Endocan and Circulating Progenitor Cells in Women with Systemic Sclerosis: Association with Inflammation and Pulmonary Hypertension
Abstract
:1. Introduction
2. Materials and Methods
2.1. Clinical Parameters and Biochemical Data
2.2. Laboratory Analyses
2.3. Statistical Analysis
3. Results
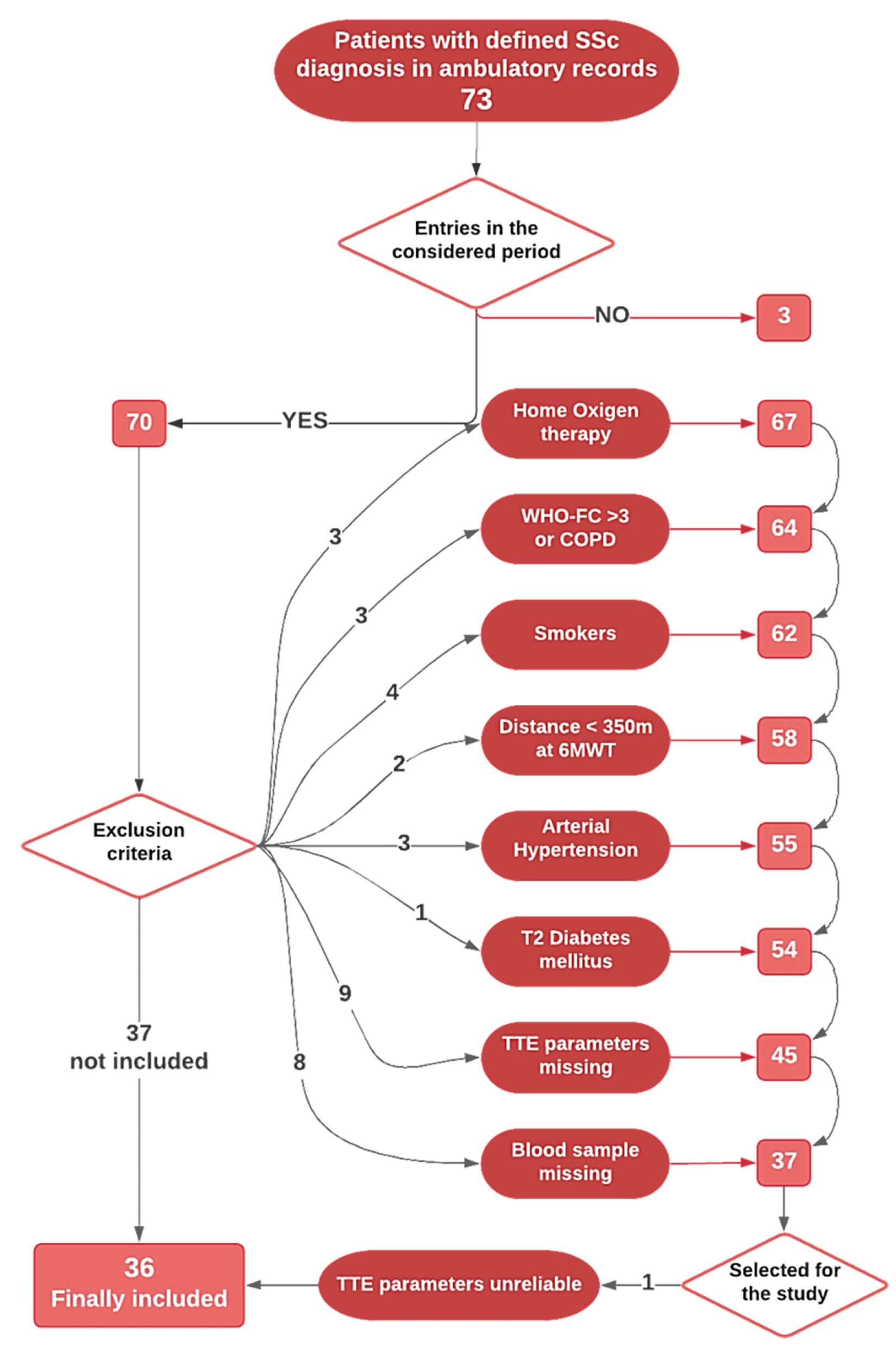
Vitamin D Supplementation and Medical Therapy
4. Discussion
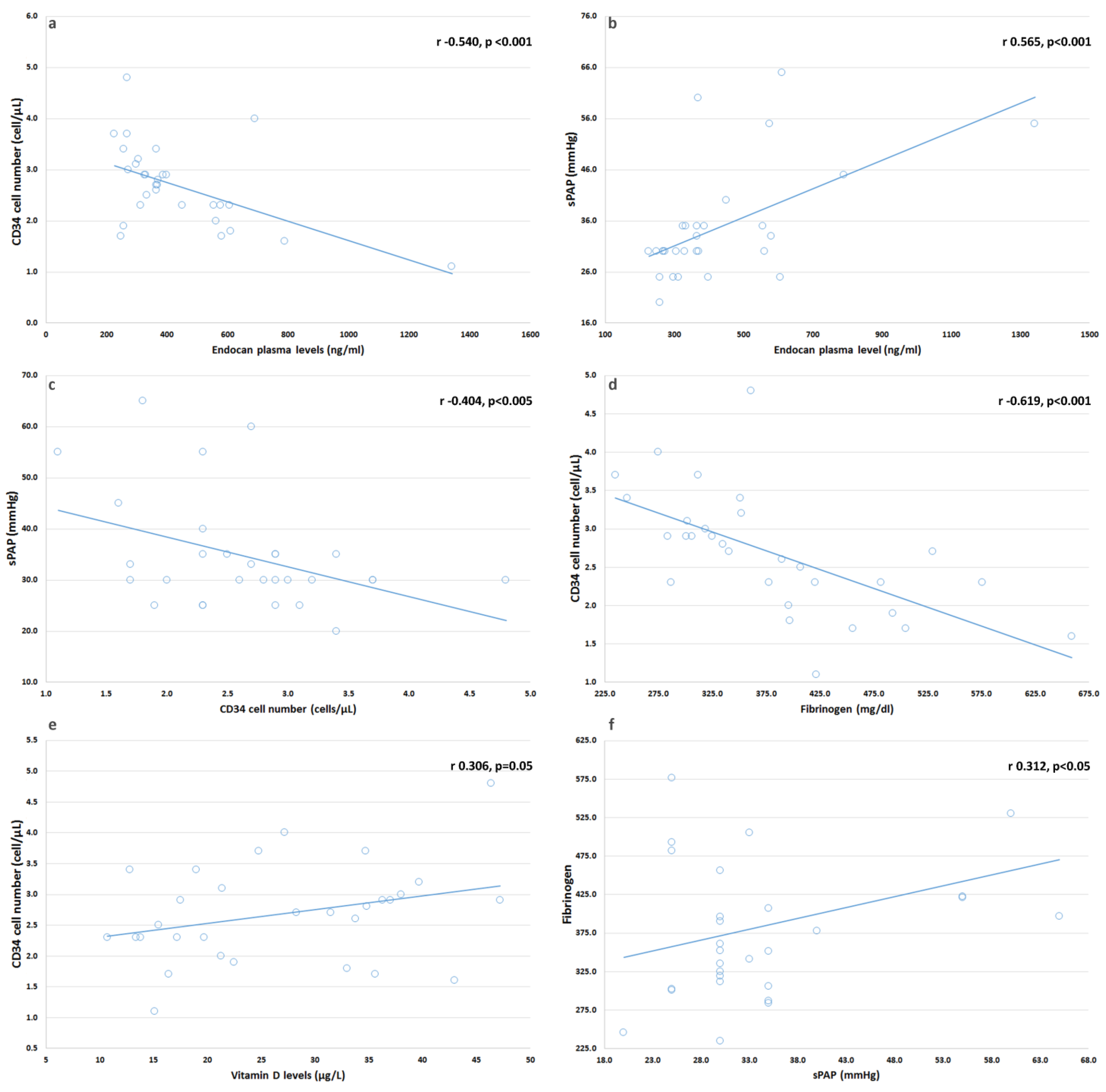
4.1. In SSc Patients, We Found Serum Endocan Levels Increased and Related with sPAP
4.2. We Found No Changes in CD34+ Cell Number in Our Patients
4.3. We Found a Correlation between CD34+ Cells and sPAP
4.4. We Found a Correlation between Vitamin D and CD34+ Suggesting That Multiple Factors Might Contribute to CD34+ Cells and Endothelium Homeostasis in SSc
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Isola, G.; Williams, R.C.; Lo Gullo, A.; Ramaglia, L.; Matarese, M.; Iorio-Siciliano, V.; Cosio, C.; Matarese, G. Risk association between scleroderma disease characteristics, periodontitis, and tooth loss. Clin. Rheumatol. 2017, 36, 2733–2741. [Google Scholar] [CrossRef] [PubMed]
- Denton, C.P.; Khanna, D. Systemic sclerosis. Lancet 2017, 390, 1685–1699. [Google Scholar] [CrossRef]
- Morrisroe, K.; Huq, M.; Stevens, W.; Rabusa, C.; Proudman, S.M.; Nikpour, M.; the Australian Scleroderma Interest Group. Risk factors for development of pulmonary arterial hypertension in Australian systemic sclerosis patients: Results from a large multicenter cohort study. BMC Pulm. Med. 2016, 16, 134. [Google Scholar] [CrossRef] [Green Version]
- Schermuly, R.T.; Ghofrani, H.A.; Wilkins, M.R.; Grimminger, F. Mechanisms of disease: Pulmonary arterial hypertension. Nat. Rev. Cardiol. 2011, 8, 443–455. [Google Scholar] [CrossRef]
- Guiducci, S.; Giacomelli, R.; Cerinic, M.M. Vascular complications of scleroderma. Autoimmun. Rev. 2007, 6, 520–523. [Google Scholar] [CrossRef]
- Hachulla, E.; Launay, D.; Boucly, A.; Mouthon, L.; de Groote, P.; Cottin, V.; Pugnet, G.; Prevot, G.; Bourlier, D.; Dauphin, C.; et al. Survival Improved in Patients Aged ≤ 70 Years with Systemic Sclerosis-Associated Pulmonary Arterial Hypertension During the Period 2006 to 2017 in France. Chest 2019, 157, 945–954. [Google Scholar] [CrossRef]
- Colletti, M.; Galardi, A.; De Santis, M.; Guidelli, G.M.; Di Giannatale, A.; Di Luigi, L.; Antinozzi, C. Exosomes in Systemic Sclerosis: Messengers Between Immune, Vascular and Fibrotic Components? Int. J. Mol. Sci. 2019, 20, 4337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aragona, C.O.; Imbalzano, E.; Mamone, F.; Cairo, V.; Lo Gullo, A.; D’Ascola, A.; Sardo, M.A.; Scuruchi, M.; Basile, G.; Saitta, A.; et al. Endothelial Progenitor Cells for Diagnosis and Prognosis in Cardiovascular Disease. Stem Cells Int. 2016, 2016, 8043792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez-Carrio, J.; Lopez, P.; Suarez, A. Endothelial Progenitor Cells as Mediators of the Crosstalk between Vascular Repair and Immunity: Lessons from Systemic Autoimmune Diseases. Curr. Med. Chem. 2018, 25, 4478–4496. [Google Scholar] [CrossRef] [PubMed]
- Spees, J.L.; Whitney, M.J.; Sullivan, D.E.; Lasky, J.A.; Laboy, M.; Ylostalo, J.; Prockop, D.J. Bone marrow progenitor cells contribute to repair and remodeling of the lung and heart in a rat model of progressive pulmonary hypertension. FASEB J. 2008, 22, 1226–1236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Papa, N.; Pignataro, F. The Role of Endothelial Progenitors in the Repair of Vascular Damage in Systemic Sclerosis. Front. Immunol. 2018, 9, 1383. [Google Scholar] [CrossRef]
- Rabquer, B.J.; Koch, A.E. Angiogenesis and vasculopathy in systemic sclerosis: Evolving concepts. Curr. Rheumatol. Rep. 2012, 14, 56–63. [Google Scholar] [CrossRef]
- Avouac, J.; Juin, F.; Wipff, J.; Couraud, P.O.; Chiocchia, G.; Kahan, A.; Boileau, C.; Uzan, G.; Allanore, Y. Circulating endothelial progenitor cells in systemic sclerosis: Association with disease severity. Ann. Rheum. Dis. 2008, 67, 1455–1460. [Google Scholar] [CrossRef] [PubMed]
- Andrigueti, F.V.; Arismendi, M.I.; Ebbing, P.C.; Kayser, C. Decreased numbers of endothelial progenitor cells in patients in the early stages of systemic sclerosis. Microvasc. Res. 2015, 98, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Kuwana, M.; Okazaki, Y.; Yasuoka, H.; Kawakami, Y.; Ikeda, Y. Defective vasculogenesis in systemic sclerosis. Lancet 2004, 364, 603–610. [Google Scholar] [CrossRef]
- Nevskaya, T.; Bykovskaia, S.; Lyssuk, E.; Shakhov, I.; Zaprjagaeva, M.; Mach, E.; Ananieva, L.; Guseva, N.; Nassonov, E. Circulating endothelial progenitor cells in systemic sclerosis: Relation to impaired angiogenesis and cardiovascular manifestations. Clin. Exp. Rheumatol. 2008, 26, 421–429. [Google Scholar]
- Lo Gullo, A.; Aragona, C.O.; Scuruchi, M.; Versace, A.G.; Saitta, A.; Imbalzano, E.; Loddo, S.; Campo, G.M.; Mandraffino, G. Endothelial progenitor cells and rheumatic disease modifying therapy. Vascul. Pharmacol. 2018, 108, 8–14. [Google Scholar] [CrossRef]
- Lo Gullo, A.; Mandraffino, G.; Bagnato, G.; Aragona, C.O.; Imbalzano, E.; D’Ascola, A.; Rotondo, F.; Cinquegrani, A.; Mormina, E.; Saitta, C.; et al. Vitamin D Status in Rheumatoid Arthritis: Inflammation, Arterial Stiffness and Circulating Progenitor Cell Number. PLoS ONE 2015, 10, e0134602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lo Gullo, A.; Mandraffino, G.; Imbalzano, E.; Mamone, F.; Aragona, C.O.; D’Ascola, A.; Loddo, S.; Cinquegrani, A.; Alibrandi, A.; Mormina, E.; et al. Toll-like receptor 3 and interleukin 1beta expression in CD34+ cells from patients with rheumatoid arthritis: Association with inflammation and vascular involvement. Clin. Exp. Rheumatol. 2014, 32, 922–929. [Google Scholar]
- Lo Gullo, A.; Mandraffino, G.; Sardo, M.A.; D’Ascola, A.; Mamone, F.; Loddo, S.; Alibrandi, A.; Imbalzano, E.; Mandraffino, R.; Mormina, E.; et al. Circulating progenitor cells in rheumatoid arthritis: Association with inflammation and oxidative stress. Scand. J. Rheumatol. 2014, 43, 184–193. [Google Scholar] [CrossRef]
- Mok, M.Y.; Yiu, K.H.; Wong, C.Y.; Qiuwaxi, J.; Lai, W.H.; Wong, W.S.; Tse, H.F.; Lau, C.S. Low circulating level of CD133+KDR+cells in patients with systemic sclerosis. Clin. Exp. Rheumatol. 2010, 28, S19–S25. [Google Scholar] [PubMed]
- Del Papa, N.; Colombo, G.; Fracchiolla, N.; Moronetti, L.M.; Ingegnoli, F.; Maglione, W.; Comina, D.P.; Vitali, C.; Fantini, F.; Cortelezzi, A. Circulating endothelial cells as a marker of ongoing vascular disease in systemic sclerosis. Arthritis Rheum. 2004, 50, 1296–1304. [Google Scholar] [CrossRef] [Green Version]
- Allanore, Y.; Batteux, F.; Avouac, J.; Assous, N.; Weill, B.; Kahan, A. Levels of circulating endothelial progenitor cells in systemic sclerosis. Clin. Exp. Rheumatol. 2007, 25, 60–66. [Google Scholar]
- Del Papa, N.; Quirici, N.; Scavullo, C.; Gianelli, U.; Corti, L.; Vitali, C.; Ferri, C.; Giuggioli, D.; Manfredi, A.; Maglione, W.; et al. Antiendothelial cell antibodies induce apoptosis of bone marrow endothelial progenitors in systemic sclerosis. J. Rheumatol. 2010, 37, 2053–2063. [Google Scholar] [CrossRef] [PubMed]
- Satoh, K.; Kagaya, Y.; Nakano, M.; Ito, Y.; Ohta, J.; Tada, H.; Karibe, A.; Minegishi, N.; Suzuki, N.; Yamamoto, M.; et al. Important role of endogenous erythropoietin system in recruitment of endothelial progenitor cells in hypoxia-induced pulmonary hypertension in mice. Circulation 2006, 113, 1442–1450. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Vitamin D and cardiovascular diseases: Causality. J. Steroid. Biochem. Mol. Biol. 2016. [Google Scholar] [CrossRef]
- Jablonski, K.L.; Chonchol, M.; Pierce, G.L.; Walker, A.E.; Seals, D.R. 25-Hydroxyvitamin D deficiency is associated with inflammation-linked vascular endothelial dysfunction in middle-aged and older adults. Hypertension 2011, 57, 63–69. [Google Scholar] [CrossRef] [Green Version]
- Grundmann, M.; Haidar, M.; Placzko, S.; Niendorf, R.; Darashchonak, N.; Hubel, C.A.; von Versen-Hoynck, F. Vitamin D improves the angiogenic properties of endothelial progenitor cells. Am. J. Physiol. Cell Physiol. 2012, 303, C954–C962. [Google Scholar] [CrossRef] [PubMed]
- Cianciolo, G.; La Manna, G.; Cappuccilli, M.L.; Lanci, N.; Della Bella, E.; Cuna, V.; Dormi, A.; Todeschini, P.; Donati, G.; Alviano, F.; et al. VDR expression on circulating endothelial progenitor cells in dialysis patients is modulated by 25(OH)D serum levels and calcitriol therapy. Blood Purif. 2011, 32, 161–173. [Google Scholar] [CrossRef]
- Uberti, F.; Lattuada, D.; Morsanuto, V.; Nava, U.; Bolis, G.; Vacca, G.; Squarzanti, D.F.; Cisari, C.; Molinari, C. Vitamin D protects human endothelial cells from oxidative stress through the autophagic and survival pathways. J. Clin. Endocrinol. Metab. 2014, 99, 1367–1374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antico, A.; Tampoia, M.; Tozzoli, R.; Bizzaro, N. Can supplementation with vitamin D reduce the risk or modify the course of autoimmune diseases? A systematic review of the literature. Autoimmun. Rev. 2012, 12, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Balta, S.; Mikhailidis, D.P.; Demirkol, S.; Ozturk, C.; Celik, T.; Iyisoy, A. Endocan: A novel inflammatory indicator in cardiovascular disease? Atherosclerosis 2015, 243, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Oktar, S.F.; Guney, I.; Eren, S.A.; Oktar, L.; Kosar, K.; Buyukterzi, Z.; Alkan, E.; Biyik, Z.; Erdem, S.S. Serum endocan levels, carotid intima-media thickness and microalbuminuria in patients with newly diagnosed hypertension. Clin. Exp. Hypertens. 2019, 1–8. [Google Scholar] [CrossRef]
- Sarrazin, S.; Adam, E.; Lyon, M.; Depontieu, F.; Motte, V.; Landolfi, C.; Lortat-Jacob, H.; Bechard, D.; Lassalle, P.; Delehedde, M. Endocan or endothelial cell specific molecule-1 (ESM-1): A potential novel endothelial cell marker and a new target for cancer therapy. Biochim. Biophys. Acta 2006, 1765, 25–37. [Google Scholar] [CrossRef]
- Lee, H.G.; Choi, H.Y.; Bae, J.S. Endocan as a potential diagnostic or prognostic biomarker for chronic kidney disease. Kidney Int. 2014, 86, 1079–1081. [Google Scholar] [CrossRef] [Green Version]
- Bechard, D.; Scherpereel, A.; Hammad, H.; Gentina, T.; Tsicopoulos, A.; Aumercier, M.; Pestel, J.; Dessaint, J.P.; Tonnel, A.B.; Lassalle, P. Human endothelial-cell specific molecule-1 binds directly to the integrin CD11a/CD18 (LFA-1) and blocks binding to intercellular adhesion molecule-1. J. Immunol. 2001, 167, 3099–3106. [Google Scholar] [CrossRef] [Green Version]
- Bessa, J.; Albino-Teixeira, A.; Reina-Couto, M.; Sousa, T. Endocan: A novel biomarker for risk stratification, prognosis and therapeutic monitoring in human cardiovascular and renal diseases. Clin. Chim. Acta 2020, 509, 310–335. [Google Scholar] [CrossRef] [PubMed]
- Balta, S.; Mikhailidis, D.P.; Demirkol, S.; Ozturk, C.; Kurtoglu, E.; Demir, M.; Celik, T.; Turker, T.; Iyisoy, A. Endocan—A novel inflammatory indicator in newly diagnosed patients with hypertension. Angiology 2014, 65, 773–777. [Google Scholar] [CrossRef]
- Icli, A.; Cure, E.; Cure, M.C.; Uslu, A.U.; Balta, S.; Mikhailidis, D.P.; Ozturk, C.; Arslan, S.; Sakiz, D.; Sahin, M.; et al. Endocan Levels and Subclinical Atherosclerosis in Patients With Systemic Lupus Erythematosus. Angiology 2016, 67, 749–755. [Google Scholar] [CrossRef] [PubMed]
- Balta, S.; Demirkol, S.; Ozturk, C.; Yildirim, A.O.; Demir, M.; Celik, T. The Relation Between Endocan Levels and Subclinic Atherosclerosis. Clin. Appl. Thromb. Hemost. 2016, 22, 495–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ying, J.; Zhou, D.; Gu, T.; Huang, J. Endocan, a Risk Factor for Developing Acute Respiratory Distress Syndrome among Severe Pneumonia Patients. Can. Respir. J. 2019, 2019, 2476845. [Google Scholar] [CrossRef] [PubMed]
- Kechagia, M.; Papassotiriou, I.; Gourgoulianis, K.I. Endocan and the respiratory system: A review. Int. J. Chron. Obstruct. Pulmon. Dis. 2016, 11, 3179–3187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, O.D.; So, E.Y.; Egan, P.C.; Goldberg, L.R.; Aliotta, J.M.; Wu, K.Q.; Dubielecka, P.M.; Ventetuolo, C.E.; Reginato, A.M.; Quesenberry, P.J.; et al. Endothelial to haematopoietic transition contributes to pulmonary arterial hypertension. Cardiovasc. Res. 2017, 113, 1560–1573. [Google Scholar] [CrossRef] [Green Version]
- Van den Hoogen, F.; Khanna, D.; Fransen, J.; Johnson, S.R.; Baron, M.; Tyndall, A.; Matucci-Cerinic, M.; Naden, R.P.; Medsger, T.A., Jr.; Carreira, P.E.; et al. 2013 classification criteria for systemic sclerosis: An American college of rheumatology/European league against rheumatism collaborative initiative. Ann. Rheum. Dis. 2013, 72, 1747–1755. [Google Scholar] [CrossRef] [Green Version]
- Clements, P.; Lachenbruch, P.; Siebold, J.; White, B.; Weiner, S.; Martin, R.; Weinstein, A.; Weisman, M.; Mayes, M.; Collier, D.; et al. Inter and intraobserver variability of total skin thickness score (modified Rodnan TSS) in systemic sclerosis. J. Rheumatol. 1995, 22, 1281–1285. [Google Scholar]
- Galie, N.; Humbert, M.; Vachiery, J.L.; Gibbs, S.; Lang, I.; Torbicki, A.; Simonneau, G.; Peacock, A.; Vonk Noordegraaf, A.; Beghetti, M.; et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur. Respir. J. 2015, 46, 903–975. [Google Scholar] [CrossRef]
- Khanna, D.; Furst, D.E.; Clements, P.J.; Allanore, Y.; Baron, M.; Czirjak, L.; Distler, O.; Foeldvari, I.; Kuwana, M.; Matucci-Cerinic, M.; et al. Standardization of the modified Rodnan skin score for use in clinical trials of systemic sclerosis. J. Scleroderma Relat. Disord. 2017, 2, 11–18. [Google Scholar] [CrossRef] [Green Version]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Hearth J. Cardiovasc. Imaging 2015, 16, 233–270. [Google Scholar] [CrossRef] [PubMed]
- Pugnet, G.; Marjanovic, Z.; Deligny, C.; Boussardon, K.; Benzidia, I.; Puyade, M.; Lansiaux, P.; Vandecasteele, E.; Smith, V.; Farge, D. Reproducibility and Utility of the 6-minute Walk Test in Systemic Sclerosis. J. Rheumatol. 2018, 45, 1273–1280. [Google Scholar] [CrossRef]
- Nagel, C.; Marra, A.M.; Benjamin, N.; Blank, N.; Cittadini, A.; Coghlan, G.; Distler, O.; Denton, C.P.; Egenlauf, B.; Fiehn, C.; et al. Reduced Right Ventricular Output Reserve in Patients With Systemic Sclerosis and Mildly Elevated Pulmonary Artery Pressure. Arthritis Rheumatol. 2019, 71, 805–816. [Google Scholar] [CrossRef]
- Mandraffino, G.; Imbalzano, E.; Sardo, M.A.; D’Ascola, A.; Mamone, F.; Lo Gullo, A.; Alibrandi, A.; Loddo, S.; Mormina, E.; David, A.; et al. Circulating progenitor cells in hypertensive patients with different degrees of cardiovascular involvement. J. Hum. Hypertens. 2014. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [Green Version]
- Yilmaz, M.I.; Siriopol, D.; Saglam, M.; Kurt, Y.G.; Unal, H.U.; Eyileten, T.; Gok, M.; Cetinkaya, H.; Oguz, Y.; Sari, S.; et al. Plasma endocan levels associate with inflammation, vascular abnormalities, cardiovascular events, and survival in chronic kidney disease. Kidney Int. 2014, 86, 1213–1220. [Google Scholar] [CrossRef] [Green Version]
- Balta, I.; Balta, S.; Demirkol, S.; Mikhailidis, D.P.; Celik, T.; Akhan, M.; Kurt, O.; Kurt, Y.G.; Aydin, I.; Kilic, S. Elevated serum levels of endocan in patients with psoriasis vulgaris: Correlations with cardiovascular risk and activity of disease. Br. J. Dermatol. 2013, 169, 1066–1070. [Google Scholar] [CrossRef] [PubMed]
- Balta, I.; Balta, S.; Koryurek, O.M.; Demirkol, S.; Mikhailidis, D.P.; Celik, T.; Cakar, M.; Kucuk, U.; Eksioglu, M.; Kurt, Y.G. Serum endocan levels as a marker of disease activity in patients with Behcet disease. J. Am. Acad. Dermatol. 2014, 70, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Balanescu, P.; Ladaru, A.; Balanescu, E.; Voiosu, T.; Baicus, C.; Dan, G.A. Endocan, Novel Potential Biomarker for Systemic Sclerosis: Results of a Pilot Study. J. Clin. Lab. Anal. 2016, 30, 368–373. [Google Scholar] [CrossRef]
- Zhao, H.; Xue, Y.; Guo, Y.; Sun, Y.; Liu, D.; Wang, X. Inhibition of endocan attenuates monocrotaline-induced connective tissue disease related pulmonary arterial hypertension. Int. Immunopharmacol. 2017, 42, 115–121. [Google Scholar] [CrossRef]
- Fujita, M.; Shannon, J.M.; Irvin, C.G.; Fagan, K.A.; Cool, C.; Augustin, A.; Mason, R.J. Overexpression of tumor necrosis factor-alpha produces an increase in lung volumes and pulmonary hypertension. Am. J. Physiol. Lung Cell Mol. Physiol. 2001, 280, L39–L49. [Google Scholar] [CrossRef]
- Tanaka, K.; Sata, M. Role of vascular progenitor cells in cardiovascular disease. Curr. Pharm. Des. 2009, 15, 2760–2768. [Google Scholar] [CrossRef]
- Westerweel, P.E.; Verhaar, M.C. Endothelial progenitor cell dysfunction in rheumatic disease. Nat. Rev. Rheumatol. 2009, 5, 332–340. [Google Scholar] [CrossRef]
- Del Papa, N.; Quirici, N.; Soligo, D.; Scavullo, C.; Cortiana, M.; Borsotti, C.; Maglione, W.; Comina, D.P.; Vitali, C.; Fraticelli, P.; et al. Bone marrow endothelial progenitors are defective in systemic sclerosis. Arthritis Rheum. 2006, 54, 2605–2615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diller, G.P.; van Eijl, S.; Okonko, D.O.; Howard, L.S.; Ali, O.; Thum, T.; Wort, S.J.; Bedard, E.; Gibbs, J.S.; Bauersachs, J.; et al. Circulating endothelial progenitor cells in patients with Eisenmenger syndrome and idiopathic pulmonary arterial hypertension. Circulation 2008, 117, 3020–3030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schiavon, M.; Fadini, G.P.; Lunardi, F.; Agostini, C.; Boscaro, E.; Calabrese, F.; Marulli, G.; Rea, F. Increased tissue endothelial progenitor cells in end-stage lung diseases with pulmonary hypertension. J. Hearth Lung Transpl. 2012, 31, 1025–1030. [Google Scholar] [CrossRef]
- Ormiston, M.L.; Deng, Y.; Stewart, D.J.; Courtman, D.W. Innate immunity in the therapeutic actions of endothelial progenitor cells in pulmonary hypertension. Am. J. Respir. Cell Mol. Biol. 2010, 43, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Conese, M.; Carbone, A.; Castellani, S.; Di Gioia, S. Paracrine effects and heterogeneity of marrow-derived stem/progenitor cells: Relevance for the treatment of respiratory diseases. Cells Tissues Organs 2013, 197, 445–473. [Google Scholar] [CrossRef]
- Xia, L.; Fu, G.S.; Yang, J.X.; Zhang, F.R.; Wang, X.X. Endothelial progenitor cells may inhibit apoptosis of pulmonary microvascular endothelial cells: New insights into cell therapy for pulmonary arterial hypertension. Cytotherapy 2009, 11, 492–502. [Google Scholar] [CrossRef]
- Smadja, D.M.; Mauge, L.; Gaussem, P.; d’Audigier, C.; Israel-Biet, D.; Celermajer, D.S.; Bonnet, D.; Levy, M. Treprostinil increases the number and angiogenic potential of endothelial progenitor cells in children with pulmonary hypertension. Angiogenesis 2011, 14, 17–27. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.C.; Kong, J.; Wei, M.; Chen, Z.F.; Liu, S.Q.; Cao, L.P. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J. Clin. Investig. 2002, 110, 229–238. [Google Scholar] [CrossRef]
- Lai, S.; Coppola, B.; Dimko, M.; Galani, A.; Innico, G.; Frassetti, N.; Mariotti, A. Vitamin D deficiency, insulin resistance, and ventricular hypertrophy in the early stages of chronic kidney disease. Ren. Fail 2014, 36, 58–64. [Google Scholar] [CrossRef]
- Covic, A.; Voroneanu, L.; Goldsmith, D. The effects of vitamin D therapy on left ventricular structure and function—Are these the underlying explanations for improved CKD patient survival? Nephron Clin. Pract. 2010, 116, c187–c195. [Google Scholar] [CrossRef]
- Gardner, D.G.; Chen, S.; Glenn, D.J. Vitamin D and the heart. Am. J. Physiol. Regul. Integr Comp. Physiol. 2013, 305, R969–R977. [Google Scholar] [CrossRef]
- Bickford, P.C.; Tan, J.; Shytle, R.D.; Sanberg, C.D.; El-Badri, N.; Sanberg, P.R. Nutraceuticals synergistically promote proliferation of human stem cells. Stem Cells Dev. 2006, 15, 118–123. [Google Scholar] [CrossRef]
- Schroder-Heurich, B.; von Hardenberg, S.; Brodowski, L.; Kipke, B.; Meyer, N.; Borns, K.; von Kaisenberg, C.S.; Brinkmann, H.; Claus, P.; von Versen-Hoynck, F. Vitamin D improves endothelial barrier integrity and counteracts inflammatory effects on endothelial progenitor cells. FASEB J. 2019, 33, 9142–9153. [Google Scholar] [CrossRef] [PubMed]
- Hammer, Y.; Soudry, A.; Levi, A.; Talmor-Barkan, Y.; Leshem-Lev, D.; Singer, J.; Kornowski, R.; Lev, E.I. Effect of vitamin D on endothelial progenitor cells function. PLoS ONE 2017, 12, e0178057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wara, A.K.; Foo, S.; Croce, K.; Sun, X.; Icli, B.; Tesmenitsky, Y.; Esen, F.; Lee, J.S.; Subramaniam, M.; Spelsberg, T.C.; et al. TGF-beta1 signaling and Kruppel-like factor 10 regulate bone marrow-derived proangiogenic cell differentiation, function, and neovascularization. Blood 2011, 118, 6450–6460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Latic, N.; Erben, R.G. Vitamin D and Cardiovascular Disease, with Emphasis on Hypertension, Atherosclerosis, and Heart Failure. Int. J. Mol. Sci. 2020, 21, 6483. [Google Scholar] [CrossRef]
- Witte, K.K.; Byrom, R.; Gierula, J.; Paton, M.F.; Jamil, H.A.; Lowry, J.E.; Gillott, R.G.; Barnes, S.A.; Chumun, H.; Kearney, L.C.; et al. Effects of Vitamin D on Cardiac Function in Patients With Chronic HF: The VINDICATE Study. J. Am. Coll. Cardiol. 2016, 67, 2593–2603. [Google Scholar] [CrossRef] [Green Version]
- Alfieri, C.; Vettoretti, S.; Ruzhytska, O.; Gandolfo, M.T.; Cresseri, D.; Campise, M.; Caldiroli, L.; Favi, E.; Binda, V.; Messa, P. Vitamin D and subclinical cardiac damage in a cohort of kidney transplanted patients: A retrospective observational study. Sci. Rep. 2020, 10, 19160. [Google Scholar] [CrossRef]
- Czaya, B.; Seeherunvong, W.; Singh, S.; Yanucil, C.; Ruiz, P.; Quiroz, Y.; Grabner, A.; Katsoufis, C.; Swaminathan, S.; Abitbol, C.; et al. Cardioprotective Effects of Paricalcitol Alone and in Combination With FGF23 Receptor Inhibition in Chronic Renal Failure: Experimental and Clinical Studies. Am. J. Hypertens. 2019, 32, 34–44. [Google Scholar] [CrossRef]
- Gluba-Brzózka, A.; Franczyk, B.; Ciałkowska-Rysz, A.; Olszewski, R.; Rysz, J. Impact of Vitamin D on the Cardiovascular System in Advanced Chronic Kidney Disease (CKD) and Dialysis Patients. Nutrients 2018, 10, 709. [Google Scholar] [CrossRef] [Green Version]
- Boucher, B.J.; Grant, W.B. Difficulties in designing randomised controlled trials of vitamin D supplementation for reducing acute cardiovascular events and in the analysis of their outcomes. Int. J. Cardiol. Hearth Vasc. 2020, 29, 100564. [Google Scholar] [CrossRef] [PubMed]
| SSc | Controls | p | |
|---|---|---|---|
| Mean ± SD | Mean ± SD | ||
| Number | 36 | 36 | ns |
| Sex (f) | 36 | 36 | ns |
| BMI (kg/m2) | 25.3 ± 4.43 | 24.9 ± 4.5 | 0.703 |
| Age (years) | 64.1 ± 12.5 | 62.3 ± 4.4 | 0.418 |
| SBP (mmHg) | 115 ± 14 | 120 ± 10 | 0.085 |
| DBP (mmHg) | 90 ± 15 | 80 ± 10 | <0.001 |
| Rodnan Skin Score | 28.9 ± 10.2 | - | - |
| Hb (g/dL) | 12.5 ± 1.7 | 13.3 ± 0.8 | 0.013 |
| Hs-CRP (mg/dL) | 0.57 ± 0.95 | 0.30 ± 0.25 | 0.11 |
| Creatinine (mg/dL) | 0.77 ± 0.38 | 0.82 ± 0.09 | 0.444 |
| Uric acid (mg/dL) | 3.86 ± 1.05 | 2.9 ± 2.60 | 0.04 |
| CD34+/μL (cells/mL) | 2.68 ± 0.79 | 2.61 ± 0.17 | 0.604 |
| Fibrinogen (mg/dL) | 381.5 ± 100.1 | 276.9 ± 21.7 | <0.001 |
| VIT D μg/L (ng/mL) | 27.34 ± 10.8 | 22.47 ± 2.6 | 0.010 |
| Endocan (ng/mL) | 365.6 ± 225.9 | 280.4 ± 68.7 | 0.034 |
| EF (%) | 59.9 ± 2.3 | 64.8 ± 0.99 | 0.001 |
| E (cm/s) | 72.9 ± 17.3 | 75.4 ± 7.3 | 0.427 |
| A (cm/s) | 71.85 ± 13.05 | 60.8 ± 5.9 | <0.001 |
| E/A (ratio) | 1.04 ± 0.41 | 1.19 ± 0.34 | 0.101 |
| E’ (cm/s) | 9.66 ± 2.1 | 10.34 ± 1.62 | 0.128 |
| S’ (cm/s) | 8.5 ± 1.4 | 8.8 ± 2.0 | 0.464 |
| E/E’ (ratio) | 7.9 ± 2.31 | 7.17 ± 1.42 | 0.111 |
| IVRT (msec) | 86.1 ± 13.3 | 81.4 ± 10.7 | 0.103 |
| RA volume (mL/m2) | 24.2 ± 5.1 | 22.1 ± 5.0 | 0.082 |
| RV wall thickness (mm) | 2.2 ± 0.6 | 2.0 ± 0.3 | 0.078 |
| RVOT prox diam (mm) | 29.1 ± 2.8 | 26.3 ± 3 | <0.001 |
| TAPSE (mm) | 15.7 ± 3.1 | 22.15 ± 4.3 | <0.001 |
| sPAP (mmHg) | 34.1 ± 10.3 | 20.4 ± 1.3 | <0.001 |
| SSc | |
|---|---|
| Pattern of SSc, diffuse/limited (%) | 24/12 (66.6/33.3%) |
| Disease duration, month | 78.3 ± 61.9 |
| Anticentromere antibodies (ACA), n (%) | 16 (44.4%) |
| Anti-topoisomerase I (Anti-Scl70), n (%) | 9 (25%) |
| Antinuclear positivity (ANA) n (%) | 22 (61.1%) |
| Interstitial lung disease, n (%) | 17 (47.2%) |
| Musculo-skeletal involvement, n (%) | 19 (52.8%) |
| Renal crisis, n (%) | 0 |
| Esophageal reflux, n (%) | 30 (83.3%) |
| Background therapy | 36 (100%) |
| Aspirin 100 mg daily, n (%) | 36 (100%) |
| Nifedipine 20 mg daily, n (%) | 36 (100%) |
| Bosentan 62, 5/125 mg daily, n (%) | 3 (8.33%) |
| Sildenafil 60 mg daily, n (%) | 4 (11.1%) |
| Standardized Coefficients | Unstandardized Coefficients | ||||
|---|---|---|---|---|---|
| Model | B | SE | Beta | t | p |
| Fibrinogen | −0.005 | 0.001 | −0.667 | −4.732 | 0.000 |
| Endocan | −0.002 | 0.001 | −0.514 | −3.171 | 0.004 |
| sPAP | −0.035 | 0.012 | −0.498 | −2.980 | 0.006 |
| SUA | −0.302 | 0.163 | −0.383 | −1.855 | 0.078 |
| CRP | −0.080 | 0.158 | −0.100 | −0.504 | 0.619 |
| Vitamin D | 0.022 | 0.014 | 0.293 | 1.621 | 0.116 |
| Standardized Coefficients | Unstandardized Coefficients | ||||
|---|---|---|---|---|---|
| Model | B | SE | Beta | t | p |
| Costant | 4.130 | 0.334 | 12.374 | 0.000 | |
| Endocan | −0.001 | 0.000 | −412 | −2.940 | 0.006 |
| Fibrinogen | −0.003 | 0.001 | −386 | −2.753 | 0.009 |
| Standardized Coefficients | Unstandardized Coefficients | ||||
|---|---|---|---|---|---|
| Model | B | SE | Beta | t | p |
| Endocan | 0.033 | 0.007 | 0.607 | 4.519 | 0.000 |
| Fibrinogen | 0.054 | 0.017 | 0.471 | 3.161 | 0.003 |
| CD34+ cells | −6.967 | 2.512 | −0.424 | −2.773 | 0.009 |
| SUA | 1.389 | 1.698 | 0.168 | 0.818 | 0.422 |
| Rodnan SS | 0.129 | 0.172 | 0.130 | 0.751 | 0.458 |
| Vitamin D | 0.007 | 0.163 | 0.007 | 0.041 | 0.967 |
| Standardized Coefficients | Unstandardized Coefficients | ||||
|---|---|---|---|---|---|
| Model | B | SE | Beta | t | p |
| Costant | 18.463 | 3.278 | 5.632 | 0.000 | |
| Endocan | 0.033 | 0.007 | 0.607 | 4.519 | 0.000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lo Gullo, A.; Mandraffino, G.; Rodríguez-Carrio, J.; Scuruchi, M.; Sinicropi, D.; Postorino, M.; Morace, C.; Giuffrida, C.; Sciortino, D.; Gallizzi, R.; et al. Endocan and Circulating Progenitor Cells in Women with Systemic Sclerosis: Association with Inflammation and Pulmonary Hypertension. Biomedicines 2021, 9, 533. https://doi.org/10.3390/biomedicines9050533
Lo Gullo A, Mandraffino G, Rodríguez-Carrio J, Scuruchi M, Sinicropi D, Postorino M, Morace C, Giuffrida C, Sciortino D, Gallizzi R, et al. Endocan and Circulating Progenitor Cells in Women with Systemic Sclerosis: Association with Inflammation and Pulmonary Hypertension. Biomedicines. 2021; 9(5):533. https://doi.org/10.3390/biomedicines9050533
Chicago/Turabian StyleLo Gullo, Alberto, Giuseppe Mandraffino, Javier Rodríguez-Carrio, Michele Scuruchi, Davide Sinicropi, Maria Postorino, Carmela Morace, Clemente Giuffrida, Davide Sciortino, Romina Gallizzi, and et al. 2021. "Endocan and Circulating Progenitor Cells in Women with Systemic Sclerosis: Association with Inflammation and Pulmonary Hypertension" Biomedicines 9, no. 5: 533. https://doi.org/10.3390/biomedicines9050533
APA StyleLo Gullo, A., Mandraffino, G., Rodríguez-Carrio, J., Scuruchi, M., Sinicropi, D., Postorino, M., Morace, C., Giuffrida, C., Sciortino, D., Gallizzi, R., Loddo, S., Zito, C., & Squadrito, G. (2021). Endocan and Circulating Progenitor Cells in Women with Systemic Sclerosis: Association with Inflammation and Pulmonary Hypertension. Biomedicines, 9(5), 533. https://doi.org/10.3390/biomedicines9050533









