Diagnostic Value of Choline PET in the Preoperative Localization of Hyperfunctioning Parathyroid Gland(s): A Comprehensive Overview
Abstract
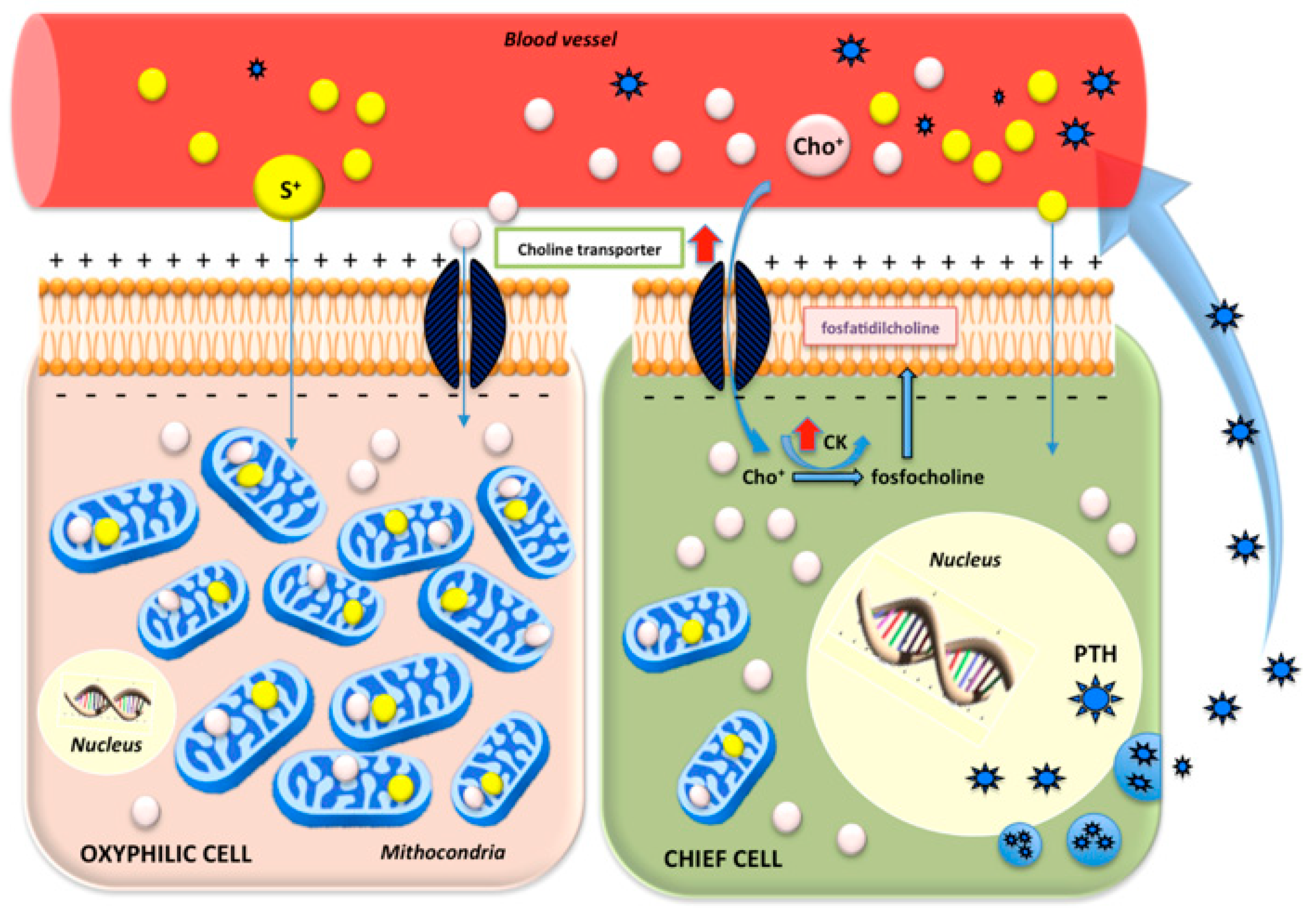
:1. Introduction
2. Materials and Methods
3. Literature Results
3.1. Positron Emission Tomography Procedure and Radiation Exposure
3.2. Diagnostics Performance
4. Discussion
4.1. Pitfalls
4.2. Semi-Quantitative Analysis and Biochemical Data Correlation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Morland, D.; Lalire, P.; Deguelte, S.; Zalzali, M.; Richard, C.; Dejust, S.; Boulagnon, C.; Ly, S.; Papathanassiou, D.; Delemer, B. Added value of 18F-fluorocholine positron emission tomography-computed tomography in presurgical localization of hyperfunctioning parathyroid glands after dual tracer subtraction scintigraphy failure: A retrospective study of 47 patients. Medicine 2020, 99, e18681. [Google Scholar] [CrossRef]
- Walker, M.D.; Silverberg, S.J. Primary hyperparathyroidism. Nat. Rev. Endocrinol. 2018, 14, 115–125. [Google Scholar] [CrossRef]
- Kunstman, J.W.; Kirsch, J.D.; Mahajan, A.; Udelsman, R. Parathyroid localization and implications for clinical management. J. Clin. Endocrinol. Metab. 2013, 98, 902–912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giordano, A.; Marozzi, P.; Meduri, G.; Ficola, U.; Calcagni, M.L.; Vaccaro, A.; Rubini, G.; Attard, M.; Li Puma, M.; Ricci, R.; et al. Quantitative comparison of technetium-99m tetrofosmin and thallium-201 images of the thyroid and abnormal parathyroid glands. Eur. J. Nucl. Med. 1999, 26, 907–911. [Google Scholar] [CrossRef]
- Taieb, D.; Hindie, E.; Grassetto, G.; Colletti, P.M.; Rubello, D. Parathyroid scintigraphy: When, how, and why? A concise systematic review. Clin. Nucl. Med. 2012, 37, 568–574. [Google Scholar] [CrossRef]
- Rubello, D.; Saladini, G.; Casara, D.; Borsato, N.; Toniato, A.; Piotto, A.; Bernante, P.; Pelizzo, M.R. Parathyroid imaging with pertechnetate plus perchlorate/MIBI subtraction scintigraphy: A fast and effective technique. Clin. Nucl. Med. 2000, 25, 527–531. [Google Scholar] [CrossRef] [PubMed]
- Grimaldi, S.; Young, J.; Kamenicky, P.; Hartl, D.; Terroir, M.; Leboulleux, S.; Berdelou, A.; Hadoux, J.; Hescot, S.; Remy, H.; et al. Challenging pre-surgical localization of hyperfunctioning parathyroid glands in primary hyperparathyroidism: The added value of 18F-Fluorocholine PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1772–1780. [Google Scholar] [CrossRef]
- Kannan, S.; Milas, M.; Neumann, D.; Parikh, R.T.; Siperstein, A.; Licata, A. Parathyroid nuclear scan. A focused review on the technical and biological factors affecting its outcome. Clin. Cases Miner. Bone Metab. 2014, 11, 25–30. [Google Scholar] [CrossRef]
- Treglia, G.; Piccardo, A.; Imperiale, A.; Strobel, K.; Kaufmann, P.A.; Prior, J.O.; Giovanella, L. Diagnostic performance of choline PET for detection of hyperfunctioning parathyroid glands in hyperparathyroidism: A systematic review and meta-analysis. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 751–765. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Kjellin, H.; Fotouhi, O.; Lee, L.; Nilsson, I.L.; Haglund, F.; Höög, A.; Lehtiö, J.; Larsson, C. Molecular profiles of oxyphilic and chief cell parathyroid adenoma. Mol. Cell. Endocrinol. 2018, 470, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Beheshti, M.; Hehenwarter, L.; Paymani, Z.; Rendl, G.; Imamovic, L.; Rettenbacher, R.; Tsybrovskyy, O.; Langsteger, W.; Pirich, C. 18F-Fluorocholine PET/CT in the assessment of primary hyperparathyroidism compared with 99mTc-MIBI or 99mTc-tetrofosmin SPECT/CT: A prospective dual-centre study in 100 patients. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1762–1771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishizuka, T.; Kajita, K.; Kamikubo, K.; Komaki, T.; Miura, K.; Nagao, S.; Nozawa, Y. Phospholipid/Ca2+-dependent Protein Kinase Activity in Human Parathyroid Adenoma. Endocrinol. Jpn. 1987, 34, 965–968. [Google Scholar] [CrossRef] [Green Version]
- Araz, M.; Soydal, Ç.; Özkan, E.; Kır, M.K.; İbiş, E.; Güllü, S.; Erdoğan, M.F.; Emral, R.; Küçük, Ö.N. The efficacy of fluorine-18-choline PET/CT in comparison with 99mTc-MIBI SPECT/CT in the localization of a hyperfunctioning parathyroid gland in primary hyperparathyroidism. Nucl. Med. Commun. 2018, 39, 989–994. [Google Scholar] [CrossRef]
- Niccoli-Asabella, A.; Ferrari, C.; Antonica, F.; Scardapane, A.; Rubini, D.; Rubini, G. Unusual 18F-FDG PET/CT finding of an oxyphil parathyroid adenoma in a patient with Hodgkin’s Lymphoma. Rev. Esp. Med. Nucl. Imagen Mol. 2014, 33, 370–373. [Google Scholar] [CrossRef] [PubMed]
- Zajíčková, K.; Zogala, D.; Kubinyi, J. Parathyroid imaging by 18F-fluorocholine PET/CT in patients with primary hyperparathyroidism and inconclusive conventional methods: Clinico-pathological correlations. Physiol. Res. 2018, 67, S551–S557. [Google Scholar] [CrossRef]
- Lezaic, L.; Rep, S.; Sever, M.J.; Kocjan, T.; Hocevar, M.; Fettich, J. 18F-Fluorocholine PET/CT for localization of hyperfunctioning parathyroid tissue in primary hyperparathyroidism: A pilot study. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 2083–2089. [Google Scholar] [CrossRef] [PubMed]
- Michaud, L.; Burgess, A.; Huchet, V.; Lefèvre, M.; Tassart, M.; Ohnona, J.; Kerrou, K.; Balogova, S.; Talbot, J.N.; Périé, S. Is 18F-fluorocholine-positron emission tomography/ computerized tomography a new imaging tool for detecting hyperfunctioning parathyroid glands in primary or secondary hyperparathyroidism? J. Clin. Endocrinol. Metab. 2014, 99, 4531–4536. [Google Scholar] [CrossRef] [Green Version]
- Orevi, M.; Freedman, N.; Mishani, E.; Bocher, M.; Jacobson, O.; Krausz, Y. Localization of parathyroid adenoma by 11C-Choline PET/CT preliminary results. Clin. Nucl. Med. 2014, 39, 1033–1038. [Google Scholar] [CrossRef]
- Rep, S.; Lezaic, L.; Kocjan, T.; Pfeifer, M.; Sever, M.J.; Simoncic, U.; Tomse, P.; Hocevar, M. Optimal scan time for evaluation of parathyroid adenoma with [18F]-fluorocholine PET/CT. Radiol. Oncol. 2015, 49, 327–333. [Google Scholar] [CrossRef] [Green Version]
- Kluijfhout, W.P.; Vorselaars, W.M.C.M.; Van Den Berk, S.A.M.; Vriens, M.R.; Borel Rinkes, I.H.M.; Valk, G.D.; Van Dalen, T.; De Klerk, J.M.H.; De Keizer, B. Fluorine-18 fluorocholine PET-CT localizes hyperparathyroidism in patients with inconclusive conventional imaging: A multicenter study from the Netherlands. Nucl. Med. Commun. 2016, 37, 1246–1252. [Google Scholar] [CrossRef] [PubMed]
- Michaud, L.; Balogova, S.; Burgess, A.; Ohnona, J.; Huchet, V.; Kerrou, K.; Lefèvre, M.; Tassart, M.; Montravers, F.; Périé, S.; et al. A pilot comparison of 18F-fluorocholine PET/CT, ultrasonography and 123I/99mTc-sestaMIBI dual-phase dual-isotope scintigraphy in the preoperative localization of hyperfunctioning parathyroid glands in primary or secondary hyperparathyroidism: Influence of. Medicine 2015, 94, e1701. [Google Scholar] [CrossRef]
- Thanseer, N.; Bhadada, S.K.; Sood, A.; Mittal, B.R.; Behera, A.; Gorla, A.K.R.; Kalathoorakathu, R.R.; Singh, P.; Dahiya, D.; Saikia, U.N.; et al. Comparative Effectiveness of Ultrasonography, 99mTc-Sestamibi, and 18F-Fluorocholine PET/CT in Detecting Parathyroid Adenomas in Patients With Primary Hyperparathyroidism. Clin. Nucl. Med. 2017, 42, e491–e497. [Google Scholar] [CrossRef]
- Hocevar, M.; Lezaic, L.; Rep, S.; Zaletel, K.; Kocjan, T.; Sever, M.J.; Zgajnar, J.; Peric, B. Focused parathyroidectomy without intraoperative parathormone testing is safe after pre-operative localization with 18F-Fluorocholine PET/CT. Eur. J. Surg. Oncol. 2017, 43, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Kluijfhout, W.P.; Pasternak, J.D.; Gosnell, J.E.; Shen, W.T.; Duh, Q.Y.; Vriens, M.R.; De Keizer, B.; Hope, T.A.; Glastonbury, C.M.; Pampaloni, M.H.; et al. 18F fluorocholine PET/MR imaging in patients with primary hyperparathyroidism and inconclusive conventional imaging: A prospective pilot study. Radiology 2017, 284, 460–467. [Google Scholar] [CrossRef] [Green Version]
- Alharbi, A.A.; Alshehri, F.M.; Albatly, A.A.; Sah, B.R.; Schmid, C.; Huber, G.F.; Huellner, M.W. [18F] Fluorocholine Uptake of Parathyroid Adenoma Is Correlated with Parathyroid Hormone Level. Mol. Imaging Biol. 2018, 20, 857–867. [Google Scholar] [CrossRef]
- Rep, S.; Hocevar, M.; Vaupotic, J.; Zdesar, U.; Zaletel, K.; Lezaic, L. 18F-choline PET/CT for parathyroid scintigraphy: Significantly lower radiation exposure of patients in comparison to conventional nuclear medicine imaging approaches. J. Radiol. Prot. 2018, 38, 343–356. [Google Scholar] [CrossRef]
- Bossert, I.; Chytiris, S.; Hodolic, M.; Croce, L.; Mansi, L.; Chiovato, L.; Mariani, G.; Trifirò, G. PETC/CT with 18 F-Choline localizes hyperfunctioning parathyroid adenomas equally well in normocalcemic hyperparathyroidism as in overt hyperparathyroidism. J. Endocrinol. Investig. 2019, 42, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Huber, G.F.; Hüllner, M.; Schmid, C.; Brunner, A.; Sah, B.; Vetter, D.; Kaufmann, P.A.; von Schulthess, G.K. Benefit of18F-fluorocholine PET imaging in parathyroid surgery. Eur. Radiol. 2018, 28, 2700–2707. [Google Scholar] [CrossRef] [PubMed]
- Quak, E.; Blanchard, D.; Houdu, B.; Le Roux, Y.; Ciappuccini, R.; Lireux, B.; de Raucourt, D.; Grellard, J.M.; Licaj, I.; Bardet, S.; et al. F18-choline PET/CT guided surgery in primary hyperparathyroidism when ultrasound and MIBI SPECT/CT are negative or inconclusive: The APACH1 study. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 658–666. [Google Scholar] [CrossRef] [Green Version]
- Prabhu, M.; Kumari, G.; Damle, N.A.; Arora, G.; Kumar, P.; Kumar, R.; Tripathi, M.; Bal, C.; Khadgawat, R.; Kumar, C.; et al. Assessment of the role of early dynamic PET/CT with 18 F-fluorocholine in detection of parathyroid lesions in patients with primary hyperparathyroidism. Nucl. Med. Commun. 2018, 39, 1190–1196. [Google Scholar] [CrossRef]
- Broos, W.A.M.; Wondergem, M.; van der Zant, F.M.; Knol, R.J.J. Dual-time-point 18F-fluorocholine PET/CT in parathyroid imaging. J. Nucl. Med. 2019, 60, 1605–1610. [Google Scholar] [CrossRef] [PubMed]
- Piccardo, A.; Trimboli, P.; Rutigliani, M.; Puntoni, M.; Foppiani, L.; Bacigalupo, L.; Crescenzi, A.; Bottoni, G.; Treglia, G.; Paparo, F.; et al. Additional value of integrated 18 F-choline PET/4D contrast-enhanced CT in the localization of hyperfunctioning parathyroid glands and correlation with molecular profile. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 766–775. [Google Scholar] [CrossRef]
- Amadou, C.; Bera, G.; Ezziane, M.; Chami, L.; Delbot, T.; Rouxel, A.; Leban, M.; Herve, G.; Menegaux, F.; Leenhardt, L.; et al. 18F-Fluorocholine PET/CT and Parathyroid 4D Computed Tomography for Primary Hyperparathyroidism: The Challenge of Reoperative Patients. World J. Surg. 2019, 43, 1232–1242. [Google Scholar] [CrossRef] [PubMed]
- Noltes, M.E.; Kruijff, S.; Noordzij, W.; Telenga, E.D.; Vállez García, D.; Trofimiuk-Müldner, M.; Opalińska, M.; Hubalewska-Dydejczyk, A.; Luurtsema, G.; Dierckx, R.A.J.O.; et al. Optimization of parathyroid 11C-choline PET protocol for localization of parathyroid adenomas in patients with primary hyperparathyroidism. EJNMMI Res. 2019, 9, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khafif, A.; Masalha, M.; Landsberg, R.; Domachevsky, L.; Bernstine, H.; Groshar, D.; Azoulay, O.; Lockman, Y. The role of F18-fluorocholine positron emission tomography/magnetic resonance imaging in localizing parathyroid adenomas. Eur. Arch. Oto Rhino Laryngol. 2019, 276, 1509–1516. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Li, W.; Xia, Z.; Lei, C.; Cao, Y.; Wang, Z.; Pang, H. The role of 18F-FCH PET/CT in patients with uremic hyperparathyroidism compared with 99mTc-sestaMIBI SPECT/CT and ultrasonography. EJNMMI Res. 2019, 9, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Broos, W.A.M.; Wondergem, M.; Knol, R.J.J.; van der Zant, F.M. Parathyroid imaging with 18F-fluorocholine PET/CT as a first-line imaging modality in primary hyperparathyroidism: A retrospective cohort study. EJNMMI Res. 2019, 9, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Dang, Y.; Huo, L.; Hu, Y.; Wang, O.; Liu, H.; Chang, X.; Liu, Y.; Xing, X.; Li, F.; et al. Preoperative Localization of Adenomas in Primary Hyperparathyroidism: The Value of 11C-Choline PET/CT in Patients with Negative or Discordant Findings on Ultrasonography and 99mTc-Sestamibi SPECT/CT. J. Nucl. Med. 2020, 61, 584–589. [Google Scholar] [CrossRef]
- Pretet, V.; Rotania, M.; Helali, M.; Ignat, M.; Vix, M.; Imperiale, A. 18F-Fluorocholine PET and Multiphase CT Integrated in Dual Modality PET/4D-CT for Preoperative Evaluation of Primary Hyperparathyroidism. J. Clin. Med. 2020, 9, 2005. [Google Scholar] [CrossRef] [PubMed]
- Cuderman, A.; Senica, K.; Rep, S.; Hocevar, M.; Kocjan, T.; Sever, M.J.; Zaletel, K.; Lezaic, L. 18F-Fluorocholine PET/CT in Primary Hyperparathyroidism: Superior Diagnostic Performance to Conventional Scintigraphic Imaging for Localization of Hyperfunctioning Parathyroid Glands. J. Nucl. Med. 2020, 61, 577–583. [Google Scholar] [CrossRef]
- Uslu-Beşli, L.; Sonmezoglu, K.; Teksoz, S.; Akgun, E.; Karayel, E.; Pehlivanoglu, H.; Khosroshahi, B.R.; Ocak, M.; Kabasakal, L.; Sager, S.; et al. Performance of f-18 fluorocholine PET/CT for detection of hyperfunctioning parathyroid tissue in patients with elevated parathyroid hormone levels and negative or discrepant results in conventional imaging. Korean J. Radiol. 2020, 21, 236–247. [Google Scholar] [CrossRef]
- Ismail, A.; Christensen, J.W.; Krakauer, M.; Søndergaard, S.B.; Zerahn, B.; Nygaard, B.; Bennedbæk, F.N.; Kristensen, B.; Jensen, L.T. 11C-Choline PET/CT vs. 99mTc-MIBI/123Iodide Subtraction SPECT/CT for Preoperative Detection of Abnormal Parathyroid Glands in Primary Hyperparathyroidism: A Prospective, Single-Centre Clinical Trial in 60 Patients. Diagnostics 2020, 10, 975. [Google Scholar] [CrossRef]
- Costa-Guda, J.; Tokura, T.; Roth, S.I.; Arnold, A. Mitochondrial DNA mutations in oxyphilic and chief cell parathyroid adenomas. BMC Endocr. Disord. 2007, 7, 8. [Google Scholar] [CrossRef] [Green Version]
- Tse, L.L.Y.; Chan, J.K.C. Thyroid and Parathyroid. In Modern Surgical Pathology; Elsevier Inc.: Amsterdam, The Netherlands, 2009; Volume 2, pp. 1597–1685. ISBN 9781416039662. [Google Scholar]
- Karakas, E.; Müller, H.H.; Schlosshauer, T.; Rothmund, M.; Bartsch, D.K. Reoperations for primary hyperparathyroidism—Improvement of outcome over two decades. Langenbeck’s Arch. Surg. 2013, 398, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Parikh, P.P.; Farra, J.C.; Allan, B.J.; Lew, J.I. Long-term effectiveness of localization studies and intraoperative parathormone monitoring in patients undergoing reoperative parathyroidectomy for persistent or recurrent hyperparathyroidism. Am. J. Surg. 2015, 210, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Xue, Y.; Zhang, Q.; Xue, T.; Yao, J.; Huang, H.; Liang, J.; Li, L.; Lin, W.; Lin, L.; et al. Is normocalcemic primary hyperparathyroidism harmful or harmless? J. Clin. Endocrinol. Metab. 2015, 100, 2420–2424. [Google Scholar] [CrossRef]
- Lowe, H.; McMahon, D.J.; Rubin, M.R.; Bilezikian, J.P.; Silverberg, S.J. Normocalcemic primary hyperparathyroidism: Further characterization of a new clinical phenotype. J. Clin. Endocrinol. Metab. 2007, 92, 3001–3005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrari, C.; Lavelli, V.; Santo, G.; Frugis, M.T.; Iuele, F.; Rubini, G.; Sardaro, A. 18F-Fluorocholine PET/CT, Tc-99m-MIBI and TC-99m-MDP SPECT/CT in Tertiary Hyperparathyroidism with Renal Osteodystrophy. Diagnostics 2020, 10, 851. [Google Scholar] [CrossRef]
- Evangelista, L.; Ravelli, I.; Magnani, F.; Iacobone, M.; Giraudo, C.; Camozzi, V.; Spimpolo, A.; Cecchin, D. 18F-choline PET/CT and PET/MRI in primary and recurrent hyperparathyroidism: A systematic review of the literature. Ann. Nucl. Med. 2020, 34, 601–619. [Google Scholar] [CrossRef]
- Vellani, C.; Hodolič, M.; Chytiris, S.; Trifirò, G.; Rubello, D.; Colletti, P.M. Early and Delayed 18F-FCH PET/CT Imaging in Parathyroid Adenomas. Clin. Nucl. Med. 2017, 42, 143–144. [Google Scholar] [CrossRef]
- Oprea-Lager, D.E.; Vincent, A.D.; van Moorselaar, R.J.A.; Gerritsen, W.R.; van den Eertwegh, A.J.M.; Eriksson, J.; Boellaard, R.; Hoekstra, O.S. Dual-Phase PET-CT to Differentiate [18F] Fluoromethylcholine Uptake in Reactive and Malignant Lymph Nodes in Patients with Prostate Cancer. PLoS ONE 2012, 7, e48430. [Google Scholar] [CrossRef]
- Aziz, A.L.; Courbon, F.; Dierickx, L.O.; Pascal, P.; Zerdoud, S. Oncocytic adenoma of thyroid incidentally detected by 18F-fluorocholine PET/CT. J. Nucl. Med. Technol. 2015, 43, 133–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carlson, D. Parathyroid pathology hyperparathyroidism and parathyroid tumors. Arch. Pathol. Lab. Med. 2010, 134, 1639–1644. [Google Scholar] [CrossRef] [PubMed]
- Kuzminski, S.J.; Sosa, J.A.; Hoang, J.K. Update in Parathyroid Imaging. Magn. Reson. Imaging Clin. N. Am. 2018, 26, 151–166. [Google Scholar] [CrossRef]
- Krátký, J.; Vítková, H.; Bartáková, J.; Telička, Z.; Antošová, M.; Límanová, Z.; Jiskra, J. Thyroid nodules: Pathophysiological insight on oncogenesis and novel diagnostic techniques. Physiol. Res. 2014, 63, S263–S275. [Google Scholar] [CrossRef]
- Boi, F.; Lombardo, C.; Cocco, M.C.; Piga, M.; Serra, A.; Lai, M.L.; Calò, P.G.; Nicolosi, A.; Mariotti, S. Thyroid diseases cause mismatch between MIBI scan and neck ultrasound in the diagnosis of hyperfunctioning parathyroids: Usefulness of FNA-PTH assay. Eur. J. Endocrinol. 2013, 168, 49–58. [Google Scholar] [CrossRef]
- Fischli, S.; Suter-Widmer, I.; Nguyen, B.T.; Müller, W.; Metzger, J.; Strobel, K.; Grünig, H.; Henzen, C. The significance of 18F-fluorocholine-PET/CT as localizing imaging technique in patients with primary hyperparathyroidism and negative conventional imaging. Front. Endocrinol. 2018, 8, 380. [Google Scholar] [CrossRef] [Green Version]
- Soelberg, K.K.; Bonnema, S.J.; Brix, T.H.; Hegedüs, L. Risk of malignancy in thyroid incidentalomas detected by 18F-fluorodeoxyglucose positron emission tomography: A systematic review. Thyroid 2012, 22, 918–925. [Google Scholar] [CrossRef]
- Peacock, M.; Bilezikian, J.P.; Klassen, P.S.; Guo, M.D.; Turner, S.A.; Shoback, D. Cinacalcet hydrochloride maintains long-term normocalcemia in patients with primary hyperparathyroidism. J. Clin. Endocrinol. Metab. 2005, 90, 135–141. [Google Scholar] [CrossRef] [Green Version]
- Marcocci, C.; Chanson, P.; Shoback, D.; Bilezikian, J.; Fernandez-Cruz, L.; Orgiazzi, J.; Henzen, C.; Cheng, S.; Sterling, L.R.; Lu, J.; et al. Cinacalcet reduces serum calcium concentrations in patients with intractable primary hyperparathyroidism. J. Clin. Endocrinol. Metab. 2009, 94, 2766–2772. [Google Scholar] [CrossRef] [PubMed]
- Quak, E.; Lasne Cardon, A.; Ciappuccini, R.; Lasnon, C.; Bastit, V.; Le Henaff, V.; Lireux, B.; Foucras, G.; Jaudet, C.; Berchi, C.; et al. Upfront F18-choline PET/CT versus Tc99m-sestaMIBI SPECT/CT guided surgery in primary hyperparathyroidism: The randomized phase III diagnostic trial APACH2. BMC Endocr. Disord. 2021, 21, 1–17. [Google Scholar] [CrossRef] [PubMed]

| Year | Isotope | Authors’ PMID | Study Type | Pts | Dose (MBq) | Time p.i. (min) | Field of View (FOV) | Scintigraphic Protocol | Performance | Main Findings |
|---|---|---|---|---|---|---|---|---|---|---|
| 2014 | 18F | Lezaic et al. [16] 25063039 | P | 24 | 100 | 5 60 | Neck and upper mediastinum | ALL | Sensitivity, specificity, PPV, NPV | PTA: [18F]FCH PET/CT sensitivity 94%; specificity 100%. Multiple parathyroid lesions: [18F]FCH PET/CT sensitivity 91% (vs. conventional imaging, 50%). |
| 2014 | 18F | Michaud et al. [17] 26469908 | P | 12 | 3/kg | Dynamic | From the skull to mid thighs (men) or to liver (women) | Dual-tracer/dual-phase SPECT/CT | Sensitivity, DR | On a per lesion level, [18F]FCH PET/CT sensitivity was 89%. On a per patient level, the DR of [18F]FCH PET/CT was 92%. |
| 2014 | 11C | Orevi et al. [18] 25290292 | P | 40 | 370 | Dynamic | Middle ear to diaphragm | Dual-tracer/dual-phase SPECT/CT | SUVmax SUVratio sensitivity | Lesions SUVmax ranged from 2.1 up to 31. The SUVratio ranged from 0.85 to 4.31. [11C]Choline yielded a sensitivity of 92.3% compared with 88.5% of [99mTc]Tc-MIBI. |
| 2015 | 18F | Rep et al. [19] 26834518 | P | 43 | 100 | 5 60 120 | Neck and upper mediastinum | \ | Sensitivity, specificity, PPV, NPV, accuracy, SUVmax, SUVratio | Accumulation of [18F]FCH PET/CT was higher in parathyroid lesions in comparison with the thyroid tissue with a significantly higher SUVmean in the second (60 min) and in the third (120 min) phase, with a better contrast lesions/thyroid tissue in the second and the third phase. |
| 2016 | 18F | Kluijfhout et al. [20] 27612033 | R | 44 | 2/kg | 30 | From the base of the skull to the mediastinum | Dual-tracer/dual-phase SPECT/CT | DR, sensitivity, PPV, SUVratio | [18F]FCH PET/CT was positive in 34/44 (77.3%) cases. In patients who underwent surgery (33), [18F]FCH PET/CT showed a sensitivity 94.3% (vs. 30% of [99mTc]Tc-MIBI) and a PPV of 97.1% (vs. 69.2%). The mean SUVmax of the 33 preoperatively identified pathological glands was 5.2, which was significantly higher than the 2.9 SUV of the thyroid. |
| 2016 | 18F | Michaud et al. [21] 26469908 | P | 17 | 3/kg | Dynamic 10 | From the skull to mid thighs (men) or to liver (women) | Dual-tracer/dual-phase SPECT/CT | Sensitivity specificity SUVmax SUVratio | Per patient-based, the scintigraphy sensitivity was 69% by open and 94% by masked reading and [18F]FCH PET/CT sensitivity was 88% by open and 94% by masked reading. Per lesion-based, the scintigraphy sensitivity was 58% by open and 83% by masked reading and [18F]FCH PET/CT sensitivity 88% by open and 96% by masked reading. |
| 2017 | 18F | Thanseer et al. [22] 28902729 | P | 54 | 150–185 | 10–15; 60 | \ | Dual-phase SPECT/CT | Sensitivity, PPV, accuracy | [18F]FCH PET/CT showed 100% sensitivity, 96.3% PPV and 96.3% accuracy in localizing parathyroid adenomas regardless of the location (eutopic or ectopic) on patient-wise analysis. In the lesion-wise analysis of 56 lesions, [18F]FCH PET/CT revealed 100% sensitivity, 92.8% PPV and 92.8% accuracy. |
| 2017 | 18F | Hocevar et al. [23] 27776943 | R | 15 | 100 | 5; 60 | Neck and upper mediastinum | Dual-phase SPECT/CT | PPV | [18F]FCH PET/CT showed a PPV of 95.2% according to the histology. |
| 2017 | 18F | Kluijfhout et al. [24] 28121522 | P | 10 | 188 ± 26 | Dynamic 10 | From the base of the skull to the mediastinum | Dual-tracer/dual-phase SPECT/CT | Sensitivity, PPV, SUVratio | [18F]FCH PET/MR reached 90% sensitivity without any false positive results (PPV: 100%). The median SUVmax was 4.9, significantly higher than the 2.7 of thyroid SUVmax (SUVratio ranging from 1.2 to 2.5). |
| 2018 | 18F | Alharbi et al. [25] 29508264 | R | 52 | 150 ± 12 | 2; 50 | Top of the head to the upper abdomen | \ | SUVmax | The majority of adenomas (71.1%) demonstrated an increase in SUVmax from early to late phase. A positive correlation was observed between SUVmax in late phase and PTH level and between ABR in late phase and PTH level. The surgical specimen volume was positively correlated with the PET/MR volume and PTH level. |
| 2018 | 18F | Rep et al. [26] 29339573 | P | 36 | 100 | 5; 60 | Neck and upper mediastinum | Dual-phase SPECT/CT | Sensitivity, specificity, ED | PSS: sensitivity 46% and specificity 98%. [99mTc]Tc-MIBI SPECT/CT sensitivity 64% and specificity 96%. [18F]FCH PET/CT sensitivity 97% and specificity 99%. The ED after administration of the RPH alone was highest for conventional dual-tracer imaging followed by [99mTc]Tc-MIBI alone (SPECT/CT); it was the lowest for [18F]FCH (PET/CT). |
| 2018 | 18F | Araz et al. [13] 30138157 | P | 35 | 100 | 45–60 | \ | Dual-phase SPECT/CT | Sensitivity, specificity, PPV, NPV, accuracy, SUVmax | The sensitivity, specificity, PPV, NPV and accuracy were 96, 100, 100, 93 and 97%, for [18F]FCH PET/CT, respectively. The mean serum PTH levels were significantly higher and lumbar and femur T scores were significantly lower in patients with an SUVmax greater than 4.4. |
| 2018 | 18F | Bossert et al. [27] 30094743 | P | 34 | 3–3.5/kg | 9; 60 | \ | Dual-tracer/dual-phase SPECT/CT | DR, sensitivity | In the whole patients group the DR was 68% for US, 71% for [18F]FCH PET/CT and 15% using [99mTc]Tc-MIBI. In the subgroup of normocalcemic, [18F]FCH PET/CT had a higher DR (71%) compared with traditional imaging. Sensitivities were 88%, 82% and 17% for [18F]FCH PET/CT, US and [99mTc]Tc-MIBI, respectively. |
| 2018 | 18F | Grimaldi et al. [7] 29680989 | P | 27 | 100 | 30 | \ | Dual-phase SPECT/CT | Sensitivity, specificity, PPV, NPV. | In patients with HPT recurrence or with a suspicion of multiple gland disease, [18F]FCH PET/CT showed a per patient sensitivity of 81%, PPV 94% and a per gland sensitivity of 76%, PPV 85%, specificity 91% and NPV 86%. |
| 2018 | 18F | Huber et al. [28] 29372312 | R | 26 | 151 ± 16 | Dynamic | From the vortex to the upper abdomen | Dual-phase SPECT/CT | Sensitivity, PPV | FCH PET sensitivity was 96.2%, PPV 100%. |
| 2018 | 18F | Quak et al. [29] 29270788 | P | 25 | 1.5/kg | 60 | \ | Dual-phase SPECT/CT | SUVratio, SUVmax, sensitivity, PPV | On per lesion and per patient [18F]FCH PET/CT sensitivities of 91.3% and 90.5%, respectively. On per lesion and per patient PPV were 87.5% and 86.4%, respectively. [18F]FCH uptake in adenomas was five and a half-fold higher than surrounding muscle background and three-fold higher than thyroid uptake. |
| 2018 | 18F | Prabhu et al. [30] 30379751 | P | 14 | 185–296 | Dynamic 45–60 | From the angle of mandible to the diaphragm | \ | A/T ratio; LN/T ratio; SUVmax | A higher SUVmax was observed in dynamic images compared with static images. A/T ratio in the dynamic and static images was comparable. The tracer may be able to adequately differentiate parathyroid adenoma from cervical LN: the difference between mean A/T and LN/T ratios in early dynamic imaging was found to be significant. |
| 2018 | 18F | Zajíčková et al. [15] 30484682 | R | 13 | 180 ± 48 | 30 ± 20 | From the base of the skull to the mediastinum | Dual-tracer/dual-phase SPECT/CT | Sensitivity, PPV | [18F]FCH yielded a per patient sensitivity of 92%. The per patient PPV of [18F]FCH was 100%. The mean size of 14 resected parathyroid lesions was 11.9 mm with a per lesion sensitivity of 93% and per gland PPV of 81%. |
| 2018 | 18F | Beheshti et al. [11] 29516131 | P | 82 | 3.2/kg | 60; ± 100–120 | From the base of the skull to the mediastinum | Dual-phase SPECT/CT | DR, sensitivity, specificity, PPV, NPV, accuracy | DR: [18F]FCH PET/CT 93% vs. [99mTc]Tc-MIBI SPECT/CT 61%. The sensitivity, specificity, PPV, NPV and overall accuracy of PET/CT were 93.7%, 96.0%, 90.2%, 97.4% and 95.3%, respectively. Corresponding values for [99mTc]Tc-MIBI SPECT/CT were 60.8%, 98.5%, 94.1%, 86.3% and 87.7%, respectively. The sizes of the parathyroid adenomas significantly impacted on [99mTc]Tc-MIBI SPECT/CT. |
| 2019 | 18F | Broos et al. [31] 30877179 | R | 64 | 150 | 5 60 | From the temporomandibular joint to the diaphragm | \ | SUVmax; SUVpeak; SUVratio | There was a significant decrease in [18F]FCH uptake in the glands on late versus early time-points but there was a significant increase in the ratio of parathyroid uptake to thyroid uptake. |
| 2019 | 18F | Piccardo et al. [32] 30219964 | P | 44 | 2.5/kg | 10 | From the upper neck to abdomen | Dual-tracer/dual-phase SPECT/CT | DR, sensitivity | [18F]FCH PET/4DCeCT detection rate was 72.7% and in surgically treated patients the sensitivity was 100%. These results were significantly better than those of [18F]FCH PET/CT (56.8% and 80%, respectively) and those of 4DCeCT (54.5 and 74%, respectively). |
| 2019 | 18F | Amadou et al. [33] 30659347 | R | 29 | 231 ± 42 | 60 | \ | Dual-phase SPECT/CT | Sensitivity specificity, PPV, NPV | On a per lesion analysis, sensitivity, specificity, PPV and NPV values were, respectively, 96%, 13%, 77% and 50% for [18F]FCH PET/CT and 75%, 40%, 80% and 33% for 4DCeCT. On a per patient analysis, sensitivity was 85% for [18F]FCH PET/CT and 63% for 4DCeCT. |
| 2019 | 11C | Noltes et al. [34] 31367792 | R | 21 | 6.3 ± 1.2 /kg | 20; | \ | \ | SUVratio SUVmax | The A/T ratio became constant from the uptake time of 20–30 min p.i. onwards. In the uptake time of 0–10 vs. 10–20 min p.i. the A/T ratio significantly increased from 1.49 to 1.65. |
| 2019 | 18F | Khafif et al. [35] 30877424 | P | 19 | 100 | Dynamic | \ | Dual-phase SPECT/CT | Sensitivity | [18F]FCH PET/MR showed a sensitivity of 84.2% and predicted the side of the disease gland in 100% of cases. |
| 2019 | 18F | Xue et al. [36] 31879808 | P | 17 | 111–185 | 10 60 | Neck and upper mediastinum | Dual-tracer/dual-phase SPECT/CT | Sensitivity, specificity, PPV, NPV, accuracy | In patients with uremic hyperparathyroidism [18F]FCH PET/CT showed a sensitivity, specificity, accuracy, PPV and NPV of 84.13%, 100%, 86.49%, 100% and 52.38%, respectively. |
| 2019 | 18F | Broos et al. [37] 31367807 | R | 271 | 150 | 5 60 | From the temporomandibular joint to the diaphragm | \ | DR; SUVmax | DR on a per patient level: 96%; DR on a per lesion level 90%. The mean SUVmax on the early images was 6.1 and decreased to a mean SUVmax of 4.9 on the late images. |
| 2020 | 18F | Morland et al. [1] 31914064 | R | 47 | 2.5/kg | Dynamic 60 | \ | Dual-tracer/dual-phase SPECT/CT | DR | DR of [18F]FCH PET/CT was 62%. [18F]FCH PET/CT positivity was associated with a higher calcium level. Cinacalcet treatment did not impact on PET positivity. |
| 2020 | 11C | Liu et al. [38] 31601698 | R | 87 | 385 ± 175 | 20 | Neck and upper chest | Dual-tracer SPECT/CT | Sensitivity, PPV, SUVmax; SUVratio(carotid); SUVratio(thyroid) | [11C]Choline sensitivity was 98.8%. The corresponding lesioned PPV was 91.3%. The mean SUVmax was 6.15 ± 4.92 in 72 lesions with a positive uptake (70 patients). |
| 2020 | 18F | Pretet et al. [39] 32604786 | R | 50 | 2/kg | 60 | From the mandible to the carina | Dual-tracer SPECT/CT | Sensitivity, DR | On a per patient analysis, sensitivity was 93%, 80% and 95% and DR% was 82%, 68% and 84%, respectively for PET/CT, 4DCeCT and PET/4DCeCT. On a per gland analysis, sensitivity PET/CT, 4DCeCT and PET/4DCeCT was 88%, 66% and 92% and DR% was 79%, 57% and 83%, respectively. PET/CT and PET/4DCeCT were more sensitive than 4DCeCT alone. |
| 2020 | 18F | Cuderman et al. [40] 31562221 | P | 103 | 100 | 5 60 | Neck and upper mediastinum | ALL | Sensitivity, specificity | [18F]FCH PET/CT demonstrated a sensitivity of 92% superior to all scintigraphic modality methods (39–56%) on a per patient level. A better sensitivity of [18F]FCH PET/CT was shown in multiple parathyroid adenoma/hyperplasia detection (88% vs. 44%). |
| 2020 | 18F | Uslu-Beşli et al. [41] 31997599 | R | 105 | 325.1 ± 86.7 | 15 45 | Neck and upper chest (15 min); whole body (45 min) | Dual-phase SPECT/CT | Sensitivity, PPV, accuracy | Sensitivity, PPV and accuracy of [18F]FCH PET/CT in the detection of HPT were 94.1%, 97.9% and 92.4%, respectively. A single time-point imaging could be associated with a potential risk of missing lesions. |
| 2020 | 11C | Ismail el al. [42] 33228254 | P | 60 | 400 | 10 ± 5 | \ | Dual-tracer SPECT/CT | Sensitivity | At the patient level, sensitivities were 0.98 for dual-tracer subtraction scintigraphy and 1.00 for [11C]Choline PET/CT. At the gland level, sensitivities were 0.88 and 0.87, respectively. |
| [18F]FCH PET/CT * | [99mTc]Tc-MIBI ** | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author-PMID | HPT | Per Lesion (%) | Per Patient (%) | ||||||||||||||
| Sen | Spe | PPV | NPV | Acc | Sen | Spe | PPV | NPV | Acc | Sen | Spe | PPV | NPV | Acc | |||
| FIRST LEVEL IMAGING | Thanseer et al., 2017 [22] | I | 100 | \ | 96.3 | \ | 96.3 | 100 | \ | 92.8 | \ | 92.8 | 80.7 | \ | 97.7 | \ | 79.6 |
| Beheshti et al., 2018 [11] | I | 93.7 | 96.0 | 90.2 | 97.4 | 95.3 | \ | \ | \ | \ | \ | 60.8 | 98.5 | 94.1 | 86.3 | 87.7 | |
| Araz et al., 2018 [13] | I | 96 | 100 | 100 | 93 | 97 | \ | \ | \ | \ | \ | 78 | 100 | 100 | 70 | 86 | |
| Lezaic et al., 2014 [16] | I | \ | \ | \ | \ | \ | 92 | 100 | 100 | 96 | 98 | 64 | 100 | 100 | 85 | 88 | |
| Rep et al., 2018 [26] | I | 97 | 99 | \ | \ | \ | \ | \ | \ | \ | \ | 64 | 96 | \ | \ | \ | |
| Cuderman et al., 2020 [40] | I | 95.5 | 99.7 | \ | \ | \ | 92 | 99.7 | \ | \ | \ | 76 | 100 | \ | \ | \ | |
| Bossert et al., 2018 [27] | I | 88 | \ | \ | \ | \ | \ | \ | \ | \ | \ | 17 | \ | \ | \ | \ | |
| Rep et al., 2015 [19] | I | \ | \ | \ | \ | \ | 93.6 | 98.2 | 96.7 | 96.4 | 96.5 | \ | \ | \ | \ | \ | |
| SECOND LEVEL IMAGING | Quak et al., 2018 [29] | I | 91.3 | \ | 87.5 | \ | \ | 90.5 | \ | 86.4 | \ | \ | \ | \ | \ | \ | \ |
| Zajíčková et al., 2018 [15] | I | 93 | \ | 81 | \ | \ | 92 | \ | 100 | \ | \ | \ | \ | \ | \ | \ | |
| Uslu-Beşli et al., 2020 [41] | I | \ | \ | \ | \ | \ | 94.1 | \ | 97.9 | \ | 92.4 | 45.1 | \ | 97.9 | \ | 45.7 | |
| Michaud et al., 2016 [21] | I; II | 96 | 56 | \ | \ | \ | \ | \ | \ | \ | \ | 83 | 56 | \ | \ | \ | |
| Kluijfhout et al., 2016 [24] | I; III | 94.3 | \ | 97.1 | \ | \ | \ | \ | \ | \ | \ | 30 | \ | 69.2 | \ | \ | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrari, C.; Santo, G.; Mammucci, P.; Pisani, A.R.; Sardaro, A.; Rubini, G. Diagnostic Value of Choline PET in the Preoperative Localization of Hyperfunctioning Parathyroid Gland(s): A Comprehensive Overview. Biomedicines 2021, 9, 231. https://doi.org/10.3390/biomedicines9030231
Ferrari C, Santo G, Mammucci P, Pisani AR, Sardaro A, Rubini G. Diagnostic Value of Choline PET in the Preoperative Localization of Hyperfunctioning Parathyroid Gland(s): A Comprehensive Overview. Biomedicines. 2021; 9(3):231. https://doi.org/10.3390/biomedicines9030231
Chicago/Turabian StyleFerrari, Cristina, Giulia Santo, Paolo Mammucci, Antonio Rosario Pisani, Angela Sardaro, and Giuseppe Rubini. 2021. "Diagnostic Value of Choline PET in the Preoperative Localization of Hyperfunctioning Parathyroid Gland(s): A Comprehensive Overview" Biomedicines 9, no. 3: 231. https://doi.org/10.3390/biomedicines9030231
APA StyleFerrari, C., Santo, G., Mammucci, P., Pisani, A. R., Sardaro, A., & Rubini, G. (2021). Diagnostic Value of Choline PET in the Preoperative Localization of Hyperfunctioning Parathyroid Gland(s): A Comprehensive Overview. Biomedicines, 9(3), 231. https://doi.org/10.3390/biomedicines9030231






