Early Dysfunction of Substantia Nigra Dopamine Neurons in the ParkinQ311X Mouse
Abstract
1. Introduction
2. Experimental Section
2.1. Animals
2.2. Mouse Genotyping
2.3. Dissection of Substantia Nigra
2.4. Stereological Cell Count in SNc
2.5. Immunofluorescence
2.6. Western Blotting
2.7. Quantification of Vacuoles in DA Neurons
2.8. In Vivo Single-Unit Extracellular Recording of SNc DA Neurons
2.9. Transmission Electron Microscopy (TEM)
2.10. Data Presentation and Statistical Analysis
3. Results
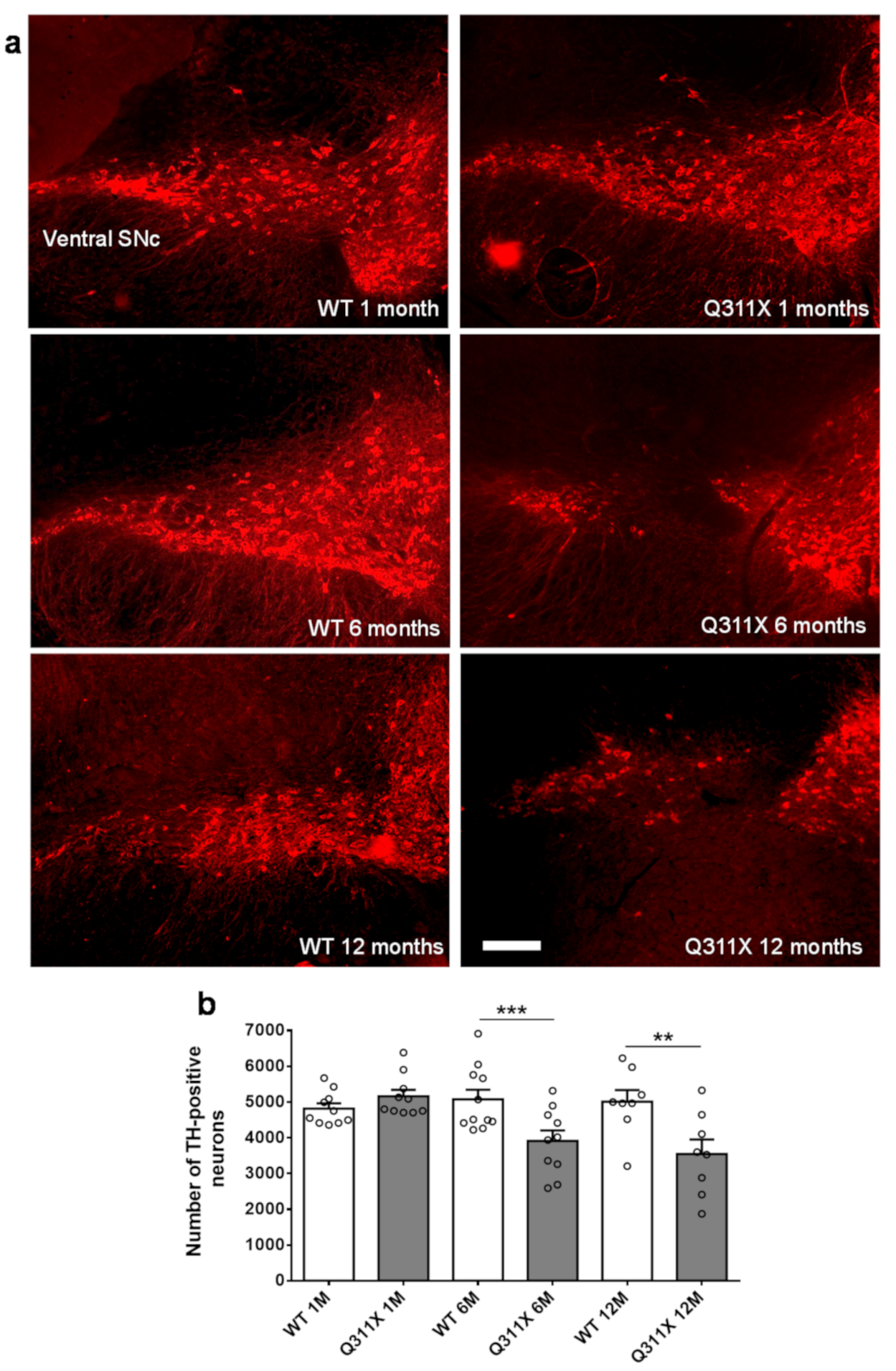
3.1. DA Neuron Loss in the SNc of ParkinQ311X Mice
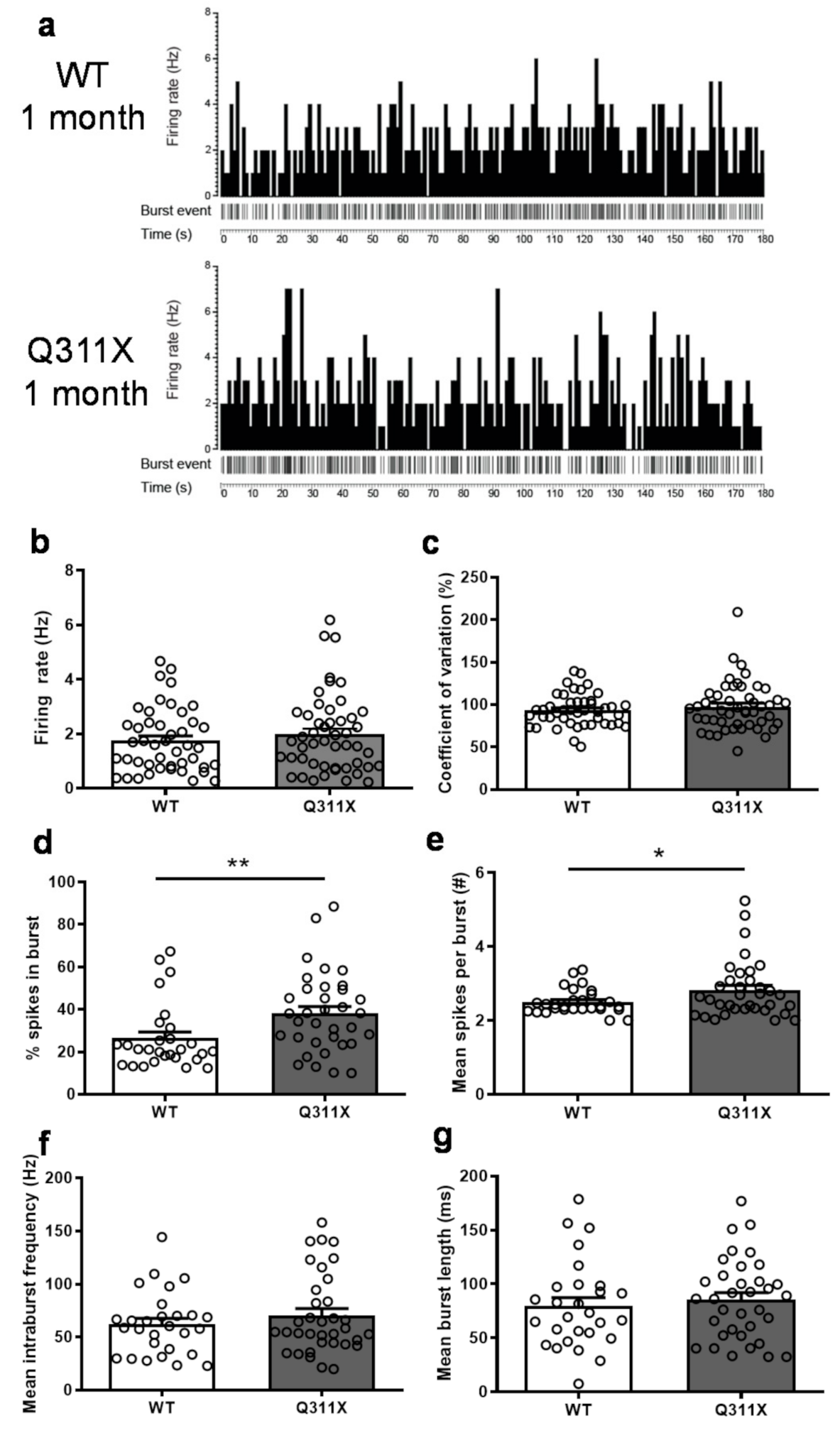
3.2. Altered Burst-Firing Activity of SNc DA Neurons in ParkinQ311X Mice
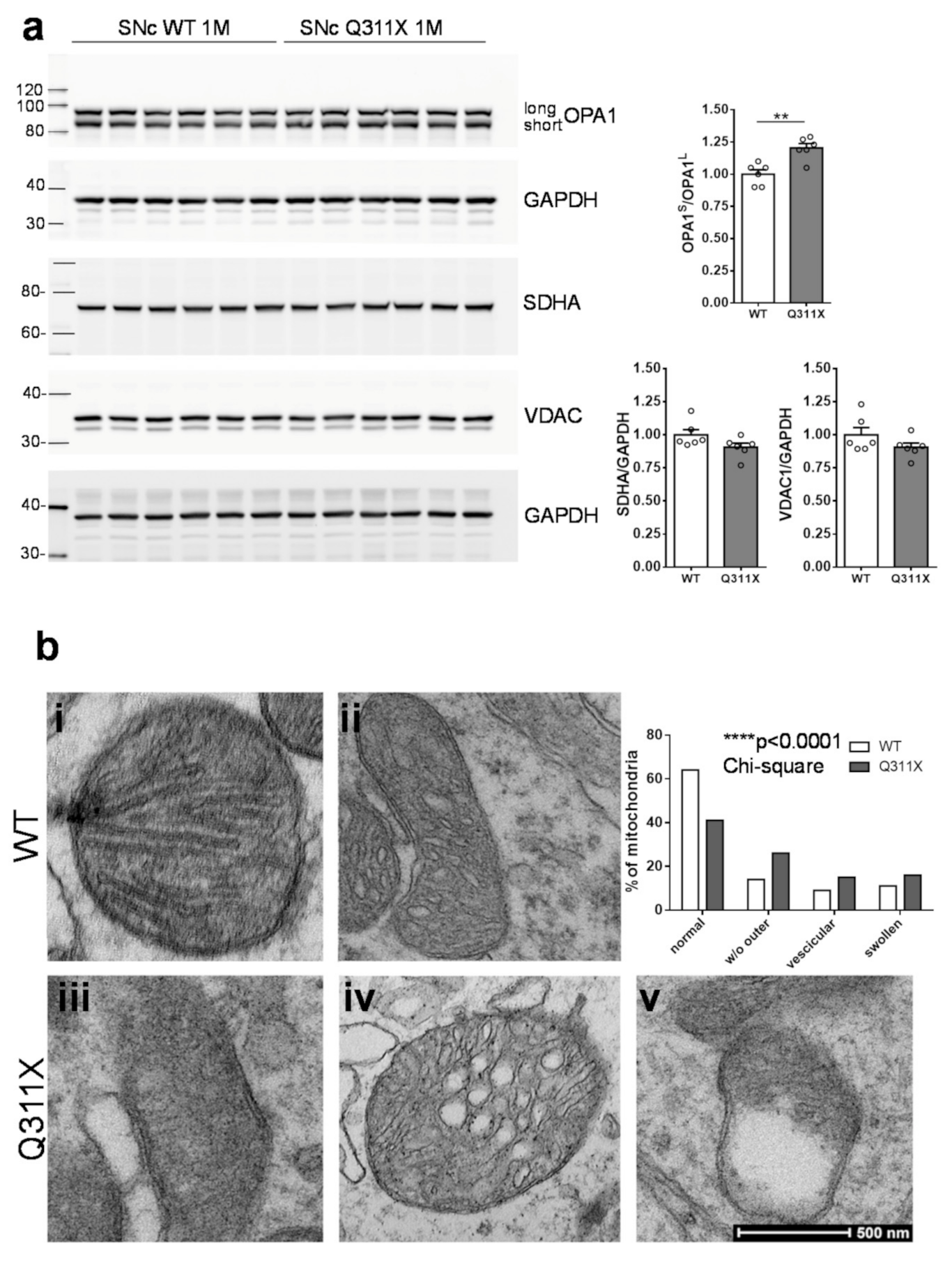
3.3. Mitochondrial Dysfunction in SNc DA Neurons of ParkinQ311X Mice
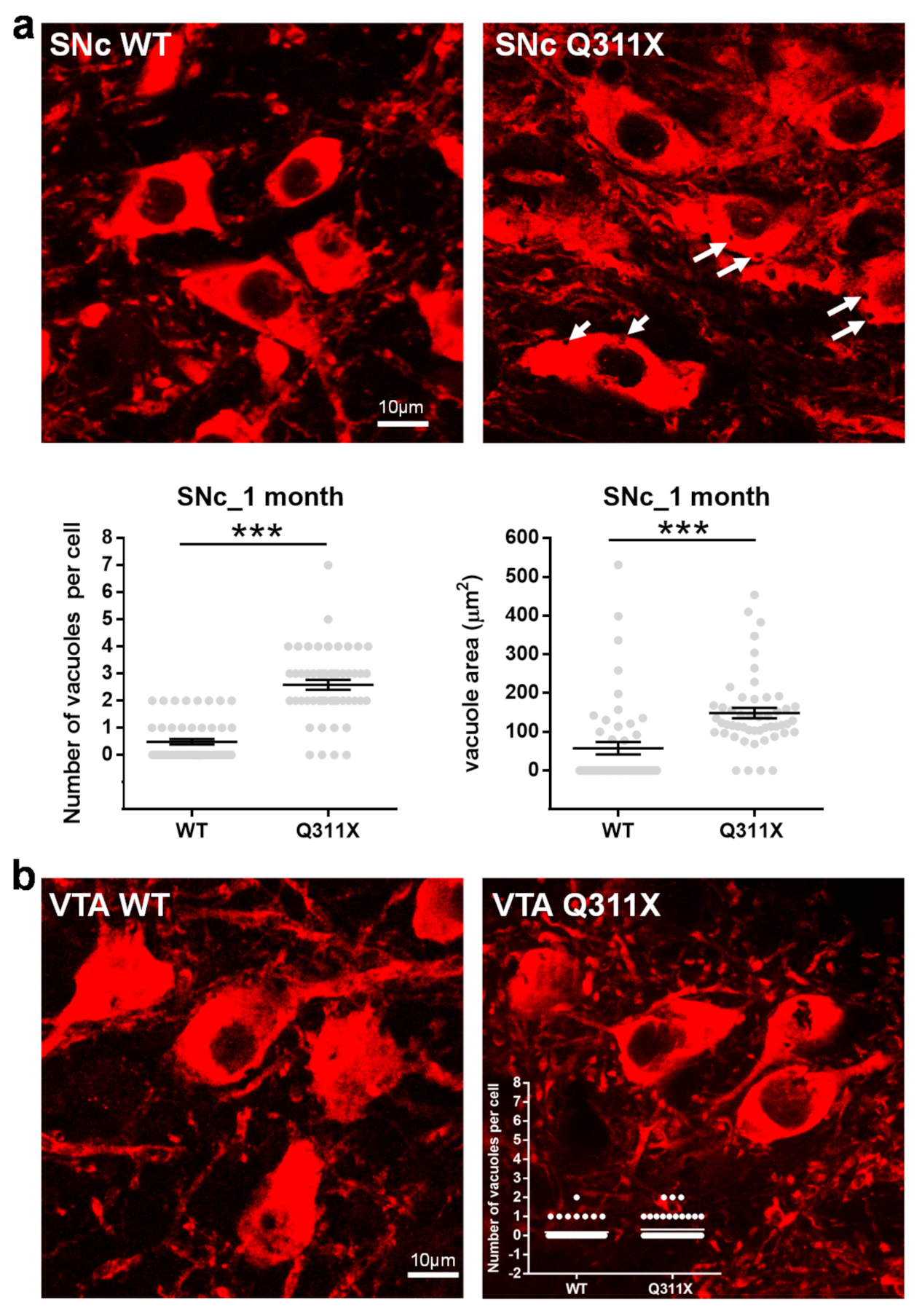
3.4. Cytoplasmic Vacuolization in SNc DA Neurons of ParkinQ311X Mice
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Mitsuyama, S.; Ohtsubo, M.; Minoshima, S.; Shimizu, N. The KM-parkin-DB: A Sub-setMutationViewDatabase Specialized forPARK2(PARKIN) Variants. Hum. Mutat. 2015, 36, E2430–E2440. [Google Scholar] [CrossRef]
- Goldberg, M.S.; Fleming, S.M.; Palacino, J.J.; Cepeda, C.; Lam, H.A.; Bhatnagar, A.; Meloni, E.G.; Wu, N.; Ackerson, L.C.; Klapstein, G.J.; et al. Parkin-deficient Mice Exhibit Nigrostriatal Deficits but Not Loss of Dopaminergic Neurons. J. Biol. Chem. 2003, 278, 43628–43635. [Google Scholar] [CrossRef] [PubMed]
- Perez, F.A.; Palmiter, R.D. Parkin-deficient mice are not a robust model of parkinsonism. Proc. Natl. Acad. Sci. 2005, 102, 2174–2179. [Google Scholar] [CrossRef] [PubMed]
- Von Coelln, R.; Thomas, B.; Savitt, J.M.; Lim, K.L.; Sasaki, M.; Hess, E.J.; Dawson, V.L.; Dawson, T.M. Loss of locus coeruleus neurons and reduced startle in parkin null mice. Proc. Natl. Acad. Sci. 2004, 101, 10744–10749. [Google Scholar] [CrossRef] [PubMed]
- Itier, J.-M.; Ibáñez, P.; Mena, M.A.; Abbas, N.; Cohen-Salmon, C.; Bohme, G.A.; Laville, M.; Pratt, J.; Corti, O.; Pradier, L.; et al. Parkin gene inactivation alters behaviour and dopamine neurotransmission in the mouse. Hum. Mol. Genet. 2003, 12, 2277–2291. [Google Scholar] [CrossRef]
- Sang, T.-K.; Chang, H.-Y.; Lawless, G.M.; Ratnaparkhi, A.; Mee, L.; Ackerson, L.C.; Maidment, N.T.; Krantz, D.E.; Jackson, G.R. A Drosophila Model of Mutant Human Parkin-Induced Toxicity Demonstrates Selective Loss of Dopaminergic Neurons and Dependence on Cellular Dopamine. J. Neurosci. 2007, 27, 981–992. [Google Scholar] [CrossRef]
- Lu, X.-H.; Fleming, S.M.; Meurers, B.; Ackerson, L.C.; Mortazavi, F.; Lo, V.; Hernandez, D.; Sulzer, D.; Jackson, G.R.; Maidment, N.T.; et al. Bacterial Artificial Chromosome Transgenic Mice Expressing a Truncated Mutant Parkin Exhibit Age-Dependent Hypokinetic Motor Deficits, Dopaminergic Neuron Degeneration, and Accumulation of Proteinase K-Resistant -Synuclein. J. Neurosci. 2009, 29, 1962–1976. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Lu, R.; Ouyang, X.; Ho, M.W.L.; Chia, W.; Yu, F.; Lim, K.-L. Drosophila Overexpressing Parkin R275W Mutant Exhibits Dopaminergic Neuron Degeneration and Mitochondrial Abnormalities. J. Neurosci. 2007, 27, 8563–8570. [Google Scholar] [CrossRef]
- Regoni, M.; Cattaneo, S.; Mercatelli, D.; Novello, S.; Passoni, A.; Bagnati, R.; Davoli, E.; Croci, L.; Consalez, G.G.; Albanese, F.; et al. Pharmacological antagonism of kainate receptor rescues dysfunction and loss of dopamine neurons in a mouse model of human parkin-induced toxicity. Cell Death Dis. 2020, 11, 1–11. [Google Scholar] [CrossRef]
- Chung, S.Y.; Kishinevsky, S.; Mazzulli, J.R.; Graziotto, J.; Mrejeru, A.; Mosharov, E.V.; Puspita, L.; Valiulahi, P.; Sulzer, D.; Milner, T.A.; et al. Parkin and PINK1 Patient iPSC-Derived Midbrain Dopamine Neurons Exhibit Mitochondrial Dysfunction and α-Synuclein Accumulation. Stem Cell Rep. 2016, 7, 664–677. [Google Scholar] [CrossRef]
- Kumar, M.; Acevedo-Cintrón, J.; Jhaldiyal, A.; Wang, H.; Andrabi, S.A.; Eacker, S.; Karuppagounder, S.S.; Brahmachari, S.; Chen, R.; Kim, H.; et al. Defects in Mitochondrial Biogenesis Drive Mitochondrial Alterations in PARKIN-Deficient Human Dopamine Neurons. Stem Cell Rep. 2020, 15, 629–645. [Google Scholar] [CrossRef] [PubMed]
- Ahfeldt, T.; Ordureau, A.; Bell, C.; Sarrafha, L.; Sun, C.; Piccinotti, S.; Grass, T.; Parfitt, G.M.; Paulo, J.A.; Yanagawa, F.; et al. Pathogenic Pathways in Early-Onset Autosomal Recessive Parkinson’s Disease Discovered Using Isogenic Human Dopaminergic Neurons. Stem Cell Rep. 2020, 14, 75–90. [Google Scholar] [CrossRef] [PubMed]
- Zhong, P.; Hu, Z.; Jiang, H.; Yan, Z.; Feng, J. Dopamine Induces Oscillatory Activities in Human Midbrain Neurons with Parkin Mutations. Cell Rep. 2017, 19, 1033–1044. [Google Scholar] [CrossRef]
- Hattori, N.; Matsuminea, H.; Asakawab, S.; Kitadaab, T.; Yoshinoa, H.; Elibol, B.; Brookes, A.J.; Yamamurae, Y.; Kobayashia, T.; Wanga, M.; et al. Point Mutations (Thr240Arg and Ala311Stop) in theParkinGene. Biochem. Biophys. Res. Commun. 1998, 249, 754–758. [Google Scholar] [CrossRef]
- Novello, S.; Arcuri, L.; Dovero, S.; Dutheil, N.; Shimshek, D.R.; Bezard, E.; Morari, M. G2019S LRRK2 mutation facilitates α-synuclein neuropathology in aged mice. Neurobiol. Dis. 2018, 120, 21–33. [Google Scholar] [CrossRef]
- Gould, T.J.; Burke, D.; Bewersdorf, J.; Booth, M.J. Adaptive optics enables 3D STED microscopy in aberrating specimens. Opt. Express 2012, 20, 20998–21009. [Google Scholar] [CrossRef]
- Arcuri, L.; Viaro, R.; Bido, S.; Longo, F.; Calcagno, M.; Fernagut, P.-O.; Zaveri, N.T.; Calò, G.; Bezard, E.; Morari, M. Genetic and pharmacological evidence that endogenous nociceptin/orphanin FQ contributes to dopamine cell loss in Parkinson’s disease. Neurobiol. Dis. 2016, 89, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Larsen, J.O.; Gundersen, H.J.; Nielsen, J. Global spatial sampling with isotropic virtual planes: Estimators of length density and total length in thick, arbitrarily orientated sections. J. Microsc. 1998, 191, 238–248. [Google Scholar] [CrossRef]
- Mazzocco, M.T.; Guarnieri, F.C.; Monzani, E.; Benfenati, F.; Valtorta, F.; Comai, S. Dysfunction of the serotonergic system in the brain of synapsin triple knockout mice is associated with behavioral abnormalities resembling synapsin-related human pathologies. Prog. Neuro-Psychopharmacology Biol. Psychiatry 2021, 105, 110135. [Google Scholar] [CrossRef]
- Grace, A.; Bunney, B. Intracellular and extracellular electrophysiology of nigral dopaminergic neurons—1. Identification and characterization. Neurosci. 1983, 10, 301–315. [Google Scholar] [CrossRef]
- Khan, N.L.; Graham, E.; Critchley, P.; Schrag, A.E.; Wood, N.W.; Lees, A.J.; Bhatia, K.P.; Quinn, N. Parkin disease: A phenotypic study of a large case series. Brain 2003, 126, 1279–1292. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.L.; Brooks, D.J.; Pavese, N.; Sweeney, M.G.; Wood, N.W.; Lees, A.J.; Piccini, P. Progression of nigrostriatal dysfunction in a parkin kindred: An [18F]dopa PET and clinical study. Brain 2002, 125, 2248–2256. [Google Scholar] [CrossRef] [PubMed]
- Poulopoulos, M.; Levy, O.A.; Alcalay, R.N. The neuropathology of genetic Parkinson’s disease. Mov. Disord. 2012, 27, 831–842. [Google Scholar] [CrossRef]
- Gantz, S.C.; Ford, C.P.; Morikawa, H.; Williams, J.T. The Evolving Understanding of Dopamine Neurons in the Substantia Nigra and Ventral Tegmental Area. Annu. Rev. Physiol. 2018, 80, 219–241. [Google Scholar] [CrossRef]
- Ge, P.; Dawson, V.L.; Dawson, T.M. PINK1 and Parkin mitochondrial quality control: A source of regional vulnerability in Parkinson’s disease. Mol. Neurodegener. 2020, 15, 1–18. [Google Scholar] [CrossRef]
- Buhlman, L.; Damiano, M.; Bertolin, G.; Ferrando-Miguel, R.; Lombès, A.; Brice, A.; Corti, O. Functional interplay between Parkin and Drp1 in mitochondrial fission and clearance. Biochim. et Biophys. Acta (BBA) - Bioenerg. 2014, 1843, 2012–2026. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.-L.; Ng, X.-H.; Grace, L.G.-Y.; Yao, T.-P. Mitochondrial Dynamics and Parkinson’s Disease: Focus on Parkin. Antioxidants Redox Signal. 2012, 16, 935–949. [Google Scholar] [CrossRef]
- MacVicar, T.; Langer, T. OPA1 processing in cell death and disease—The long and short of it. J. Cell Sci. 2016, 129, 2297–2306. [Google Scholar] [CrossRef]
- Frey, T.G.; Sun, M.G. Correlated light and electron microscopy illuminates the role of mitochondrial inner membrane remodeling during apoptosis. Biochim. et Biophys. Acta (BBA) - Bioenerg. 2008, 1777, 847–852. [Google Scholar] [CrossRef][Green Version]
- Shubin, A.V.; Demidyuk, I.V.; Komissarov, A.A.; Rafieva, L.M.; Kostrov, S.V. Cytoplasmic vacuolization in cell death and survival. Oncotarget 2016, 7, 55863–55889. [Google Scholar] [CrossRef]
- El-Brolosy, M.A.; Stainier, D.Y.R. Genetic compensation: A phenomenon in search of mechanisms. PLoS Genet. 2017, 13, e1006780. [Google Scholar] [CrossRef]
- Kreiner, G. Compensatory mechanisms in genetic models of neurodegeneration: Are the mice better than humans? Front. Cell. Neurosci. 2015, 9, 56. [Google Scholar] [CrossRef][Green Version]
- Dave, K.D.; De Silva, S.; Sheth, N.P.; Ramboz, S.; Beck, M.J.; Quang, C.; Switzer, R.C.; Ahmad, S.O.; Sunkin, S.M.; Walker, D.; et al. Phenotypic characterization of recessive gene knockout rat models of Parkinson’s disease. Neurobiol. Dis. 2014, 70, 190–203. [Google Scholar] [CrossRef] [PubMed]
- Van Rompuy, A.-S.; Lobbestael, E.; Van Der Perren, A.; Haute, C.V.D.; Baekelandt, V. Long-Term Overexpression of Human Wild-Type and T240R Mutant Parkin in Rat Substantia Nigra Induces Progressive Dopaminergic Neurodegeneration. J. Neuropathol. Exp. Neurol. 2014, 73, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Puschmann, A.; Fiesel, F.C.; Caulfield, T.R.; Hudec, R.; Ando, M.; Truban, D.; Hou, X.; Ogaki, K.; Heckman, M.G.; James, E.D.; et al. Heterozygous PINK1 p.G411S increases risk of Parkinson’s disease via a dominant-negative mechanism. Brain 2017, 140, 98–117. [Google Scholar] [CrossRef]
- Lubbe, S.J.; I Bustos, B.; Hu, J.; Krainc, D.; Joseph, T.; Hehir, J.; Tan, M.; Zhang, W.; Escott-Price, V.; Williams, N.M.; et al. Assessing the relationship between monoallelic PRKN mutations and Parkinson’s risk. Hum. Mol. Genet. 2021, 30, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.W. Structural abnormalities in neurones. J. Clin. Pathol. 1977, 11, 155–169. [Google Scholar] [CrossRef]
- Rogers-Cotrone, T.; Burgess, M.P.; Hancock, S.H.; Hinckley, J.; Lowe, K.; Ehrich, M.F.; Jortner, B.S. Vacuolation of Sensory Ganglion Neuron Cytoplasm in Rats with Long-term Exposure to Organophosphates. Toxicol. Pathol. 2010, 38, 554–559. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, Y.; Liu, Z.; Liu, C.; Zheng, J.; Qin, Q.; Huang, X. Autophagy Participates in Lysosomal Vacuolation-Mediated Cell Death in RGNNV-Infected Cells. Front. Microbiol. 2020, 11, 790. [Google Scholar] [CrossRef]
- Funakoshi, T.; Aki, T.; Tajiri, M.; Unuma, K.; Uemura, K. Necroptosis-like Neuronal Cell Death Caused by Cellular Cholesterol Accumulation. J. Biol. Chem. 2016, 291, 25050–25065. [Google Scholar] [CrossRef]
- Okamoto, K.; Hirai, S.; Iizuka, T.; Yanagisawa, T.; Watanabe, M. Reexamination of granulovacuolar degeneration. Acta Neuropathol. 1991, 82, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Cataldo, A.M.; Hamilton, D.J.; Barnett, J.L.; Paskevich, P.A.; Nixon, R.A. Properties of the endosomal-lysosomal system in the human central nervous system: Disturbances mark most neurons in populations at risk to degen-erate in Alzheimer’s disease. J. Neurosci. 1996, 16, 186–199. [Google Scholar] [CrossRef]
- Cubells, J.; Rayport, S.; Rajendran, G.; Sulzer, D. Methamphetamine neurotoxicity involves vacuolation of endocytic organelles and dopamine-dependent intracellular oxidative stress. J. Neurosci. 1994, 14, 2260–2271. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, R.A. Laminar degeneration of frontal and temporal cortex in Parkinson disease dementia. Neurol. Sci. 2017, 38, 667–671. [Google Scholar] [CrossRef]
- Anglade, P.; Vyas, S.; Javoy-Agid, F.; Herrero, M.T.; Michel, P.P.; Marquez, J.; Mouatt-Prigent, A.; Ruberg, M.; Hirsch, E.C.; Agid, Y. Apoptosis and autophagy in nigral neurons of patients with Parkinson’s disease. Histol. Histopathol. 1997, 12, 25–31. [Google Scholar]
- Sánchez-Danés, A.; Richaud-Patin, Y.; Carballo-Carbajal, I.; Jiménez-Delgado, S.; Caig, C.; Mora, S.; Di Guglielmo, C.; Ezquerra, M.; Patel, B.; Giralt, A.; et al. Disease-specific phenotypes in dopamine neurons from human iPS-based models of genetic and sporadic Parkinson’s disease. EMBO Mol. Med. 2012, 4, 380–395. [Google Scholar] [CrossRef]
- Fricker, M.; Tolkovsky, A.M.; Borutaite, V.; Coleman, M.; Brown, G.C. Neuronal Cell Death. Physiol. Rev. 2018, 98, 813–880. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.N.; Padman, B.S.; Lazarou, M. Deciphering the Molecular Signals of PINK1/Parkin Mitophagy. Trends Cell Biol. 2016, 26, 733–744. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, W.; Li, R.; Yang, H. Mitophagy in Parkinson’s Disease: From Pathogenesis to Treatment. Cells 2019, 8, 712. [Google Scholar] [CrossRef]
- Stichel, C.C.; Zhu, X.-R.; Bader, V.; Linnartz-Gerlach, B.; Schmidt, S.; Lübbert, H. Mono- and double-mutant mouse models of Parkinson’s disease display severe mitochondrial damage. Hum. Mol. Genet. 2007, 16, 2377–2393. [Google Scholar] [CrossRef] [PubMed]
- Palacino, J.J.; Sagi, D.; Goldberg, M.S.; Krauss, S.; Motz, C.; Wacker, M.; Klose, J.; Shen, J. Mitochondrial Dysfunction and Oxidative Damage in parkin-deficient Mice. J. Biol. Chem. 2004, 279, 18614–18622. [Google Scholar] [CrossRef] [PubMed]
- Maraschi, A.M.; Ciammola, A.; Folci, A.; Sassone, F.; Ronzitti, G.; Cappelletti, G.; Silani, V.; Sato, S.; Hattori, N.; Mazzanti, M.; et al. Parkin regulates kainate receptors by interacting with the GluK2 subunit. Nat. Commun. 2014, 5, 5182. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Regoni, M.; Zanetti, L.; Comai, S.; Mercatelli, D.; Novello, S.; Albanese, F.; Croci, L.; Consalez, G.G.; Ciammola, A.; Valtorta, F.; et al. Early Dysfunction of Substantia Nigra Dopamine Neurons in the ParkinQ311X Mouse. Biomedicines 2021, 9, 514. https://doi.org/10.3390/biomedicines9050514
Regoni M, Zanetti L, Comai S, Mercatelli D, Novello S, Albanese F, Croci L, Consalez GG, Ciammola A, Valtorta F, et al. Early Dysfunction of Substantia Nigra Dopamine Neurons in the ParkinQ311X Mouse. Biomedicines. 2021; 9(5):514. https://doi.org/10.3390/biomedicines9050514
Chicago/Turabian StyleRegoni, Maria, Letizia Zanetti, Stefano Comai, Daniela Mercatelli, Salvatore Novello, Federica Albanese, Laura Croci, Gian Giacomo Consalez, Andrea Ciammola, Flavia Valtorta, and et al. 2021. "Early Dysfunction of Substantia Nigra Dopamine Neurons in the ParkinQ311X Mouse" Biomedicines 9, no. 5: 514. https://doi.org/10.3390/biomedicines9050514
APA StyleRegoni, M., Zanetti, L., Comai, S., Mercatelli, D., Novello, S., Albanese, F., Croci, L., Consalez, G. G., Ciammola, A., Valtorta, F., Morari, M., & Sassone, J. (2021). Early Dysfunction of Substantia Nigra Dopamine Neurons in the ParkinQ311X Mouse. Biomedicines, 9(5), 514. https://doi.org/10.3390/biomedicines9050514










