NHS-Functionalized THP Derivative for Efficient Synthesis of Kit-Based Precursors for 68Ga Labeled PET Probes
Abstract
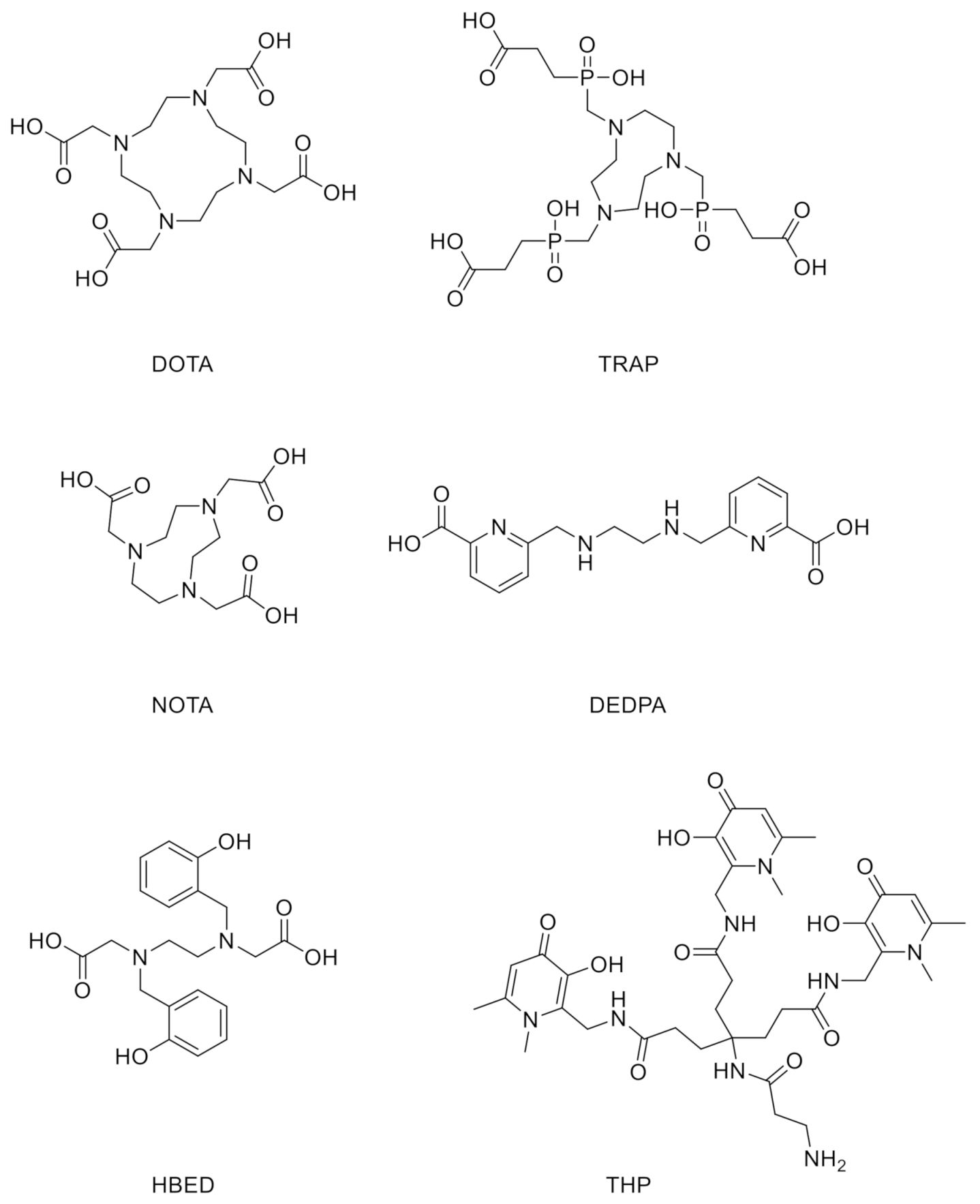
:1. Introduction
2. Experimental Section
2.1. Synthesis
2.1.1. Materials for the Synthesis
2.1.2. Synthesis of THP (5)
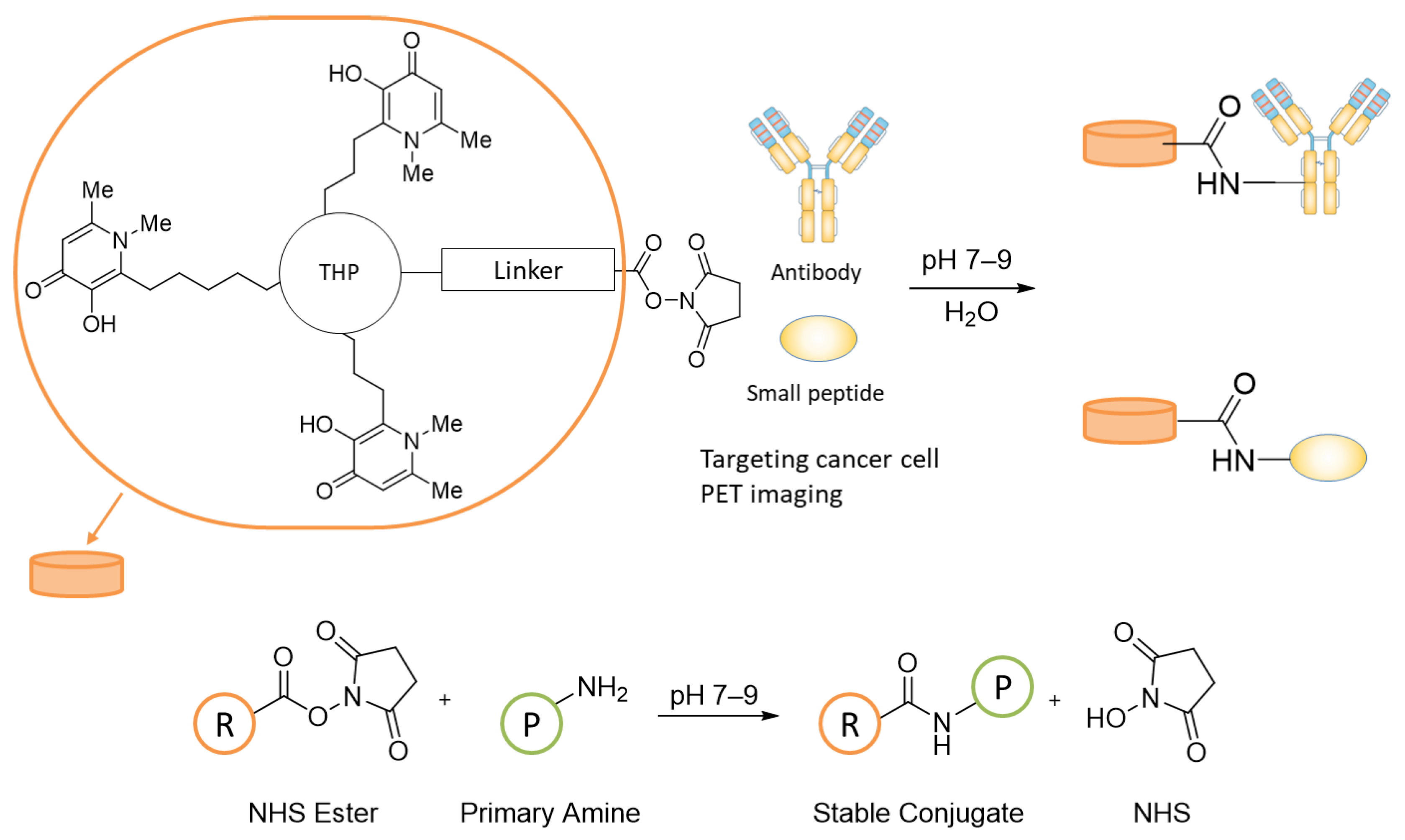
2.1.3. General Procedure for the Synthesis of bis-NHS-Succinnic/Glutaric Acid Ester (3,4)
2.1.4. General Procedure for the Synthesis of THP-Succinic/Glutaric Acid Ester (6,7)
2.1.5. Synthesis of GLP-1-Peptide-THP (8)
2.1.6. Synthesis of MY-1502-6-51-THP
2.2. Radiochemistry
2.2.1. Materials for 68Ga Radiolabeling of GLP-1-Peptide-THP
2.2.2. Sample Preparation for 68Ga Radiolabeling of GLP-1-Peptide-THP
2.2.3. GLP-1-Peptide-THP Radiolabeling
2.2.4. Materials for 68Ga Radiolabeling of the sdAb-THP Conjugate MY-1502-6-51-THP
2.2.5. MY-1502-6-51-THP Radiolabeling
3. Results and Discussion
3.1. Design and Synthesis of NHS–THP
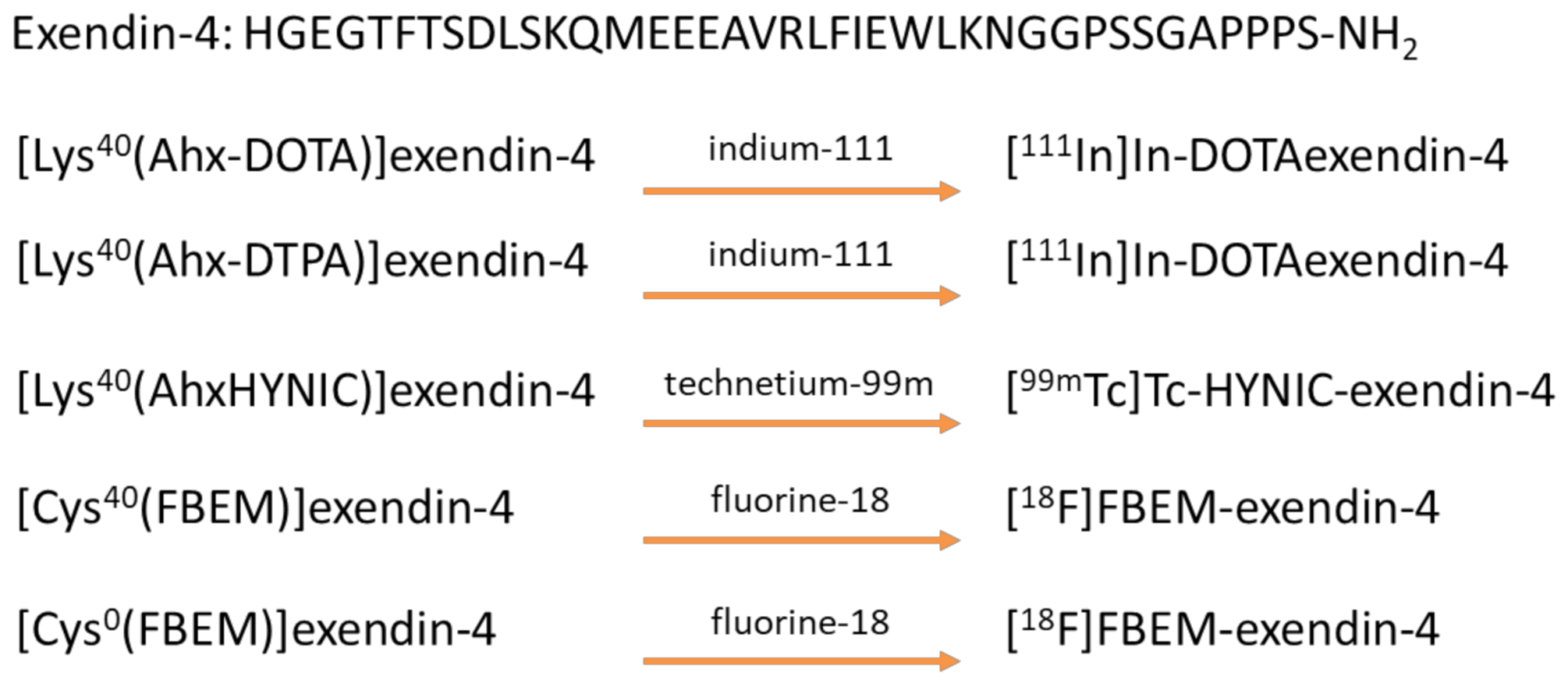
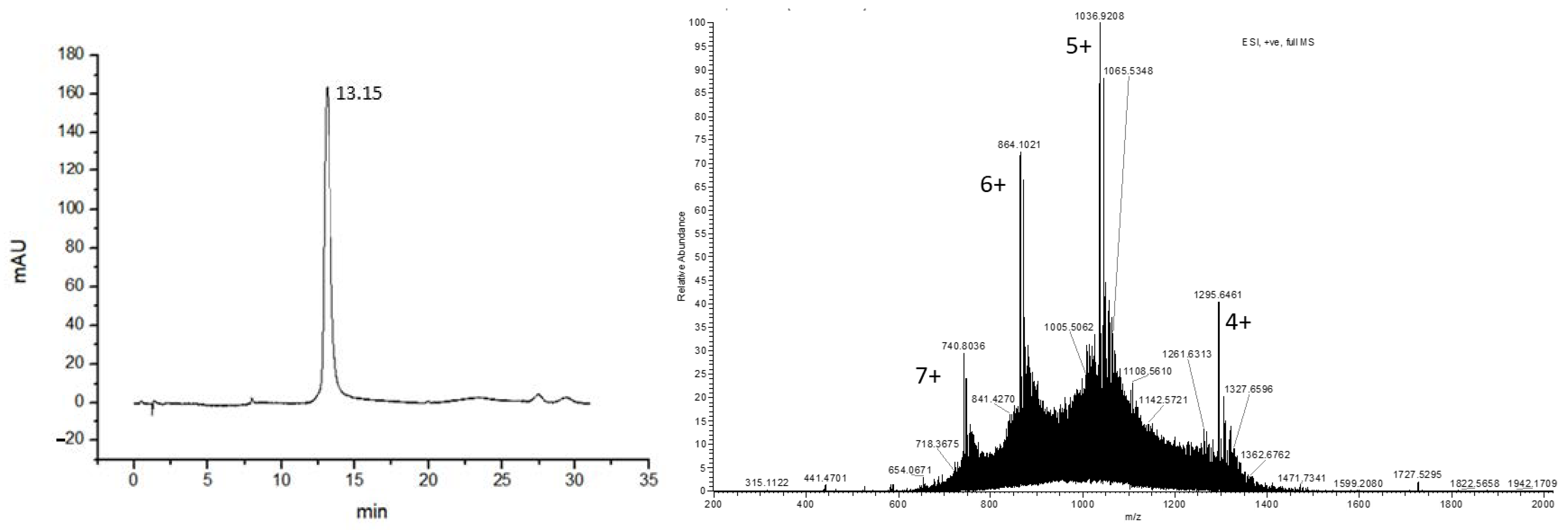
3.2. Design and Synthesis of GLP-1-Peptide–THP
3.3. 68Ga Radiolabeling of GLP-1-Peptide-THP: Characterization by Radio HPLC and ITLC
3.4. Synthesis of MY-1502-6-51-THP
3.5. 68Ga Radiolabeling of MY-1502-6-51-THP
4. Conclusions and Perspective
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Blower, P.J. A nuclear chocolate box: The periodic table of nuclear medicine. Dalton Trans. 2015, 44, 4819–4844. [Google Scholar] [CrossRef]
- Dansereau, R.N.; Line, B.R. Clinical production of pharmaceutical grade technetium-99m dextran 70 for lymphoscintigraphy. J. Nucl. Med. 1996, 37, 631. [Google Scholar]
- Chomet, M.; Provost, C.; Vega, V.; Prignon, A.; Talbot, J.; Nataf, V. Transfer of a radiolabelling process with gallium-68 from a manual method to a remote controlled method for clinical applications: The example of NODAGA-RGDfK. Eur. J. Nucl. Med. Mol. I 2016, 43, S471–S472. [Google Scholar]
- Banerjee, S.R.; Pomper, M.G. Clinical applications of Gallium-68. Appl. Radiat. Isot. 2013, 76, 2–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebenhan, T.; Vorster, M.; Marjanovic-Painter, B.; Wagener, J.; Suthiram, J.; Modiselle, M.; Mokaleng, B.; Zeevaart, J.R.; Sathekge, M. Development of a Single Vial Kit Solution for Radiolabeling of Ga-68-DKFZ-PSMA-11 and Its Performance in Prostate Cancer Patients. Molecules 2015, 20, 14860–14878. [Google Scholar] [CrossRef]
- Deutsch, E. Clinical Pet—Its Time Has Come. J. Nucl. Med. 1993, 34, 1132–1133. [Google Scholar]
- Wagner, H.N. Clinical Pet—Its Time Has Come. J. Nucl. Med. 1991, 32, 561–564. [Google Scholar]
- Benesova, M.; Schafer, M.; Bauder-Wust, U.; Afshar-Oromieh, A.; Kratochwil, C.; Mier, W.; Haberkorn, U.; Kopka, K.; Eder, M. Preclinical Evaluation of a Tailor-Made DOTA-Conjugated PSMA Inhibitor with Optimized Linker Moiety for Imaging and Endoradiotherapy of Prostate Cancer. J. Nucl. Med. 2015, 56, 914–920. [Google Scholar] [CrossRef] [Green Version]
- Notni, J.; Pohle, K.; Wester, H.J. Comparative gallium-68 labeling of TRAP-, NOTA-, and DOTA-peptides: Practical consequences for the future of gallium-68-PET. EJNMMI Res. 2012, 2. [Google Scholar] [CrossRef] [Green Version]
- Farkas, E.; Nagel, J.; Waldron, B.P.; Parker, D.; Toth, I.; Brucher, E.; Rosch, F.; Baranyai, Z. Equilibrium, Kinetic and Structural Properties of Gallium(III) and Some Divalent Metal Complexes Formed with the New DATA(m) and DATA(5m) Ligands. Chem. Eur. J. 2017, 23, 10358–10371. [Google Scholar] [CrossRef] [Green Version]
- Ramogida, C.F.; Schindler, D.; Schneider, C.; Tan, Y.L.K.; Huh, S.; Ferreira, C.L.; Adam, M.J.; Orvig, C. Synthesis and characterization of lipophilic cationic Ga(III) complexes based on the H(2)CHXdedpa and H(2)dedpa ligands and their Ga-67/68 radiolabeling studies. RSC Adv. 2016, 6, 103763–103773. [Google Scholar] [CrossRef]
- Ramogida, C.F.; Pan, J.H.; Ferreira, C.L.; Patrick, B.O.; Rebullar, K.; Yapp, D.T.T.; Lin, K.S.; Adam, M.J.; Orvig, C. Nitroimidazole-Containing H(2)dedpa and H(2)CHXdedpa Derivatives as Potential PET Imaging Agents of Hypoxia with Ga-68. Inorg. Chem. 2015, 54, 4953–4965. [Google Scholar] [CrossRef]
- Seemann, J.; Waldron, B.P.; Roesch, F.; Parker, D. Approaching ‘Kit-Type’ Labelling with Ga-68: The DATA Chelators. ChemMedChem 2015, 10, 1019–1026. [Google Scholar] [CrossRef] [Green Version]
- Cilibrizzi, A.; Abbate, V.; Chen, Y.-L.; Ma, Y.; Zhou, T.; Hider, R.C. Hydroxypyridinone Journey into Metal Chelation. Chem. Rev. 2018, 118, 7657–7701. [Google Scholar] [CrossRef]
- Berry, D.J.; Ma, Y.; Ballinger, J.R.; Tavare, R.; Koers, A.; Sunassee, K.; Zhou, T.; Nawaz, S.; Mullen, G.E.; Hider, R.C.; et al. Efficient bifunctional gallium-68 chelators for positron emission tomography: Tris(hydroxypyridinone) ligands. Chem. Commun. 2011, 47, 7068–7070. [Google Scholar] [CrossRef]
- Ma, M.T.; Cullinane, C.; Imberti, C.; Baguna Torres, J.; Terry, S.Y.; Roselt, P.; Hicks, R.J.; Blower, P.J. New Tris(hydroxypyridinone) Bifunctional Chelators Containing Isothiocyanate Groups Provide a Versatile Platform for Rapid One-Step Labeling and PET Imaging with 68Ga3+. Bioconjug. Chem. 2016, 27, 309–318. [Google Scholar] [CrossRef] [Green Version]
- Young, J.D.; Abbate, V.; Imberti, C.; Meszaros, L.K.; Ma, M.T.; Terry, S.Y.A.; Hider, R.C.; Mullen, G.E.; Blower, P.J. (68)Ga-THP-PSMA: A PET Imaging Agent for Prostate Cancer Offering Rapid, Room-Temperature, 1-Step Kit-Based Radiolabeling. J. Nucl. Med. 2017, 58, 1270–1277. [Google Scholar] [CrossRef] [Green Version]
- Keeling, G.P.; Sherin, B.; Kim, J.; San Juan, B.; Grus, T.; Eykyn, T.R.; Rösch, F.; Smith, G.E.; Blower, P.J.; Terry, S.Y.A.; et al. [68Ga]Ga-THP-Pam: A Bisphosphonate PET Tracer with Facile Radiolabeling and Broad Calcium Mineral Affinity. Bioconjug. Chem. 2020. [Google Scholar] [CrossRef]
- Hofman, M.S.; Eu, P.; Jackson, P.; Hong, E.; Binns, D.; Iravani, A.; Murphy, D.; Mitchell, C.; Siva, S.; Hicks, R.J.; et al. Cold Kit for Prostate-Specific Membrane Antigen (PSMA) PET Imaging: Phase 1 Study of (68)Ga-Tris(Hydroxypyridinone)-PSMA PET/CT in Patients with Prostate Cancer. J. Nucl. Med. 2018, 59, 625–631. [Google Scholar] [CrossRef] [Green Version]
- Eisenhut, M.; Lehmann, W.D.; Becker, W.; Behr, T.; Elser, H.; Strittmatter, W.; Steinstrasser, A.; Baum, R.P.; Valerius, T.; Repp, R.; et al. Bifunctional NHS-BAT ester for antibody conjugation and stable technetium-99m labeling: Conjugation chemistry, immunoreactivity and kit formulation. J. Nucl. Med. 1996, 37, 362–370. [Google Scholar]
- Cusnir, R.; Imberti, C.; Hider, R.C.; Blower, P.J.; Ma, M.T. Hydroxypyridinone Chelators: From Iron Scavenging to Radiopharmaceuticals for PET Imaging with Gallium-68. Int. J. Mol. Sci. 2017, 18, 116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nawaz, S.; Mullen, G.E.D.; Sunassee, K.; Bordoloi, J.; Blower, P.J.; Ballinger, J.R. Simple, mild, one-step labelling of proteins with gallium-68 using a tris(hydroxypyridinone) bifunctional chelator: A (68)Ga-THP-scFv targeting the prostate-specific membrane antigen. EJNMMI Res. 2017, 7, 86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aroda, V.R. A review of GLP-1 receptor agonists: Evolution and advancement, through the lens of randomised controlled trials. Diabetes Obes. Metab. 2018, 20 (Suppl. 1), 22–33. [Google Scholar] [CrossRef] [Green Version]
- Gallwitz, B. GLP-1 agonists and dipeptidyl-peptidase IV inhibitors. Handb. Exp. Pharmacol. 2011, 53–74. [Google Scholar] [CrossRef]
- Lugari, R.; Dei Cas, A.; Ugolotti, D.; Barilli, A.L.; Camellini, C.; Ganzerla, G.C.; Luciani, A.; Salerni, B.; Mittenperger, F.; Nodari, S.; et al. Glucagon-like peptide 1 (GLP-1) secretion and plasma dipeptidyl peptidase IV (DPP-IV) activity in morbidly obese patients undergoing biliopancreatic diversion. Horm. Metab. Res. 2004, 36, 111–115. [Google Scholar] [CrossRef]
- Gallwitz, B.; Ropeter, T.; Morys-Wortmann, C.; Mentlein, R.; Siegel, E.G.; Schmidt, W.E. GLP-1-analogues resistant to degradation by dipeptidyl-peptidase IV in vitro. Regul. Pept. 2000, 86, 103–111. [Google Scholar] [CrossRef]
- Nomiyama, T.; Kawanami, T.; Irie, S.; Hamaguchi, Y.; Terawaki, Y.; Murase, K.; Tsutsumi, Y.; Nagaishi, R.; Tanabe, M.; Morinaga, H.; et al. Exendin-4, a GLP-1 receptor agonist, attenuates prostate cancer growth. Diabetes 2014, 63, 3891–3905. [Google Scholar] [CrossRef] [Green Version]
- Ryder, R.E. The potential risks of pancreatitis and pancreatic cancer with GLP-1-based therapies are far outweighed by the proven and potential (cardiovascular) benefits. Diabet. Med. 2013, 30, 1148–1155. [Google Scholar] [CrossRef]
- Mehrabi, A.; Fischer, L.; Hafezi, M.; Dirlewanger, A.; Grenacher, L.; Diener, M.K.; Fonouni, H.; Golriz, M.; Garoussi, C.; Fard, N.; et al. A systematic review of localization, surgical treatment options, and outcome of insulinoma. Pancreas 2014, 43, 675–686. [Google Scholar] [CrossRef]
- Trujillo, J.M.; Nuffer, W.; Ellis, S.L. GLP-1 receptor agonists: A review of head-to-head clinical studies. Ther. Adv. Endocrinol. Metab. 2015, 6, 19–28. [Google Scholar] [CrossRef] [Green Version]
- Wong, M.C.; Wang, H.H.; Kwan, M.W.; Zhang, D.D.; Liu, K.Q.; Chan, S.W.; Fan, C.K.; Fong, B.C.; Li, S.T.; Griffiths, S.M. Comparative effectiveness of dipeptidyl peptidase-4 (DPP-4) inhibitors and human glucagon-like peptide-1 (GLP-1) analogue as add-on therapies to sulphonylurea among diabetes patients in the Asia-Pacific region: A systematic review. PLoS ONE 2014, 9, e90963. [Google Scholar] [CrossRef]
- Derosa, G.; Maffioli, P. GLP-1 agonists exenatide and liraglutide: A review about their safety and efficacy. Curr. Clin. Pharmacol. 2012, 7, 214–228. [Google Scholar] [CrossRef]
- Wild, D.; Wicki, A.; Mansi, R.; Behe, M.; Keil, B.; Bernhardt, P.; Christofori, G.; Ell, P.J.; Macke, H.R. Exendin-4-based radiopharmaceuticals for glucagonlike peptide-1 receptor PET/CT and SPECT/CT. J. Nucl. Med. 2010, 51, 1059–1067. [Google Scholar] [CrossRef] [Green Version]
- Brom, M.; Joosten, L.; Oyen, W.J.; Gotthardt, M.; Boerman, O.C. Radiolabelled GLP-1 analogues for in vivo targeting of insulinomas. Contrast Media Mol. Imaging 2012, 7, 160–166. [Google Scholar] [CrossRef] [Green Version]
- Pach, D.; Sowa-Staszczak, A.; Jabrocka-Hybel, A.; Stefanska, A.; Tomaszuk, M.; Mikolajczak, R.; Janota, B.; Trofimiuk-Muldner, M.; Przybylik-Mazurek, E.; Hubalewska-Dydejczyk, A. Glucagon-Like Peptide-1 Receptor Imaging with [Lys (40) (Ahx-HYNIC- (99 m) Tc/EDDA)NH 2 ]-Exendin-4 for the Diagnosis of Recurrence or Dissemination of Medullary Thyroid Cancer: A Preliminary Report. Int. J. Endocrinol. 2013, 2013, 384508. [Google Scholar] [CrossRef] [Green Version]
- Sowa-Staszczak, A.; Pach, D.; Mikolajczak, R.; Macke, H.; Jabrocka-Hybel, A.; Stefanska, A.; Tomaszuk, M.; Janota, B.; Gilis-Januszewska, A.; Malecki, M.; et al. Glucagon-like peptide-1 receptor imaging with [Lys40(Ahx-HYNIC- 99mTc/EDDA)NH2]-exendin-4 for the detection of insulinoma. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 524–531. [Google Scholar] [CrossRef] [Green Version]
- Kiesewetter, D.O.; Gao, H.; Ma, Y.; Niu, G.; Quan, Q.; Guo, N.; Chen, X. 18F-radiolabeled analogs of exendin-4 for PET imaging of GLP-1 in insulinoma. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 463–473. [Google Scholar] [CrossRef] [Green Version]
- Hubalewska-Dydejczyk, A.; Sowa-Staszczak, A.; Tomaszuk, M.; Stefanska, A. GLP-1 and exendin-4 for imaging endocrine pancreas. A review. Labelled glucagon-like peptide-1 analogues: Past, present and future. Q. J. Nucl. Med. Mol. Imaging 2015, 59, 152–160. [Google Scholar]
- Muyldermans, S. Nanobodies: Natural single-domain antibodies. Annu. Rev. Biochem. 2013, 82, 775–797. [Google Scholar] [CrossRef] [Green Version]
- Xing, Y.; Chand, G.; Liu, C.; Cook, G.J.R.; O’Doherty, J.; Zhao, L.; Wong, N.C.L.; Meszaros, L.K.; Ting, H.H.; Zhao, J. Early Phase I Study of a (99 m)Tc-Labeled Anti-Programmed Death Ligand-1 (PD-L1) Single-Domain Antibody in SPECT/CT Assessment of PD-L1 Expression in Non-Small Cell Lung Cancer. J. Nucl. Med. 2019, 60, 1213–1220. [Google Scholar] [CrossRef] [Green Version]
- Keyaerts, M.; Xavier, C.; Heemskerk, J.; Devoogdt, N.; Everaert, H.; Ackaert, C.; Vanhoeij, M.; Duhoux, F.P.; Gevaert, T.; Simon, P.; et al. Phase I Study of 68Ga-HER2-Nanobody for PET/CT Assessment of HER2 Expression in Breast Carcinoma. J. Nucl. Med. 2016, 57, 27–33. [Google Scholar] [CrossRef] [Green Version]
- Huyvetter, M.; De Vos, J.; Caveliers, V.; Vaneycken, I.; Heemskerk, J.; Duhoux, F.P.; Fontaine, C.; Vanhoeij, M.; Windhorst, A.D.; van der Aa, F.; et al. Phase I trial of 131I-GMIB-Anti-HER2-VHH1, a new promising candidate for HER2-targeted radionuclide therapy in breast cancer patients. J. Nucl. Med. 2020. [Google Scholar] [CrossRef]
- Castanar, L.; Poggetto, G.D.; Colbourne, A.A.; Morris, G.A.; Nilsson, M. The GNAT: A new tool for processing NMR data. Magn. Reason. Chem. 2018. [Google Scholar] [CrossRef] [Green Version]
- Zhou, T.; Neubert, H.; Liu, D.Y.; Liu, Z.D.; Ma, Y.M.; Kong, X.L.; Luo, W.; Mark, S.; Hider, R.C. Iron binding dendrimers: A novel approach for the treatment of haemochromatosis. J. Med. Chem. 2006, 49, 4171–4182. [Google Scholar] [CrossRef]
- Van Dongen, S.F.; Maiuri, P.; Marie, E.; Tribet, C.; Piel, M. Triggering cell adhesion, migration or shape change with a dynamic surface coating. Adv. Mater. 2013, 25, 1687–1691. [Google Scholar] [CrossRef]
- Flakus, H.T.; Hachula, B.; Holaj-Krzak, J.T. Long-distance inter-hydrogen bond coupling effects in the polarized IR spectra of succinic acid crystals. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 142, 126–134. [Google Scholar] [CrossRef]
- Velikyan, I.; Sundin, A.; Eriksson, B.; Lundqvist, H.; Sorensen, J.; Bergstrom, M.; Langstrom, B. In vivo binding of [68Ga]-DOTATOC to somatostatin receptors in neuroendocrine tumours—Impact of peptide mass. Nucl. Med. Biol. 2010, 37, 265–275. [Google Scholar] [CrossRef]
- Velikyan, I.; Rosenstrom, U.; Estrada, S.; Ljungvall, I.; Haggstrom, J.; Eriksson, O.; Antoni, G. Synthesis and preclinical evaluation of 68Ga-labeled collagelin analogs for imaging and quantification of fibrosis. Nucl. Med. Biol. 2014, 41, 728–736. [Google Scholar] [CrossRef]
- Selvaraju, R.K.; Velikyan, I.; Asplund, V.; Johansson, L.; Wu, Z.; Todorov, I.; Shively, J.; Kandeel, F.; Eriksson, B.; Korsgren, O.; et al. Pre-clinical evaluation of [(68)Ga]Ga-DO3A-VS-Cys(40)-Exendin-4 for imaging of insulinoma. Nucl. Med. Biol. 2014, 41, 471–476. [Google Scholar] [CrossRef]


| Time/Min | Solvent% | |
|---|---|---|
| A | B | |
| 0 | 95 | 5 |
| 5 | 95 | 5 |
| 20 | 5 | 95 |
| 25 | 5 | 95 |
| 25.1 | 95 | 5 |
| 30 | 95 | 5 |
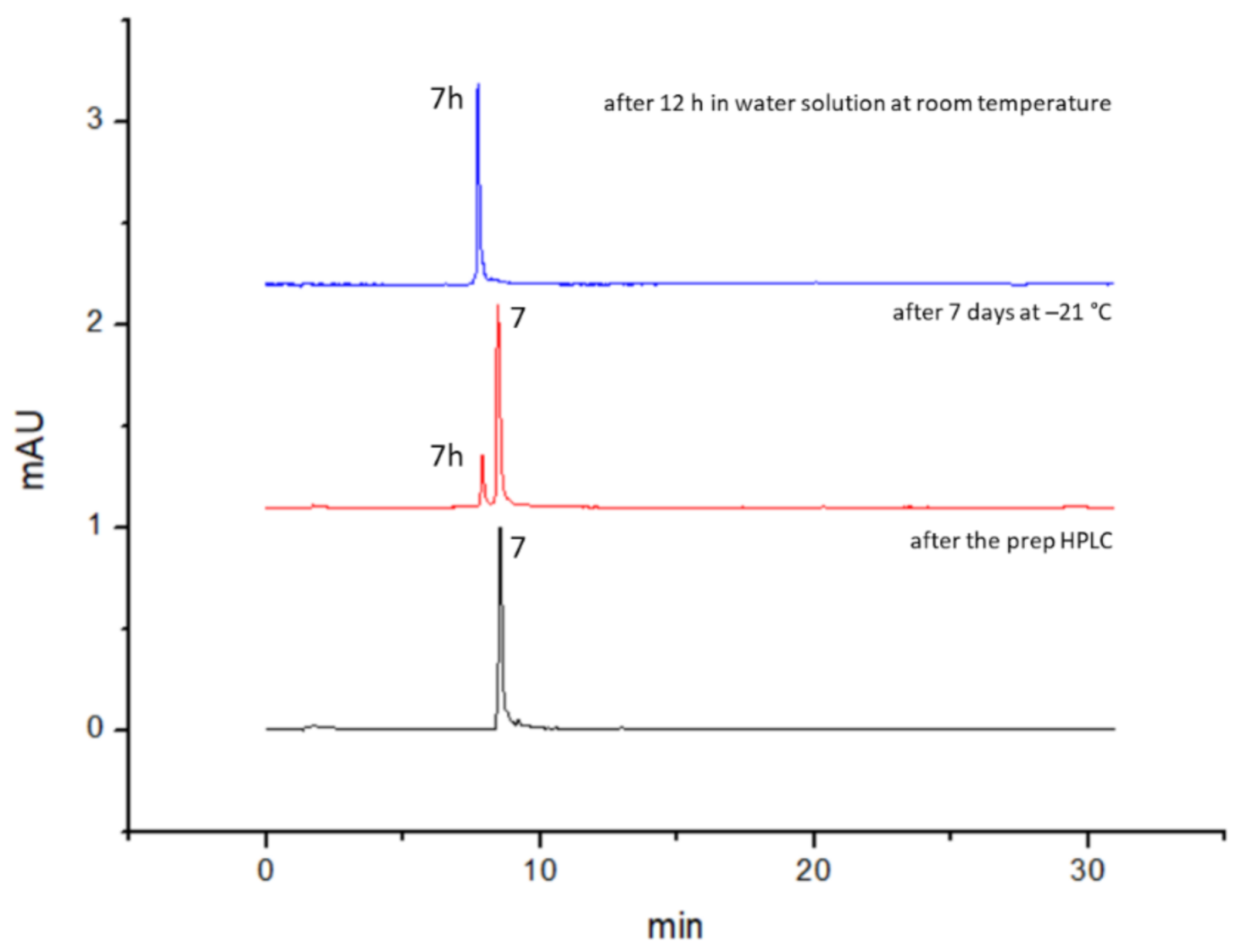
| After the Prep HPLC | After 12 h in Water Solution at RT | After 7 Days at −21 °C, Dried Compound | |||
|---|---|---|---|---|---|
| NHS ester (7) | acid der. (7h) | NHS ester (7) | acid der. (7h) | NHS ester (7) | acid der. (7h) |
| 100 | 0 | 0 | 100 | 89 | 11 |
| 1h DMSO/DMF | 1h PBS | After 30 days at −21 °C, dried compound | |||
| NHS ester (7) | acid der. (7h) | NHS ester (7) | acid der. (7h) | NHS ester (7) | acid der. (7h) |
| 97 | 3 | 91 | 9 | 88 | 12 |
| Acetate Method Rf | Citrate Method Rf | |
|---|---|---|
| 68Ga unbound | 0 | 0.8–1 |
| [68Ga]Ga-GLP-1-peptide-THP (9) | 0 | 0 |
| Reverse Phase Rt (min) | |
|---|---|
| 68Ga unbound | 1.9 |
| [68Ga]Ga-GLP-1-peptide-THP (9) | 13.7–13.9 |
| Radiochromatogram Rt (min) | UV Rt (min) | |
|---|---|---|
| 68Ga unbound | 10.7–10.8 | - |
| [68Ga]Ga-MY-1502-6-51-THP (11) | 6.7–6.8 | 6.2–6.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Floresta, G.; Keeling, G.P.; Memdouh, S.; Meszaros, L.K.; de Rosales, R.T.M.; Abbate, V. NHS-Functionalized THP Derivative for Efficient Synthesis of Kit-Based Precursors for 68Ga Labeled PET Probes. Biomedicines 2021, 9, 367. https://doi.org/10.3390/biomedicines9040367
Floresta G, Keeling GP, Memdouh S, Meszaros LK, de Rosales RTM, Abbate V. NHS-Functionalized THP Derivative for Efficient Synthesis of Kit-Based Precursors for 68Ga Labeled PET Probes. Biomedicines. 2021; 9(4):367. https://doi.org/10.3390/biomedicines9040367
Chicago/Turabian StyleFloresta, Giuseppe, George P. Keeling, Siham Memdouh, Levente K. Meszaros, Rafael T. M. de Rosales, and Vincenzo Abbate. 2021. "NHS-Functionalized THP Derivative for Efficient Synthesis of Kit-Based Precursors for 68Ga Labeled PET Probes" Biomedicines 9, no. 4: 367. https://doi.org/10.3390/biomedicines9040367
APA StyleFloresta, G., Keeling, G. P., Memdouh, S., Meszaros, L. K., de Rosales, R. T. M., & Abbate, V. (2021). NHS-Functionalized THP Derivative for Efficient Synthesis of Kit-Based Precursors for 68Ga Labeled PET Probes. Biomedicines, 9(4), 367. https://doi.org/10.3390/biomedicines9040367







