Copper Toxicity Is Not Just Oxidative Damage: Zinc Systems and Insight from Wilson Disease
Abstract
1. Introduction
1.1. Copper
1.2. Normal Copper Metabolism and Its Relationship with Liver
1.3. Copper Toxicity
2. Copper Excess and Consequences of Copper Toxicity in Humans and Animal Models
2.1. Copper in Liver Disease: Viral Hepatitis
2.2. Copper in Liver Disease: Cholestatic Liver Disorders
2.3. Copper Toxicity from Excess Consumption
2.4. Human Copper Toxicosis—Wilson Disease
2.5. Non-Wilson Copper Toxicosis
2.6. Zinc as a Therapy for Wilson Disease—Competition with Copper and Negative Copper Balance
3. Specific Targets of Copper Toxicity in Wilson Disease
3.1. Metabolic Consequences of Copper Toxicity in Wilson Disease Models
3.2. Zinc Systems in Wilson Disease: Transcriptional Regulation and Nuclear Receptors
3.3. Zinc
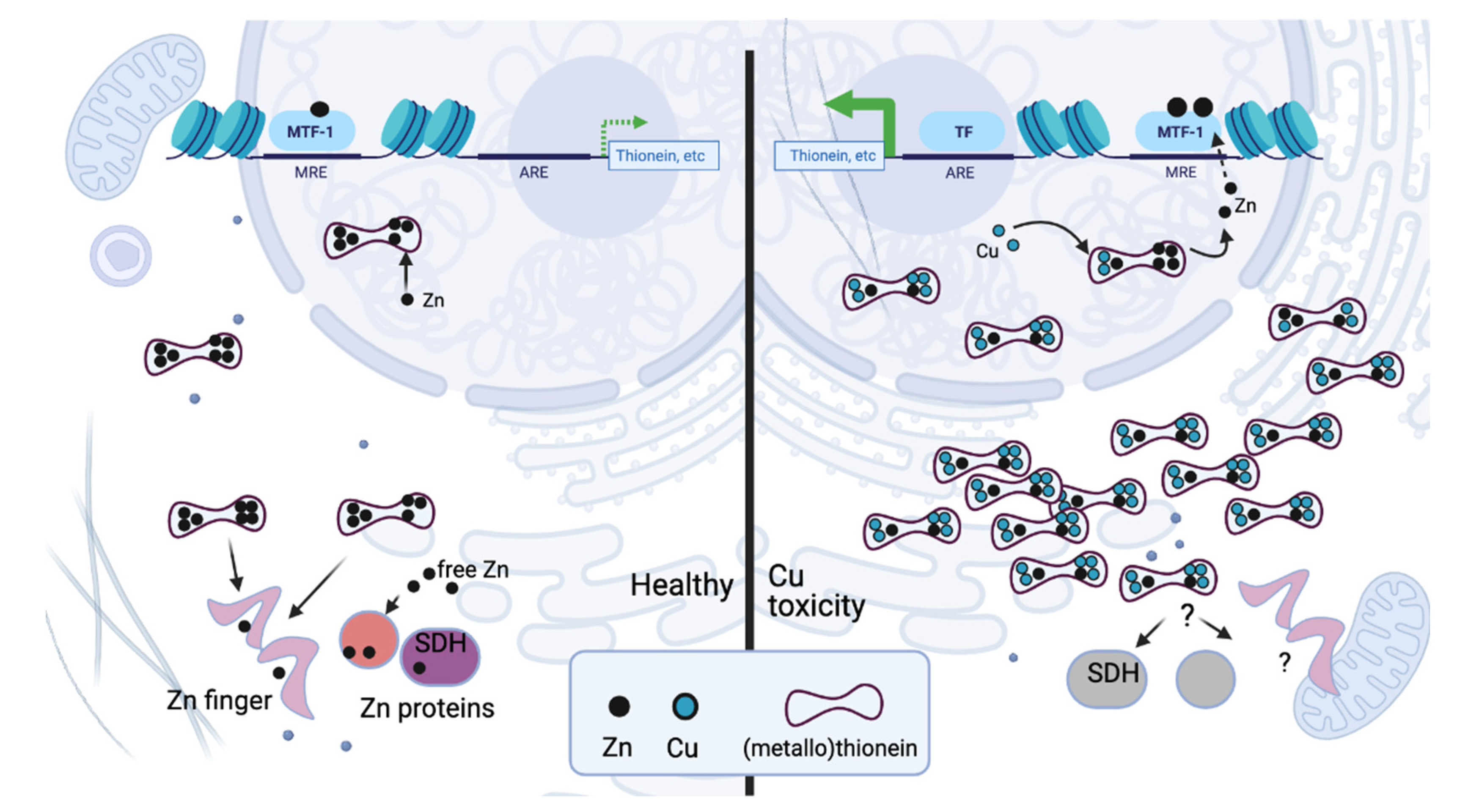
3.4. Toxic Copper and Zinc Deficiency
3.5. Copper and Zinc as Factors in Non-Wilson Pathology—Are They Linked?
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fahrni, C.J. Synthetic Fluorescent Probes for Monovalent Copper. Curr. Opin. Chem. Biol. 2013, 17, 656–662. [Google Scholar] [CrossRef] [PubMed]
- Stoj, C.; Kosman, D.J. Cuprous Oxidase Activity of Yeast Fet3p and Human Ceruloplasmin: Implication for Function. FEBS Lett. 2003, 554, 422–426. [Google Scholar] [CrossRef]
- Harris, E.D. A Requirement for Copper in Angiogenesis. Nutr. Rev. 2004, 62, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Itoh, S.; Kondo, T.; Komatsu, M.; Ohshiro, Y.; Li, C.; Kanehisa, N.; Kai, Y.; Fukuzumi, S. Functional Model of Dopamine.beta.-Hydroxylase. Quantitative Ligand Hydroxylation at the Benzylic Position of a Copper Complex by Dioxygen. J. Am. Chem. Soc. 1995, 117, 4714–4715. [Google Scholar] [CrossRef]
- Percival, S.S. Copper and Immunity. Am. J. Clin. Nutr. 1998, 67, 1064S–1068S. [Google Scholar] [CrossRef]
- Ludwig, B.; Bender, E.; Arnold, S.; Hüttemann, M.; Lee, I.; Kadenbach, B. Cytochrome c Oxidase and the Regulation of Oxidative Phosphorylation. ChemBioChem 2001, 2, 392–403. [Google Scholar] [CrossRef]
- Vest, K.E.; Hashemi, H.F.; Cobine, P.A. The Copper Metallome in Eukaryotic Cells. Met. Ions Life Sci. 2013, 12, 451–478. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, A.; Chakraborty, K.; Shukla, A. Cellular Copper Homeostasis: Current Concepts on Its Interplay with Glutathione Homeostasis and Its Implication in Physiology and Human Diseases. Metallomics 2017, 9, 1376–1388. [Google Scholar] [CrossRef]
- Nyasae, L.; Bustos, R.; Braiterman, L.; Eipper, B.; Hubbard, A. Dynamics of Endogenous ATP7A (Menkes Protein) in Intestinal Epithelial Cells: Copper-Dependent Redistribution between Two Intracellular Sites. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 292, G1181–G1194. [Google Scholar] [CrossRef]
- Polishchuk, E.V.; Concilli, M.; Iacobacci, S.; Chesi, G.; Pastore, N.; Piccolo, P.; Paladino, S.; Baldantoni, D.; van IJzendoorn, S.C.D.; Chan, J.; et al. Wilson Disease Protein ATP7B Utilizes Lysosomal Exocytosis to Maintain Copper Homeostasis. Dev. Cell 2014, 29, 686–700. [Google Scholar] [CrossRef]
- Terada, K.; Kawarada, Y.; Miura, N.; Yasui, O.; Koyama, K.; Sugiyama, T. Copper Incorporation into Ceruloplasmin in Rat Livers. Biochim. Biophys. Acta 1995, 1270, 58–62. [Google Scholar] [CrossRef]
- Linder, M.C. Copper Homeostasis in Mammals, with Emphasis on Secretion and Excretion. A Review. Int. J. Mol. Sci. 2020, 21, 4932. [Google Scholar] [CrossRef] [PubMed]
- Voskoboinik, I.; Camakaris, J. Menkes Copper-Translocating P-Type ATPase (ATP7A): Biochemical and Cell Biology Properties, and Role in Menkes Disease. J. Bioenerg. Biomembr. 2002, 34, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.-E.; Turski, M.L.; Nose, Y.; Casad, M.; Rockman, H.A.; Thiele, D.J. Cardiac Copper Deficiency Activates a Systemic Signaling Mechanism That Communicates with the Copper Acquisition and Storage Organs. Cell Metab. 2010, 11, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Goldfischer, S.; Popper, H.; Sternlieb, I. The Significance of Variations in the Distribution of Copper in Liver Disease. Am. J. Pathol. 1980, 99, 715–730. [Google Scholar]
- Huster, D.; Purnat, T.D.; Burkhead, J.L.; Ralle, M.; Fiehn, O.; Stuckert, F.; Olson, N.E.; Teupser, D.; Lutsenko, S. High Copper Selectively Alters Lipid Metabolism and Cell Cycle Machinery in the Mouse Model of Wilson Disease. J. Biol. Chem. 2007, 282, 8343–8355. [Google Scholar] [CrossRef] [PubMed]
- Muchenditsi, A.; Yang, H.; Hamilton, J.P.; Koganti, L.; Housseau, F.; Aronov, L.; Fan, H.; Pierson, H.; Bhattacharjee, A.; Murphy, R.; et al. Targeted Inactivation of Copper Transporter Atp7b in Hepatocytes Causes Liver Steatosis and Obesity in Mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 313, G39–G49. [Google Scholar] [CrossRef]
- Wooton-Kee, C.R.; Jain, A.K.; Wagner, M.; Grusak, M.A.; Finegold, M.J.; Lutsenko, S.; Moore, D.D. Elevated Copper Impairs Hepatic Nuclear Receptor Function in Wilson’s Disease. J. Clin. Investig. 2015, 125, 3449–3460. [Google Scholar] [CrossRef] [PubMed]
- Zischka, H.; Lichtmannegger, J. Pathological Mitochondrial Copper Overload in Livers of Wilson’s Disease Patients and Related Animal Models. Ann. N. Y. Acad. Sci. 2014, 1315, 6–15. [Google Scholar] [CrossRef]
- Arain, S.A.; Kazi, T.G.; Afridi, H.I.; Talpur, F.N.; Mughal, M.A.; Shah, F.; Arain, S.S.; Panhwar, A.H. Estimation of Copper and Iron Burden in Biological Samples of Various Stages of Hepatitis C and Liver Cirrhosis Patients. Biol. Trace Elem. Res. 2014, 160, 197–205. [Google Scholar] [CrossRef]
- Guo, C.H.; Chen, P.C.; Lin, K.P.; Shih, M.Y.; Ko, W.S. Trace Metal Imbalance Associated with Oxidative Stress and Inflammatory Status in Anti-Hepatitis C Virus Antibody Positive Subjects. Environ. Toxicol. Pharmacol. 2012, 33, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Petukhov, V.I.; Baumane, L.K.; Kalvinsh, I.Y.; Skesters, A.P.; Silova, A.A.; Rozentale, B.; Ivanchenko, L.A. Chronic Hepatitis C: Quantitative EPR Analysis of Nitrogen Oxide and Copper in Patients’ Blood. Bull. Exp. Biol. Med. 2008, 146, 875–877. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Huang, J.F.; Tsai, L.Y.; Huang, Y.L. Selenium, Iron, Copper, and Zinc Levels and Copper-to-Zinc Ratios in Serum of Patients at Different Stages of Viral Hepatic Diseases. Biol. Trace Elem. Res. 2006, 109, 15–24. [Google Scholar] [CrossRef]
- Afridi, H.I.; Kazi, T.G.; Kazi, N.G.; Jamali, M.K.; Sarfaraz, R.A.; Arain, M.B.; Kandhro, G.A.; Shah, A.Q.; Baig, J.A.; Jalbani, N.; et al. Determination of Copper and Iron in Biological Samples of Viral Hepatitis (A-E) Female Patients. Biol. Trace Elem. Res. 2009, 129, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, I.M.; Kaplan, H.B.; Edelson, H.S.; Weissmann, G. Ceruloplasmin: An Acute Phase Reactant That Scavenges Oxygen-Derived Free Radicals. Ann. N. Y. Acad. Sci. 1982, 389, 368–379. [Google Scholar] [CrossRef]
- Hatano, R.; Ebara, M.; Fukuda, H.; Yoshikawa, M.; Sugiura, N.; Kondo, F.; Yukawa, M.; Saisho, H. Accumulation of Copper in the Liver and Hepatic Injury in Chronic Hepatitis, C.J. Gastroenterol. Hepatol. 2000, 15, 786–791. [Google Scholar] [CrossRef]
- Ishida, M.; Nakagawara, G.; Imamura, Y.; Fukuda, M. Iron and Copper Deposition in Chronic Active Hepatitis and Liver Cirrhosis; Pathogenetic Role in Progressive Liver Cell Damage. Eur. J. Histochem. 1995, 39, 221–236. [Google Scholar]
- Ebara, M.; Fukuda, H.; Hatano, R.; Yoshikawa, M.; Sugiura, N.; Saisho, H.; Kondo, F.; Yukawa, M. Metal Contents in the Liver of Patients with Chronic Liver Disease Caused by Hepatitis C Virus. Reference to Hepatocellular Carcinoma. Oncology 2003, 65, 323–330. [Google Scholar] [CrossRef]
- Salaspuro, M.P.; Pikkarainen, P.; Sipponen, P.; Vuori, E.; Miettinen, T.A. Hepatic Copper in Primary Biliary Cirrhosis: Biliary Excretion and Response to Penicillamine Treatment. Gut 1981, 22, 901–906. [Google Scholar] [CrossRef] [PubMed]
- Benson, G.D. Hepatic Copper Accumulation in Primary Biliary Cirrhosis. Yale, J. Biol. Med. 1979, 52, 83–88. [Google Scholar]
- Gong, Y.; Klingenberg, S.L.; Gluud, C. Systematic Review and Meta-Analysis: D-Penicillamine vs. Placebo/No Intervention in Patients with Primary Biliary Cirrhosis--Cochrane Hepato-Biliary Group. Aliment. Pharmacol. Ther. 2006, 24, 1535–1544. [Google Scholar] [CrossRef]
- Eife, R.; Weiss, M.; Barros, V.; Sigmund, B.; Goriup, U.; Komb, D.; Wolf, W.; Kittel, J.; Schramel, P.; Reiter, K. Chronic Poisoning by Copper in Tap Water: I. Copper Intoxications with Predominantly Gastointestinal Symptoms. Eur. J. Med. Res. 1999, 4, 219–223. [Google Scholar] [PubMed]
- Eife, R.; Weiss, M.; Müller-Höcker, M.; Lang, T.; Barros, V.; Sigmund, B.; Thanner, F.; Welling, P.; Lange, H.; Wolf, W.; et al. Chronic Poisoning by Copper in Tap Water: II. Copper Intoxications with Predominantly Systemic Symptoms. Eur. J. Med. Res. 1999, 4, 224–228. [Google Scholar]
- Franchitto, N.; Gandia-Mailly, P.; Georges, B.; Galinier, A.; Telmon, N.; Ducassé, J.L.; Rougé, D. Acute Copper Sulphate Poisoning: A Case Report and Literature Review. Resuscitation 2008, 78, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Nastoulis, E.; Karakasi, M.V.; Couvaris, C.M.; Kapetanakis, S.; Fiska, A.; Pavlidis, P. Greenish-Blue Gastric Content: Literature Review and Case Report on Acute Copper Sulphate Poisoning. Forensic. Sci. Rev. 2017, 29, 77–91. [Google Scholar]
- Evering, W.E.; Haywood, S.; Bremner, I.; Trafford, J. The Protective Role of Metallothionein in Copper Overload: I. Differential Distribution of Immunoreactive Metallothionein in Copper-Loaded Rat Liver and Kidney. Chem. Biol. Interact. 1991, 78, 283–295. [Google Scholar] [CrossRef]
- Evering, W.E.; Haywood, S.; Bremner, I.; Wood, A.M.; Trafford, J. The Protective Role of Metallothionein in Copper-Overload: II. Transport and Excretion of Immunoreactive MT-1 in Blood, Bile and Urine of Copper-Loaded Rats. Chem. Biol. Interact. 1991, 78, 297–305. [Google Scholar] [CrossRef]
- Fuentealba, I.C.; Davis, R.W.; Elmes, M.E.; Jasani, B.; Haywood, S. Mechanisms of Tolerance in the Copper-Loaded Rat Liver. Exp. Mol. Pathol. 1993, 59, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Galhardi, C.M.; Diniz, Y.S.; Faine, L.A.; Rodrigues, H.G.; Burneiko, R.C.M.; Ribas, B.O.; Novelli, E.L.B. Toxicity of Copper Intake: Lipid Profile, Oxidative Stress and Susceptibility to Renal Dysfunction. Food Chem. Toxicol. 2004, 42, 2053–2060. [Google Scholar] [CrossRef]
- Liu, H.; Guo, H.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Copper Induces Hepatic Inflammatory Responses by Activation of MAPKs and NF-ΚB Signalling Pathways in the Mouse. Ecotoxicol. Environ. Saf. 2020, 201, 110806. [Google Scholar] [CrossRef]
- Aburto, E.M.; Cribb, A.E.; Fuentealba, I.C.; Ikede, B.O.; Kibenge, F.S.; Markham, F. Morphological and Biochemical Assessment of the Liver Response to Excess Dietary Copper in Fischer 344 Rats. Can. J. Vet. Res. 2001, 65, 97–103. [Google Scholar] [PubMed]
- Roberts, E.A.; Schilsky, M.L. Diagnosis and Treatment of Wilson Disease: An Update. Hepatology 2008, 47, 2089–2111. [Google Scholar] [CrossRef]
- Araya, M.; Núñez, H.; Pavez, L.; Arredondo, M.; Méndez, M.; Cisternas, F.; Pizarro, F.; Sierralta, W.; Uauy, R.; González, M. Administration of High Doses of Copper to Capuchin Monkeys Does Not Cause Liver Damage but Induces Transcriptional Activation of Hepatic Proliferative Responses. J. Nutr. 2012, 142, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Latorre, M.; Burkhead, J.L.; Hodar, C.; Arredondo, M.; González, M.; Araya, M. Chronic Copper Treatment Prevents the Liver Critical Balance Transcription Response Induced by Acetaminophen. J. Trace Elem. Med. Biol. 2019, 53, 113–119. [Google Scholar] [CrossRef]
- Kumar, M.; Gaharwar, U.; Paul, S.; Poojary, M.; Pandhare, K.; Scaria, V.; Bk, B. WilsonGen a Comprehensive Clinically Annotated Genomic Variant Resource for Wilson’s Disease. Sci. Rep. 2020, 10, 9037. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.B.; Chernov, I.; Zhang, H.T.; Ross, B.M.; Das, K.; Lutsenko, S.; Parano, E.; Pavone, L.; Evgrafov, O.; Ivanova-Smolenskaya, I.A.; et al. Identification and Analysis of Mutations in the Wilson Disease Gene (ATP7B): Population Frequencies, Genotype-Phenotype Correlation, and Functional Analyses. Am. J. Hum. Genet. 1997, 61, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Weiss, K.H. Wilson Disease. In GeneReviews(R); Pagon, R.A., Adam, M.P., Ardinger, H.H., Wallace, S.E., Amemiya, A., Bean, L.J.H., Bird, T.D., Fong, C.T., Mefford, H.C., Smith, R.J.H., et al., Eds.; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Lutsenko, S. Modifying Factors and Phenotypic Diversity in Wilson’s Disease. Ann. N. Y. Acad. Sci. 2014, 1315, 56–63. [Google Scholar] [CrossRef]
- Rosencrantz, R.; Schilsky, M. Wilson Disease: Pathogenesis and Clinical Considerations in Diagnosis and Treatment. Semin. Liver Dis. 2011, 31, 245–259. [Google Scholar] [CrossRef]
- Strausak, D.; Mercer, J.F.; Dieter, H.H.; Stremmel, W.; Multhaup, G. Copper in Disorders with Neurological Symptoms: Alzheimer’s, Menkes, and Wilson Diseases. Brain Res. Bull. 2001, 55, 175–185. [Google Scholar] [CrossRef]
- Litwin, T.; Gromadzka, G.; Szpak, G.M.; Jabłonka-Salach, K.; Bulska, E.; Członkowska, A. Brain Metal Accumulation in Wilson’s Disease. J. Neurol. Sci. 2013, 329, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Scheiber, I.F.; Brůha, R.; Dušek, P. Pathogenesis of Wilson Disease. Handb. Clin. Neurol. 2017, 142, 43–55. [Google Scholar] [CrossRef]
- Poujois, A.; Mikol, J.; Woimant, F. Wilson Disease: Brain Pathology. Handb. Clin. Neurol. 2017, 142, 77–89. [Google Scholar] [CrossRef]
- Brewer, G.J.; Dick, R.D.; Johnson, V.D.; Brunberg, J.A.; Kluin, K.J.; Fink, J.K. Treatment of Wilson’s Disease with Zinc: XV Long-Term Follow-up Studies. J. Lab. Clin. Med. 1998, 132, 264–278. [Google Scholar] [CrossRef]
- Brewer, G.J.; Dick, R.D.; Johnson, V.D.; Fink, J.K.; Kluin, K.J.; Daniels, S. Treatment of Wilson’s Disease with Zinc XVI: Treatment during the Pediatric Years. J. Lab. Clin. Med. 2001, 137, 191–198. [Google Scholar] [CrossRef]
- Linn, F.H.H.; Houwen, R.H.J.; van Hattum, J.; van der Kleij, S.; van Erpecum, K.J. Long-Term Exclusive Zinc Monotherapy in Symptomatic Wilson Disease: Experience in 17 Patients. Hepatology 2009, 50, 1442–1452. [Google Scholar] [CrossRef]
- Mezzaroba, L.; Alfieri, D.F.; Colado Simão, A.N.; Vissoci Reiche, E.M. The Role of Zinc, Copper, Manganese and Iron in Neurodegenerative Diseases. Neurotoxicology 2019, 74, 230–241. [Google Scholar] [CrossRef] [PubMed]
- Glass, J.D.; Reich, S.G.; DeLong, M.R. Wilson’s Disease. Development of Neurological Disease after Beginning Penicillamine Therapy. Arch. Neurol. 1990, 47, 595–596. [Google Scholar] [CrossRef] [PubMed]
- Brewer, G.J.; Askari, F.; Dick, R.B.; Sitterly, J.; Fink, J.K.; Carlson, M.; Kluin, K.J.; Lorincz, M.T. Treatment of Wilson’s Disease with Tetrathiomolybdate: V. Control of Free Copper by Tetrathiomolybdate and a Comparison with Trientine. Transl. Res. 2009, 154, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Tanner, M.S.; Portmann, B.; Mowat, A.P.; Williams, R.; Pandit, A.N.; Mills, C.F.; Bremner, I. Increased Hepatic Copper Concentration in Indian Childhood Cirrhosis. Lancet 1979, 1, 1203–1205. [Google Scholar] [CrossRef]
- Baker, A.; Gormally, S.; Saxena, R.; Baldwin, D.; Drumm, B.; Bonham, J.; Portmann, B.; Mowat, A.P. Copper-Associated Liver Disease in Childhood. J. Hepatol. 1995, 23, 538–543. [Google Scholar] [CrossRef]
- Scheinberg, I.H.; Sternlieb, I. Wilson Disease and Idiopathic Copper Toxicosis. Am. J. Clin. Nutr. 1996, 63, 842S–845S. [Google Scholar] [CrossRef] [PubMed]
- Muller, T.; Feichtinger, H.; Berger, H.; Muller, W. Endemic Tyrolean Infantile Cirrhosis: An Ecogenetic Disorder. Lancet 1996, 347, 877–880. [Google Scholar] [CrossRef]
- Müller, T.; Müller, W.; Feichtinger, H. Idiopathic Copper Toxicosis. Am. J. Clin. Nutr. 1998, 67, 1082S–1086S. [Google Scholar] [CrossRef] [PubMed]
- Müller, T.; Schäfer, H.; Rodeck, B.; Haupt, G.; Koch, H.; Bosse, H.; Welling, P.; Lange, H.; Krech, R.; Feist, D.; et al. Familial Clustering of Infantile Cirrhosis in Northern Germany: A Clue to the Etiology of Idiopathic Copper Toxicosis. J. Pediatr. 1999, 135, 189–196. [Google Scholar] [CrossRef]
- Sharda, B.; Bhandari, B.; Bhandari, L.M. Study of Copper, Zinc, Magnesium and Cadmium in ICC Patients, Parents and Siblings. Trans. R. Soc. Trop. Med. Hyg. 1982, 76, 747–750. [Google Scholar] [CrossRef]
- Bhave, S.A.; Pandit, A.N.; Tanner, M.S. Comparison of Feeding History of Children with Indian Childhood Cirrhosis and Paired Controls. J. Pediatr. Gastroenterol. Nutr. 1987, 6, 562–567. [Google Scholar] [CrossRef]
- Tanner, M.S. Role of Copper in Indian Childhood Cirrhosis. Am. J. Clin. Nutr. 1998, 67, 1074S–1081S. [Google Scholar] [CrossRef]
- Twedt, D.C.; Sternlieb, I.; Gilbertson, S.R. Clinical, Morphologic, and Chemical Studies on Copper Toxicosis of Bedlington Terriers. J. Am. Vet. Med. Assoc. 1979, 175, 269–275. [Google Scholar] [PubMed]
- Owen, C.A.; Bowie, E.J.; McCall, J.T.; Zollman, P.E. Hemostasis in the Copper-Laden Bedlington Terrier: A Possible Model of Wilson’s Disease. Haemostasis 1980, 9, 160–166. [Google Scholar] [CrossRef]
- Klomp, A.E.; van de Sluis, B.; Klomp, L.W.; Wijmenga, C. The Ubiquitously Expressed MURR1 Protein Is Absent in Canine Copper Toxicosis. J. Hepatol. 2003, 39, 703–709. [Google Scholar] [CrossRef]
- Pindar, S.; Ramirez, C. Predicting Copper Toxicosis: Relationship between the ATP7A and ATP7B Gene Mutations and Hepatic Copper Quantification in Dogs. Hum. Genet. 2019, 138, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Fieten, H.; Gill, Y.; Martin, A.J.; Concilli, M.; Dirksen, K.; van Steenbeek, F.G.; Spee, B.; van den Ingh, T.S.G.A.M.; Martens, E.C.C.P.; Festa, P.; et al. The Menkes and Wilson Disease Genes Counteract in Copper Toxicosis in Labrador Retrievers: A New Canine Model for Copper-Metabolism Disorders. Dis. Model. Mech. 2016, 9, 25–38. [Google Scholar] [CrossRef]
- Schouwink, G. De Hepato-Cerebral Degeneration (Met Een Onderzoekvan de Zinknkstofwisseling). Master’s Thesis, University of Amsterdam, Amsterdam, The Netherlands, 1961. [Google Scholar]
- Hoogenraad, T.U.; Van den Hamer, C.J.; Koevoet, R.; Korver, E.G. Oral Zinc in Wilson’s Disease. Lancet 1978, 2, 1262. [Google Scholar] [CrossRef]
- Hardyman, J.E.; Tyson, J.; Jackson, K.A.; Aldridge, C.; Cockell, S.J.; Wakeling, L.A.; Valentine, R.A.; Ford, D. Zinc Sensing by Metal-Responsive Transcription Factor 1 (MTF1) Controls Metallothionein and ZnT1 Expression to Buffer the Sensitivity of the Transcriptome Response to Zinc. Metallomics 2016, 8, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Calvo, J.; Jung, H.; Meloni, G. Copper Metallothioneins. IUBMB Life 2017, 69, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Yuzbasiyan-Gurkan, V.; Grider, A.; Nostrant, T.; Cousins, R.J.; Brewer, G.J. Treatment of Wilson’s Disease with Zinc: X. Intestinal Metallothionein Induction. J. Lab. Clin. Med. 1992, 120, 380–386. [Google Scholar] [PubMed]
- Camarata, M.A.; Ala, A.; Schilsky, M.L. Zinc Maintenance Therapy for Wilson Disease: A Comparison Between Zinc Acetate and Alternative Zinc Preparations. Hepatol. Commun. 2019, 3, 1151–1158. [Google Scholar] [CrossRef]
- Wu, Z.-Y.; Lin, M.-T.; Murong, S.-X.; Wang, N. Molecular Diagnosis and Prophylactic Therapy for Presymptomatic Chinese Patients with Wilson Disease. Arch. Neurol. 2003, 60, 737–741. [Google Scholar] [CrossRef][Green Version]
- Marcellini, M.; Di Ciommo, V.; Callea, F.; Devito, R.; Comparcola, D.; Sartorelli, M.R.; Carelli, G.; Carelli, F.; Nobili, V. Treatment of Wilson’s Disease with Zinc from the Time of Diagnosis in Pediatric Patients: A Single-Hospital, 10-Year Follow-up Study. J. Lab. Clin. Med. 2005, 145, 139–143. [Google Scholar] [CrossRef]
- Wiggelinkhuizen, M.; Tilanus, M.E.C.; Bollen, C.W.; Houwen, R.H.J. Systematic Review: Clinical Efficacy of Chelator Agents and Zinc in the Initial Treatment of Wilson Disease. Aliment. Pharmacol. Ther. 2009, 29, 947–958. [Google Scholar] [CrossRef]
- Hou, H.; Chen, D.; Liu, J.; Feng, L.; Zhang, J.; Liang, X.; Xu, Y.; Li, X. Zinc Monotherapy for Young Patients with Oligosymptomatic Wilson Disease: A Single Center, Retrospective Study. Clin. Res. Hepatol. Gastroenterol. 2021, 45, 101623. [Google Scholar] [CrossRef] [PubMed]
- Hoogenraad, T.U.; Van Hattum, J.; Van den Hamer, C.J. Management of Wilson’s Disease with Zinc Sulphate. Experience in a Series of 27 Patients. J. Neurol. Sci. 1987, 77, 137–146. [Google Scholar] [CrossRef]
- Weiss, K.H.; Gotthardt, D.N.; Klemm, D.; Merle, U.; Ferenci-Foerster, D.; Schaefer, M.; Ferenci, P.; Stremmel, W. Zinc Monotherapy Is Not as Effective as Chelating Agents in Treatment of Wilson Disease. Gastroenterology 2011, 140, 1189–1198.e1. [Google Scholar] [CrossRef] [PubMed]
- Czlonkowska, A.; Gajda, J.; Rodo, M. Effects of Long-Term Treatment in Wilson’s Disease with D-Penicillamine and Zinc Sulphate. J. Neurol. 1996, 243, 269–273. [Google Scholar] [CrossRef]
- Farinati, F.; Cardin, R.; D’inca, R.; Naccarato, R.; Sturniolo, G.C. Zinc Treatment Prevents Lipid Peroxidation and Increases Glutathione Availability in Wilson’s Disease. J. Lab. Clin. Med. 2003, 141, 372–377. [Google Scholar] [CrossRef]
- Kalita, J.; Kumar, V.; Chandra, S.; Kumar, B.; Misra, U.K. Worsening of Wilson Disease Following Penicillamine Therapy. Eur. Neurol. 2014, 71, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Medici, V.; Trevisan, C.P.; D’Incà, R.; Barollo, M.; Zancan, L.; Fagiuoli, S.; Martines, D.; Irato, P.; Sturniolo, G.C. Diagnosis and Management of Wilson’s Disease: Results of a Single Center Experience. J. Clin. Gastroenterol. 2006, 40, 936–941. [Google Scholar] [CrossRef]
- Chen, D.-B.; Feng, L.; Lin, X.-P.; Zhang, W.; Li, F.-R.; Liang, X.-L.; Li, X.-H. Penicillamine Increases Free Copper and Enhances Oxidative Stress in the Brain of Toxic Milk Mice. PLoS ONE 2012, 7, e37709. [Google Scholar] [CrossRef]
- Stuerenburg, H.J. CSF Copper Concentrations, Blood-Brain Barrier Function, and Coeruloplasmin Synthesis during the Treatment of Wilson’s Disease. J. Neural Transm. 2000, 107, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Beinhardt, S.; Leiss, W.; Stättermayer, A.F.; Graziadei, I.; Zoller, H.; Stauber, R.; Maieron, A.; Datz, C.; Steindl-Munda, P.; Hofer, H.; et al. Long-Term Outcomes of Patients with Wilson Disease in a Large Austrian Cohort. Clin. Gastroenterol. Hepatol. 2014, 12, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Bruha, R.; Marecek, Z.; Pospisilova, L.; Nevsimalova, S.; Vitek, L.; Martasek, P.; Nevoral, J.; Petrtyl, J.; Urbanek, P.; Jiraskova, A.; et al. Long-Term Follow-up of Wilson Disease: Natural History, Treatment, Mutations Analysis and Phenotypic Correlation. Liver Int. 2011, 31, 83–91. [Google Scholar] [CrossRef]
- Członkowska, A.; Litwin, T.; Karliński, M.; Dziezyc, K.; Chabik, G.; Czerska, M. D-Penicillamine versus Zinc Sulfate as First-Line Therapy for Wilson’s Disease. Eur. J. Neurol. 2014, 21, 599–606. [Google Scholar] [CrossRef]
- Dzieżyc, K.; Karliński, M.; Litwin, T.; Członkowska, A. Compliant Treatment with Anti-Copper Agents Prevents Clinically Overt Wilson’s Disease in Pre-Symptomatic Patients. Eur. J. Neurol. 2014, 21, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Merle, U.; Schaefer, M.; Ferenci, P.; Stremmel, W. Clinical Presentation, Diagnosis and Long-Term Outcome of Wilson’s Disease: A Cohort Study. Gut 2007, 56, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Svetel, M.; Pekmezović, T.; Petrović, I.; Tomić, A.; Kresojević, N.; Jesić, R.; Kazić, S.; Raicević, R.; Stefanović, D.; Delibasić, N.; et al. Long-Term Outcome in Serbian Patients with Wilson Disease. Eur. J. Neurol. 2009, 16, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Weiss, K.H.; Askari, F.K.; Czlonkowska, A.; Ferenci, P.; Bronstein, J.M.; Bega, D.; Ala, A.; Nicholl, D.; Flint, S.; Olsson, L.; et al. Bis-Choline Tetrathiomolybdate in Patients with Wilson’s Disease: An Open-Label, Multicentre, Phase 2 Study. Lancet Gastroenterol. Hepatol. 2017, 2, 869–876. [Google Scholar] [CrossRef]
- Ferenci, P. Wilson’s Disease. Clin. Gastroenterol. Hepatol. 2005, 3, 726–733. [Google Scholar] [CrossRef]
- Nair, J.; Carmichael, P.L.; Fernando, R.C.; Phillips, D.H.; Strain, A.J.; Bartsch, H. Lipid Peroxidation-Induced Etheno-DNA Adducts in the Liver of Patients with the Genetic Metal Storage Disorders Wilson’s Disease and Primary Hemochromatosis. Cancer Epidemiol. Biomark. Prev. 1998, 7, 435–440. [Google Scholar]
- Hayashi, M.; Fuse, S.; Endoh, D.; Horiguchi, N.; Nakayama, K.; Kon, Y.; Okui, T. Accumulation of Copper Induces DNA Strand Breaks in Brain Cells of Long-Evans Cinnamon (LEC) Rats, an Animal Model for Human Wilson Disease. Exp. Anim. 2006, 55, 419–426. [Google Scholar] [CrossRef]
- Yasuda, J.; Eguchi, H.; Fujiwara, N.; Ookawara, T.; Kojima, S.; Yamaguchi, Y.; Nishimura, M.; Fujimoto, J.; Suzuki, K. Reactive Oxygen Species Modify Oligosaccharides of Glycoproteins in Vivo: A Study of a Spontaneous Acute Hepatitis Model Rat (LEC Rat). Biochem. Biophys. Res. Commun. 2006, 342, 127–134. [Google Scholar] [CrossRef]
- Członkowska, A.; Gromadzka, G.; Chabik, G. Monozygotic Female Twins Discordant for Phenotype of Wilson’s Disease. Mov. Disord. Off. J. Mov. Disord. Soc. 2009, 24, 1066–1069. [Google Scholar] [CrossRef] [PubMed]
- Allen, K.J.; Buck, N.E.; Cheah, D.M.; Gazeas, S.; Bhathal, P.; Mercer, J.F. Chronological Changes in Tissue Copper, Zinc and Iron in the Toxic Milk Mouse and Effects of Copper Loading. Biometals 2006, 19, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Howell, J.M.; Mercer, J.F. The Pathology and Trace Element Status of the Toxic Milk Mutant Mouse. J. Comp. Pathol. 1994, 110, 37–47. [Google Scholar] [CrossRef]
- Buiakova, O.I.; Xu, J.; Lutsenko, S.; Zeitlin, S.; Das, K.; Das, S.; Ross, B.M.; Mekios, C.; Scheinberg, I.H.; Gilliam, T.C. Null Mutation of the Murine ATP7B (Wilson Disease) Gene Results in Intracellular Copper Accumulation and Late-Onset Hepatic Nodular Transformation. Hum. Mol. Genet. 1999, 8, 1665–1671. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Forbes, J.R.; Chen, H.S.; Cox, D.W. The LEC Rat Has a Deletion in the Copper Transporting ATPase Gene Homologous to the Wilson Disease Gene. Nat. Genet. 1994, 7, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Burkhead, J.L.; Gray, L.W.; Lutsenko, S. Systems Biology Approach to Wilson’s Disease. Biometals 2011, 24, 455–466. [Google Scholar] [CrossRef]
- Deng, D.X.; Ono, S.; Koropatnick, J.; Cherian, M.G. Metallothionein and Apoptosis in the Toxic Milk Mutant Mouse. Lab. Investig. 1998, 78, 175–183. [Google Scholar] [PubMed]
- Czachor, J.D.; Cherian, M.G.; Koropatnick, J. Reduction of Copper and Metallothionein in Toxic Milk Mice by Tetrathiomolybdate, but Not Deferiprone. J. Inorg. Biochem. 2002, 88, 213–222. [Google Scholar] [CrossRef]
- Yurkova, I.L.; Arnhold, J.; Fitzl, G.; Huster, D. Fragmentation of Mitochondrial Cardiolipin by Copper Ions in the Atp7b-/- Mouse Model of Wilson’s Disease. Chem. Phys. Lipids 2011, 164, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Polishchuk, E.V.; Merolla, A.; Lichtmannegger, J.; Romano, A.; Indrieri, A.; Ilyechova, E.Y.; Concilli, M.; Cegli, R.D.; Crispino, R.; Mariniello, M.; et al. Activation of Autophagy, Observed in Liver Tissues from Patients with Wilson Disease and from ATP7B-Deficient Animals, Protects Hepatocytes from Copper-Induced Apoptosis. Gastroenterology 2019, 156, 1173–1189.e5. [Google Scholar] [CrossRef]
- Huster, D.; Finegold, M.J.; Morgan, C.T.; Burkhead, J.L.; Nixon, R.; Vanderwerf, S.M.; Gilliam, C.T.; Lutsenko, S. Consequences of Copper Accumulation in the Livers of the Atp7b-/- (Wilson Disease Gene) Knockout Mice. Am. J. Pathol. 2006, 168, 423–434. [Google Scholar] [CrossRef]
- Yang, F.; Pei, R.; Zhang, Z.; Liao, J.; Yu, W.; Qiao, N.; Han, Q.; Li, Y.; Hu, L.; Guo, J.; et al. Copper Induces Oxidative Stress and Apoptosis through Mitochondria-Mediated Pathway in Chicken Hepatocytes. Toxicol. In Vitro 2019, 54, 310–316. [Google Scholar] [CrossRef]
- Wilmarth, P.A.; Short, K.K.; Fiehn, O.; Lutsenko, S.; David, L.L.; Burkhead, J.L. A Systems Approach Implicates Nuclear Receptor Targeting in the Atp7b(-/-) Mouse Model of Wilson’s Disease. Metallomics 2012, 4, 660–668. [Google Scholar] [CrossRef] [PubMed]
- Seessle, J.; Gohdes, A.; Gotthardt, D.N.; Pfeiffenberger, J.; Eckert, N.; Stremmel, W.; Reuner, U.; Weiss, K.H. Alterations of Lipid Metabolism in Wilson Disease. Lipids Health Dis. 2011, 10, 83. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, J.P.; Koganti, L.; Muchenditsi, A.; Pendyala, V.S.; Huso, D.; Hankin, J.; Murphy, R.C.; Huster, D.; Merle, U.; Mangels, C.; et al. Activation of Liver X Receptor/Retinoid X Receptor Pathway Ameliorates Liver Disease in Atp7B(-/-) (Wilson Disease) Mice. Hepatology 2016, 63, 1828–1841. [Google Scholar] [CrossRef]
- Song, M.O.; Freedman, J.H. Role of Hepatocyte Nuclear Factor 4alpha in Controlling Copper-Responsive Transcription. Biochim. Biophys. Acta 2011, 1813, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Laudet, V. Evolution of the Nuclear Receptor Superfamily: Early Diversification from an Ancestral Orphan Receptor. J. Mol. Endocrinol. 1997, 19, 207–226. [Google Scholar] [CrossRef] [PubMed]
- Ralle, M.; Lutsenko, S.; Blackburn, N.J. X-Ray Absorption Spectroscopy of the Copper Chaperone HAH1 Reveals a Linear Two-Coordinate Cu(I) Center Capable of Adduct Formation with Exogenous Thiols and Phosphines. J. Biol. Chem. 2003, 278, 23163–23170. [Google Scholar] [CrossRef] [PubMed]
- Barry, A.N.; Shinde, U.; Lutsenko, S. Structural Organization of Human Cu-Transporting ATPases: Learning from Building Blocks. J. Biol. Inorg. Chem. 2009, 15, 47. [Google Scholar] [CrossRef] [PubMed]
- Meacham, K.A.; Cortés, M.P.; Wiggins, E.M.; Maass, A.; Latorre, M.; Ralle, M.; Burkhead, J.L. Altered Zinc Balance in the Atp7b−/− Mouse Reveals a Mechanism of Copper Toxicity in Wilson Disease. Metallomics 2018, 10, 1595–1606. [Google Scholar] [CrossRef]
- Zhang, B.; Georgiev, O.; Hagmann, M.; Gunes, C.; Cramer, M.; Faller, P.; Vasak, M.; Schaffner, W. Activity of Metal-Responsive Transcription Factor 1 by Toxic Heavy Metals and H2O2 in Vitro Is Modulated by Metallothionein. Mol. Cell Biol. 2003, 23, 8471–8485. [Google Scholar] [CrossRef]
- Maret, W. Zinc in Cellular Regulation: The Nature and Significance of “Zinc Signals”. Int. J. Mol. Sci. 2017, 18. [Google Scholar] [CrossRef]
- Levaot, N.; Hershfinkel, M. How Cellular Zn2+ Signaling Drives Physiological Functions. Cell Calcium 2018, 75, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Kl, H.; Ja, H. Mechanism of Zinc-Mediated Inhibition of Caspase-9. Protein Sci. 2012, 21, 1056–1065. [Google Scholar] [CrossRef]
- Takeda, A.; Minami, A.; Seki, Y.; Oku, N. Differential Effects of Zinc on Glutamatergic and GABAergic Neurotransmitter Systems in the Hippocampus. J. Neurosci. Res. 2004, 75, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Kresse, W.; Sekler, I.; Hoffmann, A.; Peters, O.; Nolte, C.; Moran, A.; Kettenmann, H. Zinc Ions Are Endogenous Modulators of Neurotransmitter-Stimulated Capacitative Ca2+ Entry in Both Cultured and in Situ Mouse Astrocytes. Eur. J. Neurosci. 2005, 21, 1626–1634. [Google Scholar] [CrossRef] [PubMed]
- Huntington, C.E.; Shay, N.F.; Grouzmann, E.; Arseneau, L.M.; Beverly, J.L. Zinc Status Affects Neurotransmitter Activity in the Paraventricular Nucleus of Rats. J. Nutr. 2002, 132, 270–275. [Google Scholar] [CrossRef]
- Reilly-O’Donnell, B.; Robertson, G.B.; Karumbi, A.; McIntyre, C.; Bal, W.; Nishi, M.; Takeshima, H.; Stewart, A.J.; Pitt, S.J. Dysregulated Zn2+ Homeostasis Impairs Cardiac Type-2 Ryanodine Receptor and Mitsugumin 23 Functions, Leading to Sarcoplasmic Reticulum Ca2+ Leakage. J. Biol. Chem. 2017, 292, 13361–13373. [Google Scholar] [CrossRef] [PubMed]
- Woodier, J.; Rainbow, R.D.; Stewart, A.J.; Pitt, S.J. Intracellular Zinc Modulates Cardiac Ryanodine Receptor-Mediated Calcium Release. J. Biol. Chem. 2015, 290, 17599–17610. [Google Scholar] [CrossRef] [PubMed]
- McCall, K.A.; Huang, C.; Fierke, C.A. Function and Mechanism of Zinc Metalloenzymes. J. Nutr. 2000, 130, 1437S–1446S. [Google Scholar] [CrossRef] [PubMed]
- Krężel, A.; Maret, W. The Functions of Metamorphic Metallothioneins in Zinc and Copper Metabolism. Int. J. Mol. Sci. 2017, 18, 1237. [Google Scholar] [CrossRef]
- Bafaro, E.; Liu, Y.; Xu, Y.; Dempski, R.E. The Emerging Role of Zinc Transporters in Cellular Homeostasis and Cancer. Signal Transduct. Target. Ther. 2017, 2, 1–12. [Google Scholar] [CrossRef]
- Aydemir, T.B.; Troche, C.; Kim, M.-H.; Cousins, R.J. Hepatic ZIP14-Mediated Zinc Transport Contributes to Endosomal Insulin Receptor Trafficking and Glucose Metabolism. J. Biol. Chem. 2016, 291, 23939–23951. [Google Scholar] [CrossRef] [PubMed]
- Wooton-Kee, C.R.; Robertson, M.; Zhou, Y.; Dong, B.; Sun, Z.; Kim, K.H.; Liu, H.; Xu, Y.; Putluri, N.; Saha, P.; et al. Metabolic Dysregulation in the Atp7b-/- Wilson’s Disease Mouse Model. Proc. Natl. Acad. Sci. USA 2020, 117, 2076–2083. [Google Scholar] [CrossRef]
- Van Biervliet, S.; Kury, S.; De Bruyne, R.; Vanakker, O.M.; Schmitt, S.; Vande Velde, S.; Blouin, E.; Bezieau, S. Clinical Zinc Deficiency as Early Presentation of Wilson Disease. J. Pediatr. Gastroenterol. Nutr. 2015, 60, 457–459. [Google Scholar] [CrossRef] [PubMed]
- Theophilos, M.B.; Cox, D.W.; Mercer, J.F. The Toxic Milk Mouse Is a Murine Model of Wilson Disease. Hum. Mol. Genet. 1996, 5, 1619–1624. [Google Scholar] [CrossRef] [PubMed]
- Grimes, A.; Paynter, J.; Walker, I.D.; Bhave, M.; Mercer, J.F. Decreased Carbonic Anhydrase III Levels in the Liver of the Mouse Mutant “toxic Milk” (Tx) Due to Copper Accumulation. Biochem. J. 1997, 321 Pt 2, 341–346. [Google Scholar] [CrossRef]
- Foster, A.W.; Osman, D.; Robinson, N.J. Metal Preferences and Metallation. J. Biol. Chem. 2014, 289, 28095–28103. [Google Scholar] [CrossRef] [PubMed]
- Berry, M.N.; Kun, E.; Werner, H.V. Regulatory Role of Reducing-Equivalent Transfer from Substrate to Oxygen in the Hepatic Metabolism of Glycerol and Sorbitol. Eur. J. Biochem. 1973, 33, 407–417. [Google Scholar] [CrossRef]
- Banci, L.; Bertini, I.; Cantini, F.; Ciofi-Baffoni, S. Cellular Copper Distribution: A Mechanistic Systems Biology Approach. Cell Mol. Life Sci. 2010, 67, 2563–2589. [Google Scholar] [CrossRef]
- Jiang, L.J.; Maret, W.; Vallee, B.L. The Glutathione Redox Couple Modulates Zinc Transfer from Metallothionein to Zinc-Depleted Sorbitol Dehydrogenase. Proc. Natl. Acad. Sci. USA 1998, 95, 3483–3488. [Google Scholar] [CrossRef] [PubMed]
- Cano-Gauci, D.F.; Sarkar, B. Reversible Zinc Exchange between Metallothionein and the Estrogen Receptor Zinc Finger. FEBS Lett. 1996, 386, 1–4. [Google Scholar] [CrossRef]
- Grüngreiff, K.; Reinhold, D.; Wedemeyer, H. The Role of Zinc in Liver Cirrhosis. Ann. Hepatol. 2016, 15, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Leoni, G.; Rosato, A.; Perozzi, G.; Murgia, C. Zinc Proteome Interaction Network as a Model to Identify Nutrient-Affected Pathways in Human Pathologies. Genes Nutr. 2014, 9, 436. [Google Scholar] [CrossRef]
- Carroll, L.S.; Abdul-Rahim, A.H.; Murray, R. Zinc Containing Dental Fixative Causing Copper Deficiency Myelopathy. BMJ Case Rep. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Draine, J.; Simmons, M. A Case of Copper Deficiency Myeloneuropathy Precipitated by Zinc Ingestion and Bariatric Surgery. S. Dak. Med. 2020, 73, 178–180. [Google Scholar]
- Duncan, A.; Yacoubian, C.; Watson, N.; Morrison, I. The Risk of Copper Deficiency in Patients Prescribed Zinc Supplements. J. Clin. Pathol. 2015, 68, 723–725. [Google Scholar] [CrossRef]
- Patterson, W.P.; Winkelmann, M.; Perry, M.C. Zinc-Induced Copper Deficiency: Megamineral Sideroblastic Anemia. Ann. Intern. Med. 1985, 103, 385–386. [Google Scholar] [CrossRef]
- Minervino, A.H.H.; López-Alonso, M.; Barrêto Júnior, R.A.; Rodrigues, F.A.M.L.; Araújo, C.A.S.C.; Sousa, R.S.; Mori, C.S.; Miranda, M.; Oliveira, F.L.C.; Antonelli, A.C.; et al. Dietary Zinc Supplementation to Prevent Chronic Copper Poisoning in Sheep. Animals 2018, 8, 227. [Google Scholar] [CrossRef] [PubMed]
- Sahawneh, M.A.; Ricart, K.C.; Roberts, B.R.; Bomben, V.C.; Basso, M.; Ye, Y.; Sahawneh, J.; Franco, M.C.; Beckman, J.S.; Estévez, A.G. Cu,Zn-Superoxide Dismutase Increases Toxicity of Mutant and Zinc-Deficient Superoxide Dismutase by Enhancing Protein Stability. J. Biol. Chem. 2010, 285, 33885–33897. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Liou, I.W.; Biggins, S.W.; Yeh, M.; Jalikis, F.; Chan, L.-N.; Burkhead, J. Copper Deficiency in Liver Diseases: A Case Series and Pathophysiological Considerations. Hepatol. Commun. 2019, 3, 1159–1165. [Google Scholar] [CrossRef] [PubMed]
- Ebara, M.; Fukuda, H.; Hatano, R.; Saisho, H.; Nagato, Y.; Suzuki, K.; Nakajima, K.; Yukawa, M.; Kondo, F.; Nakayama, A.; et al. Relationship between Copper, Zinc and Metallothionein in Hepatocellular Carcinoma and Its Surrounding Liver Parenchyma. J. Hepatol. 2000, 33, 415–422. [Google Scholar] [CrossRef]
- Sengupta, S.; Wroblewski, K.; Aronsohn, A.; Reau, N.; Reddy, K.G.; Jensen, D.; Te, H. Screening for Zinc Deficiency in Patients with Cirrhosis: When Should We Start? Dig. Dis. Sci. 2015, 60, 3130–3135. [Google Scholar] [CrossRef] [PubMed]
- Hiraoka, A.; Nagamatsu, K.; Izumoto, H.; Adachi, T.; Yoshino, T.; Tsuruta, M.; Aibiki, T.; Okudaira, T.; Yamago, H.; Iwasaki, R.; et al. Zinc Deficiency as an Independent Prognostic Factor for Patients with Early Hepatocellular Carcinoma Due to Hepatitis Virus. Hepatol. Res. 2020, 50, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Askari, F.; Innis, D.; Dick, R.B.; Hou, G.; Marrero, J.; Greenson, J.; Brewer, G.J. Treatment of Primary Biliary Cirrhosis with Tetrathiomolybdate: Results of a Double-Blind Trial. Transl. Res. 2010, 155, 123–130. [Google Scholar] [CrossRef]
- Himoto, T.; Yoneyama, H.; Kurokochi, K.; Inukai, M.; Masugata, H.; Goda, F.; Haba, R.; Watanabe, S.; Senda, S.; Masaki, T. Contribution of Zinc Deficiency to Insulin Resistance in Patients with Primary Biliary Cirrhosis. Biol. Trace Elem. Res. 2011, 144, 133–142. [Google Scholar] [CrossRef]
- Katayama, K. Zinc and Protein Metabolism in Chronic Liver Diseases. Nutr. Res. 2020, 74, 1–9. [Google Scholar] [CrossRef] [PubMed]
| Author, Year, Citation | Duration | Patient Count | Treatment | Short Term Outcomes (<1 Year) | Long Term Outcomes (>1 Year) |
|---|---|---|---|---|---|
| Beinhardt et al. 2014 [92] | 14.8 years mean observation | 229 (retrospective study) | D-PEN chelation therapy (dosage not reported) | N/A | 35% stabilized, 24% improved, 26% recovered with chelation therapy, 15% deteriorated. |
| Brewer et al. 1998 [54] | 12 years | 141 | Zn: variable between 3 × 50 mg/day and 1 × 25 mg/day | Reduction in urine, plasma and (minor) hepatic Cu. Increase in urine and plasma Zn. Partial improvement of neurological symptoms. | Urine Cu above normal. High urine and plasma Zn. Gradual reduction in non-CP plasma and liver Cu to normal values at years 8–12. Gradual neurological improvement over 6 years. |
| Brewer et al. 2001 [55] | 5 years | 34–4 (pediatric) | Zn: 50–150 mg/day depending on age of patient | Reduction in urine Cu and non-CP plasma Cu (p < 0.0001 and p < 0.05, resp). Increase in urine and plasma Zn (p < 0.0001, both). Speech measures improvement (p < 0.05). Neurologic measures improve (p < 0.05). Reduction in aminotransferases ALT, AST (p < 0.01) | Urine and non-CP plasma Cu stabilizing in normal ranges. Urine and plasma Zn stabilizing at high concentrations. Little long term (3 year) improvement in dysarthria. Continuing improvement of neurologic measures. |
| Bruha et al. 2011 [93] | 15.1 years mean | 117 | Zn (17%); D-PEN (81%); 3 transplant (dosage not reported) | N/A | 82% improvement in hepatic WD; 69% improvement in neurologic WD. Long-term survival similar to reference population. |
| Członkowska et al. 1996 [86] | 12 years | 67 (34-D-PEN, 33 Zn) | Zn: variable 600–800 mg/day | N/A | Similar improvements in patients between D-PEN and Zn treatment. Zn was better tolerated and had a greater rate of continuation through the 12 year period (88% Zn vs. 56% D-PEN) |
| Członkowska et al. 2014 [94] | 5 years | 143 (neurological: 35 D-PEN, 21 Zn; hepatic: 36 D-PEN, 51 Zn) | unknown | Similar frequency of improvement in neurological symptoms and liver enzymes. | Probability of not remaining on first-line therapy was higher for Zn than D-PEN in hepatic WD but similar in neurological WD. Adverse events more common with D-PEN than Zn (15% vs. 3%) |
| Dziezyc et al. 2014 [95] | Median 12 years (range 3–52) | 87 (presymptomatic) 66.7% Zn treatment, 33.3% D-PEN | unknown | N/A | Positive treatment outcomes were similar between Zn and D-PEN with all patients. Non-compliant patients had significantly greater instances of neuro, hepatic and serum dysfunction or failure. |
| Farinati et al. 2003 [87] | 12 years | 67 | Zn: 600–800 mg/day; D-PEN: 1–1.5 g/day | N/A | Of those that continued treatment through the period, 32% and 42% improved with D-PEN and Zn, respectively. |
| Haiman Hou et al. 2021 [83] | 6 years | 36 | Zn: 2 × 25 mg/day in ages < 6 3 × 25 mg/day in ages 6–16 years 3 × 50 mg/day > 16 years | 70% of patients had significant reductions in ALT with Zn monotherapy, 30% experienced treatment failure and added D-PEN | Patients improved to normal ALT levels with Zn monotherapy or Zn and D-PEN |
| Hoogenraad et al. 1987 [84] | 27 9 patients Zn monotherapy, 8 patients developed D-PEN intolerance, 10 patients switched from D-PEN to Zn w/o developing intolerance | Zn: 3 × 200 mg/day in adults 3 × 100 mg/day in children | N/A | Eight of nine Zn patients had responded favorably to treatment, with a final patient dying in a hepatic coma. All eight patients with D-PEN intolerance improved with oral Zn, with two having deteriorating neurological symptoms during D-PEN treatment. Six of nine patients in the final group responded favorably, along with two asymptomatic patients. | |
| Linn et al. 2009 [56] | 24 years | 17 7 hepatic patients, 5 neurologic patients and 5 with both | Zn: 136–276 mg/day | N/A | (median 12 years) Consistent and significant improvement in neurological patients (p < 0.01). No significant improvement in liver biochemistry in hepatic patients. |
| Marcellini et al. 2005 [81] | 10 years | 22 (pediatric) | Zn: 50–150 mg/day depending on age of patient | N/A | 5 year: Reductions in AST, ALT and urinary Cu (p < 0.001) 10 year: No significant difference between 5- and 10-year outcomes. Significant reduction in Hepatic Cu (p = 0.001) |
| Merle et al. 2007 [96] | 5 years | 163 (retrospective) | Zn: 150–250 mg/day; D-PEN: 900–1800 mg/day; trientine: 900–2100 mg/day | N/A | 76.1% improved or stable disease. |
| Svetel et al. 2009 [97] | 15-years | 142 (prospective) | Zn or D-PEN; (dosage not reported) | 76.7% cumulative probability of survival, better prognosis with neurologic WD. Similar survival with Zn vs. D-PEN vs. combined. | |
| Weiss et al. 2011 [85] | Median 17.1 years | 288 (tertiary care centers, retrospective analysis) | Zn and D-PEN (dosage not reported) | N/A | Hepatic treatment failure more often in Zn monotherapy than with chelator or combination therapy. Zn treatment or chelators were effective in most patients; chelators were better at preventing hepatic deterioration. |
| Weiss et al. 2017 [98] | 2 years | 28 (prospective) | Bis-choline tetrathiomolybdate: 15–60 mg/day | N/A | 71% met criteria for treatment success (25% decrease in non-ceruloplasmin-bound Cu). No drug-related neurological worsening. All stable liver function. |
| Wu et al. 2003 [80] | 5 years | 17 (presymptomatic) | Zn: 2 × 50 mg/day | No significant change in serum CP or urinary Cu | Significant reduction in Serum CP and urinary Cu at 5 years. No adverse effects in any Zn treated patients. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barber, R.G.; Grenier, Z.A.; Burkhead, J.L. Copper Toxicity Is Not Just Oxidative Damage: Zinc Systems and Insight from Wilson Disease. Biomedicines 2021, 9, 316. https://doi.org/10.3390/biomedicines9030316
Barber RG, Grenier ZA, Burkhead JL. Copper Toxicity Is Not Just Oxidative Damage: Zinc Systems and Insight from Wilson Disease. Biomedicines. 2021; 9(3):316. https://doi.org/10.3390/biomedicines9030316
Chicago/Turabian StyleBarber, R. G., Zoey A. Grenier, and Jason L. Burkhead. 2021. "Copper Toxicity Is Not Just Oxidative Damage: Zinc Systems and Insight from Wilson Disease" Biomedicines 9, no. 3: 316. https://doi.org/10.3390/biomedicines9030316
APA StyleBarber, R. G., Grenier, Z. A., & Burkhead, J. L. (2021). Copper Toxicity Is Not Just Oxidative Damage: Zinc Systems and Insight from Wilson Disease. Biomedicines, 9(3), 316. https://doi.org/10.3390/biomedicines9030316







