Fetal High-Density Lipoproteins: Current Knowledge on Particle Metabolism, Composition and Function in Health and Disease
Abstract
:1. Introduction
2. Changes in Maternal Lipid Metabolism during a Normal Pregnancy
3. Importance of Cholesterol in Fetal Development
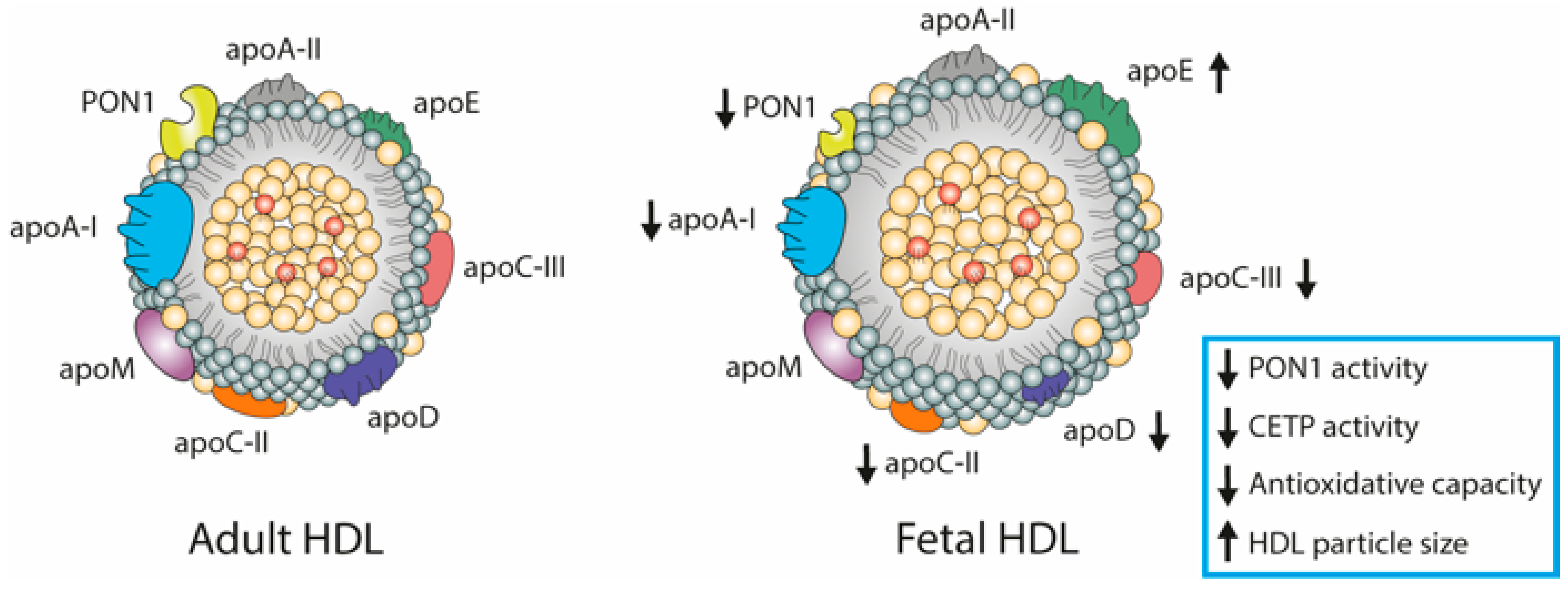
4. HDL Composition
5. HDL Functionality
5.1. Cholesterol Efflux Capacity
5.2. Anti-Inflammatory and Antioxidative Capacities
5.3. Vasodilatory Activites
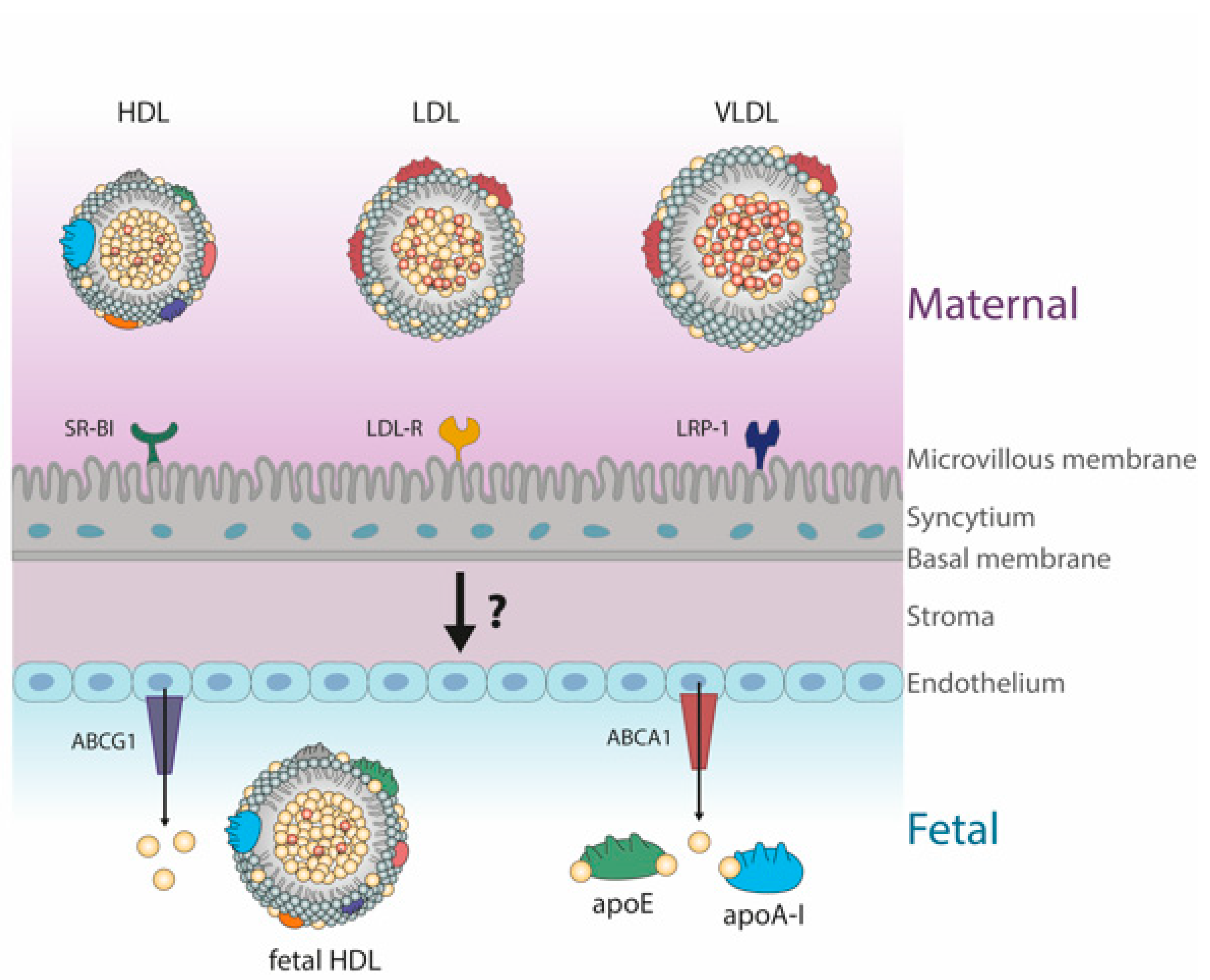
6. Fetal Lipoproteins Show Altered Concentrations and Unique Composition
HDL Metabolism in Cord Blood
7. The Role of Cord Blood-Derived HDL in Maintaining Fetoplacental Vascular Integrity
7.1. The Feto-Placental Endothelium
7.2. HDL-Sphingosine-1-Phosphate (S1P) as an Important Regulator of the Feto-Placental Vasculature
7.3. Protective Functions of Lipoprotein Associated Phospholipase A2 (LpPLA2) on the Feto-Placental Endothelium
8. Pregnancy-Related Diseases Affects HDL Metabolism and Function
8.1. Preeclampsia Associated Changes in HDL Composition and Function
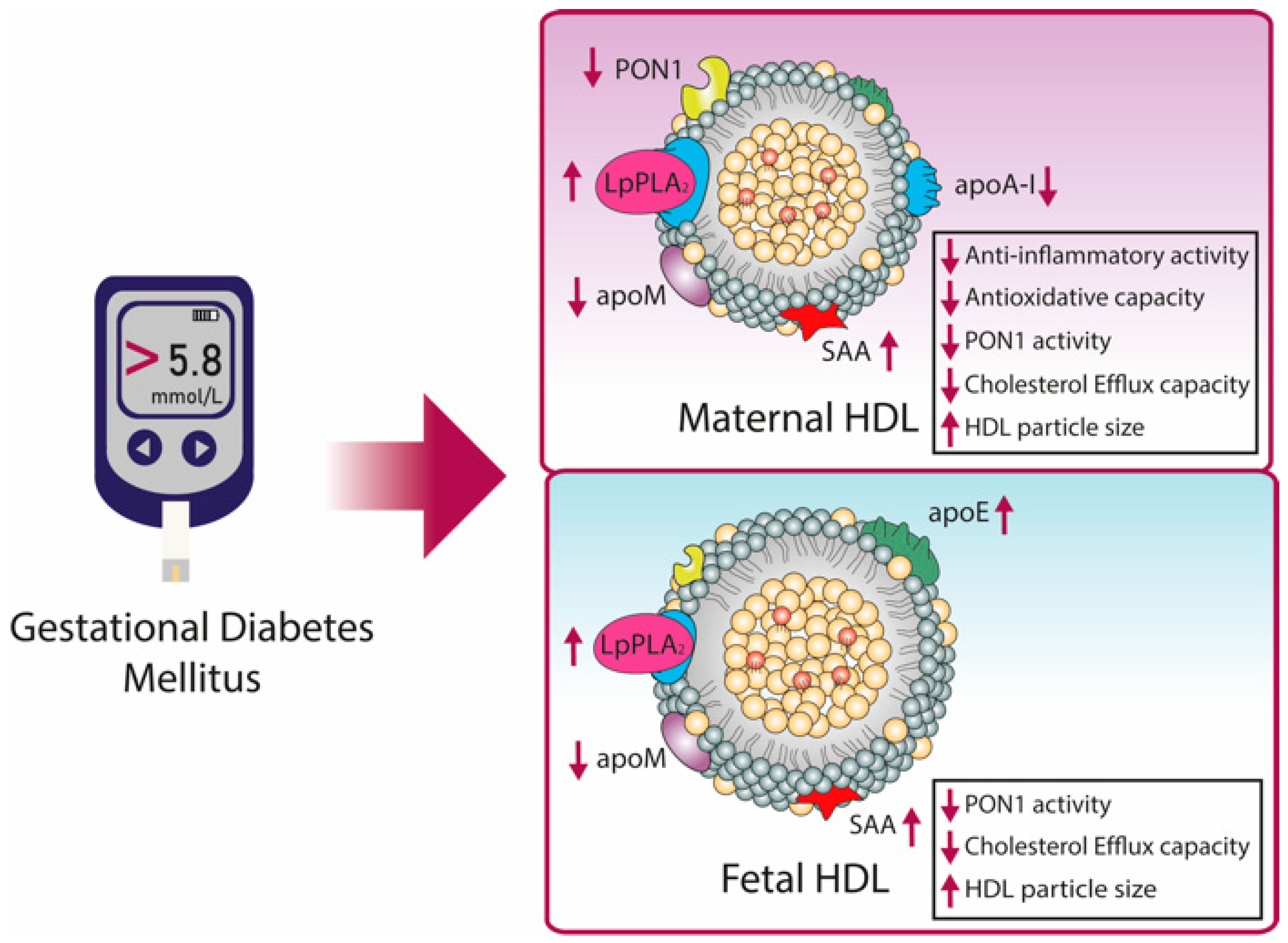
8.2. HDL in Gestational Diabetes Mellitus (GDM)
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ABCA1 | ATP-binding cassette A1 |
| ABCG1 | ATP-binding cassette G1 |
| apo | Apolipoprotein |
| CETP | Cholesteryl-ester transfer protein |
| eNOs | Endothelial nitric oxide synthase |
| HDL | High-density lipoprotein |
| HDL-C | High-density lipoprotein cholesterol |
| GDM | Gestational diabetes mellitus |
| NO | Nitric oxide |
| LCAT | Lecithin-cholesteryl acyltransferase |
| LDL | Low-density lipoprotein |
| LDL-R | Low-density lipoprotein receptor |
| LpPLA2 | Lipoprotein associated phospholipase A2 |
| LR-P1 | LDL receptor related protein 1 |
| LPL | Lipoprotein lipase |
| PAF | Platelet activating factor |
| PON1 | Paraoxonase 1 |
| SAA | Serum amyloid A |
| SR-BI | Scavenger receptor BI |
| S1P | Sphingosine-1-phosphate |
| VLDL | Very low-density lipoprotein |
References
- Roux, C.; Wolf, C.; Mulliez, N.; Gaoua, W.; Cormier, V.; Chevy, F.; Citadelle, D. Role of cholesterol in embryonic development. Am. J. Clin. Nutr. 2000, 71, 1270S–1279S. [Google Scholar] [CrossRef]
- Huang, X.; Litingtung, Y.; Chiang, C. Region-specific requirement for cholesterol modification of sonic hedgehog in patterning the telencephalon and spinal cord. Development 2007, 134, 2095–2105. [Google Scholar] [CrossRef] [Green Version]
- Woollett, L.A. Maternal cholesterol in fetal development: Transport of cholesterol from the maternal to the fetal circulation. Am. J. Clin. Nutr. 2005, 82, 1155–1161. [Google Scholar] [CrossRef] [Green Version]
- Woollett, L.A. Review: Transport of maternal cholesterol to the fetal circulation. Placenta 2011, 32, S218–S221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jayalekshmi, V.S.; Ramachandran, S. Maternal cholesterol levels during gestation: Boon or bane for the offspring? Mol. Cell. Biochem. 2021, 476, 401–416. [Google Scholar] [CrossRef] [PubMed]
- Wyne, K.L.; Woollett, L.A. Transport of maternal LDL and HDL to the fetal membranes and placenta of the Golden Syrian hamster is mediated by receptor-dependent and receptor-independent processes. J. Lipid Res. 1998, 39, 518–530. [Google Scholar] [CrossRef]
- Schmid, K.E.; Davidson, W.S.; Myatt, L.; Woollett, L.A. Transport of cholesterol across a BeWo cell monolayer. J. Lipid Res. 2003, 44, 1909–1918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wittmaack, F.M.; Gafvels, M.E.; Brönner, M.; Matsuo, H.; McCrae, K.R.; Tomaszewski, J.E.; Robinson, S.L.; Strickland, D.K.; Strauss, J.F. Localization and regulation of the human very low density lipoprotein/apolipoprotein-E receptor: Trophoblast expression predicts a role for the receptor in placental lipid transport. Endocrinology 1995, 136, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Stefulj, J.; Panzenboeck, U.; Becker, T.; Hirschmugl, B.; Schweinzer, C.; Lang, I.; Marsche, G.; Sadjak, A.; Lang, U.; Desoye, G.; et al. Human Endothelial Cells of the Placental Barrier Efficiently Deliver Cholesterol to the Fetal Circulation via ABCA1 and ABCG1. Circ. Res. 2009, 104, 600–608. [Google Scholar] [CrossRef]
- Sreckovic, I.; Birner-Gruenberger, R.; Obrist, B.; Stojakovic, T.; Scharnagl, H.; Holzer, M.; Scholler, M.; Philipose, S.; Marsche, G.; Lang, U.; et al. Distinct composition of human fetal HDL attenuates its anti-oxidative capacity. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2013, 1831, 737–746. [Google Scholar] [CrossRef]
- Murphy, S.P.; Abrams, B.F. Changes in energy intakes during pregnancy and lactation in a national sample of US women. Am. J. Public Heal. 1993, 83, 1161–1163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villar, J.; Cogswell, M.; Kestler, E.; Castillo, P.; Menendez, R.; Repke, J.T. Effect of fat and fat-free mass deposition during pregnancy on birth weight. Am. J. Obstet. Gynecol. 1992, 167, 1344–1352. [Google Scholar] [CrossRef]
- Zeng, Z.; Liu, F.; Li, S. Metabolic Adaptations in Pregnancy: A Review. Ann. Nutr. Metab. 2017, 70, 59–65. [Google Scholar] [CrossRef]
- Wiznitzer, A.; Mayer, A.; Novack, V.; Sheiner, E.; Gilutz, H.; Malhotra, A.; Novack, L. Association of lipid levels during gestation with preeclampsia and gestational diabetes mellitus: A population-based study. Am. J. Obstet. Gynecol. 2009, 201, 482.e1–482.e8. [Google Scholar] [CrossRef] [Green Version]
- Herrera, E. Lipid Metabolism during Pregnancy and its Implications for Fetal Growth. Curr. Pharm. Biotechnol. 2014, 15, 24–31. [Google Scholar] [CrossRef]
- Herrera, E.; Lasunción, M.A.; Gomez-Coronado, D.; Aranda, P.; López-Luna, P.; Maier, I. Role of lipoprotein lipase activity on lipoprotein metabolism and the fate of circulating triglycerides in pregnancy. Am. J. Obstet. Gynecol. 1988, 158, 1575–1583. [Google Scholar] [CrossRef]
- Alvarez, J.J.; Montelongo, A.; Iglesias, A.; Lasunción, M.A.; Herrera, E. Longitudinal Study on Lipoprotein Profile, High Density Lipoprotein Subclass, and Postheparin Lipases during Gestation in Women. J. Lipid Res. 1996, 37, 299–308. [Google Scholar] [CrossRef]
- Stanley, K.; Fraser, R.; Bruce, C. Physiological changes in insulin resistance in human pregnancy: Longitudinal study with the hyperinsulinaemic euglycaemic clamp technique. Br. J. Obstet. Gynaecol. 1998, 105, 756–759. [Google Scholar] [CrossRef] [Green Version]
- Ryan, E.A.; Enns, L. Role of Gestational Hormones in the Induction of Insulin Resistance. J. Clin. Endocrinol. Metab. 1988, 67, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Knopp, R.H.; Warth, M.R.; Charles, D.; Childs, M.; Li, J.R.; Mabuchi, H.; Van Allen, M.I. Lipoprotein Metabolism in Pregnancy, Fat Transport to the Fetus, and the Effects of Diabetes. Neonatology 1986, 50, 297–317. [Google Scholar] [CrossRef] [PubMed]
- Elliott, J.A. The Effect of Pregnancy on the Control of Lipolysis in Fat Cells Isolated from Human Adipose Tissue. Eur. J. Clin. Investig. 1975, 5, 159–163. [Google Scholar] [CrossRef]
- Ramos, P.; Herrera, E. Reversion of insulin resistance in the rat during late pregnancy by 72-h glucose infusion. Am. J. Physiol. Metab. 1995, 269, E858–E863. [Google Scholar] [CrossRef]
- Weinstein, I.; Soler-Argilaga, C.; Werner, H.V.; Heimberg, M. Effects of ethynyloestradiol on the metabolism of [1-14C]-oleate by perfused livers and hepatocytes from female rats. Biochem. J. 1979, 180, 265–271. [Google Scholar] [CrossRef] [Green Version]
- Iglesias, A.; Montelongo, A.; Herrera, E.; Lasunción, M.A. Changes in cholesteryl ester transfer protein activity during normal gestation and postpartum. Clin. Biochem. 1994, 27, 63–68. [Google Scholar] [CrossRef]
- Kinnunen, P.K.J.; Unnérus, H.A.; Ranta, T.; Ehnholm, C.; Nikkilä, J.E.A.; Seppälä, M. Activities of post-heparin plasma lipoprotein lipase and hepatic lipase during pregnancy and lactation. Eur. J. Clin. Investig. 1980, 10, 469–474. [Google Scholar] [CrossRef]
- Palinski, W.; Nicolaides, E.; Liguori, A.; Napoli, C. Influence of Maternal Dysmetabolic Conditions during Pregnancy on Cardiovascular Disease. J. Cardiovasc. Transl. Res. 2009, 2, 277–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schroepfer, G.J. Oxysterols: Modulators of Cholesterol Metabolism and Other Processes. Physiol. Rev. 2000, 80, 361–554. [Google Scholar] [CrossRef]
- Dietschy, J.M.; Turley, S.D.; Spady, D.K. Role of liver in the maintenance of cholesterol and low density lipoprotein homeostasis in different animal species, including humans. J. Lipid Res. 1993, 34, 1637–1659. [Google Scholar] [CrossRef]
- Wadsack, C.; Hammer, A.; Levak-Frank, S.; Desoye, G.; Kozarsky, K.; Hirschmugl, B.; Sattler, W.; Malle, E. Selective Cholesteryl Ester Uptake from High Density Lipoprotein by Human First Trimester and Term Villous Trophoblast Cells. Placenta 2003, 24, 131–143. [Google Scholar] [CrossRef]
- Weaver, D.D.; Solomon, B.D.; Akin-Samson, K.; Kelley, R.I.; Muenke, M. Cyclopia (synophthalmia) in Smith-Lemli-Opitz syndrome: First reported case and consideration of mechanism. Am. J. Med Genet. Part C: Semin. Med Genet. 2010, 154C, 142–145. [Google Scholar] [CrossRef] [Green Version]
- Lunghi, L.; Ferretti, M.E.; Medici, S.; Biondi, C.; Vesce, F. Control of human trophoblast function. Reprod. Biol. Endocrinol. 2007, 5, 6. [Google Scholar] [CrossRef] [Green Version]
- Lager, S.; Powell, T.L. Regulation of Nutrient Transport across the Placenta. J. Pregnancy 2012, 2012, 179827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desoye, G.; Gauster, M.; Wadsack, C. Placental transport in pregnancy pathologies. Am. J. Clin. Nutr. 2011, 94, 1896S–1902S. [Google Scholar] [CrossRef] [Green Version]
- Kallol, S.; Albrecht, C. Materno-fetal cholesterol transport during pregnancy. Biochem. Soc. Trans. 2020, 48, 775–786. [Google Scholar] [CrossRef] [PubMed]
- Burke, K.T.; Colvin, P.L.; Myatt, L.; Graf, G.A.; Schroeder, F.; Woollett, L.A. Transport of maternal cholesterol to the fetus is affected by maternal plasma cholesterol concentrations in the Golden Syrian hamster. J. Lipid Res. 2009, 50, 1146–1155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeşilkaya, E.; Karaer, K.; Bideci, A.; Çamurdan, O.; Perçin, E.F.; Cinaz, P. Dubowitz Syndrome: A Cholesterol Metabolism Disorder? Genet. Couns. Med Psychol. Ethical Asp. 2008, 19, 287–290. [Google Scholar]
- Maymunah, A.-O.; Kehinde, O.; Abidoye, G.; Oluwatosin, A. Hypercholesterolaemia in pregnancy as a predictor of adverse pregnancy outcome. Afr. Heal. Sci. 2015, 14, 967–973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Catov, J.M.; Newman, A.B.; Roberts, J.M.; Kelsey, S.F.; Sutton-Tyrrell, K.; Harris, T.B.; Colbert, L.; Rubin, S.M.; Satterfield, S.; Ness, R.B. Preterm Delivery and Later Maternal Cardiovascular Disease Risk. Epidemiology 2007, 18, 733–739. [Google Scholar] [CrossRef]
- Khoury, J.; Henriksen, T.; Christophersen, B.; Tonstad, S. Effect of a cholesterol-lowering diet on maternal, cord, and neonatal lipids, and pregnancy outcome: A randomized clinical trial. Am. J. Obstet. Gynecol. 2005, 193, 1292–1301. [Google Scholar] [CrossRef]
- Jan, M.R.; Nazli, R.; Shah, J.; Akhtar, T. A Study of Lipoproteins in Normal and Pregnancy Induced Hypertensive Women in Tertiary Care Hospitals of the North West Frontier Province-Pakistan. Hypertens. Pregnancy 2010, 31, 292–299. [Google Scholar] [CrossRef]
- Vrijkotte, T.G.M.; Krukziener, N.; Hutten, B.A.; Vollebregt, K.C.; Van Eijsden, M.; Twickler, M.B. Maternal Lipid Profile During Early Pregnancy and Pregnancy Complications and Outcomes: The ABCD Study. J. Clin. Endocrinol. Metab. 2012, 97, 3917–3925. [Google Scholar] [CrossRef]
- Kontush, A. HDL-mediated mechanisms of protection in cardiovascular disease. Cardiovasc. Res. 2014, 103, 341–349. [Google Scholar] [CrossRef] [Green Version]
- Feig, J.E.; Hewing, B.; Smith, J.D.; Hazen, S.L.; Fisher, E.A. High-Density Lipoprotein and Atherosclerosis Regression. Circ. Res. 2014, 114, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Pappa, E.; Elisaf, M.S.; Kostara, C.; Bairaktari, E.; Tsimihodimos, V.K. Cardioprotective Properties of HDL: Structural and Functional Considerations. Curr. Med. Chem. 2020, 27, 2964–2978. [Google Scholar] [CrossRef] [PubMed]
- Tailleux, A.; Fruchart, J.C.; Parkes, J.G. HDL Heterogeneity and Atherosclerosis. Crit. Rev. Clin. Lab. Sci. 1996, 33, 163–201. [Google Scholar] [CrossRef]
- Kostner, G.; Alaupovic, P. Composition and structure of plasma lipoproteins. Separation and quantification of the lipoprotein families occurring in the high density lipoproteins of human plasma. Biochemistry 1972, 11, 3419–3428. [Google Scholar] [CrossRef] [PubMed]
- Duriez, P.; Fruchart, J.C. High-density lipoprotein subclasses and apolipoprotein A-I. Clin. Chim. Acta 1999, 286, 97–114. [Google Scholar] [CrossRef]
- Ahnström, J.; Faber, K.; Axler, O.; Dahlbäck, B. Hydrophobic ligand binding properties of the human lipocalin apolipoprotein M. J. Lipid Res. 2007, 48, 1754–1762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christoffersen, C.; Obinata, H.; Kumaraswamy, S.B.; Galvani, S.; Ahnström, J.; Sevvana, M.; Egerer-Sieber, C.; Muller, Y.A.; Hla, T.; Nielsen, L.B.; et al. Endothelium-protective sphingosine-1-phosphate provided by HDL-associated apolipoprotein M. Proc. Natl. Acad. Sci. USA 2011, 108, 9613–9618. [Google Scholar] [CrossRef] [Green Version]
- Kontush, A.; Lindahl, M.; Lhomme, M.; Calabresi, L.; Chapman, M.J.; Davidson, W.S. Structure of HDL: Particle Subclasses and Molecular Components. Handb. Exp. Pharmacol. 2014, 224, 3–51. [Google Scholar] [CrossRef]
- Garelnabi, M.; Litvinov, D.; Mahini, H. Antioxidant and anti-inflammatory role of paraoxonase 1: Implication in arteriosclerosis diseases. N. Am. J. Med Sci. 2012, 4, 523–532. [Google Scholar] [CrossRef] [Green Version]
- Draganov, D.I.; Teiber, J.F.; Speelman, A.; Osawa, Y.; Sunahara, R.; La Du, B.N. Human paraoxonases (PON1, PON2, and PON3) are lactonases with overlapping and distinct substrate specificities. J. Lipid Res. 2005, 46, 1239–1247. [Google Scholar] [CrossRef] [Green Version]
- Kontush, A.; Lhomme, M.; Chapman, M.J. Unraveling the complexities of the HDL lipidome. J. Lipid Res. 2013, 54, 2950–2963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baker, P.W.; Rye, K.-A.; Gamble, J.R.; Vadas, M.A.; Barter, P.J. Ability of reconstituted high density lipoproteins to inhibit cytokine-induced expression of vascular cell adhesion molecule-1 in human umbilical vein endothelial cells. J. Lipid Res. 1999, 40, 345–353. [Google Scholar] [CrossRef]
- Trieb, M.; Wolf, P.; Knuplez, E.; Weger, W.; Schuster, C.; Peinhaupt, M.; Holzer, M.; Trakaki, A.; Eichmann, T.; Lass, A.; et al. Abnormal composition and function of high-density lipoproteins in atopic dermatitis patients. Allergy 2018, 74, 398–402. [Google Scholar] [CrossRef] [Green Version]
- Trakaki, A.; Marsche, G. High-Density Lipoprotein (HDL) in Allergy and Skin Diseases: Focus on Immunomodulating Functions. Biomedicines 2020, 8, 558. [Google Scholar] [CrossRef]
- Trakaki, A.; Sturm, G.J.; Pregartner, G.; Scharnagl, H.; Eichmann, T.O.; Trieb, M.; Knuplez, E.; Holzer, M.; Stadler, J.T.; Heinemann, A.; et al. Allergic rhinitis is associated with complex alterations in high-density lipoprotein composition and function. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2019, 1864, 1280–1292. [Google Scholar] [CrossRef]
- Curcic, S.; Holzer, M.; Frei, R.; Pasterk, L.; Schicho, R.; Heinemann, A.; Marsche, G. Neutrophil effector responses are suppressed by secretory phospholipase A2 modified HDL. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2015, 1851, 184–193. [Google Scholar] [CrossRef] [Green Version]
- Yetukuri, L.; Söderlund, S.; Koivuniemi, A.; Seppänen-Laakso, T.; Niemelä, P.S.; Hyvönen, M.; Taskinen, M.-R.; Vattulainen, I.; Jauhiainen, M.; Orešič, M. Composition and lipid spatial distribution of HDL particles in subjects with low and high HDL-cholesterol. J. Lipid Res. 2010, 51, 2341–2351. [Google Scholar] [CrossRef] [Green Version]
- Wiesner, P.; Leidl, K.; Boettcher, A.; Schmitz, G.; Liebisch, G. Lipid profiling of FPLC-separated lipoprotein fractions by electrospray ionization tandem mass spectrometry. J. Lipid Res. 2009, 50, 574–585. [Google Scholar] [CrossRef] [Green Version]
- Serna, J.; García-Seisdedos, D.; Alcázar, A.; Lasunción, M. Ángel; Busto, R.; Pastor, Óscar Quantitative lipidomic analysis of plasma and plasma lipoproteins using MALDI-TOF mass spectrometry. Chem. Phys. Lipids 2015, 189, 7–18. [Google Scholar] [CrossRef]
- Von Eckardstein, A.; Nofer, J.-R.; Assmann, G. High Density Lipoproteins and Arteriosclerosis. Arter. Thromb. Vasc. Biol. 2001, 21, 13–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khera, A.V.; Cuchel, M.; De La Llera-Moya, M.; Rodrigues, A.; Burke, M.F.; Jafri, K.; French, B.C.; Phillips, J.A.; Mucksavage, M.L.; Wilensky, R.L.; et al. Cholesterol Efflux Capacity, High-Density Lipoprotein Function, and Atherosclerosis. N. Engl. J. Med. 2011, 364, 127–135. [Google Scholar] [CrossRef] [Green Version]
- Li, X.-M.; Tang, W.H.W.; Mosior, M.K.; Huang, Y.; Wu, Y.; Matter, W.; Gao, V.; Schmitt, D.; DiDonato, J.A.; Fisher, E.A.; et al. Paradoxical Association of Enhanced Cholesterol Efflux With Increased Incident Cardiovascular Risks. Arter. Thromb. Vasc. Biol. 2013, 33, 1696–1705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Attie, A.D.; Kastelein, J.P.; Hayden, M.R. Pivotal role of ABCA1 in reverse cholesterol transport influencing HDL levels and susceptibility to atherosclerosis. J. Lipid Res. 2001, 42, 1717–1726. [Google Scholar] [CrossRef]
- Wang, N.; Lan, D.; Chen, W.; Matsuura, F.; Tall, A.R. ATP-binding cassette transporters G1 and G4 mediate cellular cholesterol efflux to high-density lipoproteins. Proc. Natl. Acad. Sci. USA 2004, 101, 9774–9779. [Google Scholar] [CrossRef] [Green Version]
- Tang, C.; Oram, J.F. The cell cholesterol exporter ABCA1 as a protector from cardiovascular disease and diabetes. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2009, 1791, 563–572. [Google Scholar] [CrossRef]
- Mc, P.; Wj, J.; Gh, R. Mechanisms and Consequences of Cellular Cholesterol Exchange and Transfer. Available online: https://pubmed.ncbi.nlm.nih.gov/3297153/ (accessed on 14 October 2020).
- Kennedy, M.A.; Barrera, G.C.; Nakamura, K.; Baldán, Á.; Tarr, P.; Fishbein, M.C.; Frank, J.; Francone, O.L.; Edwards, P.A. ABCG1 has a critical role in mediating cholesterol efflux to HDL and preventing cellular lipid accumulation. Cell Metab. 2005, 1, 121–131. [Google Scholar] [CrossRef] [Green Version]
- Rothblat, G.H.; Phillips, M.C. High-density lipoprotein heterogeneity and function in reverse cholesterol transport. Curr. Opin. Lipidol. 2010, 21, 229–238. [Google Scholar] [CrossRef]
- Ouimet, M.; Barrett, T.J.; Fisher, E.A. HDL and Reverse Cholesterol Transport. Circ. Res. 2019, 124, 1505–1518. [Google Scholar] [CrossRef]
- Navab, M.; Imes, S.S.; Hama, S.Y.; Hough, G.P.; Ross, L.A.; Bork, R.W.; Valente, A.J.; Berliner, J.A.; Drinkwater, D.C.; Laks, H.; et al. Monocyte transmigration induced by modification of low density lipoprotein in cocultures of human aortic wall cells is due to induction of monocyte chemotactic protein 1 synthesis and is abolished by high density lipoprotein. J. Clin. Investig. 1991, 88, 2039–2046. [Google Scholar] [CrossRef]
- Cockerill, G.W.; Rye, K.-A.; Gamble, J.R.; Vadas, M.A.; Barter, P.J. High-Density Lipoproteins Inhibit Cytokine-Induced Expression of Endothelial Cell Adhesion Molecules. Arter. Thromb. Vasc. Biol. 1995, 15, 1987–1994. [Google Scholar] [CrossRef] [PubMed]
- Calabresia, L.; Franceschinia, G.; Sirtori, C.R.; De Palma, A.; Saresellac, M.; Ferrantec, P.; Taramellib, D. Inhibition of VCAM-1 Expression in Endothelial Cells by Reconstituted High Density Lipoproteins. Biochem. Biophys. Res. Commun. 1997, 238, 61–65. [Google Scholar] [CrossRef]
- Nofer, J.-R.; Geigenmüller, S.; Göpfert, C.; Assmann, G.; Buddecke, E.; Schmidt, A. High density lipoprotein-associated lysosphingolipids reduce E-selectin expression in human endothelial cells. Biochem. Biophys. Res. Commun. 2003, 310, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Bursill, C.A.; Castro, M.L.; Beattie, D.T.; Nakhla, S.; Van Der Vorst, E.; Heather, A.K.; Barter, P.J.; Rye, K.-A. High-Density Lipoproteins Suppress Chemokines and Chemokine Receptors In Vitro and In Vivo. Arter. Thromb. Vasc. Biol. 2010, 30, 1773–1778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panzenböck, U.; Stocker, R. Formation of methionine sulfoxide-containing specific forms of oxidized high-density lipoproteins. Biochim. Biophys. Acta (BBA) Proteins Proteom. 2005, 1703, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Garner, B.; Waldeck, A.R.; Witting, P.K.; Rye, K.-A.; Stocker, R. Oxidation of High Density Lipoproteins. J. Biol. Chem. 1998, 273, 6088–6095. [Google Scholar] [CrossRef] [Green Version]
- Aviram, M.; Billecke, S.; Sorenson, R.; Bisgaier, C.; Newton, R.; Rosenblat, M.; Erogul, J.; Hsu, C.; Dunlop, C.; La Du, B. Paraoxonase Active Site Required for Protection Against LDL Oxidation Involves Its Free Sulfhydryl Group and Is Different From That Required for Its Arylesterase/Paraoxonase Activities. Arter. Thromb. Vasc. Biol. 1998, 18, 1617–1624. [Google Scholar] [CrossRef] [Green Version]
- Miyata, M.; Smith, J.D. Apolipoprotein E allele–specific antioxidant activity and effects on cytotoxicity by oxidative insults and β–amyloid peptides. Nat. Genet. 1996, 14, 55–61. [Google Scholar] [CrossRef]
- Kontush, A.; Chapman, M.J. Functionally Defective High-Density Lipoprotein: A New Therapeutic Target at the Crossroads of Dyslipidemia, Inflammation, and Atherosclerosis. Pharmacol. Rev. 2006, 58, 342–374. [Google Scholar] [CrossRef]
- Kontush, A.; Chapman, M.J. Antiatherogenic small, dense HDL—guardian angel of the arterial wall? Nat. Clin. Pr. Neurol. 2006, 3, 144–153. [Google Scholar] [CrossRef]
- Yvan-Charvet, L.; Pagler, T.A.; Seimon, T.A.; Thorp, E.; Welch, C.L.; Witztum, J.L.; Tabas, I.; Tall, A.R. ABCA1 and ABCG1 Protect Against Oxidative Stress–Induced Macrophage Apoptosis During Efferocytosis. Circ. Res. 2010, 106, 1861–1869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabet, F.; Lambert, G.; Torres, L.F.C.; Hou, L.; Sotirchos, I.; Touyz, R.M.; Jenkins, A.J.; Barter, P.J.; Rye, K.-A. Lipid-Free Apolipoprotein A-I and Discoidal Reconstituted High-Density Lipoproteins Differentially Inhibit Glucose-Induced Oxidative Stress in Human Macrophages. Arter. Thromb. Vasc. Biol. 2011, 31, 1192–1200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robbesyn, F.; Garcia, V.; Auge, N.; Vieira, O.; Frisach, M.; Salvayre, R.; Negre-Salvayre, A. HDL counterbalance the proinflammatory effect of oxidized LDL by inhibiting intracellular reactive oxygen species rise, proteasome activation, and subsequent NF-κB activation in smooth muscle cells. FASEB J. 2003, 17, 743–745. [Google Scholar] [CrossRef] [Green Version]
- De Souza, J.A.; Vindis, C.; Nègre-Salvayre, A.; Rye, K.-A.; Couturier, M.; Thérond, P.; Chantepie, S.; Salvayre, R.; Chapman, M.J.; Kontush, A. Small, dense HDL 3 particles attenuate apoptosis in endothelial cells: Pivotal role of apolipoprotein A-I. J. Cell. Mol. Med. 2009, 14, 608–620. [Google Scholar] [CrossRef]
- Nofer, J.-R.; Van Der Giet, M.; Tölle, M.; Wolinska, I.; Lipinski, K.V.W.; Baba, H.A.; Tietge, U.J.; Gödecke, A.; Ishii, I.; Kleuser, B.; et al. HDL induces NO-dependent vasorelaxation via the lysophospholipid receptor S1P3. J. Clin. Investig. 2004, 113, 569–581. [Google Scholar] [CrossRef]
- Mineo, C.; Deguchi, H.; Griffin, J.H.; Shaul, P.W. Endothelial and antithrombotic actions of HDL. Circ. Res. 2006, 98, 1352–1364. [Google Scholar] [CrossRef] [Green Version]
- Norata, G.D.; Callegari, E.; Inoue, H.; Catapano, A.L. HDL 3 Induces Cyclooxygenase-2 Expression and Prostacyclin Release in Human Endothelial Cells Via a p38 MAPK/CRE-Dependent Pathway: Effects on COX-2/PGI-Synthase Coupling. Arter. Thromb. Vasc. Biol. 2004, 24, 871–877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Movva, R.; Rader, D.J. Laboratory Assessment of HDL Heterogeneity and Function. Clin. Chem. 2008, 54, 788–800. [Google Scholar] [CrossRef] [Green Version]
- Yuhanna, I.S.; Zhu, Y.; Cox, B.E.; Hahner, L.D.; Osborne-Lawrence, S.; Lu, P.; Marcel, Y.L.; Anderson, R.G.; Mendelsohn, M.E.; Hobbs, H.H.; et al. High-density lipoprotein binding to scavenger receptor-BI activates endothelial nitric oxide synthase. Nat. Med. 2001, 7, 853–857. [Google Scholar] [CrossRef]
- Drew, B.G.; Fidge, N.H.; Gallon-Beaumier, G.; Kemp, B.E.; Kingwell, B.A. High-density lipoprotein and apolipoprotein AI increase endothelial NO synthase activity by protein association and multisite phosphorylation. Proc. Natl. Acad. Sci. USA 2004, 101, 6999–7004. [Google Scholar] [CrossRef] [Green Version]
- Van Linthout, S.; Spillmann, F.; Lorenz, M.; Meloni, M.; Jacobs, F.; Egorova, M.; Stangl, V.; De Geest, B.; Schultheiss, H.-P.; TschöpeC. Vascular-Protective Effects of High-Density Lipoprotein Include the Downregulation of the Angiotensin II Type 1 Receptor. Hypertens. 2009, 53, 682–687. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Xiao, H.; Rizzo, A.N.; Zhang, W.; Mai, Y.; Ye, M. Endothelial Nitric Oxide Synthase Dimerization Is Regulated by Heat Shock Protein 90 Rather than by Phosphorylation. PLoS ONE 2014, 9, e0105479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Averna, M.; Barbagallo, C.M.; Di Paola, G.; Labisi, M.; Pinna, G.; Marino, G.; Dimita, U.; Notarbartolo, A. Lipids, Lipoproteins and Apolipoproteins AI AII, B, CII, CIII and E in Newborns. Neonatology 1991, 60, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Saha, N.; Wong, H. Serum HDL Cholesterol and Apolipoprotein AI, All and B Levels in Singapore Newborns. Neonatology 1987, 52, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Bastida, S.; Sánchez-Muniz, F.J.; Cuesta, C.; Perea, S.; Aragonés, A. Male and female cord blood lipoprotein profile differences throughout the term-period. J. Périnat. Med. 1997, 25, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Merzouk, H.; Meghelli-Bouchenak, M.; El-Korso, N.; Belleville, J.; Prost, J. Low birth weight at term impairs cord serum lipoprotein compositions and concentrations. Eur. J. Nucl. Med. Mol. Imaging 1998, 157, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Nagasaka, H.; Chiba, H.; Kikuta, H.; Akita, H.; Takahashi, Y.; Yanai, H.; Hui, S.-P.; Fuda, H.; Fujiwara, H.; Kobayashi, K. Unique character and metabolism of high density lipoprotein (HDL) in fetus. Atherosclerosis 2002, 161, 215–223. [Google Scholar] [CrossRef]
- Dolphin, P.J.; Breckenridge, W.C.; Dolphin, M.A.; Tan, M.H. The lipoproteins of human umbilical cord blood apolipoprotein and lipid levels. Atherosclerosis 1984, 51, 109–122. [Google Scholar] [CrossRef]
- Wang, N.; Silver, D.L.; Costet, P.; Tall, A.R. Specific Binding of ApoA-I, Enhanced Cholesterol Efflux, and Altered Plasma Membrane Morphology in Cells Expressing ABC1. J. Biol. Chem. 2000, 275, 33053–33058. [Google Scholar] [CrossRef] [Green Version]
- Moore, R.E.; Navab, M.; Millar, J.S.; Zimetti, F.; Hama, S.; Rothblat, G.H.; Rader, D.J. Increased Atherosclerosis in Mice Lacking Apolipoprotein A-I Attributable to Both Impaired Reverse Cholesterol Transport and Increased Inflammation. Circ. Res. 2005, 97, 763–771. [Google Scholar] [CrossRef] [Green Version]
- Sorci-Thomas, M.G.; Bhat, S.; Thomas, M.J. Activation of lecithin:cholesterol acyltransferase by HDL ApoA-I central helices. Clin. Lipidol. 2009, 4, 113–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koo, C.; Innerarity, T.L.; Mahley, R.W. Obligatory role of cholesterol and apolipoprotein E in the formation of large cholesterol-enriched and receptor-active high density lipoproteins. J. Biol. Chem. 1985, 260, 11934–11943. [Google Scholar] [CrossRef]
- Mahley, R.W. Apolipoprotein E: Cholesterol transport protein with expanding role in cell biology. Science 1988, 240, 622–630. [Google Scholar] [CrossRef]
- Rosales, C.; Tang, D.; Gillard, B.K.; Courtney, H.S.; Pownall, H.J. Apolipoprotein E Mediates Enhanced Plasma High-Density Lipoprotein Cholesterol Clearance by Low-Dose Streptococcal Serum Opacity Factor via Hepatic Low-Density Lipoprotein Receptors In Vivo. Arter. Thromb. Vasc. Biol. 2011, 31, 1834–1841. [Google Scholar] [CrossRef] [Green Version]
- Gaidukov, L.; Viji, R.I.; Yacobson, S.; Rosenblat, M.; Aviram, M.; Tawfik, D.S. ApoE Induces Serum Paraoxonase PON1 Activity and Stability Similar to ApoA-I. Biochemistry 2010, 49, 532–538. [Google Scholar] [CrossRef]
- Zhao, Y.; Thorngate, F.E.; Weisgraber, K.H.; Williams, D.L.; Parks, J.S. Apolipoprotein E Is the Major Physiological Activator of Lecithin−Cholesterol Acyltransferase (LCAT) on Apolipoprotein B Lipoproteins. Biochemistry 2005, 44, 1013–1025. [Google Scholar] [CrossRef] [PubMed]
- Gugliucci, A.; Numaguchi, M.; Caccavello, R.; Kimura, S. Paraoxonase 1 lactonase activity and distribution in the HDL subclasses in the cord blood. Redox Rep. 2014, 19, 124–132. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Kumar, M.; Chan, W.; Berkowitz, G.; Wetmur, J.G. Increased influence of genetic variation on PON1 activity in neonates. Environ. Heal. Perspect. 2003, 111, 1403–1409. [Google Scholar] [CrossRef] [Green Version]
- Altunhan, H.; Annagür, A.; Kurban, S.; Ertuğrul, S.; Konak, M.; Örs, R. Total oxidant, antioxidant, and paraoxonase levels in babies born to pre-eclamptic mothers. J. Obstet. Gynaecol. Res. 2013, 39, 898–904. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Beamonte, R.; Lou-Bonafonte, J.M.; Martínez-Gracia, M.V.; Osada, J. Sphingomyelin in High-Density Lipoproteins: Structural Role and Biological Function. Int. J. Mol. Sci. 2013, 14, 7716–7741. [Google Scholar] [CrossRef] [Green Version]
- Zerrad-Saadi, A.; Therond, P.; Chantepie, S.; Couturier, M.; Rye, K.-A.; Chapman, M.J.; Kontush, A. HDL3-Mediated Inactivation of LDL-Associated Phospholipid Hydroperoxides Is Determined by the Redox Status of Apolipoprotein A-I and HDL Particle Surface Lipid Rigidity. Arter. Thromb. Vasc. Biol. 2009, 29, 2169–2175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellanger, N.; Julia, Z.; Villard, E.F.; El Khoury, P.; Duchene, E.; Chapman, M.J.; Fournier, N.; Le Goff, W.; Guérin, M. Functionality of postprandial larger HDL2 particles is enhanced following CETP inhibition therapy. Atherosclerosis 2012, 221, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Myatt, L. Control of vascular resistance in the human placenta. Placenta 1992, 13, 329–341. [Google Scholar] [CrossRef]
- Odum, C.U.; Pipkin, F.B. Studies on the response of isolated human chorionic plate artery strips to angiotensin II in normal pregnancy and in pregnancy induced hypertension. West Afr. J. Med. 1989, 8, 251–256. [Google Scholar] [PubMed]
- Paradis, A.; Zhang, L. Role of endothelin in uteroplacental circulation and fetal vascular function. Curr. Vasc. Pharmacol. 2013, 11, 594–605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, E.J. Role of the fetoplacental endothelium in fetal growth restriction with abnormal umbilical artery Doppler velocimetry. Am. J. Obstet. Gynecol. 2015, 213, S123–S130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boura, A.; Walters, W. Autacoids and the control of vascular tone in the human umbilical-placental circulation. Placenta 1991, 12, 453–477. [Google Scholar] [CrossRef]
- Mak, K.K.-W.; Gude, N.M.; Walters, W.A.W.; Boura, A.L.A. Effects of vasoactive autacoids on the human umbilical-fetal placental vasculature. BJOG: Int. J. Obstet. Gynaecol. 1984, 91, 99–106. [Google Scholar] [CrossRef]
- Wadsack, C.; Desoye, G.; Hiden, U. The feto-placental endothelium in pregnancy pathologies. Wien. Med. Wochenschr. 2012, 162, 220–224. [Google Scholar] [CrossRef]
- Stuart, M.; Clark, D.A.; Sunderji, S.G.; Allen, J.B.; Yambo, T.; Elrad, H.; Slott, J.H. Decreased prostacyclin production: A Characteristic of chronic placental insufficiency syndromes. Lancet 1981, 317, 1126–1128. [Google Scholar] [CrossRef]
- Giles, W. Placental Nitric Oxide Synthase Activity and Abnormal Umbilical Artery Flow Velocity Waveforms. Obstet. Gynecol. 1997, 89, 49–52. [Google Scholar] [CrossRef]
- Sattler, K.; Levkau, B. Sphingosine-1-phosphate as a mediator of high-density lipoprotein effects in cardiovascular protection. Cardiovasc. Res. 2008, 82, 201–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venkataraman, K.; Lee, Y.-M.; Michaud, J.; Thangada, S.; Ai, Y.; Bonkovsky, H.L.; Parikh, N.S.; Habrukowich, C.; Hla, T. Vascular Endothelium As a Contributor of Plasma Sphingosine 1-Phosphate. Circ. Res. 2008, 102, 669–676. [Google Scholar] [CrossRef] [Green Version]
- Gazit, S.L.; Mariko, B.; Thérond, P.; Decouture, B.; Xiong, Y.; Couty, L.; Bonnin, P.; Baudrie, V.; Le Gall, S.M.; Dizier, B.; et al. Platelet and Erythrocyte Sources of S1P Are Redundant for Vascular Development and Homeostasis, but Both Rendered Essential After Plasma S1P Depletion in Anaphylactic Shock. Circ. Res. 2016, 119, e110–e126. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Sato, K.; Kuwabara, A.; Tomura, H.; Ishiwara, M.; Kobayashi, I.; Ui, M.; Okajima, F. Sphingosine 1-Phosphate May Be a Major Component of Plasma Lipoproteins Responsible for the Cytoprotective Actions in Human Umbilical Vein Endothelial Cells. J. Biol. Chem. 2001, 276, 31780–31785. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.-J.; Van Brocklyn, J.R.; Thangada, S.; Liu, C.H.; Hand, A.R.; Menzeleev, R.; Spiegel, S.; Hla, T. Sphingosine-1-Phosphate as a Ligand for the G Protein-Coupled Receptor EDG-1. Science 1998, 279, 1552–1555. [Google Scholar] [CrossRef] [PubMed]
- Galvani, S.; Sanson, M.; Blaho, V.A.; Swendeman, S.L.; Obinata, H.; Conger, H.; Dahlbäck, B.; Kono, M.; Proia, R.L.; Smith, J.D.; et al. HDL-bound sphingosine 1-phosphate acts as a biased agonist for the endothelial cell receptor S1P1to limit vascular inflammation. Sci. Signal. 2015, 8, ra79. [Google Scholar] [CrossRef] [Green Version]
- Argraves, K.M.; Argraves, W.S. HDL serves as a S1P signaling platform mediating a multitude of cardiovascular effects. J. Lipid Res. 2007, 48, 2325–2333. [Google Scholar] [CrossRef] [Green Version]
- Brinck, J.W.; Thomas, A.; Lauer, E.; Jornayvaz, F.R.; Brulhart-Meynet, M.-C.; Prost, J.-C.; Pataky, Z.; Löfgren, P.; Hoffstedt, J.; Eriksson, M.; et al. Diabetes Mellitus Is Associated With Reduced High-Density Lipoprotein Sphingosine-1-Phosphate Content and Impaired High-Density Lipoprotein Cardiac Cell Protection. Arter. Thromb. Vasc. Biol. 2016, 36, 817–824. [Google Scholar] [CrossRef] [Green Version]
- Jing, X.-D.; Wei, X.-M.; Deng, S.-B.; Du, J.-L.; Liu, Y.-J.; She, Q. The relationship between the high-density lipoprotein (HDL)-associated sphingosine-1-phosphate (S1P) and coronary in-stent restenosis. Clin. Chim. Acta 2015, 446, 248–252. [Google Scholar] [CrossRef]
- Tong, X.; Peng, H.; Liu, D.; Ji, L.; Niu, C.; Ren, J.; Pan, B.; Hu, J.; Zheng, L.; Huang, Y. High-density lipoprotein of patients with Type 2 Diabetes Mellitus upregulates cyclooxgenase-2 expression and prostacyclin I-2 release in endothelial cells: Relationship with HDL-associated sphingosine-1-phosphate. Cardiovasc. Diabetol. 2013, 12, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Gaudio, I.; Sreckovic, I.; Zardoya-Laguardia, P.; Bernhart, E.; Christoffersen, C.; Frank, S.; Marsche, G.; Illanes, S.E.; Wadsack, C. Circulating cord blood HDL-S1P complex preserves the integrity of the feto-placental vasculature. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2020, 1865, 158632. [Google Scholar] [CrossRef] [PubMed]
- Donati, C.; Bruni, P. Sphingosine 1-phosphate regulates cytoskeleton dynamics: Implications in its biological response. Biochim. Biophys. Acta (BBA) Biomembr. 2006, 1758, 2037–2048. [Google Scholar] [CrossRef] [Green Version]
- Mehta, D.; Konstantoulaki, M.; Ahmmed, G.U.; Malik, A.B. Sphingosine 1-Phosphate-induced Mobilization of Intracellular Ca2+ Mediates Rac Activation and Adherens Junction Assembly in Endothelial Cells. J. Biol. Chem. 2005, 280, 17320–17328. [Google Scholar] [CrossRef] [Green Version]
- Wilkerson, B.A.; Grass, G.D.; Wing, S.B.; Argraves, W.S.; Argraves, K.M. Sphingosine 1-Phosphate (S1P) Carrier-dependent Regulation of Endothelial Barrier. J. Biol. Chem. 2012, 287, 44645–44653. [Google Scholar] [CrossRef] [Green Version]
- Del Gaudio, I.; Hendrix, S.; Christoffersen, C.; Wadsack, C. Neonatal HDL Counteracts Placental Vascular Inflammation via S1P–S1PR1 Axis. Int. J. Mol. Sci. 2020, 21, 789. [Google Scholar] [CrossRef] [Green Version]
- Brasier, A.R.; Recinos, A., III; Eledrisi, M.S. Vascular Inflammation and the Renin-Angiotensin System. Arter. Thromb. Vasc. Biol. 2002, 22, 1257–1266. [Google Scholar] [CrossRef] [Green Version]
- McCall, M.R.; La Belle, M.; Forte, T.M.; Krauss, R.M.; Takanami, Y.; Tribble, D.L. Dissociable and nondissociable forms of platelet-activating factor acetylhydrolase in human plasma LDL: Implications for LDL oxidative susceptibility. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 1999, 1437, 23–36. [Google Scholar] [CrossRef]
- Yost, C.C.; Weyrich, A.S.; Zimmerman, G.A. The platelet activating factor (PAF) signaling cascade in systemic inflammatory responses. Biochim. 2010, 92, 692–697. [Google Scholar] [CrossRef] [Green Version]
- Satoh, F.; Imaizumi, T.-A.; Kawamura, Y.; Yoshida, H.; Takamatsu, S.; Takamatsu, M. Increased activity of the platelet-activating factor acetylhydrolase in plasma low density lipoprotein from patients with essential hypertension. Prostaglandins 1989, 37, 673–682. [Google Scholar] [CrossRef]
- Tsimihodimos, V.; Karabina, S.-A.P.; Tambaki, A.P.; Bairaktari, E.; Miltiadous, G.; Goudevenos, J.A.; Cariolou, M.A.; Chapman, M.J.; Tselepis, A.D.; Elisaf, M. Altered distribution of platelet-activating factor-acetylhydrolase activity between LDL and HDL as a function of the severity of hypercholesterolemia. J. Lipid Res. 2002, 43, 256–263. [Google Scholar] [CrossRef]
- Kolodgie, F.D.; Burke, A.P.; Skorija, K.S.; Ladich, E.; Kutys, R.; Makuria, A.T.; Virmani, R. Lipoprotein-Associated Phospholipase A 2 Protein Expression in the Natural Progression of Human Coronary Atherosclerosis. Arter. Thromb. Vasc. Biol. 2006, 26, 2523–2529. [Google Scholar] [CrossRef] [Green Version]
- Serban, M.; Tanaseanu, C.; Kosaka, T.; Vidulescu, C.; Stoian, I.; Marta, D.S.; Tanaseanu, S.; Moldoveanu, E. Significance of platelet-activating factor acetylhydrolase in patients with non-insulin-dependent (type 2) diabetes mellitus. J. Cell. Mol. Med. 2002, 6, 643–647. [Google Scholar] [CrossRef] [Green Version]
- Schliefsteiner, C.; Hirschmugl, B.; Kopp, S.; Curcic, S.; Bernhart, E.M.; Marsche, G.; Lang, U.; Desoye, G.; Wadsack, C. Maternal Gestational Diabetes Mellitus increases placental and foetal lipoprotein-associated Phospholipase A2 which might exert protective functions against oxidative stress. Sci. Rep. 2017, 7, 12628. [Google Scholar] [CrossRef] [Green Version]
- Stadler, J.T.; Marsche, G. Obesity-Related Changes in High-Density Lipoprotein Metabolism and Function. Int. J. Mol. Sci. 2020, 21, 8985. [Google Scholar] [CrossRef]
- Rashid, S.; Genest, J. Effect of Obesity on High-density Lipoprotein Metabolism. Obesity 2007, 15, 2875–2888. [Google Scholar] [CrossRef]
- Stadler, J.; Lackner, S.; Mörkl, S.; Trakaki, A.; Scharnagl, H.; Borenich, A.; Wonisch, W.; Mangge, H.; Zelzer, S.; Meier-Allard, N.; et al. Obesity Affects HDL Metabolism, Composition and Subclass Distribution. Biomedicines 2021, 9, 242. [Google Scholar] [CrossRef]
- Farbstein, D.; Levy, A.P. HDL dysfunction in diabetes: Causes and possible treatments. Expert Rev. Cardiovasc. Ther. 2012, 10, 353–361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srivastava, R.A.K. Dysfunctional HDL in diabetes mellitus and its role in the pathogenesis of cardiovascular disease. Mol. Cell. Biochem. 2017, 440, 167–187. [Google Scholar] [CrossRef]
- Ganjali, S.; Dallinga-Thie, G.M.; Simental-Mendía, L.E.; Banach, M.; Pirro, M.; Sahebkar, A. HDL functionality in type 1 diabetes. Atherosclerosis 2017, 267, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Kosmas, C.E.; Martinez, I.; Sourlas, A.; Bouza, K.V.; Campos, F.N.; Torres, V.; Montan, P.D.; Guzman, E. High-density lipoprotein (HDL) functionality and its relevance to atherosclerotic cardiovascular disease. Drugs Context 2018, 7, 1–9. [Google Scholar] [CrossRef]
- Rader, D.J.; Hovingh, G.K. HDL and cardiovascular disease. Lancet 2014, 384, 618–625. [Google Scholar] [CrossRef]
- Holzer, M.; Schilcher, G.; Curcic, S.; Trieb, M.; Ljubojevic, S.; Stojakovic, T.; Scharnagl, H.; Kopecky, C.M.; Rosenkranz, A.R.; Heinemann, A.; et al. Dialysis Modalities and HDL Composition and Function. J. Am. Soc. Nephrol. 2015, 26, 2267–2276. [Google Scholar] [CrossRef] [Green Version]
- Holzer, M.; Birner-Gruenberger, R.; Stojakovic, T.; El-Gamal, D.; Binder, V.; Wadsack, C.; Heinemann, A.; Marsche, G. Uremia Alters HDL Composition and Function. J. Am. Soc. Nephrol. 2011, 22, 1631–1641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marsche, G.; Heine, G.H.; Stadler, J.T.; Holzer, M. Current Understanding of the Relationship of HDL Composition, Structure and Function to Their Cardioprotective Properties in Chronic Kidney Disease. Biomolecules 2020, 10, 1348. [Google Scholar] [CrossRef] [PubMed]
- Trieb, M.; Horvath, A.; Birner-Gruenberger, R.; Spindelboeck, W.; Stadlbauer, V.; Taschler, U.; Curcic, S.; Stauber, R.E.; Holzer, M.; Pasterk, L.; et al. Liver disease alters high-density lipoprotein composition, metabolism and function. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2016, 1861, 630–638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fadaei, R.; Poustchi, H.; Meshkani, R.; Moradi, N.; Golmohammadi, T.; Merat, S. Impaired HDL cholesterol efflux capacity in patients with non-alcoholic fatty liver disease is associated with subclinical atherosclerosis. Sci. Rep. 2018, 8, 11691. [Google Scholar] [CrossRef] [PubMed]
- Young, B.C.; Levine, R.J.; Karumanchi, S.A. Pathogenesis of Preeclampsia. Annu. Rev. Pathol. Mech. Dis. 2010, 5, 173–192. [Google Scholar] [CrossRef] [PubMed]
- Gathiram, P.; Moodley, J. Pre-eclampsia: Its pathogenesis and pathophysiolgy. Cardiovasc. J. Afr. 2016, 27, 71–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ACOG Committee on Obstetric Practice. Practice bulletin #33: Diagnosis and management of preeclampsia and eclampsia. Obstet. Gynecol. 2002, 99, 159–167. [Google Scholar] [CrossRef]
- Trogstad, L.; Magnus, P.; Stoltenberg, C. Pre-eclampsia: Risk factors and causal models. Best Pr. Res. Clin. Obstet. Gynaecol. 2011, 25, 329–342. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.M.; Garovic, V.D. Mechanisms and Management of Hypertension in Pregnant Women. Curr. Hypertens. Rep. 2011, 13, 338–346. [Google Scholar] [CrossRef]
- Duley, L. The Global Impact of Pre-eclampsia and Eclampsia. Semin. Perinatol. 2009, 33, 130–137. [Google Scholar] [CrossRef]
- Rugolo, L.M.S.D.S.; Bentlin, M.R.; Trindade, C.E.P. Preeclampsia: Effect on the Fetus and Newborn. NeoReviews 2011, 12, e198–e206. [Google Scholar] [CrossRef] [Green Version]
- Benschop, L.; Duvekot, J.J.; Lennep, J.E.R.V. Future risk of cardiovascular disease risk factors and events in women after a hypertensive disorder of pregnancy. Heart 2019, 105, 1273–1278. [Google Scholar] [CrossRef]
- Herrera-Garcia, G.; Contag, S. Maternal Preeclampsia and Risk for Cardiovascular Disease in Offspring. Curr. Hypertens. Rep. 2014, 16, 1–10. [Google Scholar] [CrossRef]
- Goldenberg, R.L.; Culhane, J.F.; Iams, J.D.; Romero, R. Epidemiology and causes of preterm birth. Lancet 2008, 371, 75–84. [Google Scholar] [CrossRef]
- Saarelainen, H.; Laitinen, T.; Raitakari, O.T.; Juonala, M.; Heiskanen, N.; Lyyra-Laitinen, T.; Viikari, J.S.; Vanninen, E.; Heinonen, S. Pregnancy-Related Hyperlipidemia and Endothelial Function in Healthy Women. Circ. J. 2006, 70, 768–772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stewart, F.M.; Freeman, D.J.; Ramsay, J.E.; Greer, I.A.; Caslake, M.; Ferrell, W.R. Longitudinal Assessment of Maternal Endothelial Function and Markers of Inflammation and Placental Function throughout Pregnancy in Lean and Obese Mothers. J. Clin. Endocrinol. Metab. 2007, 92, 969–975. [Google Scholar] [CrossRef] [Green Version]
- Cockell, A.P.; Poston, L. Flow-mediated vasodilatation is enhanced in normal pregnancy but reduced in preeclampsia. Hypertension 1997, 30, 247–251. [Google Scholar] [CrossRef]
- Spracklen, C.N.; Smith, C.J.; Saftlas, A.F.; Robinson, J.G.; Ryckman, K.K. Maternal Hyperlipidemia and the Risk of Preeclampsia: A Meta-Analysis. Am. J. Epidemiology 2014, 180, 346–358. [Google Scholar] [CrossRef] [Green Version]
- Brown, M.C.; Best, K.E.; Pearce, M.S.; Waugh, J.; Robson, S.C.; Bell, R. Cardiovascular disease risk in women with pre-eclampsia: Systematic review and meta-analysis. Eur. J. Epidemiol. 2013, 28, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Scantlebury, D.C.; Hayes, S.N. How Does Preeclampsia Predispose to Future Cardiovascular Disease? Curr. Hypertens. Rep. 2014, 16, 1–7. [Google Scholar] [CrossRef]
- Einbinder, Y.; Biron-Shental, T.; Agassi-Zaitler, M.; Tzadikevitch-Geffen, K.; Vaya, J.; Khatib, S.; Ohana, M.; Benchetrit, S.; Zitman-Gal, T. High-density lipoproteins (HDL) composition and function in preeclampsia. Arch. Gynecol. Obstet. 2018, 298, 405–413. [Google Scholar] [CrossRef]
- León-Reyes, G.; Maida-Claros, R.F.; Urrutia-Medina, A.X.; Jorge-Galarza, E.; Guzmán-Grenfell, A.M.; Fuentes-García, S.; Medina-Navarro, R.; Moreno-Eutimio, M.A.; Muñoz-Sánchez, J.L.; Hicks, J.J.; et al. Oxidative profiles of LDL and HDL isolated from women with preeclampsia. Lipids Heal. Dis. 2017, 16, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patil, S.B.; Kodliwadmath, M.V. Lipid peroxidation and antioxidant activity in complicated pregnancies. Clin. Exp. Obstet. Gynecol. 2009, 36, 110–112. [Google Scholar] [PubMed]
- Kumru, S.; Aydin, S.; Gursu, M.; Ozcan, Z. Changes of serum paraoxonase (an HDL-cholesterol-associated lipophilic antioxidant) and arylesterase activities in severe preeclamptic women. Eur. J. Obstet. Gynecol. Reprod. Biol. 2004, 114, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Demir, B.; Demir, S.; Atamer, Y.; Guven, S.; Atamer, A.; Kocyigit, Y.; Hekimoglu, A.; Toprak, G. Serum Levels of Lipids, Lipoproteins and Paraoxonase Activity in Pre-Eclampsia. J. Int. Med Res. 2011, 39, 1427–1431. [Google Scholar] [CrossRef] [Green Version]
- Uzun, H.; Benian, A.; Madazlı, R.; Topçuoğlu, M.; Aydın, S.; Albayrak, M. Circulating Oxidized Low-Density Lipoprotein and Paraoxonase Activity in Preeclampsia. Gynecol. Obstet. Investig. 2005, 60, 195–200. [Google Scholar] [CrossRef]
- León-Reyes, G.; Sosa, S.E.Y.; Medina-Navarro, R.; Guzmán-Grenfell, A.M.; Medina-Urrutia, A.X.; Fuentes-García, S.; Hicks, G.J.J.; Torres-Ramos, Y.D. Oxidative modifications of foetal LDL-c and HDL-c lipoproteins in preeclampsia. Lipids Heal. Dis. 2018, 17, 110. [Google Scholar] [CrossRef] [Green Version]
- Picot, M.; Croyal, M.; Duparc, T.; Combes, G.; Vayssière, C.; Perret, B.; Hamdi, S.; Martinez, L.; Genoux, A. Preeclampsia is associated with changes in the composition and dysfunction of high-density lipoproteins. Arch. Cardiovasc. Dis. Suppl. 2019, 11, e354. [Google Scholar] [CrossRef]
- Mistry, H.D.; Kurlak, L.O.; Mansour, Y.T.; Zurkinden, L.; Mohaupt, M.G.; Escher, G. Increased maternal and fetal cholesterol efflux capacity and placental CYP27A1 expression in preeclampsia. J. Lipid Res. 2017, 58, 1186–1195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, Y.; Burlison, S.; Wang, Y. PAF Levels and PAF–AH Activities in Placentas from Normal and Preeclamptic Pregnancies. Placenta 2006, 27, 744–749. [Google Scholar] [CrossRef] [PubMed]
- Maki, N.; Magness, R.R.; Miyaura, S.; Gant, N.F.; Johnston, J.M. Platelet-activating factor-acetylhydrolase activity in normotensive and hypertensive pregnancies. Am. J. Obstet. Gynecol. 1993, 168, 50–54. [Google Scholar] [CrossRef]
- Fan, P.; Liu, X.-H.; He, G.-L.; Zhang, S.; Zhang, J.-X.; Bai, H. Maternal and fetal plasma platelet-activating factor acetylhydrolase activity and distribution in pre-eclampsia. Pediatr. Res. 2012, 72, 426–431. [Google Scholar] [CrossRef] [Green Version]
- Jenum, A.K.; Mørkrid, K.; Sletner, L.; Vangen, S.; Vange, S.; Torper, J.L.; Nakstad, B.; Voldner, N.; Rognerud-Jensen, O.H.; Berntsen, S.; et al. Impact of Ethnicity on Gestational Diabetes Identified with the WHO and the Modified International Association of Diabetes and Pregnancy Study Groups Criteria: A Population-Based Cohort Study. Eur J Endocrinol 2012, 166, 317–324. [Google Scholar] [CrossRef]
- Ferrara, A. Increasing Prevalence of Gestational Diabetes Mellitus: A public health perspective. Diabetes Care 2007, 30, S141–S146. [Google Scholar] [CrossRef] [Green Version]
- Bellamy, L.; Casas, J.-P.; Hingorani, A.D.; Williams, D. Type 2 diabetes mellitus after gestational diabetes: A systematic review and meta-analysis. Lancet 2009, 373, 1773–1779. [Google Scholar] [CrossRef]
- Vrachnis, N.; Augoulea, A.; Iliodromiti, Z.; Lambrinoudaki, I.; Sifakis, S.; Creatsas, G. Previous Gestational Diabetes Mellitus and Markers of Cardiovascular Risk. Int. J. Endocrinol. 2012, 2012, 1–6. [Google Scholar] [CrossRef]
- Davis, C.L.; Gutt, M.; Llabre, M.M.; Marks, J.B.; O’Sullivan, M.J.; Potter, J.E.; Landel, J.L.; Kumar, M.; Schneiderman, N.; Gellman, M.; et al. History of Gestational Diabetes, Insulin Resistance and Coronary Risk. J. Diabetes Complicat. 1999, 13, 216–223. [Google Scholar] [CrossRef]
- Jovanovic, L. Gestational Diabetes Mellitus. JAMA 2001, 286, 2516–2518. [Google Scholar] [CrossRef]
- Yessoufou, A.; Moutairou, K. Maternal Diabetes in Pregnancy: Early and Long-Term Outcomes on the Offspring and the Concept of “Metabolic Memory”. Exp. Diabetes Res. 2011, 2011, 218598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adiels, M.; Olofsson, S.-O.; Taskinen, M.-R.; Borén, J. Diabetic dyslipidaemia. Curr. Opin. Lipidol. 2006, 17, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Dallinga-Thie, G.M.; Dullaart, R.P.; Van Tol, A. Concerted actions of cholesteryl ester transfer protein and phospholipid transfer protein in type 2 diabetes: Effects of apolipoproteins. Curr. Opin. Lipidol. 2007, 18, 251–257. [Google Scholar] [CrossRef]
- Van Deursen, D.; Jansen, H.; Verhoeven, A.J.M. Glucose increases hepatic lipase expression in HepG2 liver cells through upregulation of upstream stimulatory factors 1 and 2. Diabetologia 2008, 51, 2078–2087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baynes, C.; Henderson, A.; Anyaoku, V.; Richmond, W.; Hughes, C.L.; Johnston, D.G.; Elkeles, R.S. The Role of Insulin Insensitivity and Hepatic Lipase in the Dyslipidaemia of Type 2 Diabetes. Diabet. Med. 1991, 8, 560–566. [Google Scholar] [CrossRef]
- Lewis, G.F.; Murdoch, S.; Uffelman, K.; Naples, M.; Szeto, L.; Albers, A.; Adeli, K.; Brunzell, J.D. Hepatic lipase mRNA, protein, and plasma enzyme activity is increased in the insulin-resistant, fructose-fed Syrian golden hamster and is partially normalized by the insulin sensitizer rosiglitazone. Diabetes 2004, 53, 2893–2900. [Google Scholar] [CrossRef] [Green Version]
- Drew, B.G.; Rye, K.-A.; Duffy, S.J.; Barter, P.; Kingwell, B.A. The emerging role of HDL in glucose metabolism. Nat. Rev. Endocrinol. 2012, 8, 237–245. [Google Scholar] [CrossRef]
- Drew, B.G.; Duffy, S.J.; Formosa, M.F.; Natoli, A.K.; Henstridge, D.C.; Penfold, S.A.; Thomas, W.G.; Mukhamedova, N.; De Courten, B.; Forbes, J.M.; et al. High-Density Lipoprotein Modulates Glucose Metabolism in Patients With Type 2 Diabetes Mellitus. Circulation 2009, 119, 2103–2111. [Google Scholar] [CrossRef] [Green Version]
- Han, R.; Lai, R.; Ding, Q.; Wang, Z.; Luo, X.; Zhang, Y.; Cui, G.; He, J.; Liu, W.; Chen, Y. Apolipoprotein A-I stimulates AMP-activated protein kinase and improves glucose metabolism. Diabetologia 2007, 50, 1960–1968. [Google Scholar] [CrossRef] [Green Version]
- Brunham, L.R.; Kruit, J.K.; Pape, T.D.; Timmins, J.M.; Reuwer, A.Q.; Vasanji, Z.; Marsh, B.J.; Rodrigues, B.; Johnson, J.D.; Parks, J.S.; et al. β-cell ABCA1 influences insulin secretion, glucose homeostasis and response to thiazolidinedione treatment. Nat. Med. 2007, 13, 340–347. [Google Scholar] [CrossRef]
- Fryirs, M.A.; Barter, P.J.; Appavoo, M.; Tuch, B.E.; Tabet, F.; Heather, A.K.; Rye, K.-A. Effects of High-Density Lipoproteins on Pancreatic β-Cell Insulin Secretion. Arter. Thromb. Vasc. Biol. 2010, 30, 1642–1648. [Google Scholar] [CrossRef] [Green Version]
- Fernandez, M.-L. Small HDL Particles Are Associated with Gestational Diabetes, Providing a Potential Early Identification Tool. J. Nutr. 2019, 150, 8–9. [Google Scholar] [CrossRef]
- Mokkala, K.; Vahlberg, T.; Pellonperä, O.; Houttu, N.; Koivuniemi, E.; Laitinen, K. Distinct Metabolic Profile in Early Pregnancy of Overweight and Obese Women Developing Gestational Diabetes. J. Nutr. 2019, 150, 31–37. [Google Scholar] [CrossRef]
- Sreckovic, I.; Birner-Gruenberger, R.; Besenboeck, C.; Miljkovic, M.; Stojakovic, T.; Scharnagl, H.; Marsche, G.; Lang, U.; Kotur-Stevuljevic, J.; Jelic-Ivanovic, Z.; et al. Gestational diabetes mellitus modulates neonatal high-density lipoprotein composition and its functional heterogeneity. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2014, 1841, 1619–1627. [Google Scholar] [CrossRef]
- Mordwinkin, N.M.; Ouzounian, J.G.; Yedigarova, L.; Montoro, M.N.; Louie, S.G.; Rodgers, K.E. Alteration of endothelial function markers in women with gestational diabetes and their fetuses. J. Matern. Neonatal Med. 2012, 26, 507–512. [Google Scholar] [CrossRef] [Green Version]
- Pasternak, Y.; Biron-Shental, T.; Ohana, M.; Einbinder, Y.; Arbib, N.; Benchetrit, S.; Zitman-Gal, T. Gestational Diabetes Type 2: Variation in High-Density Lipoproteins Composition and Function. Int. J. Mol. Sci. 2020, 21, 6281. [Google Scholar] [CrossRef]
- Brites, F.; Martin, M.; Guillas, I.; Kontush, A. Antioxidative activity of high-density lipoprotein (HDL): Mechanistic insights into potential clinical benefit. BBA Clin. 2017, 8, 66–77. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stadler, J.T.; Wadsack, C.; Marsche, G. Fetal High-Density Lipoproteins: Current Knowledge on Particle Metabolism, Composition and Function in Health and Disease. Biomedicines 2021, 9, 349. https://doi.org/10.3390/biomedicines9040349
Stadler JT, Wadsack C, Marsche G. Fetal High-Density Lipoproteins: Current Knowledge on Particle Metabolism, Composition and Function in Health and Disease. Biomedicines. 2021; 9(4):349. https://doi.org/10.3390/biomedicines9040349
Chicago/Turabian StyleStadler, Julia T., Christian Wadsack, and Gunther Marsche. 2021. "Fetal High-Density Lipoproteins: Current Knowledge on Particle Metabolism, Composition and Function in Health and Disease" Biomedicines 9, no. 4: 349. https://doi.org/10.3390/biomedicines9040349
APA StyleStadler, J. T., Wadsack, C., & Marsche, G. (2021). Fetal High-Density Lipoproteins: Current Knowledge on Particle Metabolism, Composition and Function in Health and Disease. Biomedicines, 9(4), 349. https://doi.org/10.3390/biomedicines9040349







