7. [Google Scholar] [CrossRef]
- Wilke, M.; Worf, K.; Preisendörfer, B.; Heinlein, W.; Kast, T.; Bodmann, K.F. Potential savings through single-dose intravenous Dalbavancin in long-term MRSA infection treatment—A health economic analysis using German DRG data. GMS Infect. Dis. 2019, 7. [Google Scholar] [CrossRef]
- Iacovelli, V.; Gaziev, G.; Topazio, L.; Bove, P.; Vespasiani, G.; Agrò, E.F. Nosocomial urinary tract infections: A review. Urol. J. 2014, 81, 222–227. [Google Scholar] [CrossRef]
- Muder, R.R.; Brennen, C.; Rihs, J.D.; Wagener, M.M.; Obman, A.; Stout, J.E.; Yu, V.L. Isolation of Staphylococcus aureus from the urinary tract: Association of isolation with symptomatic urinary tract infection and subsequent staphylococcal bacteremia. Clin. Infect. Dis. 2006, 42, 46–50. [Google Scholar] [CrossRef] [Green Version]
- Jackson, K.A.; Bohm, M.K.; Brooks, J.T.; Asher, A.; Nadle, J.; Bamberg, W.M.; Petit, S.; Ray, S.M.; Harrison, L.H.; Lynfield, R.; et al. Invasive Methicillin-Resistant Staphylococcus aureus Infections Among Persons Who Inject Drugs—Six Sites, 2005–2016. Morb. Mortal. Wkly. Rep. 2018, 67, 625–628. [Google Scholar] [CrossRef] [Green Version]
- Bonkat, G.; Cai, T.; Veeratterapillay, R.; Bruyère, F.; Bartoletti, R.; Pilatz, A.; Köves, B.; Geerlings, S.E.; Pradere, B.; Pickard, R.; et al. Management of Urosepsis in 2018. Eur. Urol. Focus 2019, 5, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Yadav, V.; Talwar, P. Repositioning of fluoroquinolones from antibiotic to anti-cancer agents: An underestimated truth. Biomed. Pharmacother. 2019, 111, 934–946. [Google Scholar] [CrossRef]
- Jerman, U.D.; Veranič, P.; Cirman, T.; Kreft, M.E. Human Amniotic Membrane Enriched with Urinary Bladder Fibroblasts Promote the Re-Epithelization of Urothelial Injury. Cell Transplant. 2020, 29. [Google Scholar] [CrossRef] [PubMed]
- Perdikouri, E.I.A.; Arvaniti, K.; Lathyris, D.; Kiouti, F.A.; Siskou, E.; Haidich, A.B.; Papandreou, C. Infections Due to Multidrug-Resistant Bacteria in Oncological Patients: Insights from a Five-Year Epidemiological and Clinical Analysis. Microorganisms 2019, 7, 277. [Google Scholar] [CrossRef] [Green Version]
- Ramuta, T.Ž.; Tratnjek, L.; Janev, A.; Seme, K.; Starčič Erjavec, M.; Kreft, M.E. Analysis of the antibacterial activity of the human amniotic membrane homogenate against multidrug-resistant bacteria employing normal and cancerous urothelial models. Protocols.io 2021. [Google Scholar] [CrossRef]



| Strains | Relevant Genotype and/or Phenotype Features | Gram Stain | Reference/Source |
|---|---|---|---|
| Staphylococcus aureus | Reference strain; methicillin-resistant mecA-positive | Gram-positive | NCTC 12493 |
| Staphylococcus aureus | Clinical strain; methicillin-resistant | Gram-positive | Blood culture |
| Acinetobacter baumannii | Reference strain | Gram-negative | ATCC 33604 |
| Acinetobacter baumannii | Clinical strain; carbapenem-resistant | Gram-negative | Endotracheal aspirate |
| Escherichia coli | Clinical strain; extended-spectrum beta-lactamase positive | Gram-negative | Blood culture |
| Klebsiella pneumoniae | Reference strain; SHV-18 extended-spectrum beta-lactamase-producer | Gram-negative | ATCC 700603 |
| Klebsiella pneumoniae | Clinical strain; extended-spectrum beta-lactamase positive | Gram-negative | Blood culture |
| Enterococcus faecalis | Reference strain; vancomycin-resistant, vanB-positive strain | Gram-positive | ATCC 51299 |
| Enterococcus faecalis | Clinical strain; vancomycin-resistant | Gram-positive | Urine |
| Pseudomonas aeruginosa | Reference strain | Gram-negative | ATCC 27853 |
| Pseudomonas aeruginosa | Clinical strain; carbapenem-resistant | Gram-negative | Endotracheal aspirate |
| Bacterial Strain | f-hAM Homogenate | c-hAM Homogenate | ||
|---|---|---|---|---|
| 5 μL | 10 μL | 5 μL | 10 μL | |
| mean diameter of the inhibition zone ± SEM (mm) | ||||
| MRSA (reference strain) | 15.9 ± 0.5 | 18.5 ± 0.6 | 16.9 ± 0.6 | 19.6 ± 0.6 |
| MRSA (clinical strain) | 16.8 ± 0.9 | 20.1 ± 0.9 | 16.7 ± 0.9 | 20.5 ± 0.8 |
| ESBL-producing E. coli (clinical strain) | 6.4 ± 0.5 | 8.5 ± 0.6 | 5.9 ± 0.6 | 8.5 ± 0.6 |
| A. baumannii (reference strain) * | 9.6 ± 0.4 | 11.8 ± 0.4 | 8.3 ± 0.5 | 10.6 ± 0.5 |
| Carbapenem-resistant A. baumannii (clinical strain) * | 5.6 ± 0.3 | 7.4 ± 0.4 | 4.1 ± 0.6 | 5.8 ± 0.9 |
| ESBL-producing K. pneumoniae (reference strain) ** | 5.2 ± 0.3 | 7.0 ± 0.4 | 4.7 ± 0.2 | 6.3 ± 0.2 |
| ESBL-producing K. pneumoniae (clinical strain) ** | 4.7 ± 0.2 | 6.2 ± 0.3 | 4.2 ± 0.3 | 6.5 ± 0.2 |
| VRE (reference strain) | – | |||
| VRE (clinical strain) | – | |||
| P. aeruginosa (reference strain) | – | |||
| P. aeruginosa (clinical strain) | – | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramuta, T.Ž.; Tratnjek, L.; Janev, A.; Seme, K.; Starčič Erjavec, M.; Kreft, M.E. The Antibacterial Activity of Human Amniotic Membrane against Multidrug-Resistant Bacteria Associated with Urinary Tract Infections: New Insights from Normal and Cancerous Urothelial Models. Biomedicines 2021, 9, 218. https://doi.org/10.3390/biomedicines9020218
Ramuta TŽ, Tratnjek L, Janev A, Seme K, Starčič Erjavec M, Kreft ME. The Antibacterial Activity of Human Amniotic Membrane against Multidrug-Resistant Bacteria Associated with Urinary Tract Infections: New Insights from Normal and Cancerous Urothelial Models. Biomedicines. 2021; 9(2):218. https://doi.org/10.3390/biomedicines9020218
Chicago/Turabian StyleRamuta, Taja Železnik, Larisa Tratnjek, Aleksandar Janev, Katja Seme, Marjanca Starčič Erjavec, and Mateja Erdani Kreft. 2021. "The Antibacterial Activity of Human Amniotic Membrane against Multidrug-Resistant Bacteria Associated with Urinary Tract Infections: New Insights from Normal and Cancerous Urothelial Models" Biomedicines 9, no. 2: 218. https://doi.org/10.3390/biomedicines9020218






