Unexpected Role of Sterol Synthesis in RNA Stability and Translation in Leishmania
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Leishmania Culturing and Treatments
2.3. Polysome Profiling
2.4. RNA Extraction, cDNA Preparation and Real-Time Reverse-Transcription Quantitative PCR (RT-qPCR)
2.5. ER Labelling and Confocal Microscopy
2.6. Statistical Analysis
3. Results
3.1. Defects in Sterol Synthesis Lead to Global Reduction in RNA and Protein Levels in c14dm−
3.2. RNA Stability Is Compromised in c14dm−
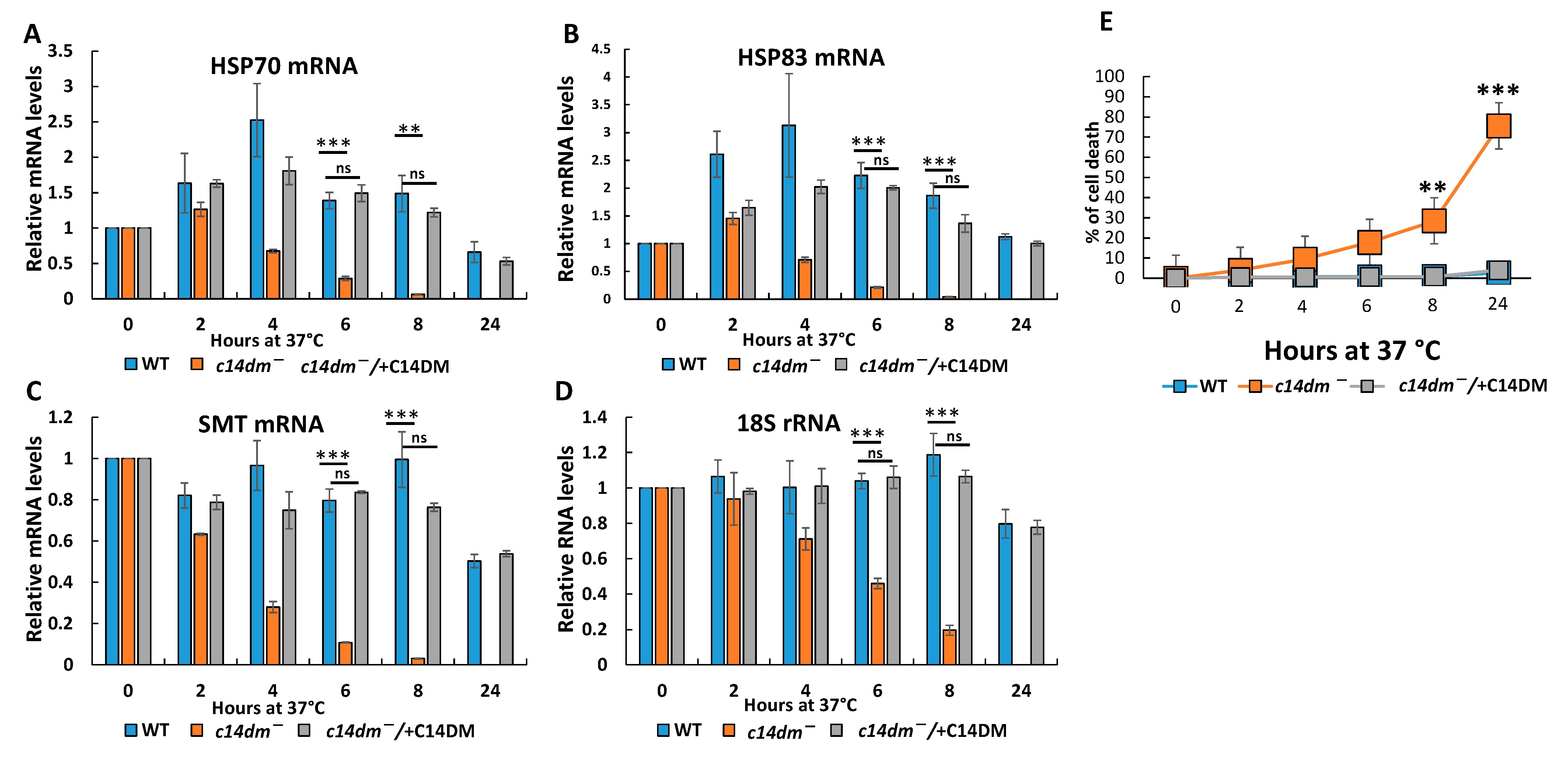
3.3. RNA Degrades Faster during Heat Shock and the Induction of Heat Shock Response Is Compromised in c14dm−
3.4. Reduced RNA Levels in c14dm− Is Not Caused by the Accumulation of Oxidants
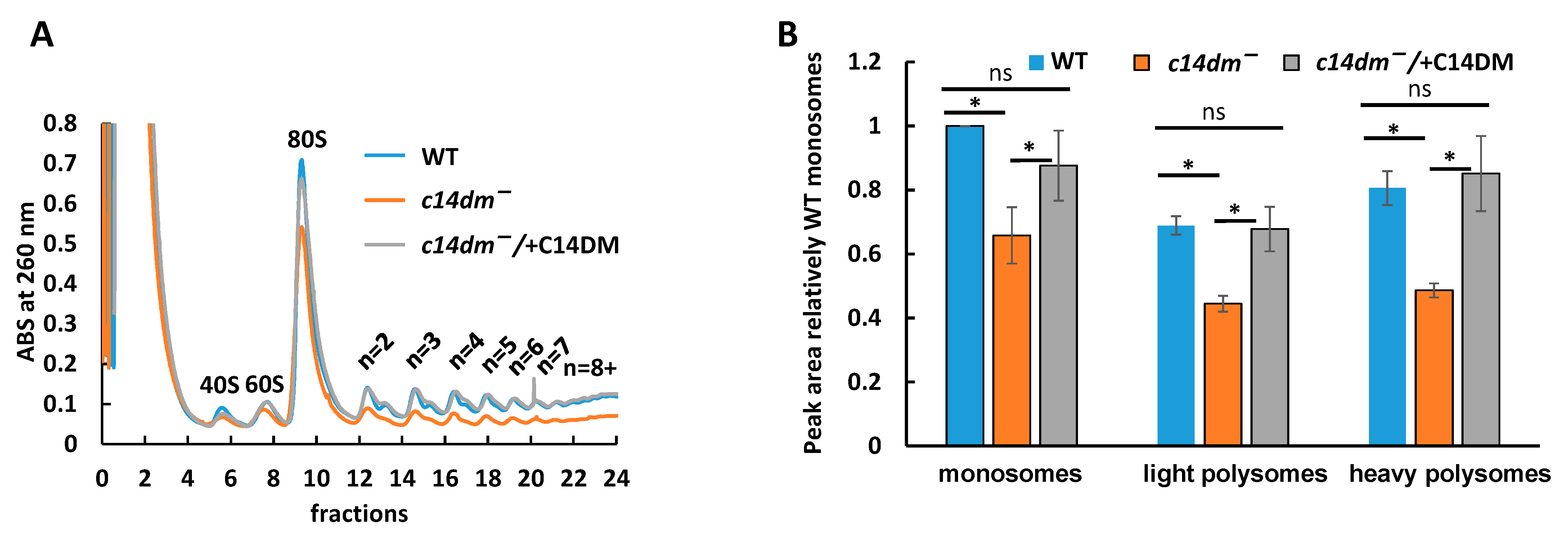
3.5. Polysome Profiling Reveals Defects in Translation in c14dm−
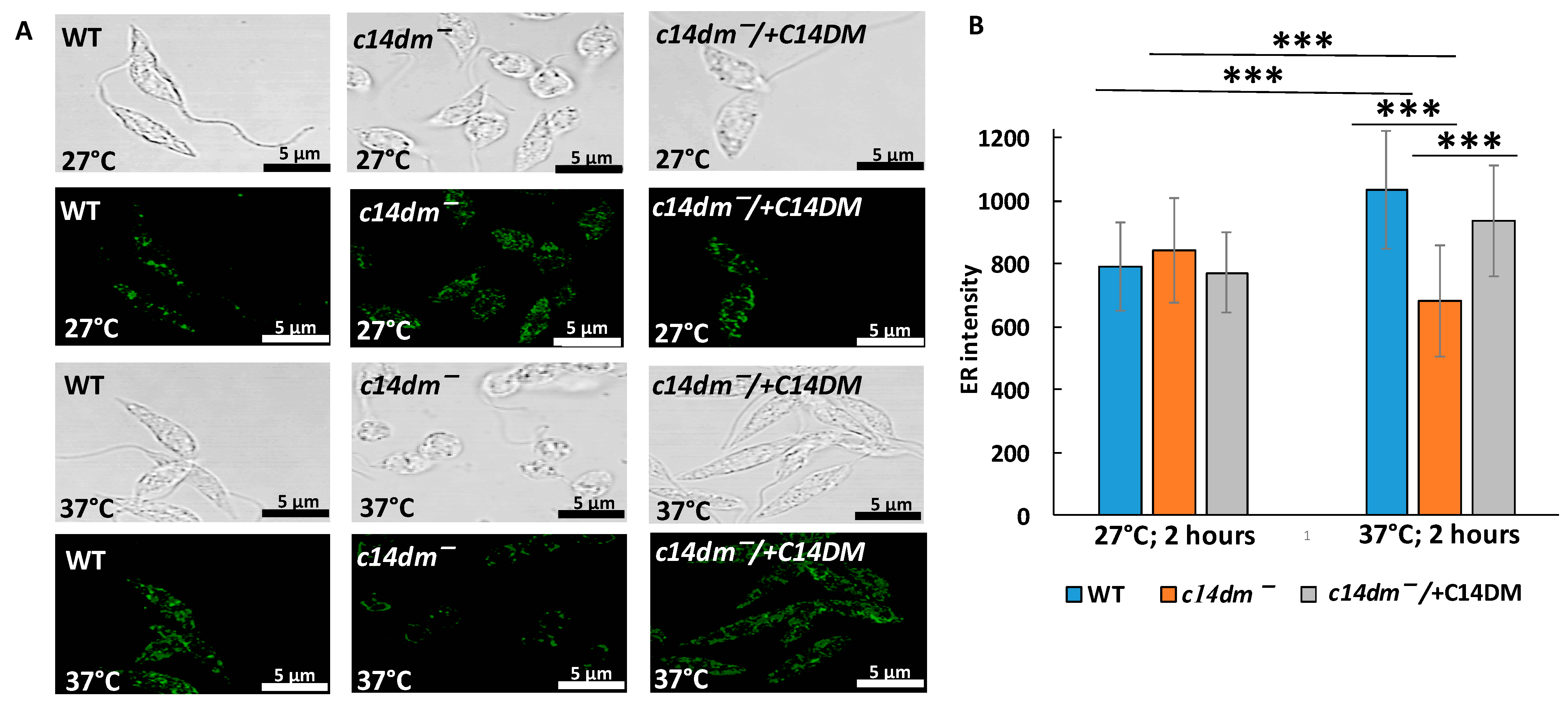
3.6. Intensity of ER Staining Is Compromised in c14dm− under Heat Shock
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alvar, J.; Vélez, I.D.; Bern, C.; Herrero, M.; Desjeux, P.; Cano, J.; Jannin, J.; den Boer, M.; WHO Leishmaniasis Control Team. Leishmaniasis Worldwide and Global Estimates of Its Incidence. PLoS ONE 2012, 7, e35671. [Google Scholar] [CrossRef] [PubMed]
- Murray, H.W.; Berman, J.D.; Davies, C.R.; Saravia, N.G. Advances in leishmaniasis. Lancet 2005, 366, 1561–1577. [Google Scholar] [CrossRef]
- Georgiadou, S.P.; Stefos, A.; Spanakos, G.; Skrimpas, S.; Makaritsis, K.; Sipsas, N.V.; Dalekos, G.N. Current clinical, laboratory, and treatment outcome characteristics of visceral leishmaniasis: Results from a seven-year retrospective study in Greece. Int. J. Infect. Dis. 2015, 34, 46–50. [Google Scholar] [CrossRef] [Green Version]
- Akhoundi, M.; Downing, T.; Votypka, J.; Kuhls, K.; Lukeš, J.; Cannet, A.; Ravel, C.; Marty, P.; Delaunay, P.; Kasbari, M.; et al. Leishmania infections: Molecular targets and diagnosis. Mol. Asp. Med. 2017, 57, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Sunter, J.; Gull, K. Shape, form, function and Leishmania pathogenicity: From textbook descriptions to biological understanding. Open Biol. 2017, 7, 7. [Google Scholar] [CrossRef] [Green Version]
- Zilberstein, D.; Shapira, M. The Role of pH and Temperature in the Development of Leishmania Parasites. Annu. Rev. Microbiol. 1994, 48, 449–470. [Google Scholar] [CrossRef] [PubMed]
- Santos, D.O.; Coutinho, C.E.R.; Madeira, M.F.; Bottino, C.G.; Vieira, R.T.; Nascimento, S.B.; Bernardino, A.M.R.; Bourguignon, S.C.; Corte-Real, S.; Pinho, R.T.; et al. Leishmaniasis treatment—a challenge that remains: A review. Parasitol. Res. 2008, 103, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Karamyshev, A.L.; Karamysheva, Z.N. Lost in Translation: Ribosome-Associated mRNA and Protein Quality Controls. Front. Genet. 2018, 9, 431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez, N.E.; Lockard, R.; Turcotte, E.A.; Araujo-Santos, T.; Bozza, P.T.; Borges, V.; Wilson, M. Lipid bodies accumulation in Leishmania infantum -infected C57BL/6 macrophages. Parasite Immunol. 2017, 39, e12443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rabhi, S.; Rabhi, I.; Trentin, B.; Piquemal, D.; Regnault, B.; Goyard, S.; Lang, T.; Descoteaux, A.; Enninga, J.; Guizani-Tabbane, L. Lipid Droplet Formation, Their Localization and Dynamics during Leishmania major Macrophage Infection. PLoS ONE 2016, 11, e0148640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Messaoud, H.B.-B.; Guichard, M.; Lawton, P.; Delton, I.; Azzouz-Maache, S. Changes in Lipid and Fatty Acid Composition During Intramacrophagic Transformation of Leishmania donovani Complex Promastigotes into Amastigotes. Lipids 2017, 52, 433–441. [Google Scholar] [CrossRef] [Green Version]
- Armitage, E.G.; Alqaisi, A.Q.I.; Godzien, J.; Peña, I.; Mbekeani, A.J.; Alonso-Herranz, V.; López-Gonzálvez, Á.; Martín, J.; Gabarro, R.; Denny, P.W.; et al. Complex Interplay between Sphingolipid and Sterol Metabolism Revealed by Perturbations to the Leishmania Metabolome Caused by Miltefosine. Antimicrob. Agents Chemother. 2018, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guarnizo, S.G.; Tikhonova, E.; Zabet-Moghaddam, M.; Zhang, K.; Muskus, C.; Karamyshev, A.; Karamysheva, Z. Drug-Induced Lipid Remodeling in Leishmania Parasites. Microorganisms 2021, 9, 790. [Google Scholar] [CrossRef] [PubMed]
- McCall, L.-I.; El Aroussi, A.; Choi, J.Y.; Vieira, D.F.; De Muylder, G.; Johnston, J.B.; Chen, S.; Kellar, D.; Siqueira-Neto, J.; Roush, W.R.; et al. Targeting Ergosterol Biosynthesis in Leishmania donovani: Essentiality of Sterol 14alpha-demethylase. PLoS Negl. Trop. Dis. 2015, 9, e0003588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, C.; Wilson, M.E. Dynamics of sterol synthesis during development of Leishmania spp. parasites to their virulent form. Parasites Vectors 2016, 9, 200. [Google Scholar] [CrossRef] [Green Version]
- Torres-Santos, E.C.; Sampaio-Santos, M.I.; Buckner, F.; Yokoyama, K.; Gelb, M.; Urbina, J.A.; Rossi-Bergmann, B. Altered sterol profile induced in Leishmania amazonensis by a natural dihydroxymethoxylated chalcone. J. Antimicrob. Chemother. 2009, 63, 469–472. [Google Scholar] [CrossRef] [Green Version]
- Drin, G. Topological Regulation of Lipid Balance in Cells. Annu. Rev. Biochem. 2014, 83, 51–77. [Google Scholar] [CrossRef]
- Woodman, B.S.; Trousdale, C.; Conover, J.; Kim, K.; Woodman, S. Yeast membrane lipid imbalance leads to trafficking defects toward the Golgi. Cell Biol. Int. 2018, 42, 890–902. [Google Scholar] [CrossRef]
- Maxfield, F.; Mondal, M. Sterol and lipid trafficking in mammalian cells. Biochem. Soc. Trans. 2006, 34, 335–339. [Google Scholar] [CrossRef]
- Georgiev, A.G.; Johansen, J.; Ramanathan, V.D.; Sere, Y.Y.; Beh, C.T.; Menon, A.K. Arv1 Regulates PM and ER Membrane Structure and Homeostasis But is Dispensable for Intracellular Sterol Transport. Traffic 2013, 14, 912–921. [Google Scholar] [CrossRef] [Green Version]
- Lepesheva, G.I.; Hargrove, T.Y.; Kleshchenko, Y.; Nes, W.D.; Villalta, F.; Waterman, M.R. CYP51: A Major Drug Target in the Cytochrome P450 Superfamily. Lipids 2008, 43, 1117–1125. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.; Hsu, F.-F.; Baykal, E.; Huang, J.; Zhang, K. Sterol Biosynthesis Is Required for Heat Resistance but Not Extracellular Survival in Leishmania. PLoS Pathog. 2014, 10, e1004427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukherjee, S.; Moitra, S.; Xu, W.; Hernandez, V.; Zhang, K. Sterol 14-α-demethylase is vital for mitochondrial functions and stress tolerance in Leishmania major. PLoS Pathog. 2020, 16, e1008810. [Google Scholar] [CrossRef]
- Kapler, G.M.; Coburn, C.M.; Beverley, S.M. Stable transfection of the human parasite Leishmania major delineates a 30-kilobase region sufficient for extrachromosomal replication and expression. Mol. Cell. Biol. 1990, 10, 1084–1094. [Google Scholar] [CrossRef] [PubMed]
- Karamysheva, Z.N.; Tikhonova, E.; Grozdanov, P.N.; Huffman, J.C.; Baca, K.R.; Karamyshev, A.; Denison, R.B.; MacDonald, C.C.; Zhang, K.; Karamyshev, A.L. Polysome Profiling in Leishmania, Human Cells and Mouse Testis. J. Vis. Exp. 2018, e57600. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Wolf, S.F.; Schlessinger, D. Nuclear metabolism of ribosomal RNA in growing, methionine-limited, and ethionine-treated HeLa cells. Biochemistry 1977, 16, 2783–2791. [Google Scholar] [CrossRef]
- Waldron, C.; Lacroute, F. Effect of growth rate on the amounts of ribosomal and transfer ribonucleic acids in yeast. J. Bacteriol. 1975, 122, 855–865. [Google Scholar] [CrossRef] [Green Version]
- Bensaude, O. Inhibiting eukaryotic transcription. Which compound to choose? How to evaluate its activity? Transcription 2011, 2, 103–108. [Google Scholar] [CrossRef] [Green Version]
- LaRiviere, F.J.; Cole, S.E.; Ferullo, D.J.; Moore, M.J. A Late-Acting Quality Control Process for Mature Eukaryotic rRNAs. Mol. Cell 2006, 24, 619–626. [Google Scholar] [CrossRef]
- Pestov, D.G.; Shcherbik, N. Rapid Cytoplasmic Turnover of Yeast Ribosomes in Response to Rapamycin Inhibition of TOR. Mol. Cell. Biol. 2012, 32, 2135–2144. [Google Scholar] [CrossRef] [Green Version]
- Kramer, S.; Queiroz, R.; Ellis, L.; Webb, H.; Hoheisel, J.D.; Clayton, C.; Carrington, M. Heat shock causes a decrease in polysomes and the appearance of stress granules in trypanosomes independently of eIF2α phosphorylation at Thr169. J. Cell Sci. 2008, 121, 3002–3014. [Google Scholar] [CrossRef] [Green Version]
- Alves, S.G.L.R. RNA-binding proteins related to stress response and differentiation in protozoa. World J. Biol. Chem. 2016, 7, 78–87. [Google Scholar] [CrossRef]
- Ennis, H.L.; Lubin, M. Cycloheximide: Aspects of Inhibition of Protein Synthesis in Mammalian Cells. Science 1964, 146, 1474–1476. [Google Scholar] [CrossRef]
- Gandin, V.; Sikström, K.; Alain, T.; Morita, M.; McLaughlan, S.; Larsson, O.; Topisirovic, I. Polysome Fractionation and Analysis of Mammalian Translatomes on a Genome-wide Scale. J. Vis. Exp. 2014, e51455. [Google Scholar] [CrossRef] [Green Version]
- Hannich, J.T.; Umebayashi, K.; Riezman, H. Distribution and Functions of Sterols and Sphingolipids. Cold Spring Harb. Perspect. Biol. 2011, 3, a004762. [Google Scholar] [CrossRef]
- Reid, D.W.; Nicchitta, C.V. Primary Role for Endoplasmic Reticulum-bound Ribosomes in Cellular Translation Identified by Ribosome Profiling. J. Biol. Chem. 2012, 287, 5518–5527. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Pompey, J.M.; Hsu, F.-F.; Key, P.; Bandhuvula, P.; Saba, J.D.; Turk, J.; Beverley, S.M. Redirection of sphingolipid metabolism toward de novo synthesis of ethanolamine in Leishmania. EMBO J. 2007, 26, 1094–1104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukherjee, S.; Xu, W.; Hsu, F.-F.; Patel, J.; Huang, J.; Zhang, K. Sterol methyltransferase is required for optimal mitochondrial function and virulence inLeishmania major. Mol. Microbiol. 2018, 111, 65–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moitra, S.; Pawlowic, M.C.; Hsu, F.-F.; Zhang, K. Phosphatidylcholine synthesis through cholinephosphate cytidylyltransferase is dispensable in Leishmania major. Sci. Rep. 2019, 9, 7602. [Google Scholar] [CrossRef] [PubMed]
- Pawlowic, M.C.; Hsu, F.-F.; Moitra, S.; Biyani, N.; Zhang, K. Plasmenylethanolamine synthesis inLeishmania major. Mol. Microbiol. 2016, 101, 238–249. [Google Scholar] [CrossRef] [Green Version]
- Moitra, S.; Basu, S.; Pawlowic, M.; Hsu, F.-F.; Zhang, K. De Novo Synthesis of Phosphatidylcholine Is Essential for the Promastigote But Not Amastigote Stage in Leishmania major. Front. Cell. Infect. Microbiol. 2021, 11, 647870. [Google Scholar] [CrossRef] [PubMed]
- Ning, Y.; Frankfater, C.; Hsu, F.-F.; Soares, R.P.; Cardoso, C.A.; Nogueira, P.M.; Lander, N.M.; Docampo, R.; Zhang, K. Lathosterol Oxidase (Sterol C-5 Desaturase) Deletion Confers Resistance to Amphotericin B and Sensitivity to Acidic Stress in Leishmania major. mSphere 2020, 5. [Google Scholar] [CrossRef] [PubMed]
- Goad, L.; Holz, G.G.; Beach, D.H. Sterols of Leishmania species, implications for biosynthesis. Mol. Biochem. Parasitol. 1984, 10, 161–170. [Google Scholar] [CrossRef]
- Urbina, J.A. Lipid biosynthesis pathways as chemotherapeutic targets in kinetoplastid parasites. Parasitology 1997, 114, 91–99. [Google Scholar] [CrossRef]
- Urbina, J.A.; Vivas, J.; Lazardi, K.; Molina, J.; Payares, G.; Pirns, M.M.; Piras, R. Antiproliferative Effects of △24(25) Sterol Methyl Transferase Inhibitors on Trypanosoma (Schizotrypanum) cruzi: In vitro and in vivo Studies. Chemotherapy 1996, 42, 294–307. [Google Scholar] [CrossRef] [PubMed]
- Maxfield, F.R.; Menon, A.K. Intracellular sterol transport and distribution. Curr. Opin. Cell Biol. 2006, 18, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Vonlaufen, N.; Kanzok, S.M.; Wek, R.C.; Jr, W.J.S. Stress response pathways in protozoan parasites. Cell. Microbiol. 2008, 10, 2387–2399. [Google Scholar] [CrossRef] [PubMed]
- Folgueira, C.; Requena, J.M. A postgenomic view of the heat shock proteins in kinetoplastids. FEMS Microbiol. Rev. 2007, 31, 359–377. [Google Scholar] [CrossRef] [PubMed]
- Mayer, M.P.; Bukau, B. Hsp70 chaperones: Cellular functions and molecular mechanism. Cell. Mol. Life Sci. 2005, 62, 670–684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shonhai, A.; Maier, A.G.; Przyborski, J.M.; Blatch, G.L. Intracellular protozoan parasites of humans: The role of molecular chaperones in development and pathogenesis. Protein Pept. Lett. 2011, 18, 143–157. [Google Scholar] [CrossRef] [Green Version]
- Minia, I.; Merce, C.; Terrao, M.; Clayton, C. Translation Regulation and RNA Granule Formation after Heat Shock of Procyclic Form Trypanosoma brucei: Many Heat-Induced mRNAs Are also Increased during Differentiation to Mammalian-Infective Forms. PLoS Negl. Trop. Dis. 2016, 10, e0004982. [Google Scholar] [CrossRef] [Green Version]
- Gitau, G.W.; Mandal, P.; Blatch, G.L.; Przyborski, J.M.; Shonhai, A. Characterisation of the Plasmodium falciparum Hsp70–Hsp90 organising protein (PfHop). Cell Stress Chaperon 2012, 17, 191–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shenton, D.; Smirnova, J.B.; Selley, J.N.; Carroll, K.; Hubbard, S.J.; Pavitt, G.; Ashe, M.P.; Grant, C.M. Global Translational Responses to Oxidative Stress Impact upon Multiple Levels of Protein Synthesis. J. Biol. Chem. 2006, 281, 29011–29021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wurtmann, E.J.; Wolin, S. RNA under attack: Cellular handling of RNA damage. Crit. Rev. Biochem. Mol. Biol. 2009, 44, 34–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Wu, J.; DeLeo, C.J. RNA damage and surveillance under oxidative stress. IUBMB Life 2006, 58, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Redman, C.M. Biosynthesis of Serum Proteins and Ferritin by Free and Attached Ribosomes of Rat Liver. J. Biol. Chem. 1969, 244, 4308–4315. [Google Scholar] [CrossRef]
- Hicks, S.J.; Drysdale, J.W.; Munro, H.N. Preferential Synthesis of Ferritin and Albumin by Different Populations of Liver Polysomes. Science 1969, 164, 584–585. [Google Scholar] [CrossRef] [PubMed]
- Casares, D.; Escribá, P.V.; Rosselló, C.A. Membrane Lipid Composition: Effect on Membrane and Organelle Structure, Function and Compartmentalization and Therapeutic Avenues. Int. J. Mol. Sci. 2019, 20, 2167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torres, M.; Rosselló, C.A.; Fernández-García, P.; Lladó, V.; Kakhlon, O.; Escribá, P.V. The Implications for Cells of the Lipid Switches Driven by Protein–Membrane Interactions and the Development of Membrane Lipid Therapy. Int. J. Mol. Sci. 2020, 21, 2322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohanian, J.; Ohanian, V. Sphingolipids in mammalian cell signalling. Cell. Mol. Life Sci. 2001, 58, 2053–2068. [Google Scholar] [CrossRef] [PubMed]
- Maceyka, M.; Harikumar, K.; Milstien, S.; Spiegel, S. Sphingosine-1-phosphate signaling and its role in disease. Trends Cell Biol. 2012, 22, 50–60. [Google Scholar] [CrossRef] [Green Version]
- Nagahashi, M.; Abe, M.; Sakimura, K.; Takabe, K.; Wakai, T. The role of sphingosine-1-phosphate in inflammation and cancer progression. Cancer Sci. 2018, 109, 3671–3678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maceyka, M.; Spiegel, S. Sphingolipid metabolites in inflammatory disease. Nat. Cell Biol. 2014, 510, 58–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Musille, P.M.; Kohn, J.A.; Ortlund, E.A. Phospholipid - Driven gene regulation. FEBS Lett. 2013, 587, 1238–1246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fessler, M.B.; Parks, J.S. Intracellular Lipid Flux and Membrane Microdomains as Organizing Principles in Inflammatory Cell Signaling. J. Immunol. 2011, 187, 1529–1535. [Google Scholar] [CrossRef] [Green Version]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karamysheva, Z.N.; Moitra, S.; Perez, A.; Mukherjee, S.; Tikhonova, E.B.; Karamyshev, A.L.; Zhang, K. Unexpected Role of Sterol Synthesis in RNA Stability and Translation in Leishmania. Biomedicines 2021, 9, 696. https://doi.org/10.3390/biomedicines9060696
Karamysheva ZN, Moitra S, Perez A, Mukherjee S, Tikhonova EB, Karamyshev AL, Zhang K. Unexpected Role of Sterol Synthesis in RNA Stability and Translation in Leishmania. Biomedicines. 2021; 9(6):696. https://doi.org/10.3390/biomedicines9060696
Chicago/Turabian StyleKaramysheva, Zemfira N., Samrat Moitra, Andrea Perez, Sumit Mukherjee, Elena B. Tikhonova, Andrey L. Karamyshev, and Kai Zhang. 2021. "Unexpected Role of Sterol Synthesis in RNA Stability and Translation in Leishmania" Biomedicines 9, no. 6: 696. https://doi.org/10.3390/biomedicines9060696
APA StyleKaramysheva, Z. N., Moitra, S., Perez, A., Mukherjee, S., Tikhonova, E. B., Karamyshev, A. L., & Zhang, K. (2021). Unexpected Role of Sterol Synthesis in RNA Stability and Translation in Leishmania. Biomedicines, 9(6), 696. https://doi.org/10.3390/biomedicines9060696





