SARS-CoV-2 Rapid Antigen Testing of Symptomatic and Asymptomatic Individuals on the University of Arizona Campus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Biospecimen Collection
2.3. PCR Testing
2.4. Rapid Antigen Testing
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CAP | College of American Pathology |
| CDC | Center for Disease Control |
| CLIA | Clinical Laboratory Improvement Amendments |
| ICU | Intensive care unit |
References
- WHO Coronavirus (COVID-19) Dashboard. Available online: www.COVID19.who.int (accessed on 1 February 2021).
- Wee, S.K.; Sivalingam, S.P.; Yap, E.P.H. Rapid Direct Nucleic Acid Amplification Test without RNA Extraction for SARS-CoV-2 Using a Portable PCR Thermocycler. Genes 2020, 11, 664. [Google Scholar] [CrossRef] [PubMed]
- Mackay, I.M. Real-time PCR in virology. Nucleic Acids Res. 2002, 30, 1292–1305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernández-Huerta, M.T.; Mayoral, L.P.; Navarro, L.M.S.; Mayoral-Andrade, G.; Mayoral, E.P.; Zenteno, E.; Pérez-Campos, E. Should RT-PCR be considered a gold standard in the diagnosis of COVID-19? J. Med. Virol. 2021, 93, 137–138. [Google Scholar] [CrossRef] [PubMed]
- HCP Fact Sheet. Available online: www.fda.gov/media/137884/download (accessed on 1 February 2021).
- Research Use Only 2019-Novel Coronavirus (2019-nCoV) Real-Time RT-PCR Primers and Probes. Available online: www.cdc.gov/coronavirus/2019-ncov/lab/rt-pcr-panel-primer-probes.html (accessed on 1 February 2021).
- CDC 2019-Novel Coronavirus (2019-nCoV) Real-Time RT-PCR Diagnostic Panel. Available online: www.fda.gov/media/134922/download (accessed on 1 February 2021).
- Kociolek, L.K.; Muller, W.J.; Yee, R.; Bard, J.D.; Brown, C.A.; Revell, P.A.; Wardell, H.; Savage, T.J.; Jung, S.; Dominguez, S.; et al. Comparison of Upper Respiratory Viral Load Distributions in Asymptomatic and Symptomatic Children Diagnosed with SARS-CoV-2 Infection in Pediatric Hospital Testing Programs. J. Clin. Microbiol. 2020, 59. [Google Scholar] [CrossRef] [PubMed]
- Mina, M.J.; Parker, R.; Larremore, D.B. Rethinking Covid-19 Test Sensitivity—A Strategy for Containment. N. Engl. J. Med. 2020, 383, e120. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.-D.; Chang, S.-Y.; Wang, J.-T.; Tsai, M.-J.; Hung, C.-C.; Hsu, C.-L.; Chang, S.-C. Prolonged virus shedding even after seroconversion in a patient with COVID-19. J. Infect. 2020, 81, 318–356. [Google Scholar] [CrossRef] [PubMed]
- Larremore, D.B.; Wilder, B.; Lester, E.; Shehata, S.; Burke, J.M.; Hay, H.A.; Tambe, M.; Mina, M.H.; Parker, R. Test sensitivity is secondary to frequency and turnaround time for COVID-19 surveillance. medRxiv 2020. [Google Scholar] [CrossRef]
- SARS-CoV-2 Infectious Dose. Available online: http://www.clinlabnavigator.com/sars-cov-2-infectious-dose.html (accessed on 1 February 2021).
- Pekosz, A.; Cooper, C.K.; Parvu, V.; Li, M.; Andrews, J.C.; Manabe, Y.; Kodsi, S.; Leitch, J.; Gary, D.S.; Roger-Dalbert, C. Antigen-based testing but not real time PCR correlates with SARS-CoV-2 virus culture. medRxiv 2020. [Google Scholar] [CrossRef]
| ID# | Type of Sample | Expected Result | Ag Test Result |
|---|---|---|---|
| 1 | SPK | POS | POS |
| 2 | SPK | NEG | NEG |
| 3 | SPK | POS | POS |
| 4 | SPK | POS | POS |
| 5 | SPK | POS | POS |
| 6 | SPK | POS | POS |
| 7 | SPK | NEG | NEG |
| 8 | SPK | POS | POS |
| 9 | SPK | POS | POS |
| 10 | SPK | POS | POS |
| 11 | SPK | POS | POS |
| 12 | SPK | NEG | NEG |
| 13 | SPK | NEG | NEG |
| 14 | SPK | POS | POS |
| 15 | SPK | POS | POS |
| 16 | SPK | POS | POS |
| 17 | SPK | POS | POS |
| 18 | SPK | NEG | NEG |
| 19 | SPK | POS | POS |
| 20 | SPK | POS | POS |
| ID# | Type of Sample | Ag Test | RT-PCR (ct) |
| BF00043 | SYMP | POS | POS (ct 23) |
| BF00044 | SYMP | POS | POS (ct 14) |
| BF00081 | SYMP | POS | POS (na) |
| BF00094 | SYMP | POS | POS (na) |
| BF00106 | SYMP | POS | POS (na) |
| BF00112 | SYMP | POS | POS (na) |
| 22 | ASYM | POS | POS (ct 27) |
| 34 | ASYM | POS | POS (ct 22) |
| 113 | ASYM | POS | POS (ct 25) |
| 127 | ASYM | POS | POS (ct 17) |
| 156 | ASYM | POS | POS (ct 22) |
| 112 | ASYM | NEG | POS (ct 36) |
| 139 | ASYM | NEG | POS (ct 31) |
| 24524 | ASYM | POS | POS (ct 23) |
| 21448 | ASYM | NEG | POS (ct 35) |
| ID# | Ag Test | RT-PCR (Cycle Threshold) |
|---|---|---|
| 22 | POS | POS (ct27) |
| NEG | POS (ct33) | |
| NEG | POS (ct34) | |
| 113 | POS | POS (ct25) |
| NEG | POS (ct35) | |
| 127 | POS | POS (ct17) |
| NEG | POS (ct36) | |
| NEG | POS (ct35) | |
| 112 | NEG | POS (ct36) |
| NEG | POS (ct36) | |
| 139 | NEG | POS (ct31) |
| NEG | POS (ct36) |
| Symptomatic Subjects | ||
|---|---|---|
| PCR RESULTS | ||
| POS (average ct = 20) | NEG | |
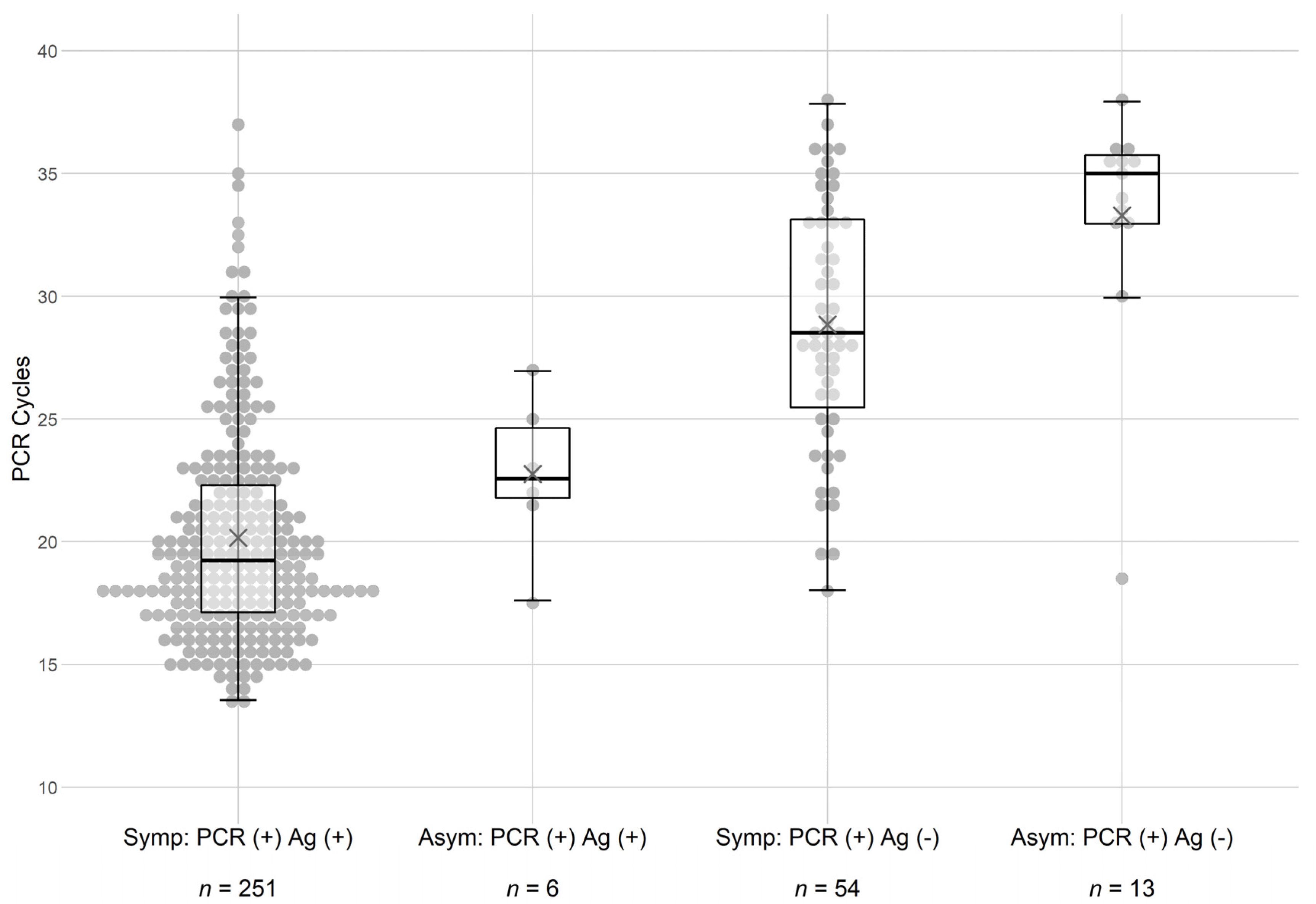
| Ag Pos | n = 251 | n = 7 |
| Ct < 30, n = 243, X = 20 | ||
| Ct > 30, n = 8, X = 33 | ||
| Ag Neg | n = 54 | n = 573 |
| Ct < 30, n = 34, X = 26 | ||
| Ct > 30, n = 20, X = 34 | ||
| Asymptomatic Subjects | ||
| PCR RESULTS | ||
| POS (average ct = 23) | NEG | |
| Ct < 30, n = 6, X = 23 | ||
| Ct > 30, n = 0 | ||
| Ag Neg | n = 13 | n = 1525 |
| Ct < 30, n = 1, X = 18 | ||
| Ct > 30, n = 12, X = 35 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harris, D.T.; Badowski, M.; Jernigan, B.; Sprissler, R.; Edwards, T.; Cohen, R.; Paul, S.; Merchant, N.; Weinkauf, C.C.; Bime, C.; et al. SARS-CoV-2 Rapid Antigen Testing of Symptomatic and Asymptomatic Individuals on the University of Arizona Campus. Biomedicines 2021, 9, 539. https://doi.org/10.3390/biomedicines9050539
Harris DT, Badowski M, Jernigan B, Sprissler R, Edwards T, Cohen R, Paul S, Merchant N, Weinkauf CC, Bime C, et al. SARS-CoV-2 Rapid Antigen Testing of Symptomatic and Asymptomatic Individuals on the University of Arizona Campus. Biomedicines. 2021; 9(5):539. https://doi.org/10.3390/biomedicines9050539
Chicago/Turabian StyleHarris, David T., Michael Badowski, Brandon Jernigan, Ryan Sprissler, Taylor Edwards, Randall Cohen, Stephen Paul, Nirav Merchant, Craig C. Weinkauf, Christian Bime, and et al. 2021. "SARS-CoV-2 Rapid Antigen Testing of Symptomatic and Asymptomatic Individuals on the University of Arizona Campus" Biomedicines 9, no. 5: 539. https://doi.org/10.3390/biomedicines9050539
APA StyleHarris, D. T., Badowski, M., Jernigan, B., Sprissler, R., Edwards, T., Cohen, R., Paul, S., Merchant, N., Weinkauf, C. C., Bime, C., Erickson, H. E., Bixby, B., Parthasarathy, S., Chaudhary, S., Natt, B., Cristan, E., El Aini, T., Rischard, F., Campion, J., ... Dake, M. D. (2021). SARS-CoV-2 Rapid Antigen Testing of Symptomatic and Asymptomatic Individuals on the University of Arizona Campus. Biomedicines, 9(5), 539. https://doi.org/10.3390/biomedicines9050539







