Excessive Intake of High-Fructose Corn Syrup Drinks Induces Impaired Glucose Tolerance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Oral Glucose and Fructose Tolerance Tests (OGTT and OFTT) and Insulin Tolerance Test (ITT)
2.3. Measurement of Tissue Weight and Serum Parameters
2.4. RNA Isolation and Quantitative PCR (qPCR)
2.5. Histological and Immunohistochemical Analyses
2.6. Measurement of Cytokine Levels in the Serum
2.7. Statistical Analysis
3. Results
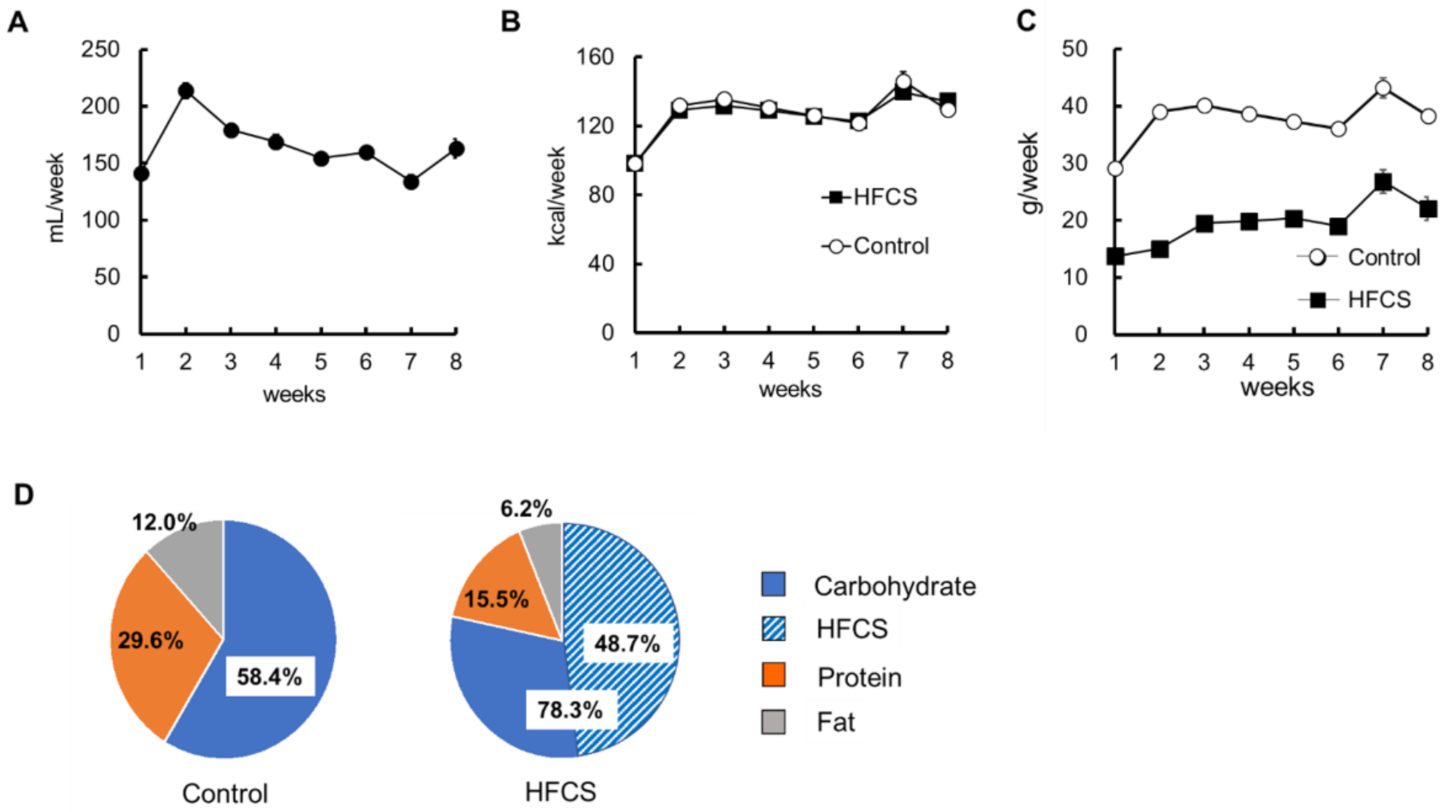
3.1. Excess HFCS–Water Intake and its Nutrient Intake Rate under Conditions of Controlled Caloric Intake
3.2. Excess HFCS–Water Intake under Conditions of Controlled Caloric Intake Did Not Lead to Obesity
3.3. Excess HFCS–Water Intake under Conditions of Controlled Caloric Intake Led to Impaired Glucose Tolerance (IGT)
3.4. Serum Biochemical Parameters
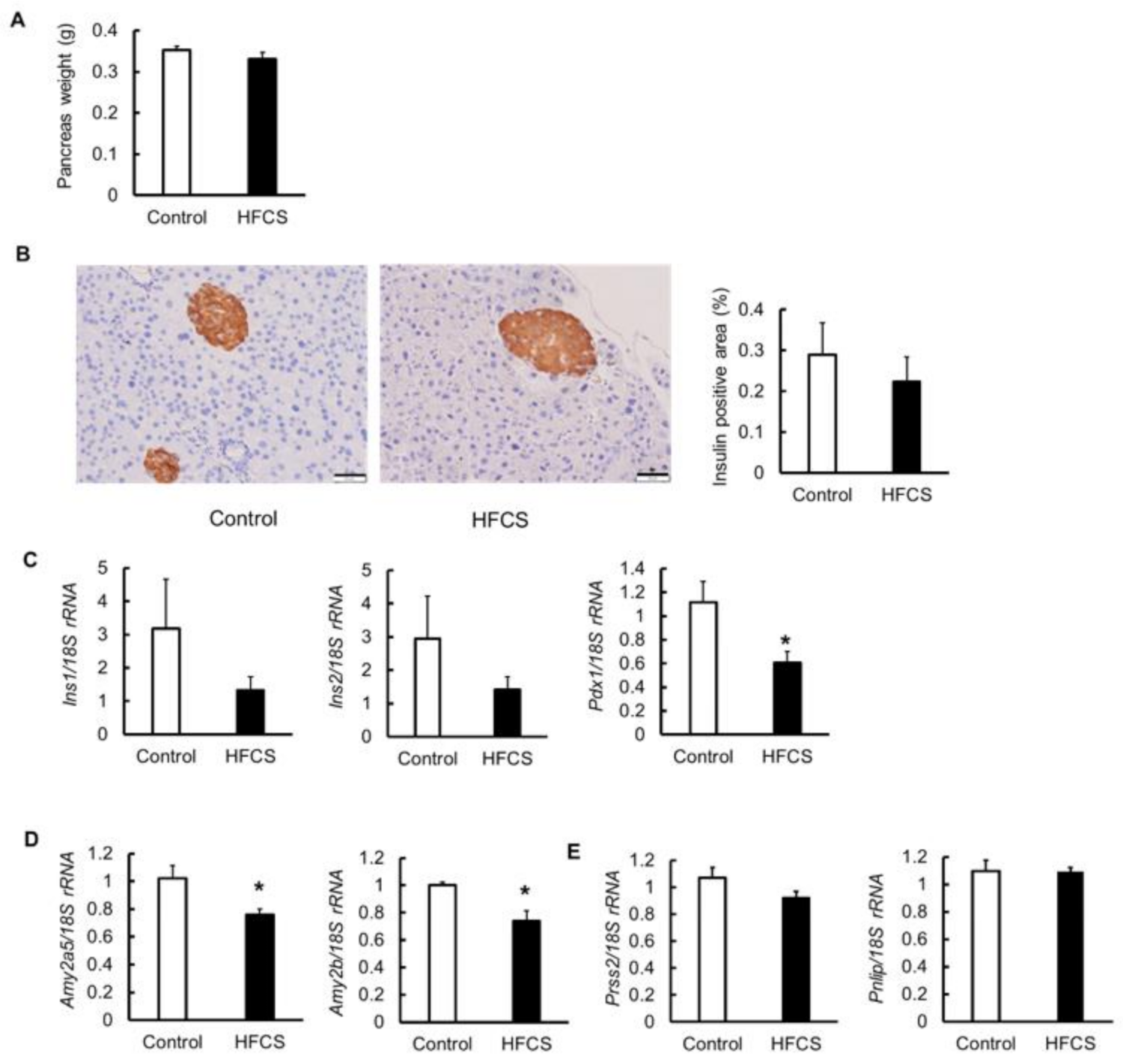
3.5. Effects on Pancreas
3.6. MRNA Expression of Glucose Transporter 2 (Glut2), Glycolytic Enzymes, Amino Acid Transporter, and Glutaminolysis in the Pancreas
3.7. Inflammatory Risk
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- International Diabetes Federation. IDF Diabetes Atlas, 9th ed.; International Diabetes Federation: Brussels, Belgium, 2019; Available online: https://www.diabetesatlas.org/en/resources/ (accessed on 12 May 2021).
- Yaghootkar, H.; Whitcher, B.; Bell, J.D.; Thomas, E.L. Ethnic differences in adiposity and diabetes risk—insights from genetic studies. J. Intern. Med. 2020, 288, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Yoon, K.-H.; Lee, J.-H.; Kim, J.-W.; Cho, J.H.; Choi, Y.-H.; Ko, S.-H.; Zimmet, P.; Son, H.-Y. Epidemic obesity and type 2 diabetes in Asia. Lancet 2006, 368, 1681–1688. [Google Scholar] [CrossRef]
- Ma, R.C.; Chan, J.C. Type 2 diabetes in East Asians: Similarities and differences with populations in Europe and the United States. Ann. N. Y. Acad. Sci. 2013, 1281, 64–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okura, T.; Nakamura, R.; Fujioka, Y.; Kawamoto-Kitao, S.; Ito, Y.; Matsumoto, K.; Shoji, K.; Sumi, K.; Matsuzawa, K.; Izawa, S.; et al. Body mass index ≥23 is a risk factor for insulin resistance and diabetes in Japanese people: A brief report. PLoS ONE 2018, 13, e0201052. [Google Scholar] [CrossRef] [PubMed]
- Sone, H.; Ito, H.; Ohashi, Y.; Akanuma, Y.; Yamada, N. Obesity and type 2 diabetes in Japanese patients. Lancet 2003, 361, 85. [Google Scholar] [CrossRef]
- Fukushima, M.; Suzuki, H.; Seino, Y. Insulin secretion capacity in the development from normal glucose tolerance to type 2 diabetes. Diabetes Res. Clin. Pr. 2004, 66, S37–S43. [Google Scholar] [CrossRef]
- Kim, J.A.; Kim, D.H.; Kim, S.M.; Park, Y.G.; Kim, N.H.; Baik, S.H.; Choi, K.M.; Han, K.; Yoo, H.J. Impact of the Dynamic Change of Metabolic Health Status on the Incident Type 2 Diabetes: A Nationwide Population-Based Cohort Study. Endocrinol. Metab. 2019, 34, 406–414. [Google Scholar] [CrossRef]
- Teixeira, M.G.; Mill, J.G.; Pereira, A.C.; Molina, M.D.C.B. Consumo de antioxidantes em participantes do ELSA-Brasil: Resultados da linha de base. Rev. Bras. Epidemiologia 2016, 19, 149–159. [Google Scholar] [CrossRef] [Green Version]
- Summary Results of the National Health and Nutrition Survey Japan 2020. Available online: https://www.mhlw.go.jp/content/10900000/000687163.pdf (accessed on 12 May 2021).
- Bray, G.A.; Nielsen, S.J.; Popkin, B.M. Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am. J. Clin. Nutr. 2004, 79, 537–543. [Google Scholar] [CrossRef]
- Montonen, J.; Järvinen, R.; Knekt, P.; Heliövaara, M.; Reunanen, A. Consumption of Sweetened Beverages and Intakes of Fructose and Glucose Predict Type 2 Diabetes Occurrence. J. Nutr. 2007, 137, 1447–1454. [Google Scholar] [CrossRef] [Green Version]
- Hu, F.B.; Malik, V.S. Sugar-sweetened beverages and risk of obesity and type 2 diabetes: Epidemiologic evidence. Physiol. Behav. 2010, 100, 47–54. [Google Scholar] [CrossRef] [Green Version]
- Sheludiakova, A.; Rooney, K.; Boakes, R.A. Metabolic and behavioural effects of sucrose and fructose/glucose drinks in the rat. Eur. J. Nutr. 2012, 51, 445–454. [Google Scholar] [CrossRef]
- Shintani, T.; Yamada, T.; Hayashi, N.; Iida, T.; Nagata, Y.; Ozaki, N.; Toyoda, Y. Rare Sugar Syrup Containingd-Allulose but Not High-Fructose Corn Syrup Maintains Glucose Tolerance and Insulin Sensitivity Partly via Hepatic Glucokinase Translocation in Wistar Rats. J. Agric. Food Chem. 2017, 65, 2888–2894. [Google Scholar] [CrossRef]
- Takata, T.; Sakasai-Sakai, A.; Takino, J.-I.; Takeuchi, M. Evidence for Toxic Advanced Glycation End-Products Generated in the Normal Rat Liver. Nutrients 2019, 11, 1612. [Google Scholar] [CrossRef] [Green Version]
- Mock, K.; Lateef, S.; Benedito, V.A.; Tou, J.C. High-fructose corn syrup-55 consumption alters hepatic lipid metabolism and promotes triglyceride accumulation. J. Nutr. Biochem. 2017, 39, 32–39. [Google Scholar] [CrossRef]
- Tsilas, C.S.; de Souza, R.J.; Mejia, S.B.; Mirrahimi, A.; Cozma, A.I.; Jayalath, V.H.; Ha, V.; Tawfik, R.; Di Buono, M.; Jenkins, A.L.; et al. Relation of total sugars, fructose and sucrose with incident type 2 diabetes: A systematic review and meta-analysis of prospective cohort studies. Can. Med. Assoc. J. 2017, 189, E711–E720. [Google Scholar] [CrossRef] [Green Version]
- Sadowska, J.; Rygielska, M. The effect of high fructose corn syrup on the plasma insulin and leptin concentration, body weight gain and fat accumulation in rat. Adv. Clin. Exp. Med. 2019, 28, 879–884. [Google Scholar] [CrossRef]
- Aughsteen, A.A.; Abu-Umair, M.S.; A Mahmoud, S. Biochemical analysis of serum pancreatic amylase and lipase enzymes in patients with type 1 and type 2 diabetes mellitus. Saudi Med. J. 2005, 26, 73–77. [Google Scholar]
- Madole, M.B.; Iyer, C.M.; Madivalar, M.T.; Wadde, S.K.; Howale, D.S. Evaluation of Biochemical Markers Serum Amylase and Serum Lipase for the Assessment of Pancreatic Exocrine Function in Diabetes Mellitus. J. Clin. Diagn. Res. 2016, 10, BC01–BC04. [Google Scholar] [CrossRef]
- Remedi, M.S.; Koster, J.C.; Patton, B.L.; Nichols, C.G. ATP-Sensitive K+ Channel Signaling in Glucokinase-Deficient Diabetes. Diabetes 2005, 54, 2925–2931. [Google Scholar] [CrossRef]
- Osbak, K.K.; Colclough, K.; Saint-Martin, C.; Beer, N.L.; Bellanné-Chantelot, C.; Ellard, S.; Gloyn, A.L. Update on mutations in glucokinase (GCK), which cause maturity-onset diabetes of the young, permanent neonatal diabetes, and hyperinsulinemic hypoglycemia. Hum. Mutat. 2009, 30, 1512–1526. [Google Scholar] [CrossRef]
- Nakamura, A.; Terauchi, Y. Present status of clinical deployment of glucokinase activators. J. Diabetes Investig. 2015, 6, 124–132. [Google Scholar] [CrossRef]
- Giroix, M.-H.; Jijakli, H.; Courtois, P.; Zhang, Y.; Sener, A.; Malaisse, W.J. Fructokinase activity in rat liver, ileum, parotid gland, pancreas, pancreatic islet, B and non-B islet cell homogenates. Int. J. Mol. Med. 2006, 17, 517–522. [Google Scholar] [CrossRef] [Green Version]
- Helsley, R.N.; Moreau, F.; Gupta, M.K.; Radulescu, A.; DeBosch, B.; Softic, S. Tissue-Specific Fructose Metabolism in Obesity and Diabetes. Curr. Diabetes Rep. 2020, 20, 64. [Google Scholar] [CrossRef]
- Javed, K.; Fairweather, S.J. Amino acid transporters in the regulation of insulin secretion and signalling. Biochem. Soc. Trans. 2019, 47, 571–590. [Google Scholar] [CrossRef] [Green Version]
- Lowndes, J.; Sinnett, S.S.; Rippe, J.M. No Effect of Added Sugar Consumed at Median American Intake Level on Glucose Tolerance or Insulin Resistance. Nutrients 2015, 7, 8830–8845. [Google Scholar] [CrossRef]
- Goncalves, M.D.; Lu, C.; Tutnauer, J.; Hartman, T.E.; Hwang, S.-K.; Murphy, C.J.; Pauli, C.; Morris, R.; Taylor, S.; Bosch, K.; et al. High-fructose corn syrup enhances intestinal tumor growth in mice. Science 2019, 363, 1345–1349. [Google Scholar] [CrossRef]
- Imamura, F.; O’Connor, L.; Ye, Z.; Mursu, J.; Hayashino, Y.; Bhupathiraju, S.N.; Forouhi, N.G. Consumption of sugar sweetened beverages, artificially sweetened beverages, and fruit juice and incidence of type 2 diabetes: Systematic review, meta-analysis, and estimation of population attributable fraction. BMJ 2015, 351, h3576. [Google Scholar] [CrossRef] [Green Version]
- Morgan, R.E. Does consumption of high-fructose corn syrup beverages cause obesity in children? Pediatr. Obes. 2013, 8, 249–254. [Google Scholar] [CrossRef]
- Taskinen, M.-R.; Packard, C.J.; Borén, J. Dietary Fructose and the Metabolic Syndrome. Nutrients 2019, 11, 1987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Softic, S.; Gupta, M.K.; Wang, G.-X.; Fujisaka, S.; O’Neill, B.T.; Rao, T.N.; Willoughby, J.; Harbison, C.; Fitzgerald, K.; Ilkayeva, O.; et al. Divergent effects of glucose and fructose on hepatic lipogenesis and insulin signaling. J. Clin. Investig. 2017, 127, 4059–4074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Douard, V.; Ferraris, R.P. Regulation of the fructose transporter GLUT5 in health and disease. Am. J. Physiol. Metab. 2008, 295, E227–E237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jegatheesan, P.; De Bandt, J. Fructose and NAFLD: The Multifaceted Aspects of Fructose Metabolism. Nutrients 2017, 9, 230. [Google Scholar] [CrossRef] [Green Version]
- Jang, C.; Hui, S.; Lu, W.; Cowan, A.J.; Morscher, R.J.; Lee, G.; Liu, W.; Tesz, G.J.; Birnbaum, M.J.; Rabinowitz, J.D. The Small Intestine Converts Dietary Fructose into Glucose and Organic Acids. Cell Metab. 2018, 27, 351–361.e3. [Google Scholar] [CrossRef] [Green Version]
- Ishimoto, T.; Lanaspa, M.A.; Le, M.T.; Garcia, G.E.; Diggle, C.P.; MacLean, P.S.; Jackman, M.R.; Asipu, A.; Roncal-Jimenez, C.A.; Kosugi, T.; et al. Opposing effects of fructokinase C and A isoforms on fructose-induced metabolic syndrome in mice. Proc. Natl. Acad. Sci. USA 2012, 109, 4320–4325. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Tong, X.; Vandommelen, K.; Gupta, N.; Stamper, K.; Brady, G.F.; Meng, Z.; Lin, J.; Rui, L.; Omary, M.B.; et al. Lipogenic transcription factor ChREBP mediates fructose-induced metabolic adaptations to prevent hepatotoxicity. J. Clin. Investig. 2017, 127, 2855–2867. [Google Scholar] [CrossRef] [Green Version]
- Stanhope, K.L.; Schwarz, J.M.; Keim, N.L.; Griffen, S.C.; Bremer, A.A.; Graham, J.L.; Hatcher, B.; Cox, C.L.; Dyachenko, A.; Zhang, W.; et al. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J. Clin. Investig. 2009, 119, 1322–1334. [Google Scholar] [CrossRef] [Green Version]
- Zubiría, M.G.; Alzamendi, A.; Moreno, G.; Rey, M.A.; Spinedi, E.; Giovambattista, A. Long-Term Fructose Intake Increases Adipogenic Potential: Evidence of Direct Effects of Fructose on Adipocyte Precursor Cells. Nutrients 2016, 8, 198. [Google Scholar] [CrossRef] [Green Version]
- Melloul, D.; Marshak, S.; Cerasi, E. Regulation of insulin gene transcription. Diabetologia 2002, 45, 309–326. [Google Scholar] [CrossRef] [Green Version]
- Kulkarni, R.N.; Jhala, U.S.; Winnay, J.N.; Krajewski, S.; Montminy, M.; Kahn, C.R. PDX-1 haploinsufficiency limits the compensatory islet hyperplasia that occurs in response to insulin resistance. J. Clin. Investig. 2004, 114, 828–836. [Google Scholar] [CrossRef] [Green Version]
- Ahlgren, U.; Jonsson, J.; Jonsson, L.; Simu, K.; Edlund, H. beta -Cell-specific inactivation of the mouse Ipf1/Pdx1 gene results in loss of the beta -cell phenotype and maturity onset diabetes. Genes Dev. 1998, 12, 1763–1768. [Google Scholar] [CrossRef] [Green Version]
- Gao, T.; McKenna, B.; Li, C.; Reichert, M.; Nguyen, J.; Singh, T.; Yang, C.; Pannikar, A.; Doliba, N.; Zhang, T.; et al. Pdx1 Maintains β Cell Identity and Function by Repressing an α Cell Program. Cell Metab. 2014, 19, 259–271. [Google Scholar] [CrossRef] [Green Version]
- Kurahashi, M.; Nakamura, H.; Inomata, K. Effect of streptozotocin-induced diabetes on amylase activity in rat pancreas and parotid glands. Jpn. J. Oral Biol. 1985, 27, 992–995. [Google Scholar] [CrossRef] [Green Version]
- Murakami, H.; Matsumoto, M.; Inoue, H.; Kaji, Y. Digestive enzyme activities of pancreas and intestinal digesta in streptozotocin-induced diabetic piglets. Anim. Sci. J. 2007, 78, 55–60. [Google Scholar] [CrossRef]
- Fujimoto, S.; Mukai, E.; Inagaki, N. Role of endogenous ROS production in impaired metabolism-secretion coupling of diabetic pancreatic β cells. Prog. Biophys. Mol. Biol. 2011, 107, 304–310. [Google Scholar] [CrossRef]
- Tappy, L.; Lê, K.-A. Metabolic Effects of Fructose and the Worldwide Increase in Obesity. Physiol. Rev. 2010, 90, 23–46. [Google Scholar] [CrossRef] [Green Version]
- Bartley, C.; Brun, T.; Oberhauser, L.; Grimaldi, M.; Molica, F.; Kwak, B.R.; Bosco, D.; Chanson, M.; Maechler, P. Chronic fructose renders pancreatic β-cells hyper-responsive to glucose-stimulated insulin secretion through extracellular ATP signaling. Am. J. Physiol. Metab. 2019, 317, E25–E41. [Google Scholar] [CrossRef] [Green Version]
- Thorens, B. GLUT2 in pancreatic and extra-pancreatic gluco-detection. Mol. Membr. Biol. 2001, 18, 265–273. [Google Scholar] [CrossRef]
- Holman, G.D. Structure, function and regulation of mammalian glucose transporters of the SLC2 family. Pflügers Arch. Eur. J. Physiol. 2020, 472, 1155–1175. [Google Scholar] [CrossRef]
- Ohtsubo, K.; Takamatsu, S.; Minowa, M.T.; Yoshida, A.; Takeuchi, M.; Marth, J.D. Dietary and Genetic Control of Glucose Transporter 2 Glycosylation Promotes Insulin Secretion in Suppressing Diabetes. Cell 2005, 123, 1307–1321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- German, M.S. Glucose sensing in pancreatic islet beta cells: The key role of glucokinase and the glycolytic intermediates. Proc. Natl. Acad. Sci. USA 1993, 90, 1781–1785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henquin, J.-C. Triggering and amplifying pathways of regulation of insulin secretion by glucose. Diabetes 2000, 49, 1751–1760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ježek, P.; Jabůrek, M.; Plecitá-Hlavatá, L. Contribution of Oxidative Stress and Impaired Biogenesis of Pancreatic β-Cells to Type 2 Diabetes. Antioxidants Redox Signal. 2019, 31, 722–751. [Google Scholar] [CrossRef] [Green Version]
- Marrano, N.; Biondi, G.; Cignarelli, A.; Perrini, S.; Laviola, L.; Giorgino, F.; Natalicchio, A. Functional loss of pancreatic islets in type 2 diabetes: How can we halt it? Metabolism 2020, 110, 154304. [Google Scholar] [CrossRef] [PubMed]
- Otter, S.; Lammert, E.; Information, P.E.K.F.C. Exciting Times for Pancreatic Islets: Glutamate Signaling in Endocrine Cells. Trends Endocrinol. Metab. 2016, 27, 177–188. [Google Scholar] [CrossRef]
- Gammelsaeter, R.; Jenstad, M.; Bredahl, M.; Gundersen, V.; Chaudhry, F. Complementary expression of SN1 and SAT2 in the islets of Langerhans suggests concerted action of glutamine transport in the regulation of insulin secretion. Biochem. Biophys. Res. Commun. 2009, 381, 378–382. [Google Scholar] [CrossRef]
- Rubio-Aliaga, I.; Wagner, C.A. Regulation and function of the SLC38A3/SNAT3 glutamine transporter. Channels 2016, 10, 440–452. [Google Scholar] [CrossRef]
- Catalán, V.; Gómez-Ambrosi, J.; Ramirez, B.; Rotellar, F.; Pastor, C.; Silva, C.; Rodríguez, A.; Gil, M.J.; Cienfuegos, J.A.; Frühbeck, G. Proinflammatory Cytokines in Obesity: Impact of Type 2 Diabetes Mellitus and Gastric Bypass. Obes. Surg. 2007, 17, 1464–1474. [Google Scholar] [CrossRef]
- Xu, H.; Barnes, G.T.; Yang, Q.; Tan, G.; Yang, D.; Chou, C.J.; Sole, J.; Nichols, A.; Ross, J.S.; Tartaglia, L.A.; et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Investig. 2003, 112, 1821–1830. [Google Scholar] [CrossRef]
- Pajvani, U.B.; Scherer, P.E. Adiponectin: Systemic contributor to insulin sensitivity. Curr. Diabetes Rep. 2003, 3, 207–213. [Google Scholar] [CrossRef]
- Duncan, B.B.; Schmidt, M.I.; Pankow, J.S.; Bang, H.; Couper, D.; Ballantyne, C.M.; Hoogeveen, R.C.; Heiss, G. Adiponectin and the Development of Type 2 Diabetes: The Atherosclerosis Risk in Communities Study. Diabetes 2004, 53, 2473–2478. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Meng, R.-W.; Kunutsor, S.K.; Chowdhury, R.; Yuan, J.-M.; Koh, W.-P.; Pan, A. Plasma adiponectin levels and type 2 diabetes risk: A nested case-control study in a Chinese population and an updated meta-analysis. Sci. Rep. 2018, 8, 406. [Google Scholar] [CrossRef]
- Arora, A.; Behl, T.; Sehgal, A.; Singh, S.; Sharma, N.; Bhatia, S.; Sobarzo-Sanchez, E.; Bungau, S. Unravelling the involvement of gut microbiota in type 2 diabetes mellitus. Life Sci. 2021, 273, 119311. [Google Scholar] [CrossRef]
- Boulangé, C.L.; Neves, A.L.; Chilloux, J.; Nicholson, J.K.; Dumas, M.-E. Impact of the gut microbiota on inflammation, obesity, and metabolic disease. Genome Med. 2016, 8, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Bhat, S.F.; Pinney, S.E.; Kennedy, K.M.; McCourt, C.R.; Mundy, M.A.; Surette, M.G.; Sloboda, D.M.; Simmons, R.A. Exposure to high fructose corn syrup during adolescence in the mouse alters hepatic metabolism and the microbiome in a sex-specific manner. J. Physiol. 2021, 599, 1487–1511. [Google Scholar] [CrossRef] [PubMed]
- Mazzoli, A.; Gatto, C.; Crescenzo, R.; Cigliano, L.; Iossa, S. Prolonged Changes in Hepatic Mitochondrial Activity and Insulin Sensitivity by High Fructose Intake in Adolescent Rats. Nutrients 2021, 13, 1370. [Google Scholar] [CrossRef] [PubMed]
- Wang, C. Sex-specific metabolic changes induced by high fructose corn syrup during adolescence: Novel evidence from metabolomic and microbiome analyses in mice. J. Physiol. 2021, 599, 2143–2144. [Google Scholar] [CrossRef] [PubMed]




| Gene | Forward | Reverse |
|---|---|---|
| Ins1 | TCAGAGACCATCAGCAAGCA | AGAGAGCCTCTACCAGGTGG |
| Ins2 | CACCCAGGCTTTTGTCAAGC | TGCCAAGGTCTGAAGGTCAC |
| Pdx1 | AAATCCACCAAAGCTCACGC | GCAGTACGGGTCCTCTTGTT |
| Amy2a5 | CAAAATGGTTCTCCCAAGGA | CATCTTCTCGCCATTCCACT |
| Amy2b | GATGCTTATCAGGTTATTGATCTGG | TCTCTCCATTCCACTTGCGG |
| Prss2 | TGATCTGTGTTGGCTTCCTG | CCAGTCCACGTAGTTGCAGA |
| Pnlip | TTCCCGAACGACATATACCC | GCTCCAAAGGTCCTCTTTCC |
| Glut2 | GACTGGAGCCCTCTTGATGG | GTGTGGTTGGAGCGATCTCT |
| Gck | CAGGACAGTGGAGCGTGAA | TCCAGGAAGTCAGAGATGCAC |
| Khk | GCGTGGATGTGTCTCAAGTG | GCAGGTTCGTGTCGTAGAGT |
| SLC38A3 | CGACAGACAGAGATGGTGGA | CCTCGAAATCGGTGAAGTGT |
| Gls | GCGGGCGACAATAAAATAAA | CACTCTTTCAACCTGGGATCA |
| Glud1 | TGGCCTACACAATGGAGAGA | TCAGGTCCAATCCCAGGTTA |
| 18S rRNA | AACGAACGAGACTCTGGCAT | CGGACATCTAAGGGCATCACAG |
| Parameters | Control Group (n = 6) | HFCS Group (n = 6) |
|---|---|---|
| TG (mg/dL) | 39.72 ± 6.81 | 45.58 ± 6.80 |
| T-Cho (mg/dL) | 84.18 ± 3.66 | 94.04 ± 2.77 |
| UA (mg/dL) | 1.24 ± 0.24 | 1.04 ± 0.14 |
| BUN (mg/dL) | 25.05 ± 1.44 | 20.49 ± 1.43 * |
| AST (U/L) | 112.46 ± 16.50 | 76.74 ± 9.28 |
| ALT (U/L) | 39.2 ± 3.51 | 29.58 ± 1.46 ** |
| TP (g/L) | 4.67 ± 0.09 | 4.38 ± 0.06 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hattori, H.; Hanai, Y.; Oshima, Y.; Kataoka, H.; Eto, N. Excessive Intake of High-Fructose Corn Syrup Drinks Induces Impaired Glucose Tolerance. Biomedicines 2021, 9, 541. https://doi.org/10.3390/biomedicines9050541
Hattori H, Hanai Y, Oshima Y, Kataoka H, Eto N. Excessive Intake of High-Fructose Corn Syrup Drinks Induces Impaired Glucose Tolerance. Biomedicines. 2021; 9(5):541. https://doi.org/10.3390/biomedicines9050541
Chicago/Turabian StyleHattori, Hidemi, Yuma Hanai, Yuto Oshima, Hiroaki Kataoka, and Nozomu Eto. 2021. "Excessive Intake of High-Fructose Corn Syrup Drinks Induces Impaired Glucose Tolerance" Biomedicines 9, no. 5: 541. https://doi.org/10.3390/biomedicines9050541
APA StyleHattori, H., Hanai, Y., Oshima, Y., Kataoka, H., & Eto, N. (2021). Excessive Intake of High-Fructose Corn Syrup Drinks Induces Impaired Glucose Tolerance. Biomedicines, 9(5), 541. https://doi.org/10.3390/biomedicines9050541






