MyD88 Is Not Required for Muscle Injury-Induced Endochondral Heterotopic Ossification in a Mouse Model of Fibrodysplasia Ossificans Progressiva
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Husbandry and Breeding
2.2. Tamoxifen Treatment and Induction of Heterotopic Ossification in FOP Mice by Pinch Injury
2.3. Radiographs and Microcomputed Tomography (microCT)
2.4. Histology
2.5. Isolation and Culture of Intramuscular Fibroadipoprogenitors
2.5.1. Skeletal Muscle Digestion
2.5.2. MACS and FACS
2.5.3. FAP Plating and Culture during Expansion
2.6. FAP Treatments and Measurement of Smad1/5 Phosphorylation by Western Blot
2.7. Statistical Analysis
3. Results
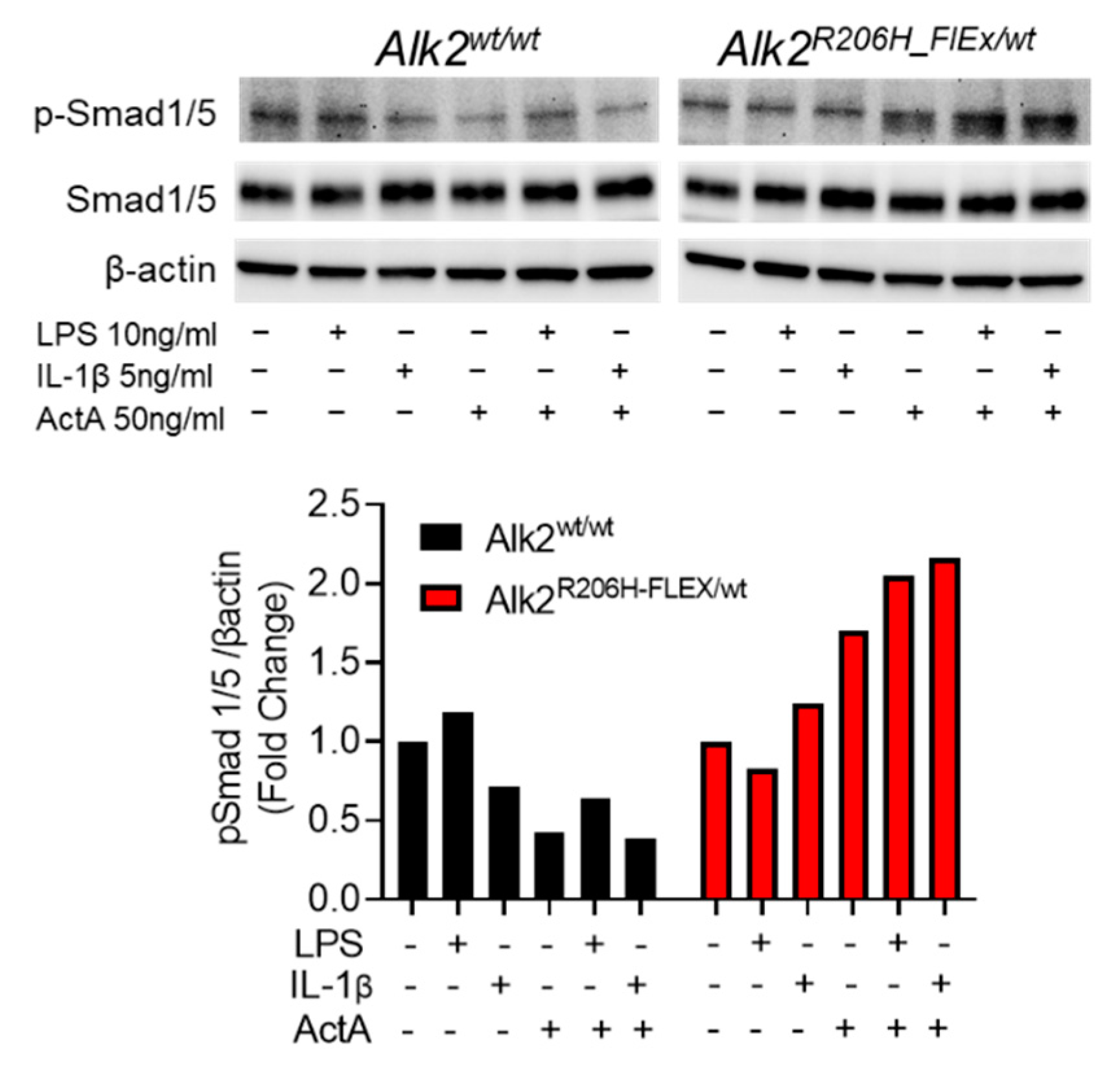
3.1. Activation of MyD88-Dependent Signaling Pathways Enhances Aberrant ActA-Induced Smad1/5 Phosphorylation in FOP FAPs
3.2. Conditional Deletion of MyD88 in Pdfgrα-Positive Cells Does Not Alter the Volume of Injury-Induced EHO
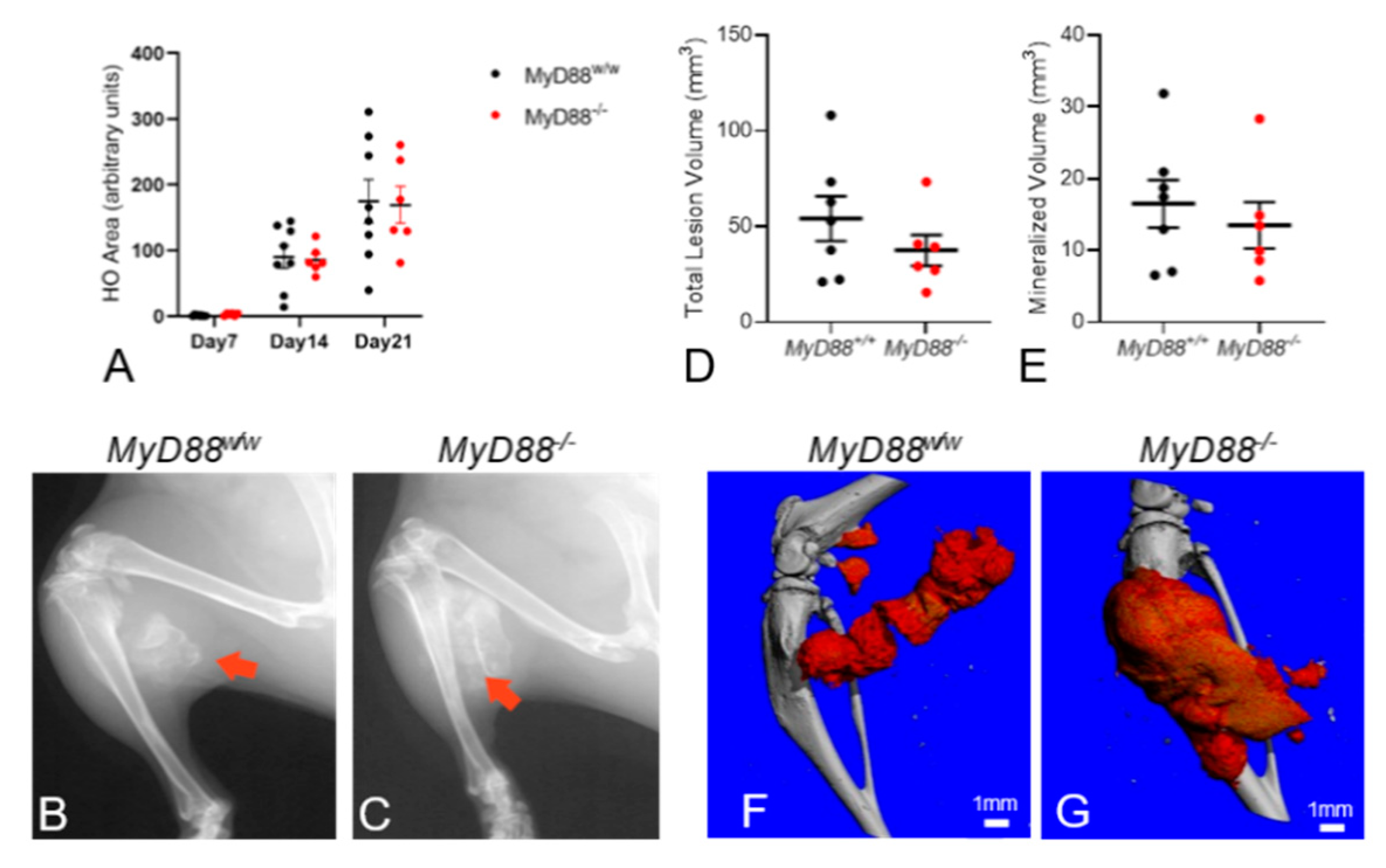
3.3. Global Deletion of MyD88 does Not Alter Muscle Injury-Induced EHO Formation in FOP Mice
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shore, E.M.; Xu, M.; Feldman, G.J.; Fenstermacher, D.A.; Cho, T.J.; Choi, I.H.; Kaplan, F.S. A recurrent mutation in the BMP type I receptor Acvr1 causes inherited and sporadic fibrodysplasia ossificans progressiva. Nat. Genet. 2006, 38, 525–527. [Google Scholar] [CrossRef]
- Huning, I.; Gillessen-Kaesbach, G. Fibrodysplasia Ossificans Progressiva: Clinical Course, Genetic Mutations and Genotype-Phenotype Correlation. Mol. Syndromol. 2014, 5, 201–211. [Google Scholar] [CrossRef]
- Smith, R.; Athanasou, N.A.; Vipond, S.E. Fibrodysplasia (myositis) ossificans progressiva: Clinicopathological features and natural history. QJM 1996, 89, 445–446. [Google Scholar] [CrossRef] [PubMed]
- Virdi, A.S.; Shore, E.M.; Oreffo, R.O.; Li, M.; Connor, J.M.; Smith, R.; Kaplan, F.S.; Triffitt, J.T. Phenotypic and molecular heterogeneity in fibrodysplasia ossificans progressiva. Calcif. Tissue Int. 1999, 65, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Pignolo, R.J.; Shore, E.M.; Kaplan, F.S. Fibrodysplasia ossificans progressiva: Clinical and genetic aspects. Orphanet J. Rare Dis. 2011, 6, 80. [Google Scholar] [CrossRef] [PubMed]
- Pignolo, R.J.; Bedford-Gay, C.; Liljesthrom, M.; Durbin-Johnson, B.P.; Shore, E.M.; Rocke, D.M.; Kaplan, F.S. The Natural History of Flare-Ups in Fibrodysplasia Ossificans Progressiva (FOP): A Comprehensive Global Assessment. J. Bone Miner. Res. 2016, 31, 650–656. [Google Scholar] [CrossRef]
- Kaplan, F.S.; Al Mukaddam, M.; Pignolo, R.J. Longitudinal Patient-Reported Mobility Assessment In Fibrodysplasia Ossificans Progressiva (Fop). Bone 2017. [Google Scholar] [CrossRef] [PubMed]
- Pignolo, R.J.; Durbin-Johnson, B.P.; Rocke, D.M.; Kaplan, F.S. Joint-Specific Risk Of Impaired Function In Fibrodysplasia Ossificans Progressiva (Fop). Bone 2017. [Google Scholar] [CrossRef]
- Dey, D.; Bagarova, J.; Hatsell, S.J.; Armstrong, K.A.; Huang, L.; Ermann, J.; Vonner, A.J.; Shen, Y.; Mohedas, A.H.; Lee, A.; et al. Two tissue-resident progenitor lineages drive distinct phenotypes of heterotopic ossification. Sci. Transl. Med. 2016, 8, 366ra163. [Google Scholar] [CrossRef]
- Lees-Shepard, J.B.; Yamamoto, M.; Biswas, A.A.; Stoessel, S.J.; Nicholas, S.E.; Cogswell, C.A.; Devarakonda, P.M.; Schneider, M.J., Jr.; Cummins, S.M.; Legendre, N.P.; et al. Activin-dependent signaling in fibro/adipogenic progenitors causes fibrodysplasia ossificans progressiva. Nat. Commun. 2018, 9, 471. [Google Scholar] [CrossRef]
- Pulik, L.; Mierzejewski, B.; Ciemerych, M.A.; Brzoska, E.; Legosz, P. The Survey of Cells Responsible for Heterotopic Ossification Development in Skeletal Muscles-Human and Mouse Models. Cells 2020, 9, 1324. [Google Scholar] [CrossRef]
- Feng, H.; Xing, W.; Han, Y.; Sun, J.; Kong, M.; Gao, B.; Yang, Y.; Yin, Z.; Chen, X.; Zhao, Y.; et al. Tendon-derived cathepsin K-expressing progenitor cells activate Hedgehog signaling to drive heterotopic ossification. J. Clin. Investig. 2020, 130, 6354–6365. [Google Scholar] [CrossRef] [PubMed]
- Kan, C.; Ding, N.; Yang, J.; Tan, Z.; McGuire, T.L.; Lu, H.; Zhang, K.; Berger, D.M.P.; Kessler, J.A.; Kan, L. BMP-dependent, injury-induced stem cell niche as a mechanism of heterotopic ossification. Stem Cell Res. Ther. 2019, 10, 14. [Google Scholar] [CrossRef] [PubMed]
- Kan, C.; Chen, L.; Hu, Y.; Ding, N.; Li, Y.; McGuire, T.L.; Lu, H.; Kessler, J.A.; Kan, L. Gli1-labeled adult mesenchymal stem/progenitor cells and hedgehog signaling contribute to endochondral heterotopic ossification. Bone 2018, 109, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Olmsted-Davis, E.A.; Salisbury, E.A.; Hoang, D.; Davis, E.L.; Lazard, Z.; Sonnet, C.; Davis, T.A.; Forsberg, J.A.; Davis, A.R. Progenitors in Peripheral Nerves Launch Heterotopic Ossification. Stem Cells Transl. Med. 2017, 6, 1109–1119. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Loder, S.J.; Sorkin, M.; Li, S.; Shrestha, S.; Zhao, B.; Mishina, Y.; James, A.W.; Levi, B. Analysis of Bone-Cartilage-Stromal Progenitor Populations in Trauma Induced and Genetic Models of Heterotopic Ossification. Stem Cells 2016, 34, 1692–1701. [Google Scholar] [CrossRef]
- Medina, A.; Ma, Z.; Varkey, M.; Liu, H.; Iwashina, T.; Ding, J.; Tredget, E.E. Fibrocytes participate in the development of heterotopic ossification. J. Burn Care Res. 2015, 36, 394–404. [Google Scholar] [CrossRef] [PubMed]
- Downey, J.; Lauzier, D.; Kloen, P.; Klarskov, K.; Richter, M.; Hamdy, R.; Faucheux, N.; Scime, A.; Balg, F.; Grenier, G. Prospective heterotopic ossification progenitors in adult human skeletal muscle. Bone 2015, 71, 164–170. [Google Scholar] [CrossRef]
- Kan, L.; Liu, Y.; McGuire, T.L.; Berger, D.M.; Awatramani, R.B.; Dymecki, S.M.; Kessler, J.A. Dysregulation of local stem/progenitor cells as a common cellular mechanism for heterotopic ossification. Stem Cells 2009, 27, 150–156. [Google Scholar] [CrossRef]
- Wosczyna, M.N.; Biswas, A.A.; Cogswell, C.A.; Goldhamer, D.J. Multipotent Progenitors Resident In The Skeletal Muscle Interstitium Exhibit Robust Bmp-Dependent Osteogenic Activity And Mediate Heterotopic Ossification. J. Bone Miner. Res. 2012, 27, 1004–1017. [Google Scholar] [CrossRef]
- Alessi Wolken, D.M.; Idone, V.; Hatsell, S.J.; Yu, P.B.; Economides, A.N. The Obligatory Role of Activin a in the Formation of Heterotopic Bone in Fibrodysplasia Ossificans Progressiva. Bone 2017. [Google Scholar] [CrossRef]
- Hatsell, S.J.; Idone, V.; Wolken, D.M.; Huang, L.; Kim, H.J.; Wang, L.; Wen, X.; Nannuru, K.C.; Jimenez, J.; Xie, L.; et al. ACVR1R206H receptor mutation causes fibrodysplasia ossificans progressiva by imparting responsiveness to activin A. Sci. Transl. Med. 2015, 7, 303ra137. [Google Scholar] [CrossRef]
- Upadhyay, J.; Xie, L.; Huang, L.; Das, N.; Stewart, R.C.; Lyon, M.C.; Palmer, K.; Rajamani, S.; Graul, C.; Lobo, M.; et al. The Expansion of Heterotopic Bone in Fibrodysplasia Ossificans Progressiva is Activin A-Dependent. J. Bone Miner. Res. 2017, 32, 2489–2499. [Google Scholar] [CrossRef] [PubMed]
- Genet, F.; Kulina, I.; Vaquette, C.; Torossian, F.; Millard, S.; Pettit, A.R.; Sims, N.A.; Anginot, A.; Guerton, B.; Winkler, I.G.; et al. Neurological heterotopic ossification following spinal cord injury is triggered by macrophage-mediated inflammation in muscle. J. Pathol. 2015, 236, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Convente, M.R.; Chakkalakal, S.A.; Yang, E.; Caron, R.J.; Zhang, D.; Kambayashi, T.; Kaplan, F.S.; Shore, E.M. Depletion of Mast Cells and Macrophages Impairs Heterotopic Ossification in an Acvr1(R206H) Mouse Model of Fibrodysplasia Ossificans Progressiva. J. Bone Miner. Res. 2018, 33, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Levesque, J.P.; Sims, N.A.; Pettit, A.R.; Alexander, K.A.; Tseng, H.W.; Torossian, F.; Genet, F.; Lataillade, J.J.; Le Bousse-Kerdiles, M.C. Macrophages Driving Heterotopic Ossification: Convergence of Genetically-Driven and Trauma-Driven Mechanisms. J. Bone Miner. Res. 2018, 33, 365–366. [Google Scholar] [CrossRef] [PubMed]
- Moustakas, A.; Heldin, C.H. Ecsit-ement on the crossroads of Toll and BMP signal transduction. Genes Dev. 2003, 17, 2855–2859. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Ao, L.; Shi, Y.; Johnson, T.R.; Fullerton, D.A.; Meng, X. Oxidized low density lipoprotein induces bone morphogenetic protein-2 in coronary artery endothelial cells via Toll-like receptors 2 and 4. J. Biol. Chem. 2011, 286, 12213–12220. [Google Scholar] [CrossRef]
- Yang, X.; Fullerton, D.A.; Su, X.; Ao, L.; Cleveland, J.C., Jr.; Meng, X. Pro-osteogenic phenotype of human aortic valve interstitial cells is associated with higher levels of Toll-like receptors 2 and 4 and enhanced expression of bone morphogenetic protein 2. J. Am. Coll. Cardiol. 2009, 53, 491–500. [Google Scholar] [CrossRef]
- Huang, R.L.; Yuan, Y.; Zou, G.M.; Liu, G.; Tu, J.; Li, Q. LPS-stimulated inflammatory environment inhibits BMP-2-induced osteoblastic differentiation through crosstalk between TLR4/MyD88/NF-κB and BMP/Smad signaling. Stem. Cells Dev. 2014, 23, 277–289. [Google Scholar] [CrossRef]
- Wang, H.; Behrens, E.M.; Pignolo, R.J.; Kaplan, F.S. ECSIT links TLR and BMP signaling in FOP connective tissue progenitor cells. Bone 2018, 109, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Ebert, S.; Zeretzke, M.; Nau, R.; Michel, U. Microglial cells and peritoneal macrophages release activin A upon stimulation with Toll-like receptor agonists. Neurosci. Lett. 2007, 413, 241–244. [Google Scholar] [CrossRef]
- Liu, J.; Zhuang, Z.J.; Bian, D.X.; Ma, X.J.; Xun, Y.H.; Yang, W.J.; Luo, Y.; Liu, Y.L.; Jia, L.; Wang, Y.; et al. Toll-like receptor-4 signalling in the progression of non-alcoholic fatty liver disease induced by high-fat and high-fructose diet in mice. Clin. Exp. Pharmacol. Physiol. 2014, 41, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Winnall, W.R.; Muir, J.A.; Hedger, M.P. Differential responses of epithelial Sertoli cells of the rat testis to Toll-like receptor 2 and 4 ligands: Implications for studies of testicular inflammation using bacterial lipopolysaccharides. Innate Immun. 2011, 17, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Yamin, M.; Holbrook, E.H.; Gray, S.T.; Busaba, N.Y.; Lovett, B.; Hamilos, D.L. Profibrotic transforming growth factor beta 1 and activin A are increased in nasal polyp tissue and induced in nasal polyp epithelium by cigarette smoke and Toll-like receptor 3 ligation. Int. Forum. Allergy Rhinol. 2015, 5, 573–582. [Google Scholar] [CrossRef]
- Zeng, Q.; Song, R.; Ao, L.; Xu, D.; Venardos, N.; Fullerton, D.A.; Meng, X. Augmented Osteogenic Responses In Human Aortic Valve Cells Exposed to Oxldl And Tlr4 Agonist: A Mechanistic Role of Notch1 And Nf-Kappab Interaction. PLoS ONE 2014, 9, e95400. [Google Scholar] [CrossRef]
- Zhan, Q.; Song, R.; Zeng, Q.; Yao, Q.; Ao, L.; Xu, D.; Meng, X. Activation Of Tlr3 Induces Osteogenic Responses In Human Aortic Valve Interstitial Cells Through The Nf-Kappab And Erk1/2 Pathways. Int. J. Biol. Sci. 2015, 11, 482–493. [Google Scholar] [CrossRef]
- Elkins, C.E.; Lyu, H.; Dave, S.; Roberts, R.; Hohl, M.; Perrien, D.S. Characterization of an inducible mouse model of fibrodysplasia ossificans progressiva utilizing both cre-lox and dre-rox recombinase systems. PLoS Biol. 2021. submitted. [Google Scholar]
- Uezumi, A.; Ito, T.; Morikawa, D.; Shimizu, N.; Yoneda, T.; Segawa, M.; Yamaguchi, M.; Ogawa, R.; Matev, M.M.; Miyagoe-Suzuki, Y.; et al. Fibrosis and adipogenesis originate from a common mesenchymal progenitor in skeletal muscle. J. Cell Sci. 2011, 124 Pt 21, 3654–3664. [Google Scholar] [CrossRef]
- Pan, H.; Fleming, N.; Hong, C.C.; Mishina, Y.; Perrien, D.S. Methods for the reliable induction of heterotopic ossification in the conditional Alk2(Q207D) mouse. J. Musculoskelet. Neuronal Interact. 2020, 20, 149–159. [Google Scholar]
- Buie, H.R.; Campbell, G.M.; Klinck, R.J.; MacNeil, J.A.; Boyd, S.K. Automatic segmentation of cortical and trabecular compartments based on a dual threshold technique for in vivo micro-CT bone analysis. Bone 2007, 41, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Cho, H.H.; Joo, H.J.; Bae, Y.C.; Jung, J.S. Role of MyD88 in TLR agonist-induced functional alterations of human adipose tissue-derived mesenchymal stem cells. Mol. Cell. Biochem. 2008, 317, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Hanke, M.L.; Angle, A.; Kielian, T. MyD88-dependent signaling influences fibrosis and alternative macrophage activation during Staphylococcus aureus biofilm infection. PLoS ONE 2012, 7, e42476. [Google Scholar] [CrossRef]
- Nasi, S.; Ea, H.K.; Chobaz, V.; van Lent, P.; Lioté, F.; So, A.; Busso, N. Dispensable role of myeloid differentiation primary response gene 88 (MyD88) and MyD88-dependent toll-like receptors (TLRs) in a murine model of osteoarthritis. Jt. Bone Spine 2014, 81, 320–324. [Google Scholar] [CrossRef]
- Leite, F.R.; de Aquino, S.G.; Guimarães, M.R.; Cirelli, J.A.; Zamboni, D.S.; Silva, J.S.; Junior, C.R. Relevance of the myeloid differentiation factor 88 (MyD88) on RANKL, OPG, and nod expressions induced by TLR and IL-1R signaling in bone marrow stromal cells. Inflammation 2015, 38, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chu, X.; Xu, B.; Gao, H.; Li, B.Y.; Liu, Y.; Reiter, J.L.; Wang, Y. Lipopolysaccharides Improve Mesenchymal Stem Cell-Mediated Cardioprotection by MyD88 and stat3 Signaling in a Mouse Model of Cardiac Ischemia/Reperfusion Injury. Stem. Cells Dev. 2019, 28, 620–631. [Google Scholar] [CrossRef]
- Gong, J.; Li, J.; Dong, H.; Chen, G.; Qin, X.; Hu, M.; Yuan, F.; Fang, K.; Wang, D.; Jiang, S.; et al. Inhibitory effects of berberine on proinflammatory M1 macrophage polarization through interfering with the interaction between TLR4 and MyD88. BMC Complement. Altern. Med. 2019, 19, 314. [Google Scholar] [CrossRef] [PubMed]
- Lounev, V.Y.; Ramachandran, R.; Wosczyna, M.N.; Yamamoto, M.; Maidment, A.D.; Shore, E.M.; Kaplan, F.S. Identification of Progenitor Cells That Contribute To Heterotopic Skeletogenesis. J. Bone Jt. Surg. Am. 2009, 91, 652–663. [Google Scholar] [CrossRef]
- Moraes, J.R.; Moraes, F.R. Effect of a persistent inflammatory process on experimental heterotopic ossification. The influence of macrophages. Braz. J. Med. Biol. Res. 1993, 26, 53–66. [Google Scholar]
- Barruet, E.; Morales, B.M.; Cain, C.J.; Ton, A.N.; Wentworth, K.L.; Chan, T.V.; Moody, T.A.; Haks, M.C.; Ottenhoff, T.H.; Hellman, J.; et al. NF-kappaB/MAPK activation underlies ACVR1-mediated inflammation in human heterotopic ossification. JCI Insight 2018, 3. [Google Scholar] [CrossRef]
- Sorkin, M.; Huber, A.K.; Hwang, C.; Carson, W.F.t.; Menon, R.; Li, J.; Vasquez, K.; Pagani, C.; Patel, N.; Li, S.; et al. Regulation of heterotopic ossification by monocytes in a mouse model of aberrant wound healing. Nat. Commun. 2020, 11, 722. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.M.; Yoo, D.G.; Kim, M.C.; Song, J.M.; Park, M.K.; Quan, F.S.; Akira, S.; Compans, R.W. MyD88 plays an essential role in inducing B cells capable of differentiating into antibody-secreting cells after vaccination. J. Virol. 2011, 85, 11391–11400. [Google Scholar] [CrossRef]
- Li, C.; Huang, X.; Liu, Y.; Yang, T.; Zhang, L.; Li, M.; Jiang, F.; Huang, W.; Zhou, P. Dendritic cells play an essential role in transplantation responses via myeloid differentiation factor 88 signaling. Transpl. Proc. 2013, 45, 1842–1845. [Google Scholar] [CrossRef] [PubMed]
- Ruud, J.; Wilhelms, D.B.; Nilsson, A.; Eskilsson, A.; Tang, Y.J.; Ströhle, P.; Caesar, R.; Schwaninger, M.; Wunderlich, T.; Bäckhed, F.; et al. Inflammation- and tumor-induced anorexia and weight loss require MyD88 in hematopoietic/myeloid cells but not in brain endothelial or neural cells. FASEB J. 2013, 27, 1973–1980. [Google Scholar] [CrossRef] [PubMed]
- Fiedler, K.; Kokai, E.; Bresch, S.; Brunner, C. MyD88 is involved in myeloid as well as lymphoid hematopoiesis independent of the presence of a pathogen. Am. J. Blood Res. 2013, 3, 124–140. [Google Scholar]
- Fuchs, A.; Monlish, D.A.; Ghosh, S.; Chang, S.W.; Bochicchio, G.V.; Schuettpelz, L.G.; Turnbull, I.R. Trauma Induces Emergency Hematopoiesis through IL-1/MyD88-Dependent Production of G-CSF. J. Immunol. 2019, 202, 3020–3032. [Google Scholar] [CrossRef]
- Brennan, T.A.; Lindborg, C.M.; Bergbauer, C.R.; Wang, H.; Kaplan, F.S.; Pignolo, R.J. Mast cell inhibition as a therapeutic approach in fibrodysplasia ossificans progressiva (FOP). Bone 2017. [Google Scholar] [CrossRef]
- Gannon, F.H.; Glaser, D.; Caron, R.; Thompson, L.D.; Shore, E.M.; Kaplan, F.S. Mast cell involvement in fibrodysplasia ossificans progressiva. Hum. Pathol. 2001, 32, 842–848. [Google Scholar] [CrossRef]
- Kan, L.; Lounev, V.Y.; Pignolo, R.J.; Duan, L.; Liu, Y.; Stock, S.R.; McGuire, T.L.; Lu, B.; Gerard, N.P.; Shore, E.M.; et al. Substance P signaling mediates BMP-dependent heterotopic ossification. J. Cell. Biochem. 2011, 112, 2759–2772. [Google Scholar] [CrossRef]
- Federico, S.; Pozzetti, L.; Papa, A.; Carullo, G.; Gemma, S.; Butini, S.; Campiani, G.; Relitti, N. Modulation of the Innate Immune Response by Targeting Toll-like Receptors: A Perspective on Their Agonists and Antagonists. J. Med. Chem. 2020, 63, 13466–13513. [Google Scholar] [CrossRef]
- Jiang, L.; Shao, Y.; Tian, Y.; Ouyang, C.; Wang, X. Nuclear Alarmin Cytokines in Inflammation. J. Immunol. Res. 2020, 2020, 7206451. [Google Scholar] [CrossRef] [PubMed]
- Murao, A.; Aziz, M.; Wang, H.; Brenner, M.; Wang, P. Release mechanisms of major DAMPs. Apoptosis 2021, 26, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Dimicoli, S.; Wei, Y.; Bueso-Ramos, C.; Yang, H.; Dinardo, C.; Jia, Y.; Zheng, H.; Fang, Z.; Nguyen, M.; Pierce, S.; et al. Overexpression of the toll-like receptor (TLR) signaling adaptor MYD88, but lack of genetic mutation, in myelodysplastic syndromes. PLoS ONE 2013, 8, e71120. [Google Scholar] [CrossRef]
- Sato, N.; Takahashi, N.; Suda, K.; Nakamura, M.; Yamaki, M.; Ninomiya, T.; Kobayashi, Y.; Takada, H.; Shibata, K.; Yamamoto, M.; et al. MyD88 but not TRIF is essential for osteoclastogenesis induced by lipopolysaccharide, diacyl lipopeptide, and IL-1alpha. J. Exp. Med. 2004, 200, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Kawao, N.; Yano, M.; Tamura, Y.; Okumoto, K.; Okada, K.; Kaji, H. Role of osteoclasts in heterotopic ossification enhanced by fibrodysplasia ossificans progressiva-related activin-like kinase 2 mutation in mice. J. Bone Miner. Metab. 2016, 34, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Yano, M.; Kawao, N.; Okumoto, K.; Tamura, Y.; Okada, K.; Kaji, H. Fibrodysplasia ossificans progressiva-related activated activin-like kinase signaling enhances osteoclast formation during heterotopic ossification in muscle tissues. J. Biol. Chem. 2014, 289, 16966–16977. [Google Scholar] [CrossRef] [PubMed]
- El-Zayat, S.R.; Sibaii, H.; Mannaa, F.A. Toll-like receptors activation, signaling, and targeting: An overview. Bull. Natl. Res. Cent. 2019, 43, 187. [Google Scholar] [CrossRef]
- Wesche, H.; Korherr, C.; Kracht, M.; Falk, W.; Resch, K.; Martin, M.U. The interleukin-1 receptor accessory protein (IL-1RAcP) is essential for IL-1-induced activation of interleukin-1 receptor-associated kinase (IRAK) and stress-activated protein kinases (SAP kinases). J. Biol. Chem. 1997, 272, 7727–7731. [Google Scholar] [CrossRef]
- Hoffmann, E.; Ashouri, J.; Wolter, S.; Doerrie, A.; Dittrich-Breiholz, O.; Schneider, H.; Wagner, E.F.; Troppmair, J.; Mackman, N.; Kracht, M. Transcriptional regulation of EGR-1 by the interleukin-1-JNK-MKK7-c-Jun pathway. J. Biol. Chem. 2008, 283, 12120–12128. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lyu, H.; Elkins, C.M.; Pierce, J.L.; Serezani, C.H.; Perrien, D.S. MyD88 Is Not Required for Muscle Injury-Induced Endochondral Heterotopic Ossification in a Mouse Model of Fibrodysplasia Ossificans Progressiva. Biomedicines 2021, 9, 630. https://doi.org/10.3390/biomedicines9060630
Lyu H, Elkins CM, Pierce JL, Serezani CH, Perrien DS. MyD88 Is Not Required for Muscle Injury-Induced Endochondral Heterotopic Ossification in a Mouse Model of Fibrodysplasia Ossificans Progressiva. Biomedicines. 2021; 9(6):630. https://doi.org/10.3390/biomedicines9060630
Chicago/Turabian StyleLyu, Huili, Cody M. Elkins, Jessica L. Pierce, C. Henrique Serezani, and Daniel S. Perrien. 2021. "MyD88 Is Not Required for Muscle Injury-Induced Endochondral Heterotopic Ossification in a Mouse Model of Fibrodysplasia Ossificans Progressiva" Biomedicines 9, no. 6: 630. https://doi.org/10.3390/biomedicines9060630
APA StyleLyu, H., Elkins, C. M., Pierce, J. L., Serezani, C. H., & Perrien, D. S. (2021). MyD88 Is Not Required for Muscle Injury-Induced Endochondral Heterotopic Ossification in a Mouse Model of Fibrodysplasia Ossificans Progressiva. Biomedicines, 9(6), 630. https://doi.org/10.3390/biomedicines9060630




