Biogenic Ferrihydrite Nanoparticles: Synthesis, Properties In Vitro and In Vivo Testing and the Concentration Effect
Abstract
:1. Introduction
2. Materials and Methods
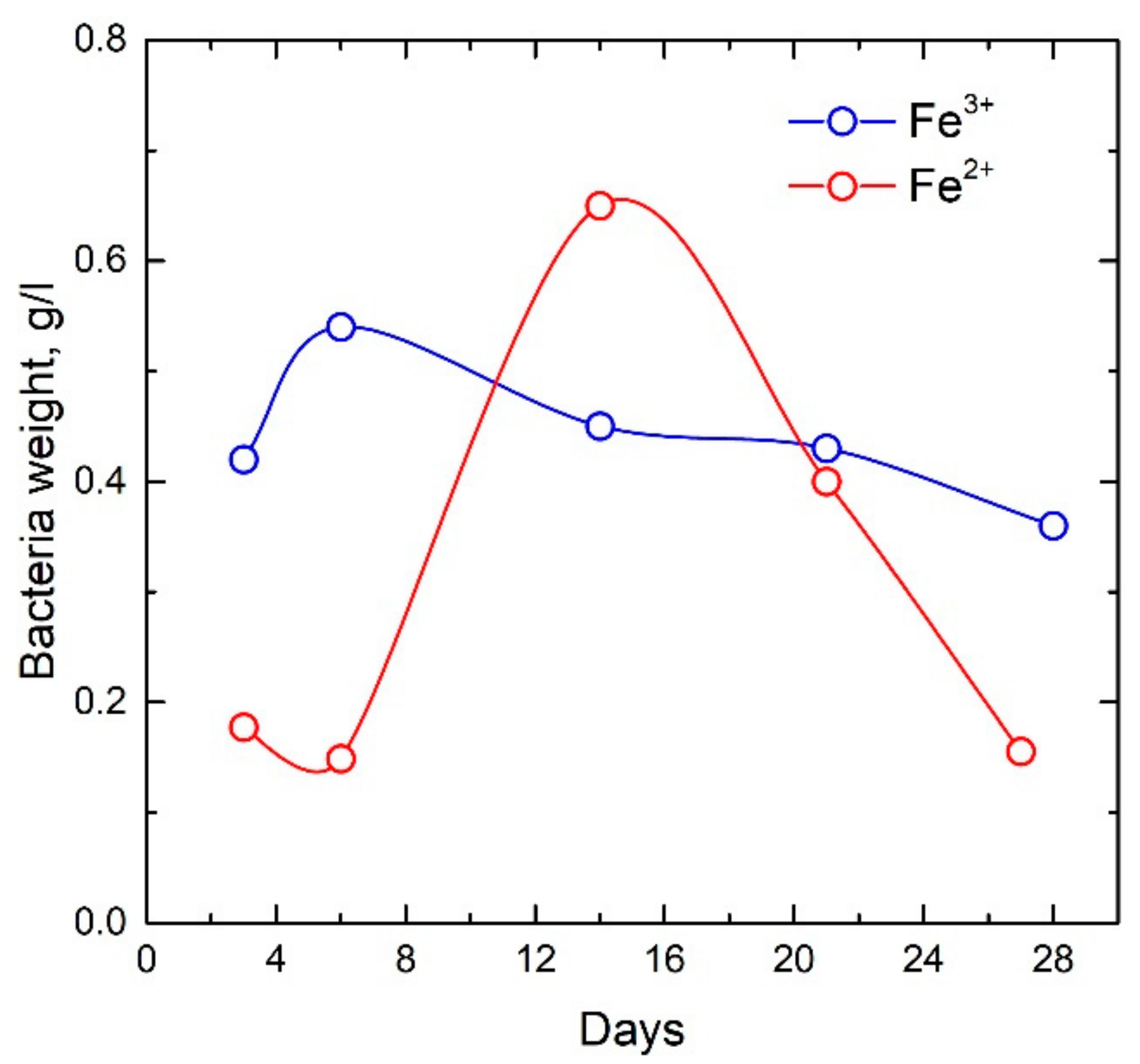
2.1. Production Technology
2.2. FTIR Spectroscopy
2.3. Mossbauer Spectroscopy
2.4. Magnetometric Measurements
2.5. Biological Research In Vitro
2.6. Biological Research In Vivo
3. Results
3.1. Dynamic Light Scattering
3.2. FTIR Spectroscopy
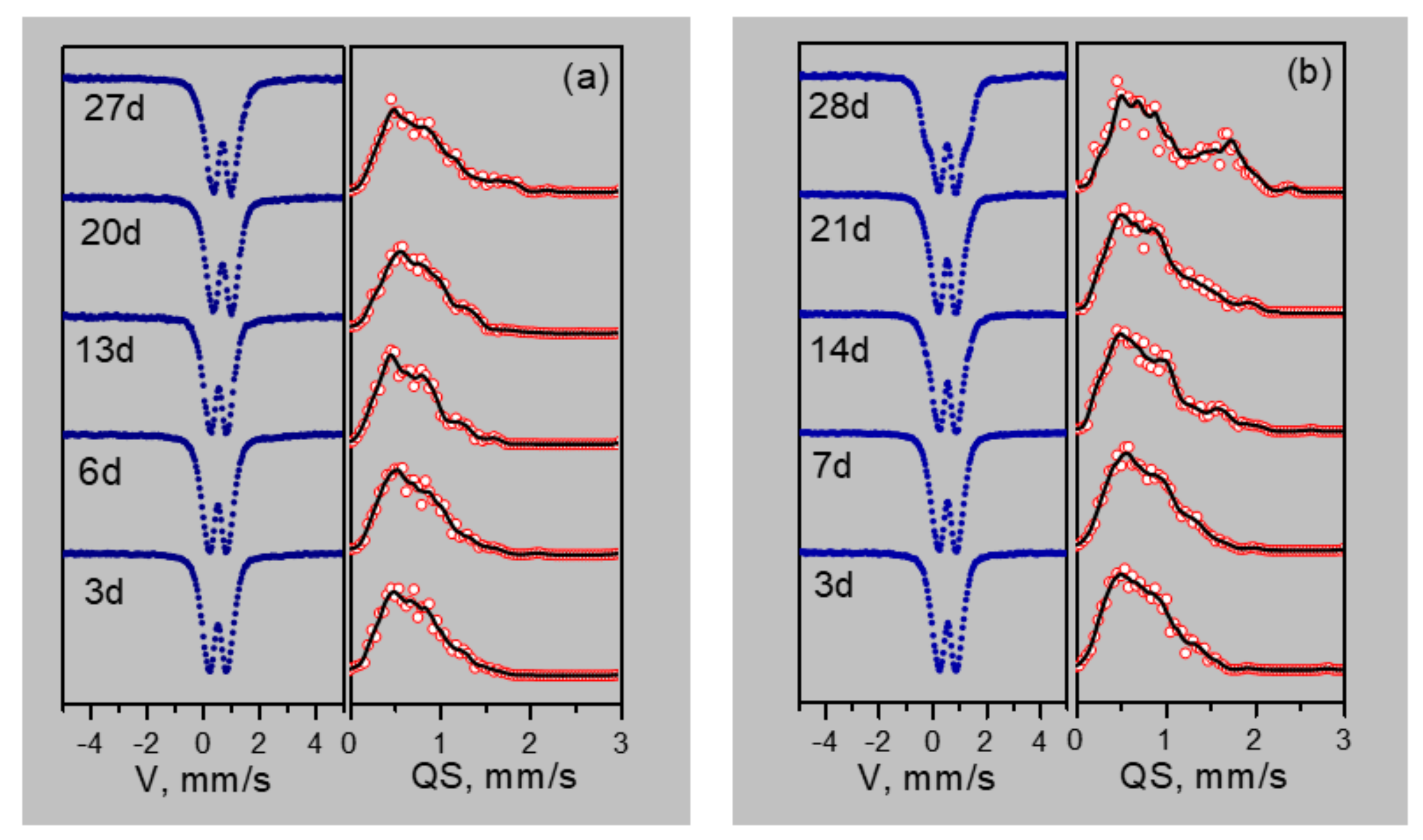
3.3. Mössbauer Spectroscopy
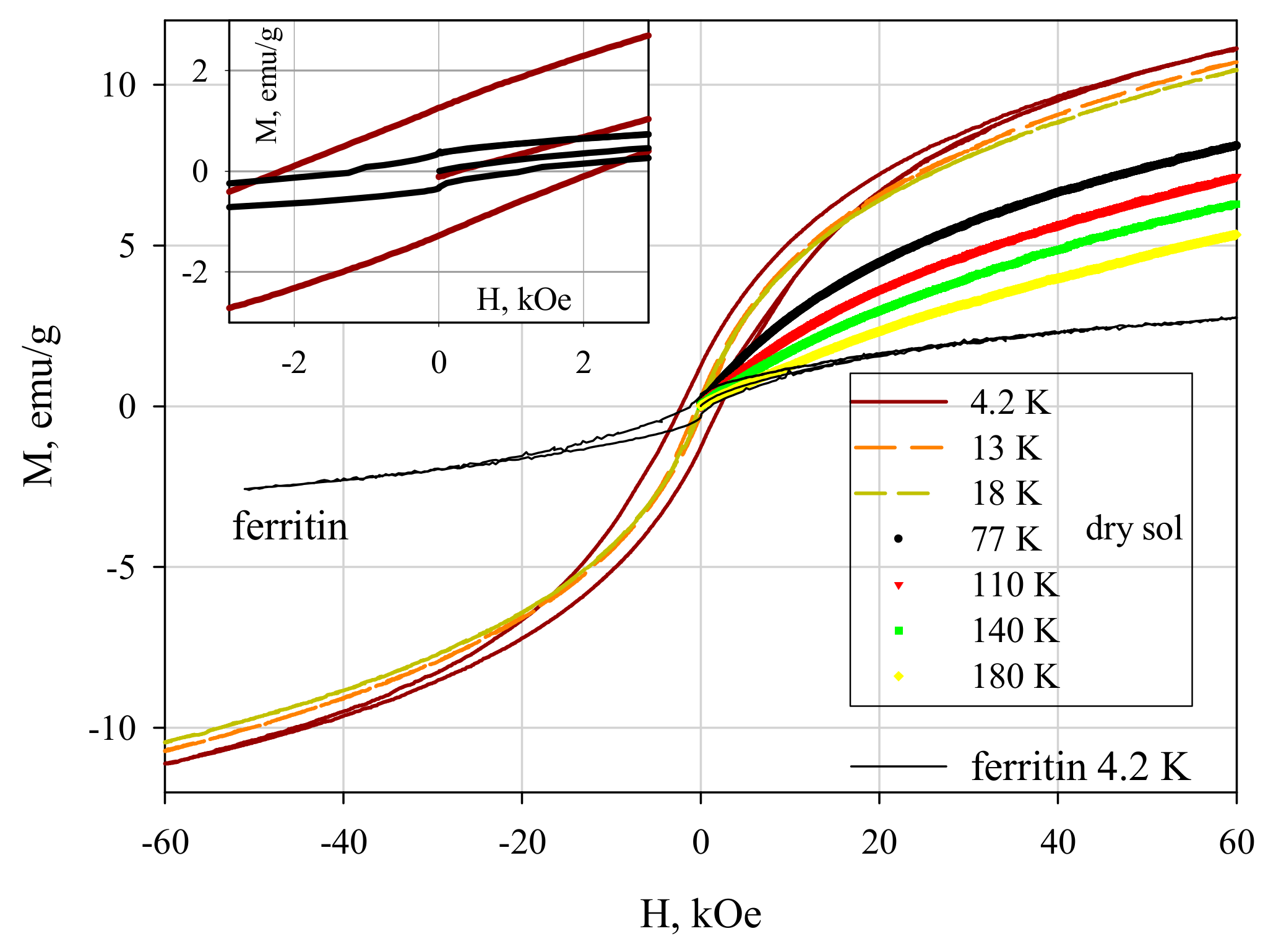
3.4. Magnetic Properties of Ferrihydrite Nanoparticles
3.5. Investigation of the Effect of Ferrihydrite Nanoparticles In Vitro
3.6. Investigation of the Effect of Ferrihydrite Nanoparticles In Vivo
3.6.1. Morphological Changes in the Liver
3.6.2. Morphological Changes in the Spleen
3.6.3. Morphological Changes in the Kidneys
3.6.4. Morphological Changes in the Lungs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hiemstra, T. Formation, stability, and solubility of metal oxide nanoparticles: Surface entropy, enthalpy, and free energy of ferrihydrite. Geochim. Cosmochim. Acta 2015, 158, 179–198. [Google Scholar] [CrossRef]
- Baldi, F.; Minacci, A.; Pepi, M.; Scozzafava, A. Gel sequestration of heavy metals by Klebsiella oxytoca isolated from iron mat. FEMS Microbiol. Ecol. 2001, 36, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Leone, S.; De Castro, C.; Parrilli, M.; Baldi, F.; Lanzetta, R. Structure of the Iron-Binding Exopolysaccharide Produced Anaerobically by the Gram-Negative BacteriumKlebsiella oxytoca BAS-10. Eur. J. Org. Chem. 2007, 2007, 5183–5189. [Google Scholar] [CrossRef] [Green Version]
- Gallo, G.; Baldi, F.; Renzone, G.; Gallo, M.; Cordaro, A.; Scaloni, A.; Puglia, A. Adaptative biochemical pathways and regulatory networks in Klebsiella oxytoca BAS-10 producing a biotechnologically relevant exopolysaccharide during Fe(III)-citrate fermentation. Microb. Cell Fact. 2012, 11, 152. [Google Scholar] [CrossRef] [Green Version]
- Kianpour, S.; Ebrahiminezhad, A.; Mohkam, M.; Tamaddon, A.M.; Dehshahri, A.; Heidari, R.; Ghasemi, Y. Physicochemical and biological characteristics of the nanostructured polysaccharide-iron hydrogel produced by microorganism Klebsiella oxytoca. J. Basic Microbiol. 2017, 57, 132–140. [Google Scholar] [CrossRef]
- Silva, N.J.O.; Amaral, V.S.; Carlos, L.D. Relevance of magnetic moment distribution and scaling law methods to study the magnetic behavior of antiferromagnetic nanoparticles: Application to ferritin. Phys. Rev. B 2005, 71, 184408. [Google Scholar] [CrossRef] [Green Version]
- Seehra, M.S.; Singh, V.; Song, X.; Bali, S.; Eyring, E.M. Synthesis, structure and magnetic properties of non-crystalline ferrihydrite nanoflakes. J. Phys. Chem. Solids 2010, 71, 1362–1366. [Google Scholar] [CrossRef]
- Balaev, D.A.; Krasikov, A.A.; Dubrovskiy, A.A.; Popkov, S.I.; Stolyar, S.V.; Iskhakov, R.S.; Ladygina, V.P.; Yaroslavtsev, R.N. Exchange bias in nano-ferrihydrite. J. Appl. Phys. 2016, 120, 183903. [Google Scholar] [CrossRef]
- Balaev, D.A.; Krasikov, A.A.; Dubrovskii, A.A.; Semenov, S.V.; Bayukov, O.A.; Stolyar, S.V.; Iskhakov, R.S.; Ladygina, V.P.; Ishchenko, L.A. Magnetic properties and the mechanism of formation of the uncompensated magnetic moment of antiferromagnetic ferrihydrite nanoparticles of a bacterial origin. J. Exp. Theor. Phys. 2014, 119, 479–487. [Google Scholar] [CrossRef]
- Stolyar, S.V.; Balaev, D.A.; Ladygina, V.P.; Dubrovskiy, A.A.; Krasikov, A.A.; Popkov, S.I.; Bayukov, O.A.; Knyazev, Y.V.; Yaroslavtsev, R.N.; Volochaev, M.N.; et al. Bacterial Ferrihydrite Nanoparticles: Preparation, Magnetic Properties, and Application in Medicine. J. Supercond. Nov. Magn. 2018, 31, 2297–2304. [Google Scholar] [CrossRef] [Green Version]
- Chilom, C.G.; Sandu, N.; Bălăşoiu, M.; Yaroslavtsev, R.N.; Stolyar, S.V.; Rogachev, A.V. Ferrihydrite nanoparticles insights: Structural characterization, lactate dehydrogenase binding and virtual screening assay. Int. J. Biol. Macromol. 2020, 164, 3559–3567. [Google Scholar] [CrossRef]
- Picceri, G.G.; Leonardi, P.; Iotti, M.; Gallo, M.; Baldi, F.; Zambonelli, A.; Amicucci, A.; Vallorani, L.; Piccoli, G.; Ciccimarra, G.; et al. Bacteria-produced ferric exopolysaccharide nanoparticles as iron delivery system for truffles (Tuber borchii). Appl. Microbiol. Biotechnol. 2018, 102, 1429–1441. [Google Scholar] [CrossRef] [PubMed]
- Baldi, F.; Marchetto, D.; Battistel, D.; Daniele, S.; Faleri, C.; De Castro, C.; Lanzetta, R. Iron-binding characterization and polysaccharide production by Klebsiella oxytoca strain isolated from mine acid drainage. J. Appl. Microbiol. 2009, 107, 1241–1250. [Google Scholar] [CrossRef] [Green Version]
- Casentini, B.; Gallo, M.; Baldi, F. Arsenate and arsenite removal from contaminated water by iron oxides nanoparticles formed inside a bacterial exopolysaccharide. J. Environ. Chem. Eng. 2019, 7, 102908. [Google Scholar] [CrossRef] [Green Version]
- Kharazian, B.; Hadipour, N.L.; Ejtehadi, M.R. Understanding the nanoparticle–protein corona complexes using computational and experimental methods. Int. J. Biochem. Cell Biol. 2016, 75, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Ge, C.; Tian, J.; Zhao, Y.; Chen, C.; Zhou, R.; Chai, Z. Towards understanding of nanoparticle–protein corona. Arch. Toxicol. 2015, 89, 519–539. [Google Scholar] [CrossRef] [PubMed]
- Ke, P.C.; Lin, S.; Parak, W.J.; Davis, T.P.; Caruso, F. A Decade of the Protein Corona. ACS Nano 2017, 11, 11773–11776. [Google Scholar] [CrossRef] [PubMed]
- del Pino, P.; Pelaz, B.; Zhang, Q.; Maffre, P.; Nienhaus, G.U.; Parak, W.J. Protein corona formation around nanoparticles—From the past to the future. Mater. Horiz. 2014, 1, 301–313. [Google Scholar] [CrossRef]
- Barbero, F.; Russo, L.; Vitali, M.; Piella, J.; Salvo, I.; Borrajo, M.L.; Busquets-Fité, M.; Grandori, R.; Bastús, N.G.; Casals, E.; et al. Formation of the Protein Corona: The Interface between Nanoparticles and the Immune System. Semin. Immunol. 2017, 34, 52–60. [Google Scholar] [CrossRef]
- Saptarshi, S.R.; Duschl, A.; Lopata, A.L. Interaction of nanoparticles with proteins: Relation to bio-reactivity of the nanoparticle. J. Nanobiotechnol. 2013, 11, 26. [Google Scholar] [CrossRef] [Green Version]
- Fleischer, C.C.; Payne, C.K. Nanoparticle–Cell Interactions: Molecular Structure of the Protein Corona and Cellular Outcomes. Acc. Chem. Res. 2014, 47, 2651–2659. [Google Scholar] [CrossRef]
- Salvati, A.; Pitek, A.S.; Monopoli, M.P.; Prapainop, K.; Bombelli, F.B.; Hristov, D.R.; Kelly, P.M.; Åberg, C.; Mahon, E.; Dawson, K.A. Transferrin-functionalized nanoparticles lose their targeting capabilities when a biomolecule corona adsorbs on the surface. Nat. Nanotechnol. 2013, 8, 137–143. [Google Scholar] [CrossRef] [Green Version]
- Nel, A.E.; Mädler, L.; Velegol, D.; Xia, T.; Hoek, E.M.V.; Somasundaran, P.; Klaessig, F.; Castranova, V.; Thompson, M. Understanding biophysicochemical interactions at the nano–bio interface. Nat. Mater. 2009, 8, 543–557. [Google Scholar] [CrossRef]
- Lunova, M.; Prokhorov, A.; Jirsa, M.; Hof, M.; Olżyńska, A.; Jurkiewicz, P.; Kubinová, Š.; Lunov, O.; Dejneka, A. Nanoparticle core stability and surface functionalization drive the mTOR signaling pathway in hepatocellular cell lines. Sci. Rep. 2017, 7, 16049. [Google Scholar] [CrossRef] [Green Version]
- Mirshafiee, V.; Mahmoudi, M.; Lou, K.; Cheng, J.; Kraft, M.L. Protein corona significantly reduces active targeting yield. Chem. Commun. 2013, 49, 2557. [Google Scholar] [CrossRef]
- Chilom, C.G.; Bălan, A.; Sandu, N.; Bălăşoiu, M.; Stolyar, S.; Orelovich, O. Exploring the Conformation and Thermal Stability of Human Serum Albumin Corona of Ferrihydrite Nanoparticles. Int. J. Mol. Sci. 2020, 21, 9734. [Google Scholar] [CrossRef] [PubMed]
- Simberg, D.; Park, J.-H.; Karmali, P.P.; Zhang, W.-M.; Merkulov, S.; McCrae, K.; Bhatia, S.N.; Sailor, M.; Ruoslahti, E. Differential proteomics analysis of the surface heterogeneity of dextran iron oxide nanoparticles and the implications for their in vivo clearance. Biomaterials 2009, 30, 3926–3933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lovley, D.R.; Phillips, E.J. Novel mode of microbial energy metabolism: Organic carbon oxidation coupled to dissimilatory reduction of iron or manganese. Appl. Environ. Microbiol. 1988, 54, 1472–1480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balaev, A.D.; Boyarshinov, Y.V.; Karpenko, M.M.; Khrustalev, B.P. Automated magnetometer with superconducting solenoid. Instrum. Exp. Tech. 1985, 26. Available online: https://www.osti.gov/biblio/5496232 (accessed on 22 March 2021).
- Kolenchukova, O.A.; Stolyar, S.V.; Ladygina, V.P.; Biryukova, E.A. Chemiluminescence activity of neutrophil granulocytes under the influence of magnetic nanoparticles of ferrihydrite (in vitro). Med. Immunol. 2020, 22, 533–538. [Google Scholar] [CrossRef]
- European Convention for Protection of Vertebrate Animals Used for Experimental and Ether Scientific Purpose; Council of Europe: Strasbourg, Paris, 1986; Volume 52.
- Stolyar, S.V.; Ladygina, V.P.; Boldyreva, A.V.; Kolenchukova, O.A.; Vorotynov, A.M.; Bairmani, M.S.; Yaroslavtsev, R.N.; Iskhakov, R.S. Synthesis, Properties, and in vivo Testing of Biogenic Ferrihydrite Nanoparticles. Bull. Russ. Acad. Sci. Phys. 2020, 84, 1366–1369. [Google Scholar] [CrossRef]
- Inzhevatkin, E.V.; Kolenchukova, O.A.; Dobretsov, K.G.; Ladygina, V.P.; Boldyreva, A.V.; Stolyar, S.V. Efficiency of Ampicillin-Associated Biogenic Ferrihydrite Nanoparticles in Combination with a Magnetic Field for Local Treatment of Burns. Bull. Exp. Biol. Med. 2020, 169, 683–686. [Google Scholar] [CrossRef] [PubMed]
- Russell, J.D. Infrared spectroscopy of ferrihydrite: Evidence for the presence of structural hydroxyl groups. Clay Miner. 1979, 14, 109–114. [Google Scholar] [CrossRef]
- del Carmen Prieto, M.; Amores, J.M.G.; Escribano, V.S.; Busca, G. Characterization of coprecipitated Fe 2 O 3 –Al 2 O 3 powders. J. Mater. Chem. 1994, 4, 1123–1130. [Google Scholar] [CrossRef]
- Chukhrov, F.V.; Zvyagin, B.B.; Gorshkov, A.I. Ferrihydrite. Izv. Akad. Nauk SSSR Ser. Geol. 1973, 4, 23–34. [Google Scholar]
- Stolyar, S.V.; Bayukov, O.A.; Gurevich, Y.L.; Ladygina, V.P.; Iskhakov, R.S.; Pustoshilov, P.P. Mössbauer study of bacterial ferrihydrite. Inorg. Mater. 2007, 43, 638–641. [Google Scholar] [CrossRef]
- Balaev, D.A.; Krasikov, A.A.; Stolyar, S.V.; Iskhakov, R.S.; Ladygina, V.P.; Yaroslavtsev, R.N.; Bayukov, O.A.; Vorotynov, A.M.; Volochaev, M.N.; Dubrovskiy, A.A. Change in the magnetic properties of nanoferrihydrite with an increase in the volume of nanoparticles during low-temperature annealing. Phys. Solid State 2016, 58, 1782–1791. [Google Scholar] [CrossRef]
- Balaev, D.A.; Krasikov, A.A.; Dubrovskiy, A.A.; Popkov, S.I.; Stolyar, S.V.; Bayukov, O.A.; Iskhakov, R.S.; Ladygina, V.P.; Yaroslavtsev, R.N. Magnetic properties of heat treated bacterial ferrihydrite nanoparticles. J. Magn. Magn. Mater. 2016, 410, 171–180. [Google Scholar] [CrossRef]
- Mørup, S.; Madsen, D.E.; Frandsen, C.; Bahl, C.R.H.; Hansen, M.F. Experimental and theoretical studies of nanoparticles of antiferromagnetic materials. J. Phys. Condens. Matter 2007, 19, 213202. [Google Scholar] [CrossRef]
- Anghel, L.; Balasoiu, M.; Ishchenko, L.A.; Stolyar, S.V.; Kurkin, T.S.; Rogachev, A.V.; Kuklin, A.I.; Kovalev, Y.S.; Raikher, Y.L.; Iskhakov, R.S.; et al. Characterization of bio-synthesized nanoparticles produced by Klebsiella oxytoca. J. Phys. Conf. Ser. 2012, 351, 012005. [Google Scholar] [CrossRef]
- Bellamy, L.J. The Infra-Red Spectra of Complex Molecules; Springer: Dordrecht, The Netherlands, 1975; ISBN 978-94-011-6019-3. [Google Scholar]
- Rani, C.; Tiwari, S.D. Estimation of particle magnetic moment distribution for antiferromagnetic ferrihydrite nanoparticles. J. Magn. Magn. Mater. 2015, 385, 272–276. [Google Scholar] [CrossRef]
- Parmar, C.; Parmar, G.S. Structural and Magnetic Properties of Six-Line Ferrihydrite Nanoparticles. J. Supercond. Nov. Magn. 2019. [Google Scholar] [CrossRef]
- Balaev, D.A.; Popkov, S.I.; Krasikov, A.A.; Balaev, A.D.; Dubrovskiy, A.A.; Stolyar, S.V.; Yaroslavtsev, R.N.; Ladygina, V.P.; Iskhakov, R.S. Temperature behavior of the antiferromagnetic susceptibility of nanoferrihydrite from the measurements of the magnetization curves in fields of up to 250 kOe. Phys. Solid State 2017, 59, 1940–1946. [Google Scholar] [CrossRef]
- Silva, N.J.O.; Millán, A.; Palacio, F.; Kampert, E.; Zeitler, U.; Rakoto, H.; Amaral, V.S. Temperature dependence of antiferromagnetic susceptibility in ferritin. Phys. Rev. B 2009, 79, 104405. [Google Scholar] [CrossRef] [Green Version]
- Popkov, S.I.; Krasikov, A.A.; Velikanov, D.A.; Kirillov, V.L.; Martyanov, O.N.; Balaev, D.A. Formation of the magnetic subsystems in antiferromagnetic NiO nanoparticles using the data of magnetic measurements in fields up to 250 kOe. J. Magn. Magn. Mater. 2019, 483, 21–26. [Google Scholar] [CrossRef]
- Popkov, S.I.; Krasikov, A.A.; Dubrovskiy, A.A.; Volochaev, M.N.; Kirillov, V.L.; Martyanov, O.N.; Balaev, D.A. Size effects in the formation of an uncompensated ferromagnetic moment in NiO nanoparticles. J. Appl. Phys. 2019, 126, 103904. [Google Scholar] [CrossRef]
- Néel, L.; Acad, C.R. Superparamagnétisme des grains très fins antiferromagnétiques. CR Sci. Paris 1961, 252, 4075. [Google Scholar]
- Makhlouf, S.A.; Parker, F.T.; Berkowitz, A.E. Magnetic hysteresis anomalies in ferritin. Phys. Rev. B 1997, 55, R14717–R14720. [Google Scholar] [CrossRef]
- Zhang, Y.-N.; Poon, W.; Tavares, A.J.; McGilvray, I.D.; Chan, W.C.W. Nanoparticle–liver interactions: Cellular uptake and hepatobiliary elimination. J. Control. Release 2016, 240, 332–348. [Google Scholar] [CrossRef] [PubMed]
- Zamay, G.S.; Zamay, T.N.; Lukyanenko, K.A.; Kichkailo, A.S. Aptamers Increase Biocompatibility and Reduce the Toxicity of Magnetic Nanoparticles Used in Biomedicine. Biomedicines 2020, 8, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]









| Characteristics 1 | Control | Ferrihydrite Nanoparticles (25 mg/mL) | Ferrihydrite Nanoparticles (50 mg/mL) |
| 1 | 2 | 3 | |
| Luminol-dependent reaction | |||
| Imax | 20,060 (12,451–32,490) | 3321 (988–7673) | 11,384 (2807–19,278) P1 = 0.014 |
| Smax × 105 | 7.1 (3.8–10.4) | 1.2 (0.3–2.7) | 3.4 (1.02–5.9) P1 = 0.006 |
| IA | 3.1 (2.2–4.3) | 5.7 (1.4–9.5) | 9.3 (6.7–11.5) P1 = 0.009 |
| Lucigenin-dependent reaction | |||
| Imax | 2009 (919–2640) | 8170 (7580–9030) P1 < 0.001 | 183 (886–3044) |
| IA | 2.3 (1.1–3.4) | 6.5 (4.3–7.0) P1 < 0.001 | 3.1 (2.4–4.3) |
| Characteristics 1 | Control | Ferrihydrite Nanoparticles (25 mg/mL) | Ferrihydrite Nanoparticles (50 mg/mL) |
|---|---|---|---|
| 1 | 2 | 3 | |
| Luminol-dependent reaction | |||
| Spontaneous reaction | |||
| Tmax | 7680 (5580–127,300) | 8230 (5040–10,560) | 791 (496–1992) P1 < 0.001 |
| Zymosan-induced reaction | |||
| Imax | 32,061 (15,452–34,571) | 14,234 (4917–16,348) | 5454 (1071–6684) P1 = 0.014 |
| Smax × 105 | 9.3 (5.7–10.8) | 6.4 (4.8–8.9) | 1.7 (0.5–2.4) P1 < 0.001 |
| IA | 1.0 (0.6–1.2) | 1.3 (1.0–2.5) | 3.1 (2.3–4.1) P1 < 0.001 |
| Lucigenin-dependent reaction | |||
| Imax | 1619 (1219–2568) | 4860 (3571–7123) P1 < 0.001 | 1454 (796–2064) |
| Smax × 104 | 2.8 (1.9–3.3) | 10.8 (9.1–12.1) P1 < 0.001 | 3.0 (2.3–3.3) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stolyar, S.V.; Kolenchukova, O.A.; Boldyreva, A.V.; Kudryasheva, N.S.; Gerasimova, Y.V.; Krasikov, A.A.; Yaroslavtsev, R.N.; Bayukov, O.A.; Ladygina, V.P.; Birukova, E.A. Biogenic Ferrihydrite Nanoparticles: Synthesis, Properties In Vitro and In Vivo Testing and the Concentration Effect. Biomedicines 2021, 9, 323. https://doi.org/10.3390/biomedicines9030323
Stolyar SV, Kolenchukova OA, Boldyreva AV, Kudryasheva NS, Gerasimova YV, Krasikov AA, Yaroslavtsev RN, Bayukov OA, Ladygina VP, Birukova EA. Biogenic Ferrihydrite Nanoparticles: Synthesis, Properties In Vitro and In Vivo Testing and the Concentration Effect. Biomedicines. 2021; 9(3):323. https://doi.org/10.3390/biomedicines9030323
Chicago/Turabian StyleStolyar, Sergey V., Oksana A. Kolenchukova, Anna V. Boldyreva, Nadezda S. Kudryasheva, Yulia V. Gerasimova, Alexandr A. Krasikov, Roman N. Yaroslavtsev, Oleg A. Bayukov, Valentina P. Ladygina, and Elena A. Birukova. 2021. "Biogenic Ferrihydrite Nanoparticles: Synthesis, Properties In Vitro and In Vivo Testing and the Concentration Effect" Biomedicines 9, no. 3: 323. https://doi.org/10.3390/biomedicines9030323
APA StyleStolyar, S. V., Kolenchukova, O. A., Boldyreva, A. V., Kudryasheva, N. S., Gerasimova, Y. V., Krasikov, A. A., Yaroslavtsev, R. N., Bayukov, O. A., Ladygina, V. P., & Birukova, E. A. (2021). Biogenic Ferrihydrite Nanoparticles: Synthesis, Properties In Vitro and In Vivo Testing and the Concentration Effect. Biomedicines, 9(3), 323. https://doi.org/10.3390/biomedicines9030323









