Serum Amyloid A1/Toll-Like Receptor-4 Axis, an Important Link between Inflammation and Outcome of TBI Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Approval
2.2. Human Samples
2.3. Intracranial Clinical Severity Evaluation
2.4. Outcome
2.5. ELISA Assay
2.6. Animals
2.7. Mixed Glial Cultures
2.8. Closed Head Injury (CHI) Model
2.9. Neurological Severity Score (NSS) Test
2.10. Blood–Brain Barrier Integrity Assessment
2.11. RNA Extraction and Quantitative Real-Time PCR
2.12. Immunoblotting and Image Analysis
2.13. Immunofluorescence
2.14. Statistics
3. Results
3.1. Expression Profile of SAA1 and S100B and Their Correlation with Injury Severity
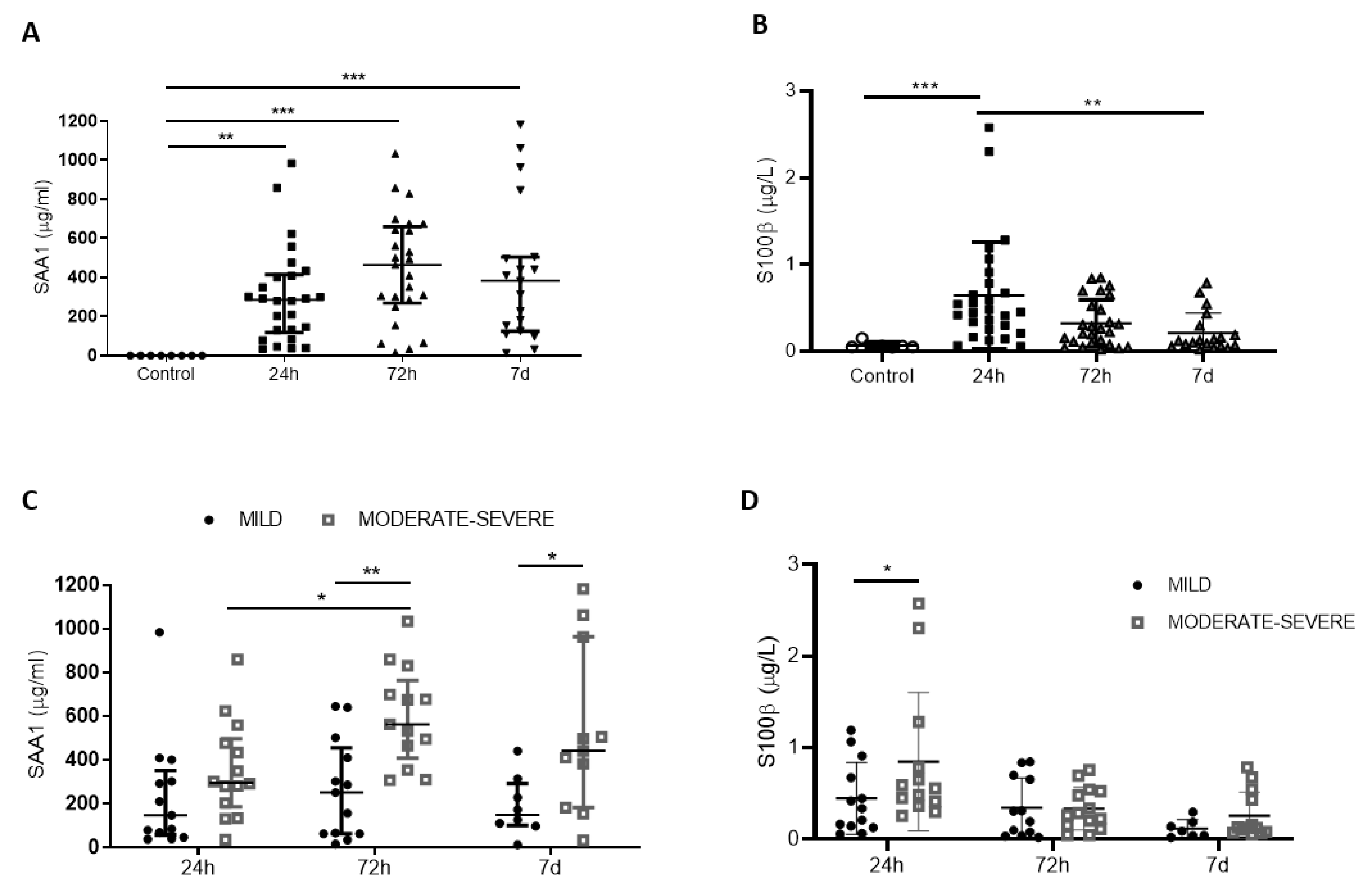
3.2. Correlation of SAA1 Alone or in Combination with S100B with Neurological Outcome 6 Months after TBI
| Cut-Off (µg/mL) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|
| >381.2 | 84.62 | 69.2 | 73.3 | 80 |

3.3. Positive Correlation between SAA1 Serum Levels and TLR4 mRNA in White Blood Cells from Human TBI Patients
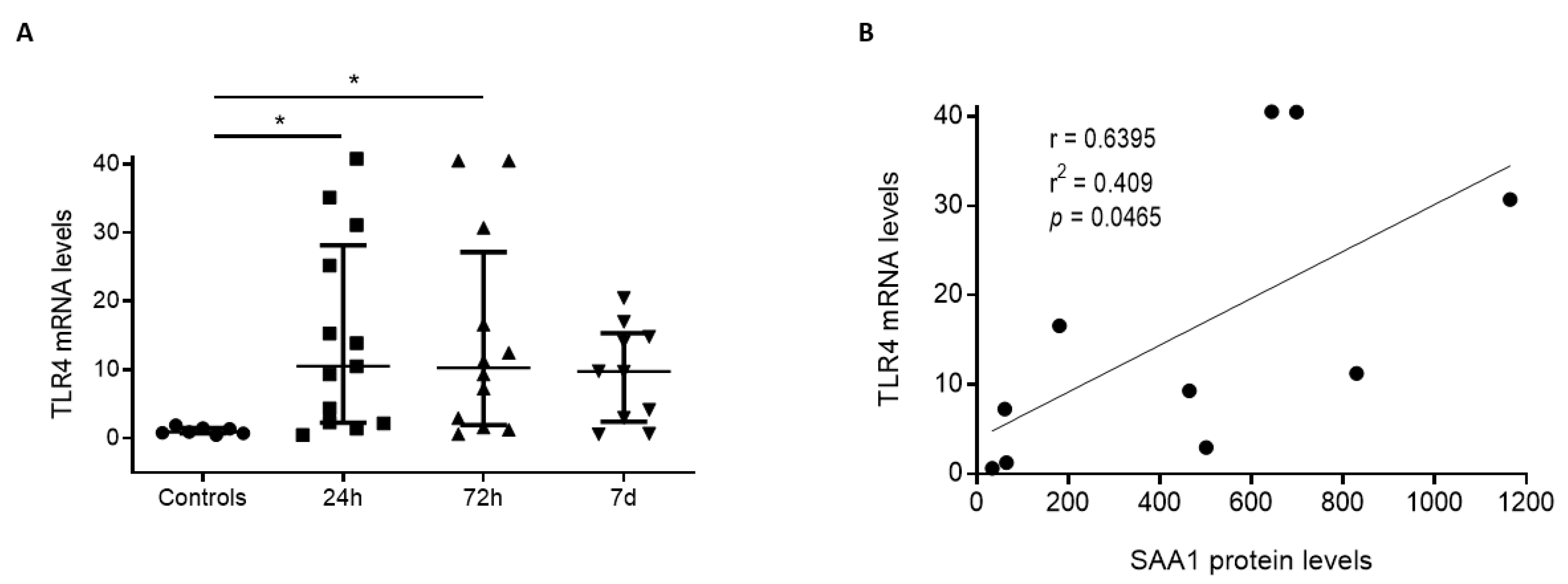
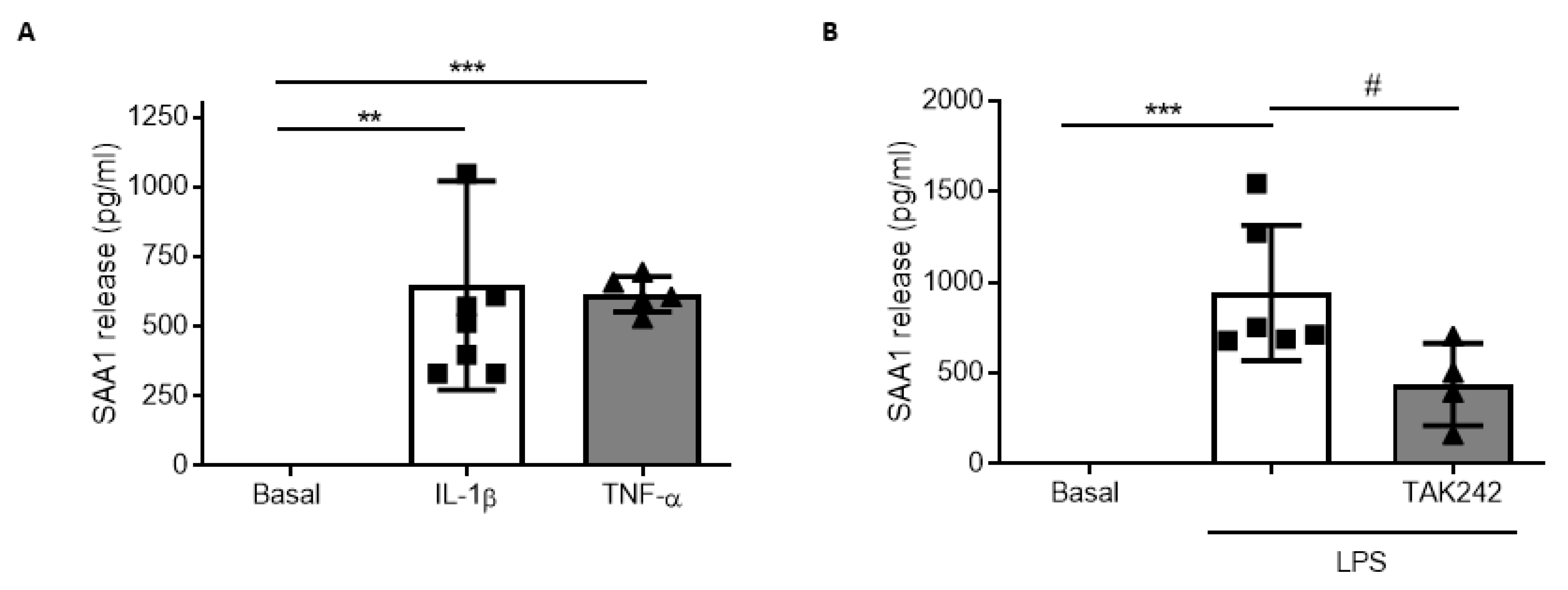
3.4. SAA1 Contributes to Inflammation in Glial Cultures via TLR4 Activation

3.5. Post-TBI Treatment with the TLR4 Antagonist TAK242 Reduces SAA1 Levels
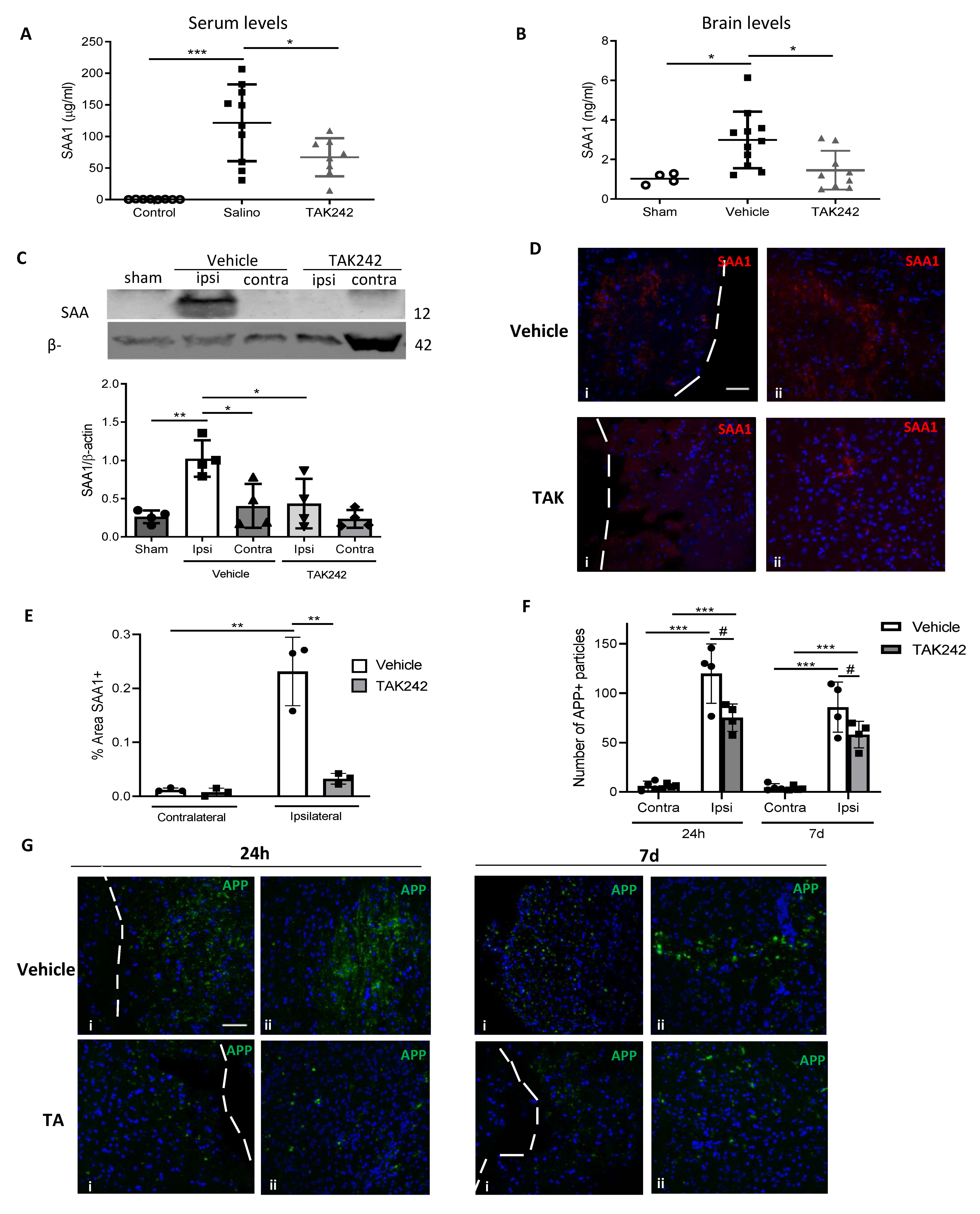
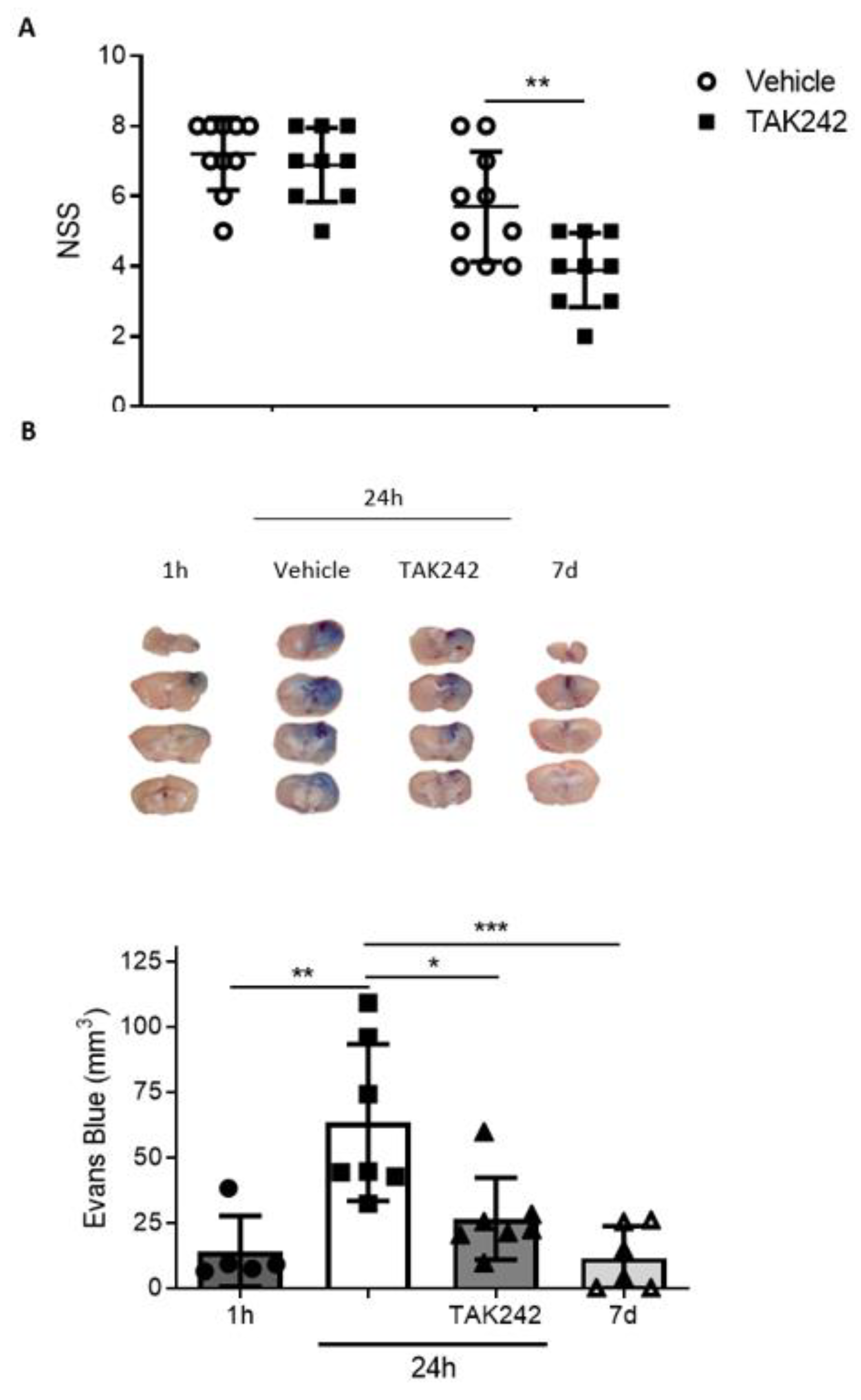
3.6. Treatment with TAK242 Improves Neurobehavioral Outcome and Prevents Blood-Brain Barrier Disruption
4. Discussion
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Dixon, K.J. Pathophysiology of Traumatic Brain Injury. Phys. Med. Rehabil. Clin. N. Am. 2017, 28, 215–225. [Google Scholar] [CrossRef]
- Jarrahi, A.; Braun, M.; Ahluwalia, M.; Gupta, R.V.; Wilson, M.; Munie, S.; Ahluwalia, P.; Vender, J.R.; Vale, F.L.; Dhandapani, K.M.; et al. Revisiting traumatic brain injury: From molecular mechanisms to therapeutic interventions. Biomedicines 2020, 8, 389. [Google Scholar] [CrossRef]
- Prins, M.; Greco, T.; Alexander, D.; Giza, C.C. The pathophysiology of traumatic brain injury at a glance. DMM Dis. Model. Mech. 2013, 6, 1307–1315. [Google Scholar] [CrossRef] [Green Version]
- Capizzi, A.; Woo, J.; Verduzco-Gutierrez, M. Traumatic Brain Injury: An Overview of Epidemiology, Pathophysiology, and Medical Management. Med. Clin. N. Am. 2020, 104, 213–238. [Google Scholar] [CrossRef]
- Needham, E.J.; Helmy, A.; Zanier, E.R.; Jones, J.L.; Coles, A.J.; Menon, D.K. The immunological response to traumatic brain injury. J. Neuroimmunol. 2019, 332, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Hinson, H.E.; Rowell, S.; Schreiber, M. Clinical evidence of inflammation driving secondary brain injury: A systematic review. J. Trauma Acute Care Surg. 2015, 78, 184–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janssens, S.; Beyaert, R. Role of Toll-Like Receptors in Pathogen Recognition. Clin. Microbiol. Rev. 2003, 16, 637–646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaure, C.; Liu, Y. A comparative review of toll-like receptor 4 expression and functionality in different animal species. Front. Immunol. 2014, 5, 316. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.K.; Liu, J.T.; Peng, Z.W.; Fan, H.; Yao, A.H.; Cheng, P.; Liu, L.; Ju, G.; Kuang, F. Different TLR4 expression and microglia/macrophage activation induced by hemorrhage in the rat spinal cord after compressive injury. J. Neuroinflamm. 2013, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, X.; Liu, S.; Ding, W.; Yue, P.; Jiang, Q.; Zhao, M.; Hu, F.; Zhang, H. TLR4 signal ablation attenuated neurological deficits by regulating microglial M1/M2 phenotype after traumatic brain injury in mice. J. Neuroimmunol. 2017, 310, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Manivannan, S.; Makwana, M.; Ahmed, A.I.; Zaben, M. Profiling biomarkers of traumatic axonal injury: From mouse to man. Clin. Neurol. Neurosurg. 2018, 171, 6–20. [Google Scholar] [CrossRef] [PubMed]
- Blennow, K.; Brody, D.L.; Kochanek, P.M.; Levin, H.; McKee, A.; Ribbers, G.M.; Yaffe, K.; Zetterberg, H. Traumatic brain injuries. Nat. Rev. Dis. Prim. 2016, 2. [Google Scholar] [CrossRef]
- Zetterberg, H.; Blennow, K. Fluid biomarkers for mild traumatic brain injury and related conditions. Nat. Rev. Neurol. 2016, 12, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Gabay, C.; Kushner, I. Acute-Phase Proteins and Other Systemic Responses to Inflammation. N. Engl. J. Med. 1999, 340, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Carabias, C.S.; Castaño-León, A.M.; Blanca Navarro, B.; Panero, I.; Eiriz, C.; Gómez, P.A.; Egea, J.; Lagares, A. Serum Amyloid A1 as a Potential Intracranial and Extracranial Clinical Severity Biomarker in Traumatic Brain Injury. J. Intensive Care Med. 2020, 35, 1180–1195. [Google Scholar] [CrossRef]
- Parada, E.; Buendia, I.; Navarro, E.; Avendaño, C.; Egea, J.; López, M.G. Microglial HO-1 induction by curcumin provides antioxidant, antineuroinflammatory, and glioprotective effects. Mol. Nutr. Food Res. 2015, 59, 1690–1700. [Google Scholar] [CrossRef]
- Flierl, M.A.; Stahel, P.F.; Beauchamp, K.M.; Morgan, S.J.; Smith, W.R.; Shohami, E. Mouse closed head injury model induced by a weight-drop device. Nat. Protoc. 2009, 4, 1328–1337. [Google Scholar] [CrossRef]
- Wilson, J.T.L.; Pettigrew, L.E.L.; Teasdale, G.M. Structured interviews for the glasgow outcome scale and the extended glasgow outcome scale: Guidelines for their use. J. Neurotrauma 1998, 15, 573–580. [Google Scholar] [CrossRef]
- Sandri, S.; Rodriguez, D.; Gomes, E.; Monteiro, H.P.; Russo, M.; Campa, A. Is serum amyloid A an endogenous TLR4 agonist? J. Leukoc. Biol. 2008, 83, 1174–1180. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Zhu, S.; Li, J.; D’Amore, J.; D’Angelo, J.; Yang, H.; Wang, P.; Tracey, K.J.; Wang, H. Serum amyloid a stimulates PKR expression and HMGB1 release possibly through TLR4/RAGE receptors. Mol. Med. 2015, 21, 515–525. [Google Scholar] [CrossRef]
- Hahn, A.; Kny, M.; Pablo-Tortola, C.; Todiras, M.; Willenbrock, M.; Schmidt, S.; Schmoeckel, K.; Jorde, I.; Nowak, M.; Jarosch, E.; et al. Serum amyloid A1 mediates myotube atrophy via Toll-like receptors. J. Cachexia Sarcopenia Muscle 2020, 11, 103–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, S.; Oh, J.H.; Shin, C.Y.; Yoon, H.S.; Lee, D.H.; Chung, J.H. Serum amyloid A1 is induced by UV irradiation and detected by toll-like receptor 4 to causes skin inflammation. J. Dermatol. Sci. 2016, 84, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Hirai, K.; Furusho, H.; Kawashima, N.; Xu, S.; de Beer, M.C.; Battaglino, R.; Van Dyke, T.; Stashenko, P.; Sasaki, H. Serum Amyloid A Contributes to Chronic Apical Periodontitis via TLR2 and TLR4. J. Dent. Res. 2019, 98, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Brea, D.; Blanco, M.; Ramos-Cabrer, P.; Moldes, O.; Arias, S.; Pérez-Mato, M.; Leira, R.; Sobrino, T.; Castillo, J. Toll-like receptors 2 and 4 in ischemic stroke: Outcome and therapeutic values. J. Cereb. Blood Flow Metab. 2011, 31, 1424–1431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Seny, D.; Cobraiville, G.; Charlier, E.; Neuville, S.; Esser, N.; Malaise, D.; Malaise, O.; Calvo, F.Q.; Relic, B.; Malaise, M.G. Acute-Phase Serum Amyloid A in Osteoarthritis: Regulatory Mechanism and Proinflammatory Properties. PLoS ONE 2013, 8, e66769. [Google Scholar] [CrossRef] [Green Version]
- Yu, N.; Liu, S.; Yi, X.; Zhang, S.; Ding, Y. Serum amyloid A induces interleukin-1β secretion from keratinocytes via the NACHT, LRR and PYD domains-containing protein 3 inflammasome. Clin. Exp. Immunol. 2015, 179, 344–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agoston, D.V.; Vink, R.; Helmy, A.; Risling, M.; Nelson, D.; Prins, M. How to Translate Time: The Temporal Aspects of Rodent and Human Pathobiological Processes in Traumatic Brain Injury. J. Neurotrauma 2019, 36, 1724–1737. [Google Scholar] [CrossRef]
- Zhang, D.; Li, H.; Li, T.; Zhou, M.; Hao, S.; Yan, H.; Yu, Z.; Li, W.; Li, K.; Hang, C. TLR4 inhibitor resatorvid provides neuroprotection in experimental traumatic brain injury: Implication in the treatment of human brain injury. Neurochem. Int. 2014, 75, 11–18. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Li, J.; Ye, Q.; Dong, Y.; Bao, G.M.; Shen, Y.K.; Weng, J.F.; Luo, L.F.; Cen, M. Usefulness of serum interleukin-33 as a prognostic marker of severe traumatic brain injury. Clin. Chim. Acta 2019, 497, 6–12. [Google Scholar] [CrossRef]
- Mahan, M.Y.; Thorpe, M.; Ahmadi, A.; Abdallah, T.; Casey, H.; Sturtevant, D.; Judge-Yoakam, S.; Hoover, C.; Rafter, D.; Miner, J.; et al. Glial Fibrillary Acidic Protein (GFAP) Outperforms S100 Calcium-Binding Protein B (S100B) and Ubiquitin C-Terminal Hydrolase L1 (UCH-L1) as Predictor for Positive Computed Tomography of the Head in Trauma Subjects. World Neurosurg. 2019, 128, e434–e444. [Google Scholar] [CrossRef]
- Lagerstedt, L.; Egea-Guerrero, J.J.; Bustamante, A.; Montaner, J.; Rodríguez-Rodríguez, A.; El Rahal, A.; Turck, N.; Quintana, M.; García-Armengol, R.; Prica, C.M.; et al. H-FABP: A new biomarker to differentiate between CT-positive and CT-negative patients with mild traumatic brain injury. PLoS ONE 2017, 12, e0175572. [Google Scholar] [CrossRef] [PubMed]
- Thelin, E.; Al Nimer, F.; Frostell, A.; Zetterberg, H.; Blennow, K.; Nyström, H.; Svensson, M.; Bellander, B.M.; Piehl, F.; Nelson, D.W. A Serum protein biomarker panel improves outcome prediction in human traumatic brain injury. J. Neurotrauma 2019, 36, 2850–2862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez-Yáñez, M.; Brea, D.; Arias, S.; Blanco, M.; Pumar, J.M.; Castillo, J.; Sobrino, T. Increased expression of Toll-like receptors 2 and 4 is associated with poor outcome in intracerebral hemorrhage. J. Neuroimmunol. 2012, 247, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Chitnis, T.; Weiner, H.L. CNS inflammation and neurodegeneration. J. Clin. Investig. 2017, 127, 3577–3587. [Google Scholar] [CrossRef] [Green Version]
- Labzin, L.I.; Heneka, M.T.; Latz, E. Innate Immunity and Neurodegeneration. Annu. Rev. Med. 2018, 69, 437–449. [Google Scholar] [CrossRef] [PubMed]
- Sevenich, L. Brain-resident microglia and blood-borne macrophages orchestrate central nervous system inflammation in neurodegenerative disorders and brain cancer. Front. Immunol. 2018, 9, 697. [Google Scholar] [CrossRef] [PubMed]
- Passey, S.L.; Bozinovski, S.; Vlahos, R.; Anderson, G.P.; Hansen, M.J. Serum amyloid A induces toll-like receptor 2-dependent inflammatory cytokine expression and atrophy in C2C12 skeletal muscle myotubes. PLoS ONE 2016, 11, e0146882. [Google Scholar] [CrossRef]
- Jang, W.Y.; Lee, B.R.; Jeong, J.; Sung, Y.; Choi, M.; Song, P.; Kim, H.; Jang, S.; Kim, H.; Joo, K., II; et al. Overexpression of serum amyloid a 1 induces depressive-like behavior in mice. Brain Res. 2017, 1654, 55–65. [Google Scholar] [CrossRef]
- Jang, S.; Jang, W.Y.; Choi, M.; Lee, J.; Kwon, W.; Yi, J.; Park, S.J.; Yoon, D.; Lee, S.; Kim, M.O.; et al. Serum amyloid A1 is involved in amyloid plaque aggregation and memory decline in amyloid beta abundant condition. Transgenic Res. 2019, 28, 499–508. [Google Scholar] [CrossRef]
- Kindy, M.S.; Yu, J.; Guo, J.T.; Zhu, H. Apolipoprotein serum amyloid A in Alzheimer’s disease. J. Alzheimer’s Dis. 1999, 1, 155–167. [Google Scholar] [CrossRef]
| Controls | 24 h | 72 h | 7 d | |
|---|---|---|---|---|
| N (%) * | 8 | 29 (85.3) | 28 (82) | 21 (61.7) |
| Sex, male/female | 4/4 | 22/7 | 21/7 | 16/5 |
| Age, mean | 44 ± 17.6 | 52.8 ± 18.3 | ||
| Severity (GCS) | ||||
| mild | 15 | 15 | 10 | |
| moderate | 4 | 3 | 4 | |
| severe | 10 | 10 | 7 | |
| Outcome at 6 months (GOSE) | ||||
| Favorable | 13 | 13 | 8 | |
| Unfavorable | 13 | 13 | 11 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farré-Alins, V.; Palomino-Antolín, A.; Narros-Fernández, P.; Lopez-Rodriguez, A.B.; Decouty-Perez, C.; Muñoz-Montero, A.; Zamorano-Fernández, J.; Mansilla-Fernández, B.; Giner-García, J.; García-Feijoo, P.; et al. Serum Amyloid A1/Toll-Like Receptor-4 Axis, an Important Link between Inflammation and Outcome of TBI Patients. Biomedicines 2021, 9, 599. https://doi.org/10.3390/biomedicines9060599
Farré-Alins V, Palomino-Antolín A, Narros-Fernández P, Lopez-Rodriguez AB, Decouty-Perez C, Muñoz-Montero A, Zamorano-Fernández J, Mansilla-Fernández B, Giner-García J, García-Feijoo P, et al. Serum Amyloid A1/Toll-Like Receptor-4 Axis, an Important Link between Inflammation and Outcome of TBI Patients. Biomedicines. 2021; 9(6):599. https://doi.org/10.3390/biomedicines9060599
Chicago/Turabian StyleFarré-Alins, Víctor, Alejandra Palomino-Antolín, Paloma Narros-Fernández, Ana Belen Lopez-Rodriguez, Céline Decouty-Perez, Alicia Muñoz-Montero, Jorge Zamorano-Fernández, Beatriz Mansilla-Fernández, Javier Giner-García, Pablo García-Feijoo, and et al. 2021. "Serum Amyloid A1/Toll-Like Receptor-4 Axis, an Important Link between Inflammation and Outcome of TBI Patients" Biomedicines 9, no. 6: 599. https://doi.org/10.3390/biomedicines9060599






