Interaction of Full-Length Glycosylphosphatidylinositol-Anchored Proteins with Serum Proteins and Their Translocation to Cells In Vitro Depend on the (Pre-)Diabetic State in Rats and Humans
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animal Handling
2.3. GPLD1 Activity Assay Using Micelle-Like Bovine Erythrocyte AChE (bAChE) Complexes and Chip-Based Sensing, Preparation, and Enrichment of hCD73 from Human Erythrocytes, Measurement of Cholesterol and HDL Concentrations in Human Plasma, and Reconstitution of Micelle-Like Complexes without bAChE
2.4. Determination of the Amount of GPLD1 Protein in Serum Using Chip-Based Sensing
2.5. Interaction of GPLD1 and Serum Proteins with Micelle-Like bAChE Complexes
2.6. Isolation of HDLs (High-Density Lipoproteins) from Human Plasma
2.7. Reconstitution of HDL Harboring Human CD73 (hCD73-recHDL)
2.8. Translocation of bAChE from Micelle-Like Complexes, Liposomes, and HDLs to Rat Adipocytes
2.9. Effect of Micelle-Like bAChE Complexes on LDH Release of Rat Adipocytes
2.10. Reconstitution of Erythrocyte Band-3 Protein into Liposomes
2.11. Pretreatment of Serum
2.12. GPLD1 Treatment of Micelle-Like bAChE Complexes
2.13. Statistical Analysis
2.14. Miscellaneous
3. Results
3.1. Strong and Weak Dependence on Metabolic Derangement of Serum GPI-PLD Activity and GPLD1 Amount, Respectively, in Rats
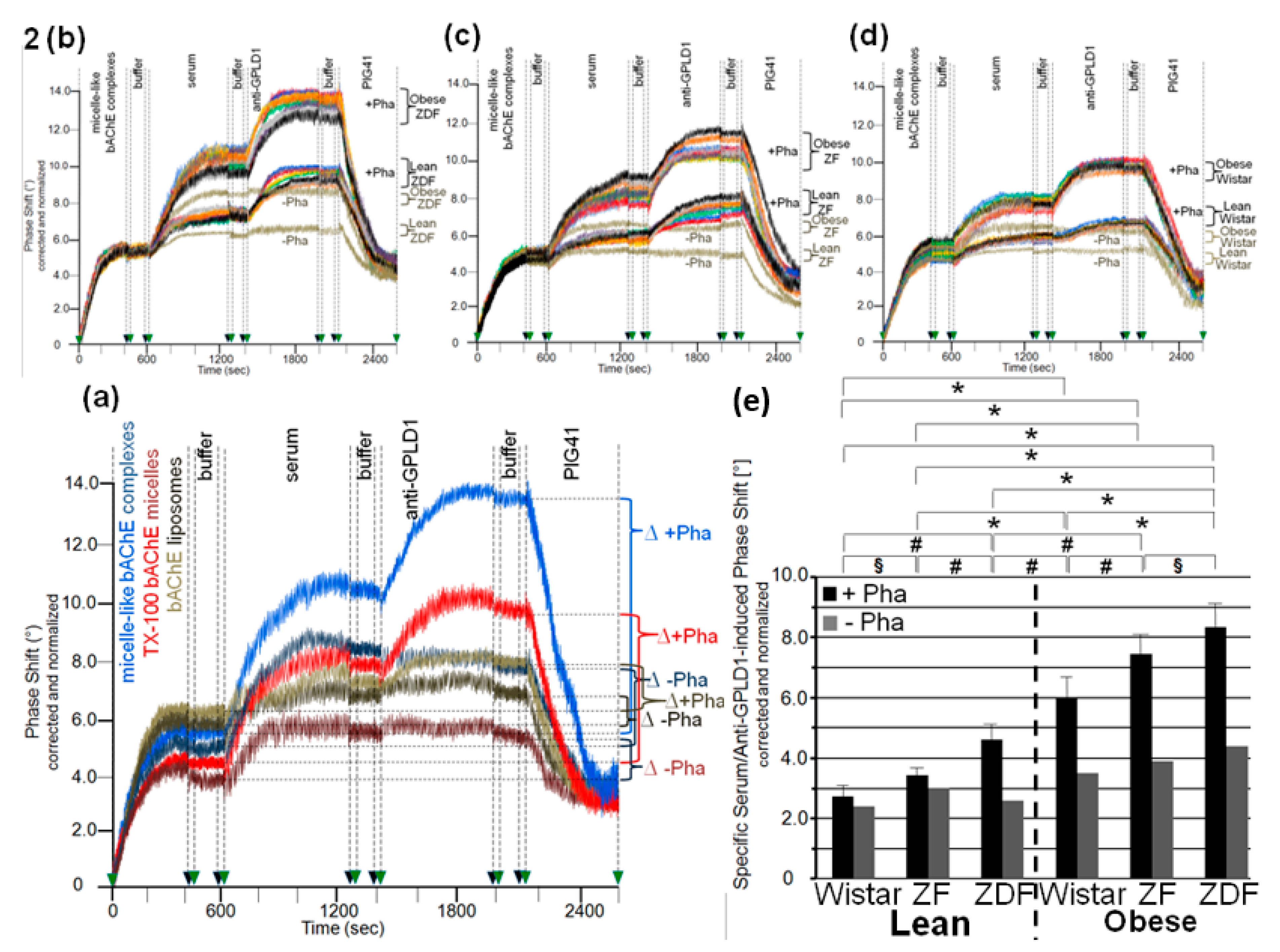
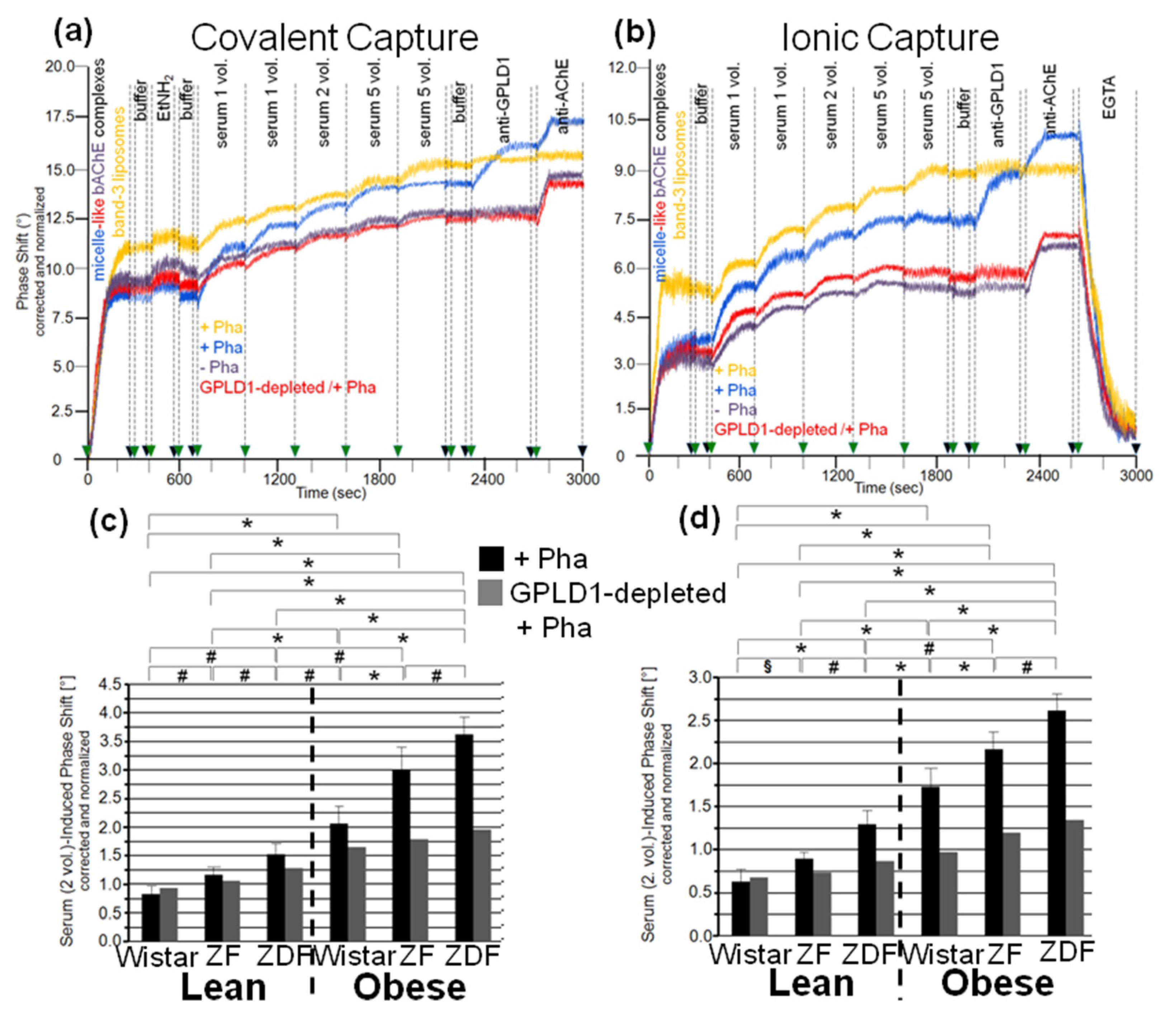
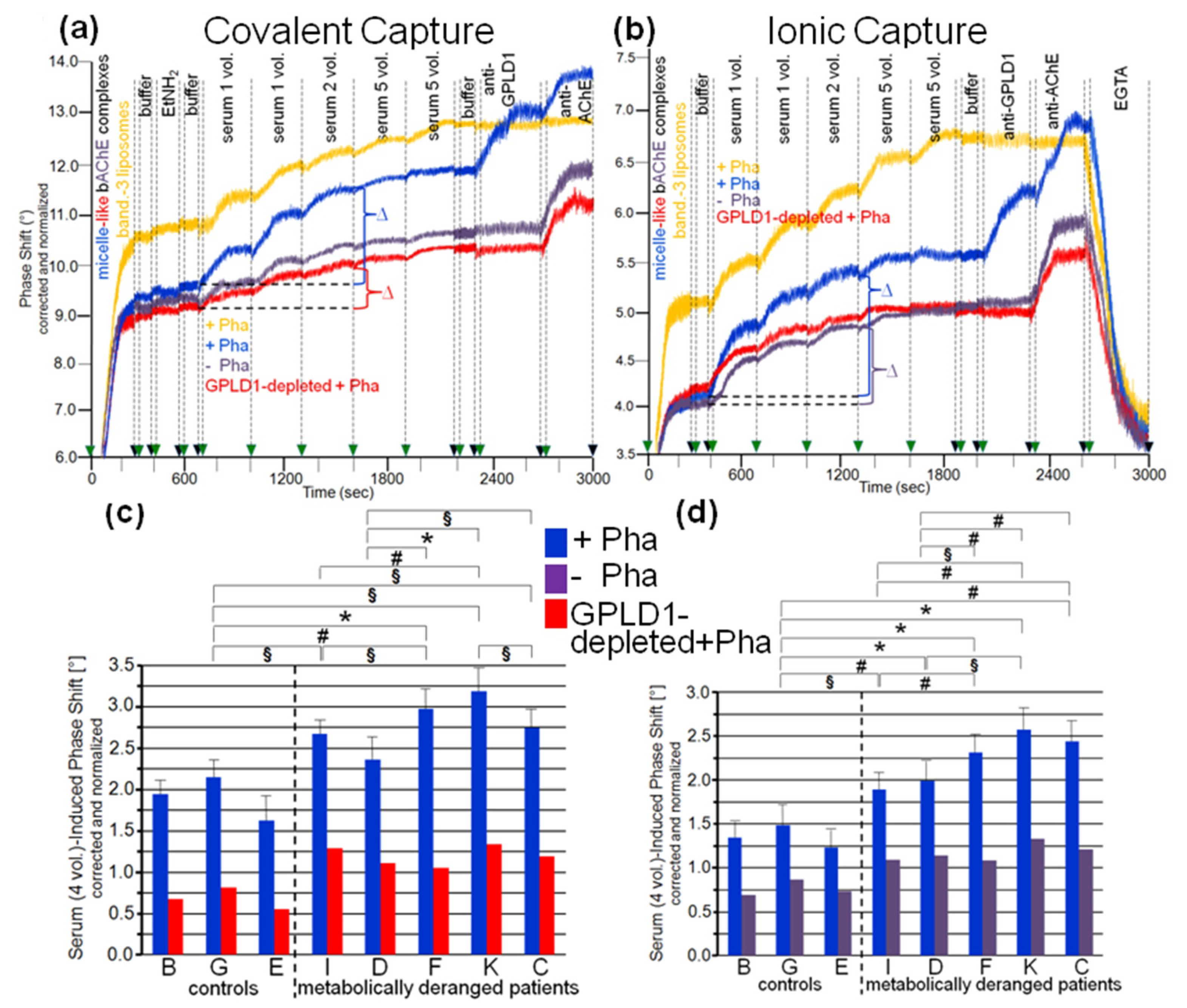
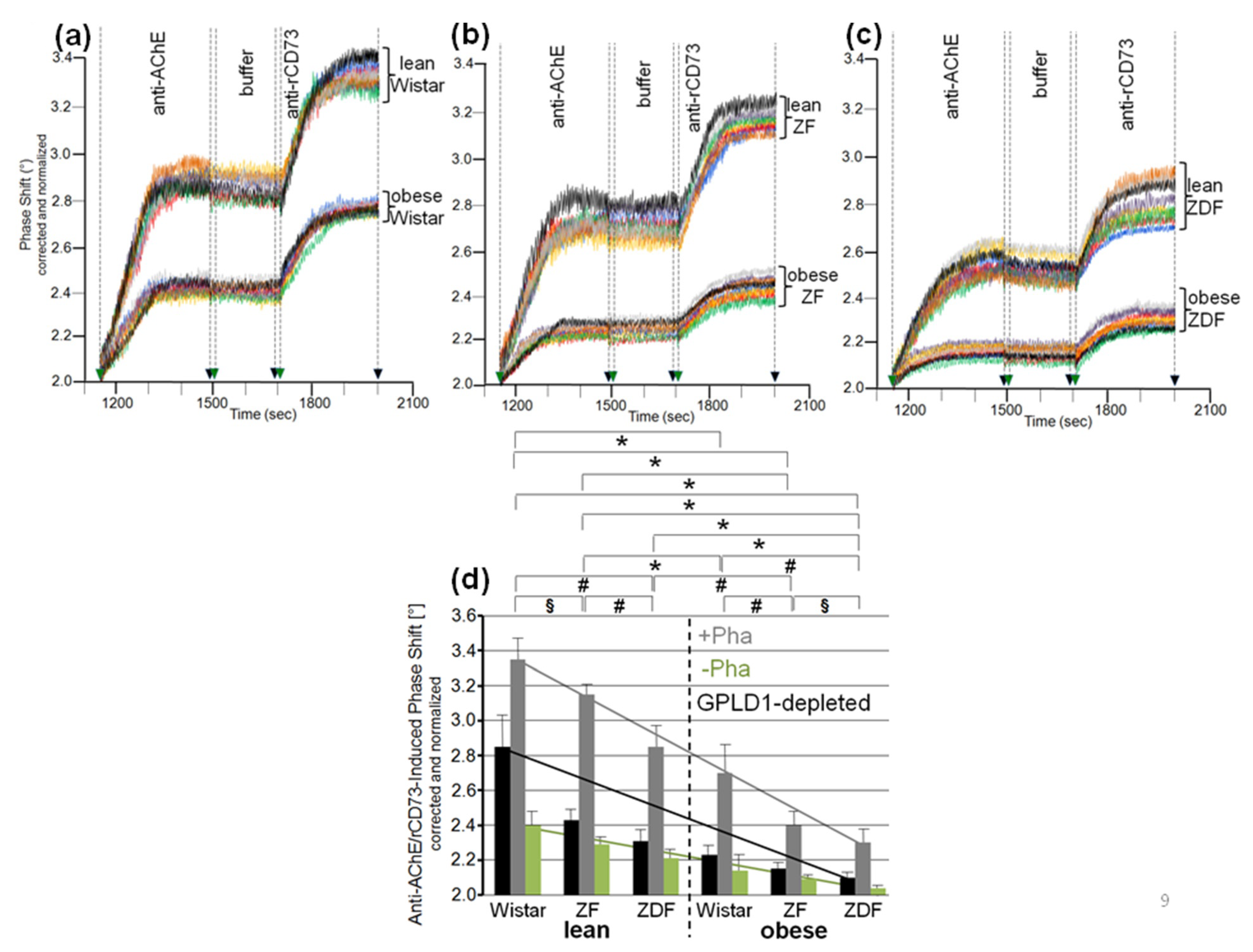
3.2. Dependence of the Interaction of Serum GPLD1 with Micelle-Like bAChE Complexes on Metabolic Derangement in Rats and Humans
3.3. Dependence of the Interaction between Serum Proteins, Not Identical with GPLD1, and the GPI Inositolglycan of Micelle-Like bAChE Complexes on Metabolic Derangement in Rats and Humans
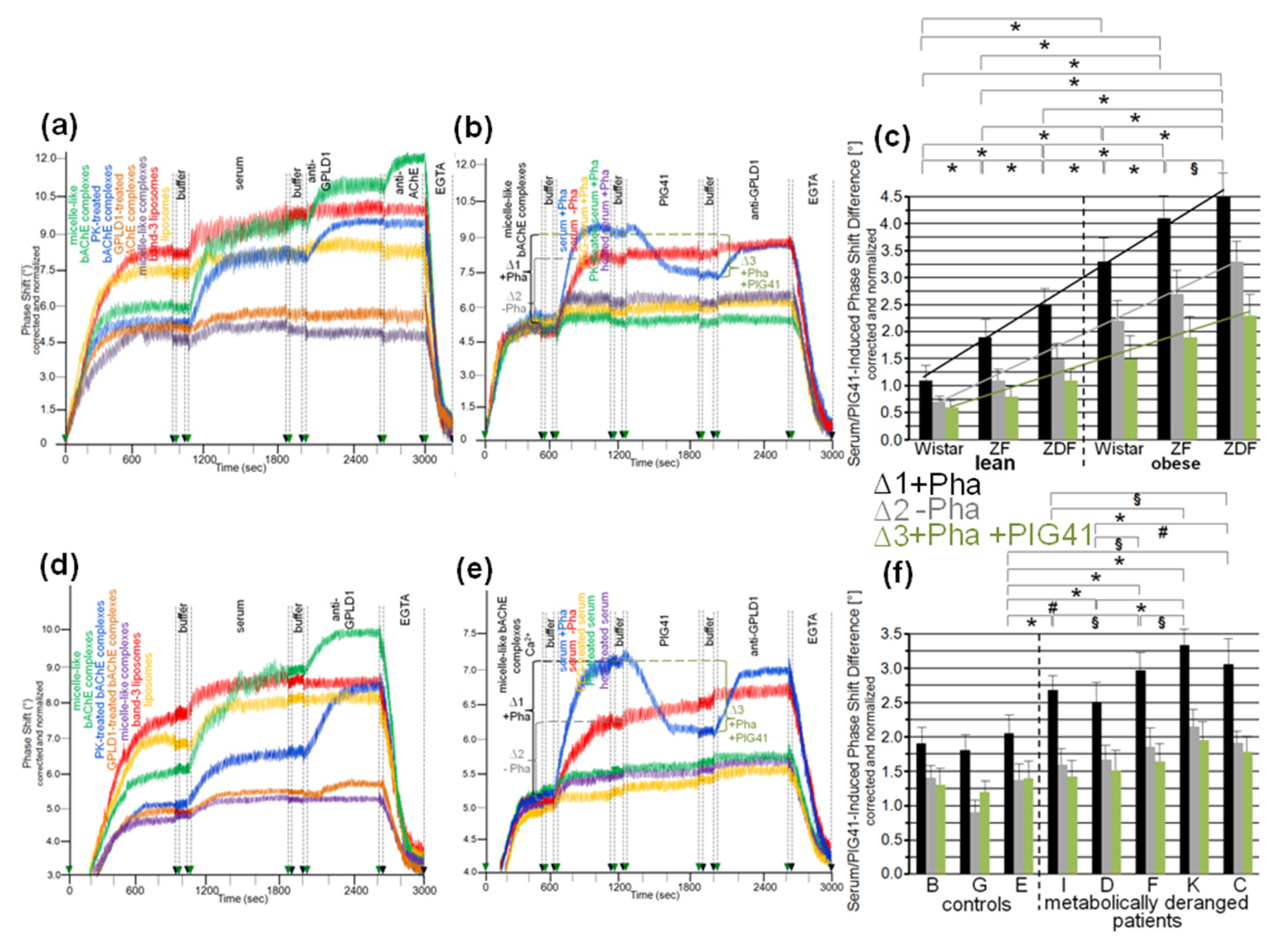
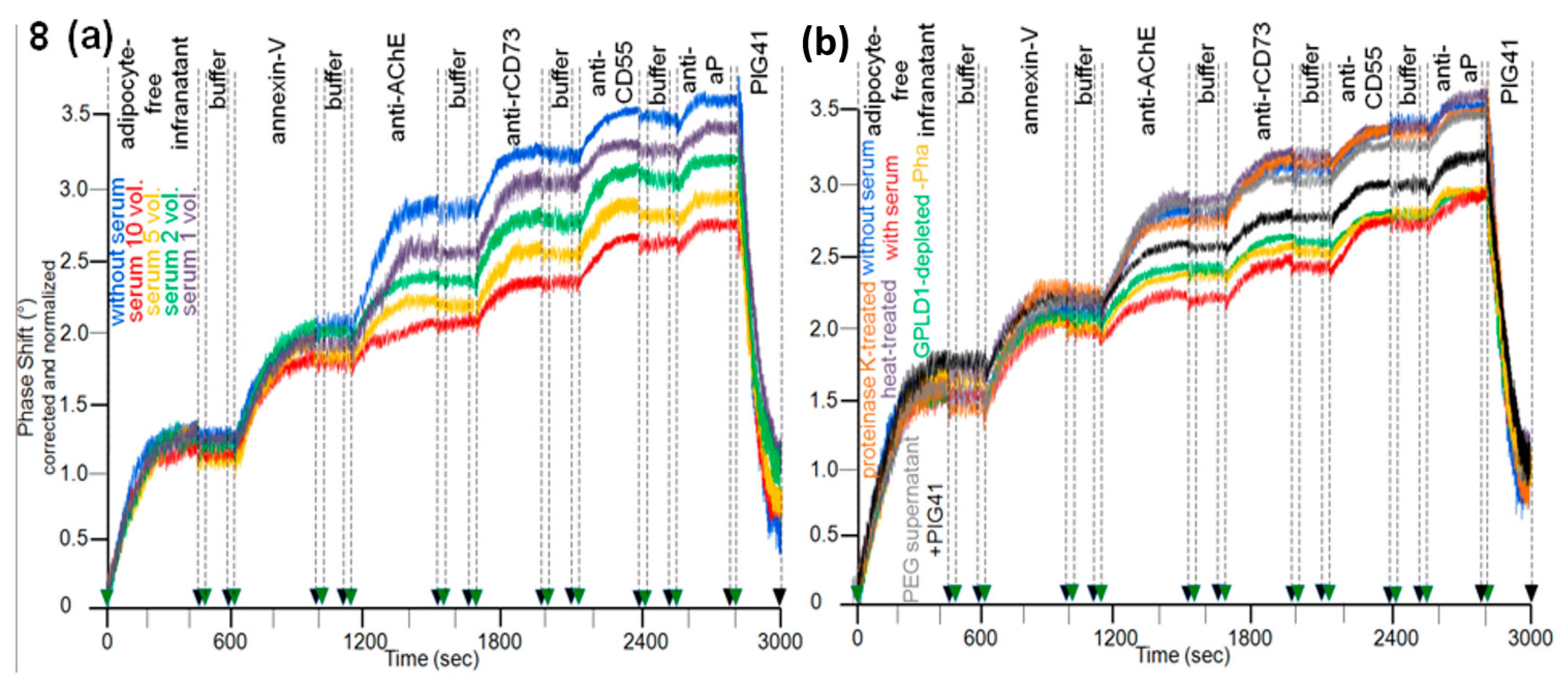
3.4. Impairment of Translocation of Full-Length GPI-APs from Micelle-Like Complexes to Rat Adipocytes by GPLD1 and Other Serum Proteins
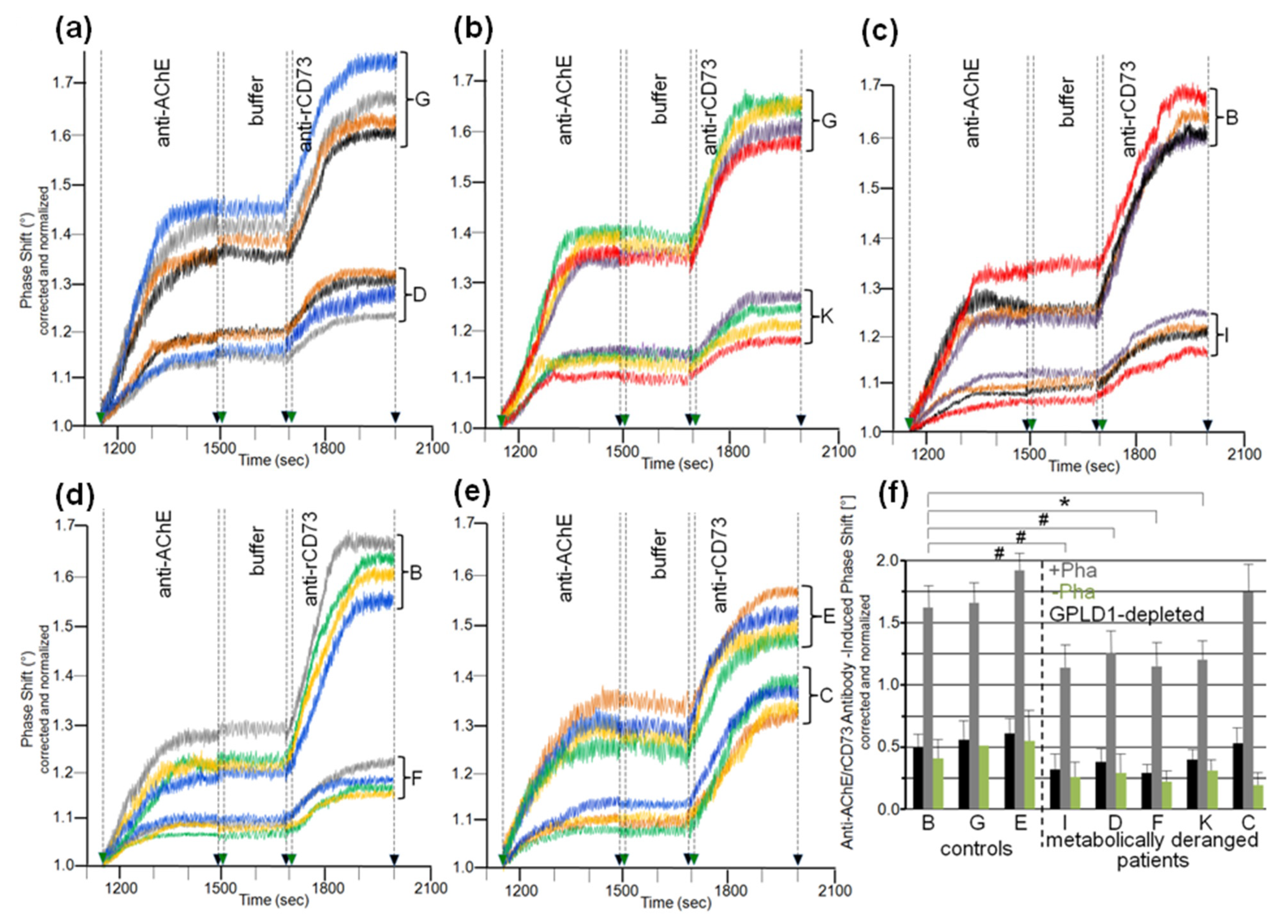
3.5. Dependence of the Impairment of GPI-AP Translocation by Serum Proteins on the Metabolic Derangement in Rats and Humans
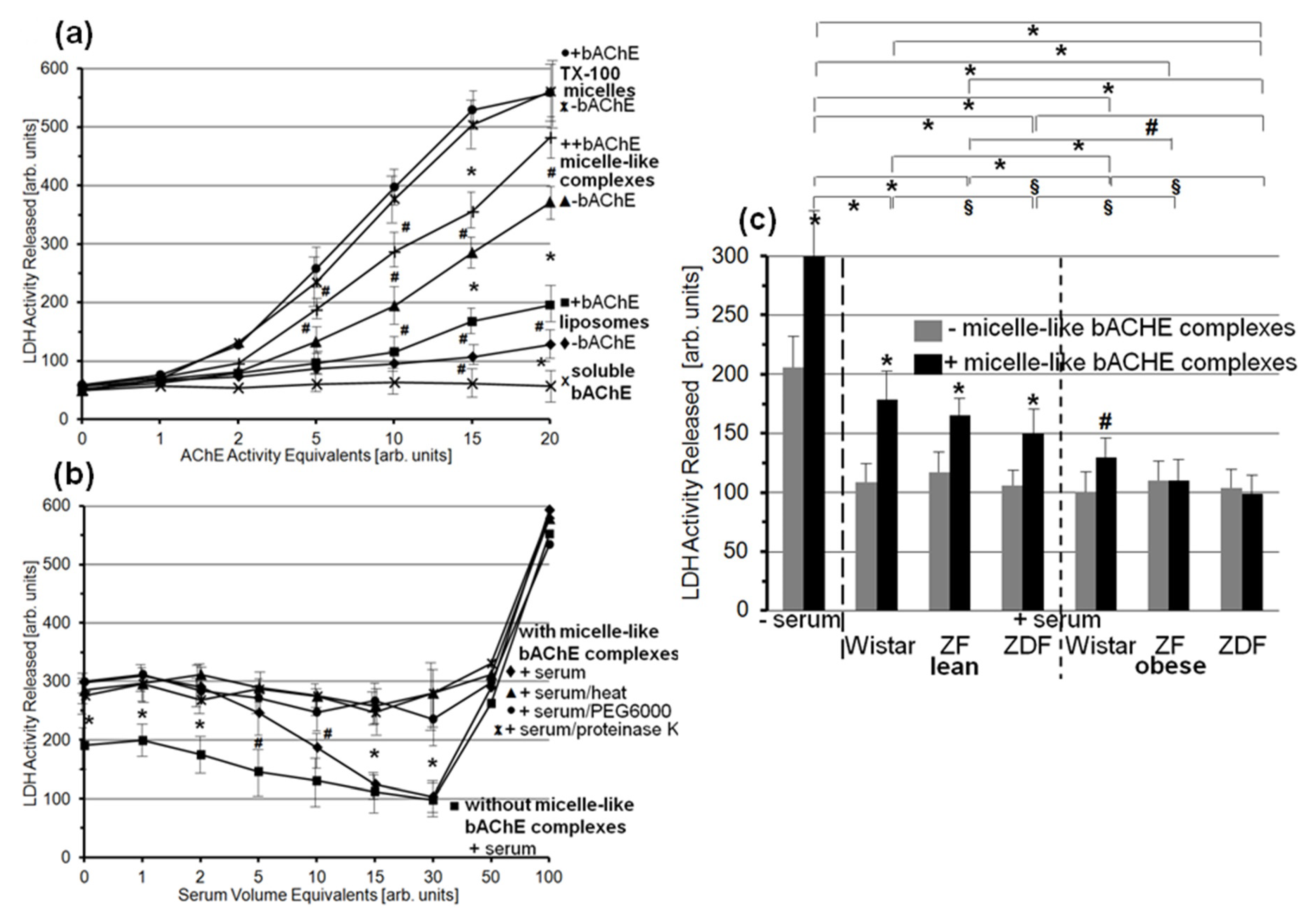
3.6. Lysis of Rat Adipocytes by Micelle-Like bAChE Complexes and Its Prevention by Serum Proteins from Metabolically Deranged Rats
4. Discussion
4.1. GPLD1 as Metabolic Stress-Induced GPI-Interacting and Degrading Protein
4.2. Metabolic Stress-Induced GPI-Interacting Proteins in Serum
4.3. Prevention of Metabolic Stress-Induced Translocation of GPI-APs to and Lysis of Acceptor Cells by GPLD1 and Other Serum Proteins
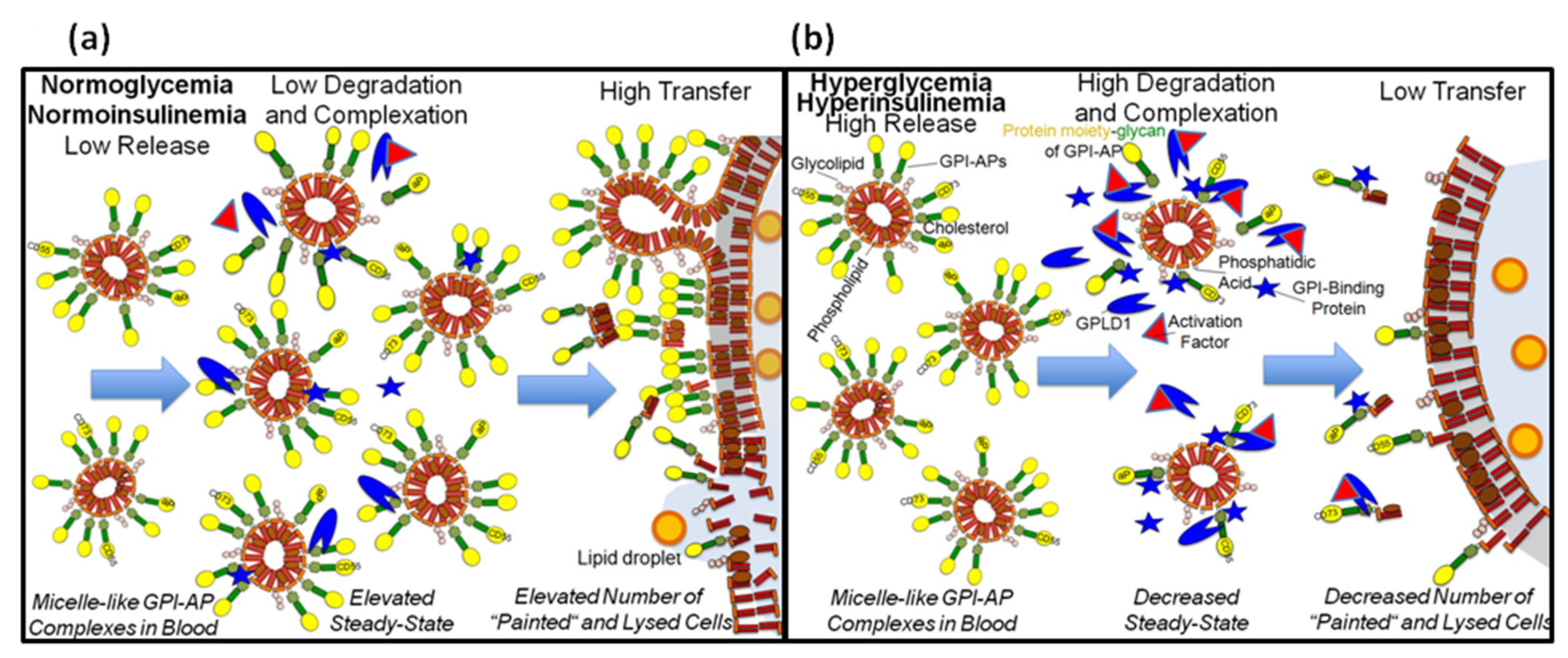
4.4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nosjean, O.; Briolay, A.; Roux, B. Mammalian GPI proteins: Sorting, membrane residence and functions. Biochim. Biophys. Acta 1997, 1331, 153–186. [Google Scholar] [CrossRef]
- Eisenhaber, B.; Bork, P.; Eisenhaber, B. Post-translational GPI lipid anchor modification of proteins in kingdoms of life: Analysis of protein sequence data from complete genomes. Protein Eng. 2001, 14, 17–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orlean, P.; Menon, A.K. GPI anchoring of protein in yeast and mammalian cells, or: How we learned to stop worrying and love glycophospholipids. J. Lipid Res. 2007, 48, 993–1011. [Google Scholar] [PubMed] [Green Version]
- Muniz, M.; Riezman, H. Trafficking of glycosylphosphatidylinositol anchored proteins from the endoplasmic reticulum to the cell surface. J. Lipid Res. 2016, 57, 352–360. [Google Scholar] [CrossRef] [Green Version]
- Kinoshita, T.; Fujita, M. Biosynthesis of GPI-anchored proteins: Special emphasis on GPI lipid remodeling. J. Lipid Res. 2016, 57, 6–24. [Google Scholar] [CrossRef] [Green Version]
- Kinoshita, T.; Fujita, M.; Maeda, Y. Biosynthesis, remodelling and functions of mammalian GPI-anchored proteins: Recent progress. J. Biochem. 2008, 144, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, M.A.J.; Haldar, K.; Cross, G.A.M. Trypanosoma brucei variant surface glycoprotein has a sn-1,2-dimyristyl glycerol membrane anchor at its COOH terminus. J. Biol. Chem. 1985, 260, 4963–4968. [Google Scholar] [CrossRef]
- Haldar, K.; Ferguson, M.A.J.; Cross, G.A.M. Acylation of a Plasmodium falciparum merozoite surface antigen via sn-1,2-diacyl glycerol. J. Biol. Chem. 1985, 260, 4969–4974. [Google Scholar] [CrossRef]
- Müller, G.A. The release of glycosylphosphatidylinositol-anchored proteins from the cell surface. Arch. Biochem. Biophys. 2018, 656, 1–18. [Google Scholar] [CrossRef]
- Müller, G.A. Glycosylphosphatidylinositol-Anchored Proteins and Their Release from Cells—From Phenomenon to Meaning, 1st ed.; Nova Science Publishers Inc.: New York, NY, USA, 2018; pp. 39–91. [Google Scholar]
- Sio, Y.Y.; Anantharaman, R.; Lee, S.Q.E.; Matta, S.A.; Ng, Y.T.; Chew, F.T. The asthma-associated PER1-like domain-containing protein 1 (PERLD1) haplotype influences soluble glycosylphosphatidylinositol anchor protein (sGPI-AP) levels in serum and immune cell proliferation. Sci. Rep. 2020. [Google Scholar] [CrossRef] [Green Version]
- Fujihara, Y.; Ikawa, M. GPI-AP release in cellular, developmental, and reproductive biology. J. Lipid Res. 2016, 57, 538–545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rieu, J.-P.; Ronzon, F.; Place, C.; Dekkiche, F.; Cross, B.; Roux, B. Insertion of GPI-anchored alkaline phosphatase into supported membranes: A combined AFM and fluorescence microscopy study. Acta Biochim. Pol. 2004, 51, 189–197. [Google Scholar] [CrossRef] [Green Version]
- Cross, B.; Ronzon, F.; Roux, B.; Rieu, J.-P. Measurement of the anchorage force between GPI-anchored alkaline phosphatase and supported membranes by AFM force spectroscopy. Langmuir 2005, 21, 5149–5153. [Google Scholar] [CrossRef] [PubMed]
- Caseli, L.; Masui, D.C.; Furriel, R.P.M.; Leone, F.A.; Zaniquelli, M.E.D.; Orbulescu, J.; Leblanc, R.M. Rat osseous plate alkaline phosphatase as Langmuir monolayer—An infrared study at the air-water interface. J. Colloid Interface Sci. 2008, 320, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Kouzayha, A.; Besson, F. GPI-alkaline phosphatase insertion into phosphatidylcholine monolayers: Phase behavior and morphology changes. Biochem. Biophys. Res. Commun. 2005, 333, 1315–1321. [Google Scholar] [CrossRef] [PubMed]
- Ronzon, F.; Rieu, J.-P.; Chauvet, J.-P.; Roux, B. A thermodynamic study of GPI-anchored and soluble form of alkaline phosphatase films at the air-water interface. J. Colloid Interface Sci. 2006, 301, 493–502. [Google Scholar] [CrossRef]
- Rooney, I.A.; Heuser, J.E.; Atkinson, J.P. GPI-anchored complement regulatory proteins in seminal plasma. An analysis of their physical condition and the mechanisms of their binding to exogenous cells. J. Clin. 1996, 97, 1675–1686. [Google Scholar] [CrossRef] [Green Version]
- Rabesandratana, H.; Toutant, J.P.; Reggio, H.; Vidal, M. Decay-accelerating factor (CD55) and membrane inhibitor of reactive lysis (CD59) are released within exosomes during In vitro maturation of reticulocytes. Blood 1998, 91, 2573–2580. [Google Scholar] [CrossRef] [Green Version]
- Clayton, A.; Al-Taei, S.; Webber, J.; Mason, M.D.; Tabi, H.Z. Cancer exosomes express CD39 and CD73, which suppress T cells through adenosine production. J. Immunol. 2011, 187, 676–683. [Google Scholar] [CrossRef]
- Robertson, C.; Booth, S.A.; Beniac, D.R.; Coulthart, M.B.; Booth, T.F.; McNicol, A. Cellular prion protein is released on exosomes from activated platelets. Blood 2006, 107, 3907–3911. [Google Scholar] [CrossRef]
- Müller, G.; Schneider, M.; Biemer-Daub, G.; Wied, S. Microvesicles released from rat adipocytes and harboring glycosylphosphatidylinositol-anchored proteins transfer RNA stimulating lipid synthesis. Cell. Signal. 2011, 23, 1207–1223. [Google Scholar] [CrossRef] [PubMed]
- Müller, G. Microvesicles/exosomes as potential novel biomarkers of metabolic diseases. Diabetes Metab. Syndr. Obes. 2012, 5, 247–282. [Google Scholar] [CrossRef] [Green Version]
- Ceriani, R.L.; Blank, E.W. Experimental therapy of human breast tumors with 131I-labeled monoclonal antibodies prepared against the human milk fat globule. Cancer Res. 1988, 48, 4664–4672. [Google Scholar]
- Mahmood, A.; Yamagishi, F.; Eliakim, K.; DeSchryver-Kecskemeti, T.L.; Gramlich, T.L.; Alpers, D.H. A possible role for rat intestinal surfactant-like particles in transepithelial triacylglycerol transport. J. Clin. Investig. 1994, 93, 70–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olofsson, S.O.; Asp, L.; Boren, J. The assembly and secretion of apolipoprotein B-containing lipoproteins. Curr. Opin. Lipidol. 1999, 10, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Väkevä, A.; Jauhiainen, M.; Ehnholm, C.; Lehto, T.; Meri, S. High-density lipoproteins can act a carriers of glycophosphoinositol lipid-anchored CD59 in human plasma. Immunology 1994, 82, 28–33. [Google Scholar]
- Müller, G.A.; Herling, A.W.; Stemmer, K.; Lechner, A.; Tschöp, M.H. Chip-based sensing for release of unprocessed cell surface proteins in vitro and in serum and its (patho)physiological relevance. Am. J. Physiol. Endocrinol. Metab. 2019, 317, E212–E233. [Google Scholar] [CrossRef] [PubMed]
- Müller, G.A.; Ussar, S.; Tschöp, M.H.; Müller, T.D. Age-dependent membrane release and degradation of full-length glycosylphosphatidylinositol-anchored proteins in rats. Mech. Ageing Dev. 2020, 190, 111307. [Google Scholar] [CrossRef] [PubMed]
- Müller, G.A.; Tschöp, M.H.; Müller, T.D. Upregulated phospholipase D activity toward glycosylphosphatidylinositol-anchored proteins in micelle-like serum complexes in metabolically deranged rats and humans. Am. J. Physiol. Endocrinol. Metab. 2020, 318, E462–E479. [Google Scholar] [CrossRef] [PubMed]
- Bouma, S.R.; Drislane, F.W.; Huestis, W.H. Selective extraction of membrane-bound proteins by phospholipid vesicles. Circulation 1977, 104, 2649–2652. [Google Scholar]
- Nakamura, M.; Tsujii, K.; Katsuragi, Y.; Kurihara, K.; Sunamoto, J. Taste receptor proteins directly extracted by liposome from intact epithelium of bullfrog tongue. Biochem. Biophys. Res. Commun. 1994, 201, 415–422. [Google Scholar] [CrossRef]
- Okumura, Y.; Ishitobi, M.; Sobel, M.; Akiyoshi, K.; Sunamoto, J. Transfer of membrane proteins from human platelet to liposomal fraction by interaction with liposomes containing an articifical boundary lipid. Biochem. Biophys. Acta 1994, 1194, 335–340. [Google Scholar] [CrossRef]
- Kogure, K.; Nakamura, C.; Okuda, O.; Hayashi, K.; Ueno, M. Effect of dicetylphosphate or stearic acid on spontaneous transfer of protein from influenza virus-infected cells to dimyristoylphosphatidylcholine liposomes. Biochim. Biophys. Acta 1997, 1329, 174–182. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, K.; Okumura, Y. Mechanism of selective release of membrane proteins from human erythrocytes in the presence of liposomes. Arch. Biochem. Biophys. 2000, 379, 344–352. [Google Scholar] [CrossRef]
- Huang, K.-S.; Li, S.; Fung, W.-J.C.; Hulmes, J.D.; Reik, L.; Pan, Y.-C.E.; Low, M.G. Purification and characterization of glycosyl-phosphatidylinositol-specific phospholipase D. J. Biol. Chem. 1990, 265, 17738–17745. [Google Scholar] [CrossRef]
- Stieger, S.; Diem, S.; Jakob, A.; Brodbeck, U. Enzymatic properties of phosphatidylinositol-glycan-specific phospholipase C from rat liver and phosphatidylinositol-glycan-specific phospholipase D from rat serum. Eur. J. Biochem. 1991, 197, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-Y.; Hollfelder, K.; Huang, K.-S.; Low, M.G. Structural features of GPI-specific phospholipase D revealed by proteolytic fragmentation and Ca2+ binding studies. J. Biol. Chem. 1994, 269, 28963–28971. [Google Scholar] [CrossRef]
- Gordon, V.M.; Nelson, K.L.; Buckley, J.T.; Stevens, V.L.; Tweten, R.K.; Elwood, P.C.; Leppla, S.H. Clostridium septicum alpha toxin uses glycosylphosphatidylinositol-anchored protein receptors. J. Biol. Chem. 1999, 274, 27274–27280. [Google Scholar] [CrossRef] [Green Version]
- Dolezal, S.; Hester, S.; Kirby, P.S.; Nairn, A.; Pierce, M.; Abbott, K.L. Elevated levels of glycosylphosphatidylinositol (GPI) anchored proteins in plasma from human cancers detected by C. septicum alpha toxin. Cancer Biomark. 2014, 14, 55–62. [Google Scholar] [CrossRef] [Green Version]
- Gronewold, T.M.A.; Schlecht, U.; Quandt, E. Analysis of proteolytic degradation of a crude protein mixture using a surface acoustic wave sensor. Biosens. Bioelectron. 2005, 22, 2360–2365. [Google Scholar] [CrossRef]
- Andrä, J.; Böhling, A.; Gronewold, T.M.A.; Schlecht, U.; Perpeet, M.; Gutsmann, T. Surface acoustic wave biosensor as a tool to study the interaction of antimicrobial peptides with phospholipid and lipopolysaccharide model membranes. Langmuir 2008, 24, 9148–9153. [Google Scholar] [CrossRef] [PubMed]
- Havel, R.J.; Eder, H.A.; Bragdon, J.H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J. Clin. Investig. 1955, 34, 1345–1358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levy, R.I.; Fredrickson, D.S. Heterogeneity of plasma high density lipoproteins. J. Clin. Investig. 1965, 44, 426–441. [Google Scholar] [CrossRef]
- Jauhiainen, M.; Metso, J.; Pahlman, R.; Blomqvist, S.; van Tol, A.; Ehnholm, C. Human plasma phospholipid transfer protein causes high density lipoprotein conversion. J. Biol. Chem. 1993, 6, 4032–4039. [Google Scholar] [CrossRef]
- Mills, G.L.; Lane, P.A.; Weech, P.K. A Guidebook to Lipoprotein Technique; Burdon, R.H., Knippenberg, P.H., Eds.; Elsevier: Amsterdam, The Netherlands, 1989; pp. 55–60. [Google Scholar]
- Brewer, H.B.; Lux, S.E.; Ronan, R.; John, K.M. Amino acid sequence of human apoLp-Gln-II (apoA-II), an apolipoprotein isolated from the high-density lipoprotein complex. Proc. Natl. Acad. Sci. USA 1972, 69, 1304–1308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, Y.; Kim, D.; Yagi, M.; Yamasaki, Y.; Kurita, J.; Iida, T.; Matsuyama, Y.; Yamaguchi, K.; Oda, T. Application of LDH-release assay to cellular-level evaluation of the toxic potential of harmful algal species. Biosci. Biotechnol. Biochem. 2013, 77, 345–352. [Google Scholar] [CrossRef] [Green Version]
- Sekler, I.; Lo, R.S.; Kopito, R.R. A conserved glutamate is responsible for ion selectivity and pH dependence of the mammalian anion exchangers AE1 and AE2. J. Biol. Chem. 1995, 270, 28751–28758. [Google Scholar] [CrossRef] [Green Version]
- Gronewold, T.M.A.; Glass, S.; Quandt, E.; Famulok, M. Monitoring complex formation in the blood-coagulation cascade using aptamer-coated SAW sensors. Biosens. Bioelectron. 2005, 20, 2044–2052. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, T. Biosynthesis and biology of mammalian GPI-anchored proteins. Open Biol. 2020, 10, 190290. [Google Scholar] [CrossRef] [Green Version]
- Frick, W.; Bauer, A.; Bauer, J.; Wied, S.; Müller, G. Structure-activity relationship of synthetic phosphoinositolglycans mimicking metabolic insulin action. Biochemistry 1998, 37, 13421–13436. [Google Scholar] [CrossRef]
- Hoener, M.C.; Brodbeck, U. Phosphatidylinositol-glycan-specific phospholipase D is an amphiphilic glycoprotein that in serum is associated with high-density lipoproteins. Eur. J. Biochem. 1992, 206, 747–757. [Google Scholar] [CrossRef]
- Deeg, M.A.; Bierman, E.L.; Cheung, M.C. GPI-specific phospholipase D associates with an apoA-I- and apoA-IV-containing complex. J. Lipid Res. 2001, 42, 442–451. [Google Scholar] [CrossRef]
- Hoener, M.C.; Bolli, R.; Brodbeck, U. Glycosyl-phosphatidyl--specific phospholipase D. Interaction with and stimulation by apolipoprotein A-! FEBS Lett. 1993, 327, 203–206. [Google Scholar] [CrossRef] [Green Version]
- Ratnoff, W.D.; McCord, M.C.; Meri, S.; Okada, H.; Lackman, P.J. Functional, immunoreactive, soluble CD59 is present in human plasma. Mol. Immunol. 1993, 30 (Suppl. 1), 46. [Google Scholar]
- Kennedy, A.L.; Lappin, T.R.J.; Lavery, T.D.; Hadden, D.R.; Weaver, J.A.; Montgomery, D.A.D. Relation of high-density lipoprotein cholesterol concentration to type of diabetes and its control. Br. Med. J. 1978, 2, 1191–1194. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.D.; Jeng, C.Y.; Reaven, G.M. HDL metabolism in diabetes. Diabetes Metab. Rev. 1987, 3, 653–668. [Google Scholar] [CrossRef] [PubMed]
- Owecki, M.; Nikisch, E.; Miczke, A.; Pupek-Musialik, D.; Sowinski, J. Serum resistin is related to plasma HDL cholesterol and inversely correlated with LDL cholesterol in diabetic and obese humans. Neuro. Endocrinol. Lett. 2010, 31, 673–678. [Google Scholar]
- Fukui, T.; Hirano, T. High-density lipoprotein subspecis between patients with type 1 diabetes and type 2 diabetes without/with intensive insulin therapy. Endocr. J. 2012, 59, 561–569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Low, H.; Hoang, A.; Forbes, J.; Thomas, M.; Lyons, J.G.; Nestel, P.; Bach, L.A.; Sviridov, D. Advanced glycation end-products (AGEs) and functionality of reverse cholsterol transport in patients with type 2 diabetes and in mouse models. Diabetologia 2012, 55, 2513–2521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, N.K.P.; Nicholls, S.J.; Tan, J.T.M.; Bursill, C.A. The role of high-density lipoproteins in diabetes and its vascular complications. Int. J. Mol. Sci. 2018, 19, 1680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bitzur, R.; Cohen, H.; Kamari, Y.; Shaish, A.; Harats, D. Triglycerides and HDL cholesterol. Stars or second label in diabetes? Diabetes Care 2009, 32 (Suppl. 2), S373–S377. [Google Scholar] [CrossRef] [Green Version]
- Brader, L.; Overgaard, A.; Christensen, L.P.; Jeppesen, P.B.; Hermansen, K. Polyphenol-rich Bilberry ameliorates total cholesterol and LDL-cholesterol when implemented in the diet of Zucker Diabetic Fatty rats. Rev. Diabet. Stud. 2013, 10, 270–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macedo de Almeida, M.; Potente Dutra Luquetti, S.C.; Sabarense, C.M.; do Amaral Correa, J.O.; dos Reis, L.G.; Santos da Conceicao, E.P.; Lisboa, P.C.; Gaspar de Moura, E.; Gameiro, J.; Sunfeld da Gama, M.A.; et al. Butter naturally enriched in cis-9, trans-11 CLA prevents hyperinsulinemia and increases both serum HDL cholesterol and triacylglycerol levels in rats. Lipids Health Dis. 2015, 13, 200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steven, S.; Oelze, M.; Hanf, A.; Kröller-Schön, S.; Kashani, F.; Roohani, S.; Welschof, P.; Kopp, M.; Gödtel-Armbrust, U.; Xia, N.; et al. The SGLT2 inhibitor empagliflozin improves the primary diabetic complications in ZDF rats. Redox Biol. 2017, 13, 370–385. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.-L.; Hu, J.-W.; Liu, G.-L.; Lu, S.-Y. Comparative medical characteristics of ZDF-T2DM rats during the course of development to late stage disease. Anim. Model. Exp. Med. 2018, 1, 203–211. [Google Scholar] [CrossRef]
- Misumi, Y.; Ogata, S.; Ohkuba, K.; Hirose, S.; Ikehara, Y. Primary structure of human placental 5′-nucleotidase and identification of the glycolipid anchor in the mature form. Eur. J. Biochem. 1990, 191, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Airas, L.; Niemelä, J.; Salmi, M.; Puurunen, T.; Smith, D.J.; Jalkanen, S. Differential regulation and function of CD73, a glycosyl-phosphatidylinositol-linked 70-kDa adhesion molecule, on lymphocytes and endothelial cells. J. Cell Biol. 1997, 136, 421–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miwa, T.; Sun, X.; Ohta, R.; Okada, N.; Harris, C.L.; Morgan, B.P.; Song, W.C. Characterization of glycosylphosphatidylinositol-anchored decay accelerating factor (GPI-DAF) and transmembrane DAF gene expression in wild-type and GPI-DAF gene knockout mice using polyclonal and monoclonal antibodies with dual or single specificity. Immunology 2001, 104, 207–214. [Google Scholar] [CrossRef]
- Vainer, E.D.; Meir, K.; Furman, M.; Semenenko, I.; Konikoff, F.; Vainer, G.W. Characterization of novel CD55 isoforms expression in normal and neoplastic tissues. Tissue Antigens 2013, 82, 26–34. [Google Scholar] [CrossRef]
- Picher, M.; Burch, L.H.; Hirsh, A.J.; Spychala, J.; Boucher, R.C. Ecto 5′-nucleotidase and non-specific phosphatase. Two AMP-hydrolyzing ectoenzymes with distinct roles in human airways. J. Biol. Chem. 2003, 278, 13468–13479. [Google Scholar] [CrossRef] [Green Version]
- Savinov, A.Y.; Salehi, M.; Yadav, M.C.; Radichev, I.; Luis Millan, J.; Savinova, J. Transgenic overexpression of tissue-non-specific alkaline phosphatase (TNAP) in vascular endothelium results in generalized artial calcification. J. Am. Heart Assoc. 2015, 4, e002499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aluigi, M.G.; Coradeghini, R.; Guida, C.; Scanarotti, C.; Bassi, A.M.; Falugi, C.; Santi, P.L.; Raposio, E. Pre-adipocytes commitment to neurogenesis 1: Preliminary localisation of cholinergic molecules. Cell Biol. Int. 2009, 33, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Flores-Borja, F.; Kieszkievicz, J.; Church, V.; Francis-West, P.H.; Schofield, J.; Rademacher, T.W.; Lund, T. Genetic regulation of mouse glycosylphosphatidylinositol-phospholipase D. Biochimie 2004, 86, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, S.; Fujishima, Y.; Maeda, N.; Tsugawa-Shimizu, Y.; Nakamura, Y.; Tanaka, Y.; Obata, Y.; Fukuda, S.; Nagao, H.; Kita, S.; et al. Impact of glycosylphosphatidylinositol-specific phospholipase D on hepatic diacylglycerol accumulation, steatosis, and insulin resistance in diet-induced obesity. Am. J. Physiol. Endocrinol. Metab. 2019, 316, E239–E250. [Google Scholar] [CrossRef] [PubMed]
- Schofield, J.N.; Stephens, J.W.; Hurel, S.J.; Bell, K.M.; deSouza, J.B.; Rademacher, T.W. Insulin reduces serum glycosylphosphatidylinositol phospholipase D levels in human type I diabetic patients and Streptozotocin diabetic rats. Mol. Genet. Metab. 2002, 75, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Abdolmaleki, F.; Heidarianpour, A. Endurance exercise training restores diabetes-induced alteration in circulating glycosylphosphatidylinositol-specific phospholipase D levels in rats. Diabetol. Metab. Syndr. 2020, 12, 43. [Google Scholar] [CrossRef]
- Chalasani, N.; Vuppalanchi, R.; Raikwar, N.S.; Deeg, M.A. Glycosylphosphatidylinositol-specific phospholipase D in nonalcoholic fatty liver disease: A preliminary study. J. Clin. Endocrinol. Metab. 2006, 91, 2279–2285. [Google Scholar] [CrossRef] [Green Version]
- Qin, W.; Liang, Y.-Z.; Qin, B.-Y.; Zhang, J.-L.; Xia, N. The clinical significance of glycoprotein phospholipase D levels in distinguishing early stage latent autoimmune diabetes in adults and type 2 diabetes. PLoS ONE 2016, 11, e0156959. [Google Scholar] [CrossRef] [Green Version]
- Kurtz, T.A.; Fineberg, N.S.; Considine, R.V.; Deeg, M.A. Insulin resistance is associated with increased serum levels of glycosylphosphatidylinositol-specific phospholipase D. Metabolism 2004, 53, 138–139. [Google Scholar] [CrossRef]
- von Toerne, C.; Huth, C.; de las Heras, T.; Kronenberg, F.; Herder, C.; Koenig, W.; Meisinger, C.; Rathmann, W.; Waldenberger, M.; Roden, M.; et al. MASP1, THB1, GPLD1 and Apo-IV are novel biomarkers associated with prediabetes: The KORA F4 study. Diabetologia 2016, 59, 1882–1892. [Google Scholar] [CrossRef]
- Gray, D.L.; O’Brien, K.D.; D’Alessio, D.A.; Brehm, B.J.; Deeg, M.A. Plasma glycosylphosphatidylinositol-specific phospholipase D predicts the change in insulin sensitivity in response to a low-fat but not a low-carbohydrate diet in obese women. Metabolism 2008, 57, 473–478. [Google Scholar] [CrossRef] [Green Version]
- Raymond, F.D.; Fortunato, G.; Moss, D.W.; Castaldo, G.; Salvatore, F.; Impallomeni, M. Inositol-specific phospholipase D activity in health and disease. Clin. Sci. 1994, 86, 447–451. [Google Scholar] [CrossRef] [PubMed]
- Maguire, G.A.; Gossner, A. Glycosyl phosphatidyl inositol phospholipase D activity in human serum. Ann. Clin. Biochem. 1995, 32, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Deeg, M.A.; Bowen, R.F.; Williams, M.D.; Olson, L.K.; Kirk, E.A.; Leboeuf, R.C. Increased expression of GPI-specific phospholipase D in mouse models of type 1 diabetes. Am. J. Physiol. Endocrinol. Metab. 2001, 281, E147–E154. [Google Scholar] [CrossRef] [PubMed]
- Raikwar, N.S.; Bowen-Deeg, R.F.; Du, X.S.; Low, M.G.; Deeg, M.A. Glycosylphosphatidylinositol-specific phospholipase D improves glucose tolerance. Metab. Clin. Exp. 2010, 59, 1413–1420. [Google Scholar] [CrossRef]
- Raikwar, N.S.; Cho, W.K.; Bowen, R.F.; Deeg, M.A. Glycosylphosphatidylinositol-specific phospholipase D influences triglyceride-rich lipoprotein metabolism. Am. J. Physiol. Endocrinol. Metab. 2006, 290, E463–E470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davitz, M.A.; Hom, J.; Schenkman, S. Purification of a glycosyl-phosphatidylinositol-specific phospholipase D from human plasma. J. Biol. Chem. 1989, 264, 13760–13764. [Google Scholar] [CrossRef]
- Low, M.G.; Huang, K.-S. Factors affecting the ability of glycosylphosphatidylinositol-specific phospholipase D to degrade the membrane anchors of cell surface proteins. Biochem. J. 1991, 279, 483–493. [Google Scholar] [CrossRef]
- Low, M.G.; Prasad, A.R. A phospholipase D specific for the phosphatidylinositol anchor of cell-surface proteins is abundant in plasma. Proc. Natl. Acad. Sci. USA 1988, 85, 980–984. [Google Scholar] [CrossRef] [Green Version]
- Metz, C.N.; Brunner, G.; Choi-Muira, N.H.; Nguyen, H.Y.; Gabrilove, J.; Caras, I.W.; Altszuler, N.; Rifkin, D.B.; Wilson, E.L.; Davitz, M.A. Release of GPI-anchored membrane proteins by a cell-associated GPI-specific phospholipase D. EMBO J. 1994, 13, 1741–1751. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, O.G.; Wilhelm, S.; Escott, G.M.; Lutz, V.; Magdolen, V.; Schmitt, M.; Rifkin, D.B.; Wilson, E.L.; Graeff, H.; Brunner, G. Cellular glycosylphosphatidylinositol-specific phospholipase D regulates urokinase receptor shedding and cell surface expression. J. Cell. Physiol. 1999, 180, 225–235. [Google Scholar] [CrossRef]
- Küng, M.; Bütikofer, P.; Brodbeck, U.; Stadelmann, B. Expression of intracellular and GPI-anchored forms of GPI-specific phospholipase D in COS-1 cells. Biochim. Biophys. Acta 1997, 1357, 329–338. [Google Scholar] [CrossRef] [Green Version]
- Mann, K.J.; Hepworth, M.R.; Raikwar, N.S.; Deeg, M.A.; Sevlever, D. Effect of glycosylphosphatidylinositol (GPI)-phospholipase D overexpression on GPI metabolism. Biochem. J. 2004, 378, 641–648. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.-Y.; Hofmann, S.L. Lysosomal metabolism of lipid-modified proteins. J. Lipid Res. 2006, 47, 1352–1357. [Google Scholar] [CrossRef] [Green Version]
- Bergman, A.S.; Carlsson, S.R. Saponin-induced release of cell-surface-anchored Thy-1 by serum glycosylphosphatidylinositol-specific phospholipase D. Biochem. J. 1994, 298, 661–668. [Google Scholar] [CrossRef] [Green Version]
- Tujioka, H.; Misumi, Y.; Takami, N.; Ikehara, Y. Posttranslational modification of glycosylphosphatidylinositol (GPI)-specific phospholipase D and its activity in cleavage of GPI anchors. Biochem. Biophys. Res. Commun. 1998, 251, 737–743. [Google Scholar] [CrossRef] [PubMed]
- Civenni, G.; Bütikofer, P.; Stadelmann, B.; Brodbeck, U. In vitro phosphoryation of purified glycosylphosphatidylinositol-specific phospholipase D. Biol. Chem. 1999, 380, 585–588. [Google Scholar] [CrossRef] [PubMed]
- Rhode, H.; Schulze, M.; Cumme, G.A.; Göhlert, A.; Blume, E.; Bublitz, R.; Schilling, K.; Horn, A. Glycosylphosphatidylinositol-specific phospholipase D of human serum-activity modulation by naturally occurring amphiphiles. Biol. Chem. 2000, 381, 471–485. [Google Scholar] [CrossRef] [PubMed]
- Low, M.G.; Huang, K.-S. Phosphatidic acid, lysophosphatidic acid, and lipid A are inhibitors of glycosylphosphatidylinositol-specific phospholipase D. J. Biol. Chem. 1993, 268, 8480–8490. [Google Scholar] [CrossRef]
- Low, M.G.; Stütz, P. Inhibition of the plasma glycosylphosphatidylinositol-specific phospholipase D by synthetic analogs of lipid A and phosphatidic acid. Arch. Biochem. Biophys. 1999, 371, 332–339. [Google Scholar] [CrossRef]
- Medof, M.E.; Kinoshita, T.; Nussenzweig, V. Inhibition of complement activation on the surface of cells after incorporation of decay-accelerating factor (DAF) into their membranes. J. Exp. Med. 1984, 160, 1558–1578. [Google Scholar] [CrossRef] [PubMed]
- Medof, M.E.; Kinoshita, T.; Silber, R.; Nussenzweig, V. Amelioration of lytic abnormalities of paroxysmale nocturnal hemoglobinuria with decay-accelerating factor. Proc. Natl. Acad. Sci. USA 1985, 82, 2980–2984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zalman, L.S.; Wood, L.M.; Frank, M.M.; Müller-Eberhard, H.J. Deficiency of the homologous restriction factor in paroxysmal nocturnal hemoglobinuria. J. Exp. Med. 1987, 165, 572–577. [Google Scholar] [CrossRef] [Green Version]
- Wilcox, L.A.; Ezzel, J.L.; Bernshaw, N.J.; Parker, C.J. Molecular basis of the enhanced susceptibility of the erythrocytes of paroxysmal nocturnal hemoglobinuria to hemolysis in acidified serum. Blood 1991, 78, 820–829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van den Berg, C.W.; Cinek, T.; Hallett, M.B.; Horejsi, V.; Morgan, B.P. Exogenous glycosyl phosphatidylinositol-anchored CD59 associates with kinases in membrane clusters on U937 cells and becomes Ca(2+)-signaling competent. J. Cell Biol. 1995, 131, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Premkumar, D.R.D.; Fukuoka, Y.; Sevlever, D.; Brunschwig, E.; Rosenberry, T.L.; Tykocinski, M.L.; Medof, M.E. Properties of exogenously added GPI-anchored proteins following their incorporation into cells. J. Cell. Biochem. 2001, 82, 234–245. [Google Scholar] [CrossRef]
- Müller, G.A. Membrane insertion and intercellular transfer of glycosylphosphatidylinositol-anchored proteins: Potential therapeutic applications. Arch. Physiol. Biochem. 2020, 126, 139–156. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, S.; Anderson, M.; Ahmed, S.N.; Sell, K.W.; Selvaraj, P. Purification and optimization of functional reconstitution on the surface of leukemic cell lines of GPI-anchored Fc gamma receptor III. J. Immunol. Methods 1995, 184, 241–251. [Google Scholar] [CrossRef]
- Zhang, F.; Schmidt, W.G.; Hou, Y.; Williams, A.F.; Jacobson, K. Spontaneous incorporation of the glycosyl-phosphatidylinositol-linked protein Thy-1 into cell membranes. Proc. Natl. Acad. Sci. USA 1992, 89, 5231–5235. [Google Scholar] [CrossRef] [Green Version]
- Ilangumaran, S.; Arni, S.; Poincelet, M.; Theler, J.M.; Brennan, P.J.; Nasir-ud-Din; Hoessli, D.C. Integration of mycobacterial lipoarabinomannans into glycosylphosphatidylinositol-rich domains of lymphomonocytic cell plasma membranes. J. Immunol. 1995, 155, 1334–1342. [Google Scholar] [PubMed]
- Walter, E.I.; Ratnoff, W.D.; Long, K.E.; Kazura, J.W.; Medof, M.E. Effect of glycoinositolphospholipid anchor lipid groups on functional properties of decay-accelerating factor proteins in cells. J. Biol. Chem. 1992, 267, 1245–1252. [Google Scholar] [CrossRef]
- Suzuki, K.; Okumura, Y. GPI-linked proteins do not transfer spontaneously from erythrocytes to liposomes. New aspects of reorganization of the cell membrane. Biochemistry 2000, 39, 9477–9485. [Google Scholar] [CrossRef] [PubMed]
- Heider, S.; Dangerfield, J.A.; Metzner, C. Biomedical applications of glycosylphosphatidylinositol-anchored proteins. J. Lipid Res. 2016, 57, 1778–1788. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Geno-Type | Feeding | Weight [g] | Age [Weeks] | Fasting Blood Glucose [mM] | Fasting Plasma Insulin [µg/L] | Pheno-Type |
|---|---|---|---|---|---|---|
| Wistar | lean | 309.7 356.4 336.8 321.5 360.7 376.3 331.8 358.9 344.0 | 10 10 10 10 10 10 10 10 | 5.49 5.87 5.94 6.23 6.10 6.81 7.25 7.87 6.44 | 0.56 0.48 0.59 0.65 0.74 0.86 1.46 1.69 0.88 | normo-glycemic normo-insulinemic |
| obese | 509.6 469.3 496.1 481.4 561.0 482.1 523.9 580.4 512.0 | 10 10 10 10 10 10 10 10 | 6.36 6.08 6.75 6.97 6.44 6.82 7.45 7.90 6.85 | 0.89 1.74 2.73 1.97 1.63 2.94 1.83 2.19 1.99 | normo-glycemic mildly hyper-insulinemic | |
| ZF | lean | 435.3 496.5 473.2 450.8 505.2 479.3 499.5 421.7 470.2 | 40 40 40 40 40 40 40 40 | 6.27 5.75 5.42 5.99 6.34 5.81 5.12 5.93 5.83 | 1.24 0.87 0.55 0.70 0.62 0.75 0.71 1.12 0.82 | normo-glycemic normo-insulinemic |
| obese | 589.4 606.5 635.1 609.6 687.5 669.8 727.3 698.9 653.0 | 40 40 40 40 40 40 40 40 | 5.11 5.84 5.97 6.25 5.80 6.49 5.62 5.33 5.80 | 3.25 3.44 2.94 2.61 3.50 2.57 3.75 3.29 3.17 | normo-glycemic hyper-insulinemic | |
| ZDF | lean | 376.2 327.8 385.1 395.6 342.0 328.3 401.7 340.5 362.1 | 16 16 16 16 16 16 16 16 | 6.09 5.79 5.23 5.12 5.53 5.97 6.16 5.38 5.65 | 1.44 1.14 1.99 0.79 0.85 0.57 1.03 1.30 1.14 | normo-glycemic mildly hyper-insulinemic |
| obese | 355.6 397.2 337.1 438.0 463.8 451.2 425.9 474.4 417.9 | 16 16 16 16 16 16 16 16 | 20.41 26.87 19.40 24.23 17.94 25.18 21.07 23.82 22.37 | 1.56 2.98 2.41 3.07 1.84 1.22 2.19 2.63 2.24 | hyper-glycemic hyper-insulinemic |
| Proband | Age (Years) | Gender | HbA1c (%) | Body Weight | Metabolic State |
|---|---|---|---|---|---|
| G | 29 | F | 4.8 | lean | control |
| B | 50 | F | 5.4 | lean | control |
| E | 42 | M | 5.1 | lean | control |
| D | 64 | F | 5.9 | overweight | T1D |
| I | 68 | M | 6.6 | overweight | T2D |
| F | 49 | F | 7.1 | obese | T2D |
| C | 41 | F | 5.5 | obese | T1D |
| K | 51 | F | 7.5 | obese | T1D |
| (a) | ||||||
| Proband | HDL-Chol. (mM) | apo A-I (mg/dL) | CD73 (μg/mL) | CD55 (μg/mL) | AChE (μg/dL) | Total Chol. (mM) |
| G | 1.45 ± 0.13 | 161.27 ± 10.40 | 1.31 ± 0.29 | 0.12 ± 0.03 | 0.57 ± 0.19 | 4.96 ± 0.13 |
| B | 1.35 ± 0.18 | 147.39 ± 8.06 | 1.37 ± 0.25 | 0.09 ± 0.03 | 0.44 ± 0.16 | 5.04 ± 0.19 |
| E | 1.51 ± 0.20 | 153.84 ± 11.92 | 1.46 ± 0.19 | 0.06 ± 0.02 | 0.35 ± 0.14 | 4.84 ± 0.24 |
| D | 1.79 ± 0.19 | 174.77 ± 12.51 | 1.69 ± 0.21 | 0.14 ± 0.03 | 0.66 ± 0.14 | 5.25 ± 0.18 |
| I | 1.21 ± 0.23 | 137.11 ± 10.08 | 1.82 ± 0.24 | 0.11 ± 0.02 | 0.76 ± 0.13 | 5.16 ± 0.20 |
| F | 1.06 ± 0.11 | 129.63 ± 9.65 | 1.94 ± 0.31 | 0.16 ± 0.04 | 0.70 ± 0.15 | 5.29 ± 0.23 |
| C | 1.85 ± 0.22 | 180.70 ± 7.91 | 1.51 ± 0.29 | 0.10 ± 0.03 | 0.50 ± 0.11 | 4.89 ± 0.15 |
| K | 1.59 ± 0.18 | 183.36 ± 8.87 | 1.77 ± 0.25 | 0.18 ± 0.02 | 0.59 ± 0.13 | 4.74 ± 0.31 |
| (b) | ||||||
| Proband | HDL-Chol. (mM) | apo A-I (mg/dL) | CD73 (μg/mL) | CD55 (μg/mL) | AChE (μg/dL) | Total Chol. (mM) |
| Control | 1.44 ± 0.20 | 154.17 ± 14.87 | 1.38 ± 0.26 | 0.09 ± 0.02 | 0.45 ± 0.17 | 4.95 ± 0.13 |
| T1D/T2D | 1.50 ± 0.18 | 161.11 ± 13.95 | 1.75 § ± 0.22 | 0.14 § ± 0.03 | 0.63 ± 0.15 | 5.07 ± 0.20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Müller, G.A.; Lechner, A.; Tschöp, M.H.; Müller, T.D. Interaction of Full-Length Glycosylphosphatidylinositol-Anchored Proteins with Serum Proteins and Their Translocation to Cells In Vitro Depend on the (Pre-)Diabetic State in Rats and Humans. Biomedicines 2021, 9, 277. https://doi.org/10.3390/biomedicines9030277
Müller GA, Lechner A, Tschöp MH, Müller TD. Interaction of Full-Length Glycosylphosphatidylinositol-Anchored Proteins with Serum Proteins and Their Translocation to Cells In Vitro Depend on the (Pre-)Diabetic State in Rats and Humans. Biomedicines. 2021; 9(3):277. https://doi.org/10.3390/biomedicines9030277
Chicago/Turabian StyleMüller, Günter A., Andreas Lechner, Matthias H. Tschöp, and Timo D. Müller. 2021. "Interaction of Full-Length Glycosylphosphatidylinositol-Anchored Proteins with Serum Proteins and Their Translocation to Cells In Vitro Depend on the (Pre-)Diabetic State in Rats and Humans" Biomedicines 9, no. 3: 277. https://doi.org/10.3390/biomedicines9030277
APA StyleMüller, G. A., Lechner, A., Tschöp, M. H., & Müller, T. D. (2021). Interaction of Full-Length Glycosylphosphatidylinositol-Anchored Proteins with Serum Proteins and Their Translocation to Cells In Vitro Depend on the (Pre-)Diabetic State in Rats and Humans. Biomedicines, 9(3), 277. https://doi.org/10.3390/biomedicines9030277






