Optimization and Development of Albumin–Biopolymer Bioconjugates with Solubility-Improving Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Albumin Conjugates with Biopolymers
2.2.2. FTIR and DSC Measurements
2.2.3. Rheological Measurements
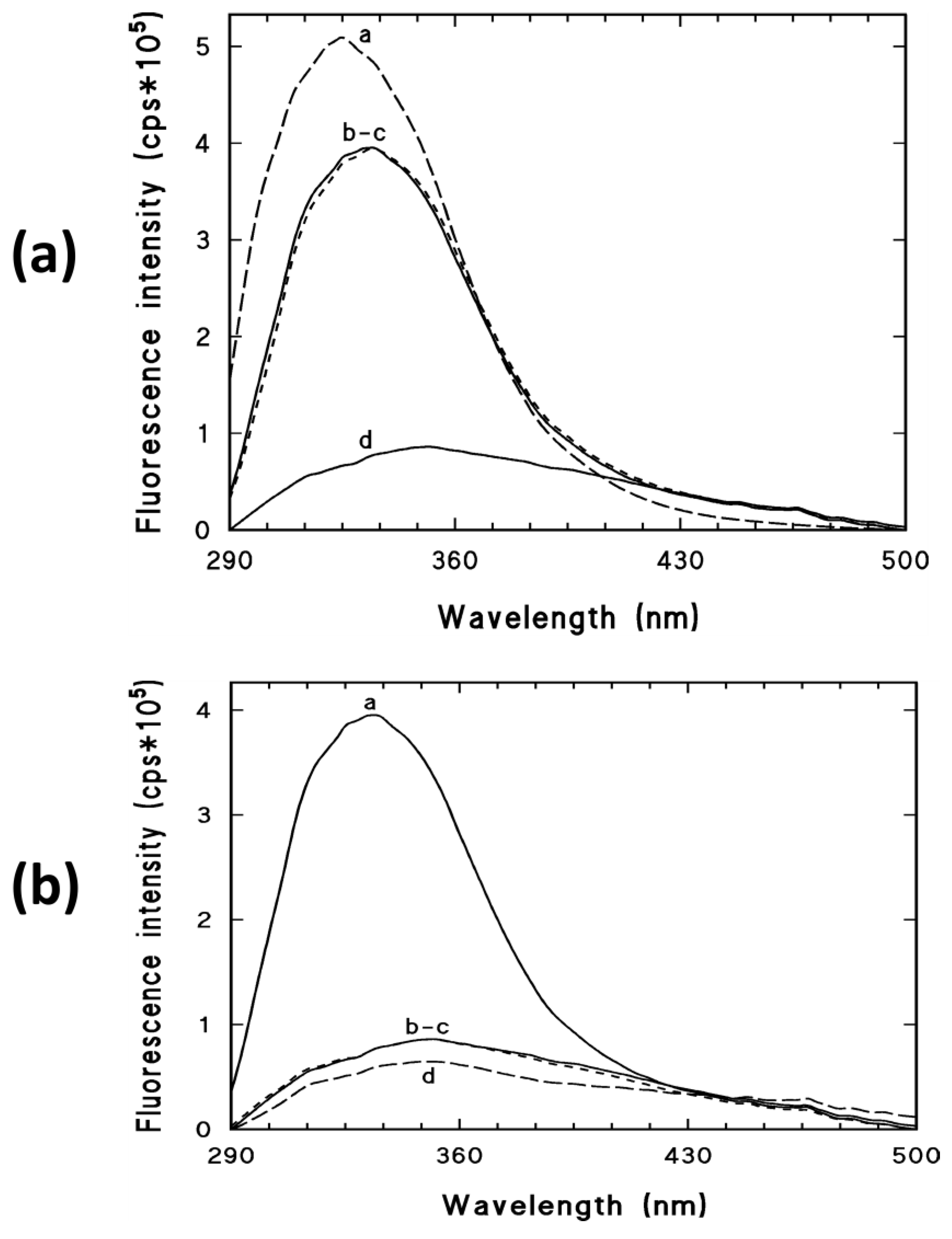
2.2.4. Fluorescence Spectroscopy Measurements
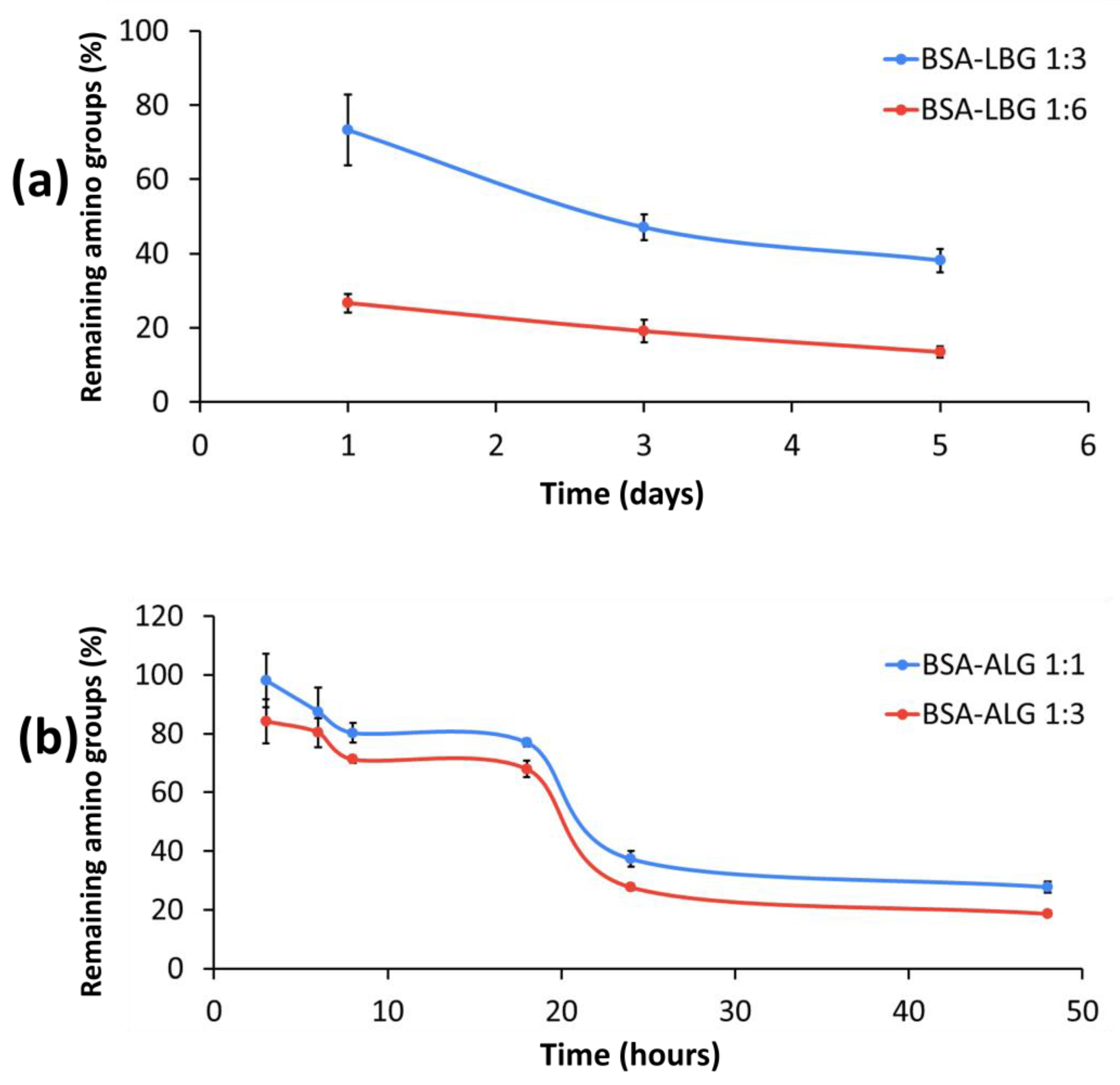
2.2.5. Determination of Available Amino Groups
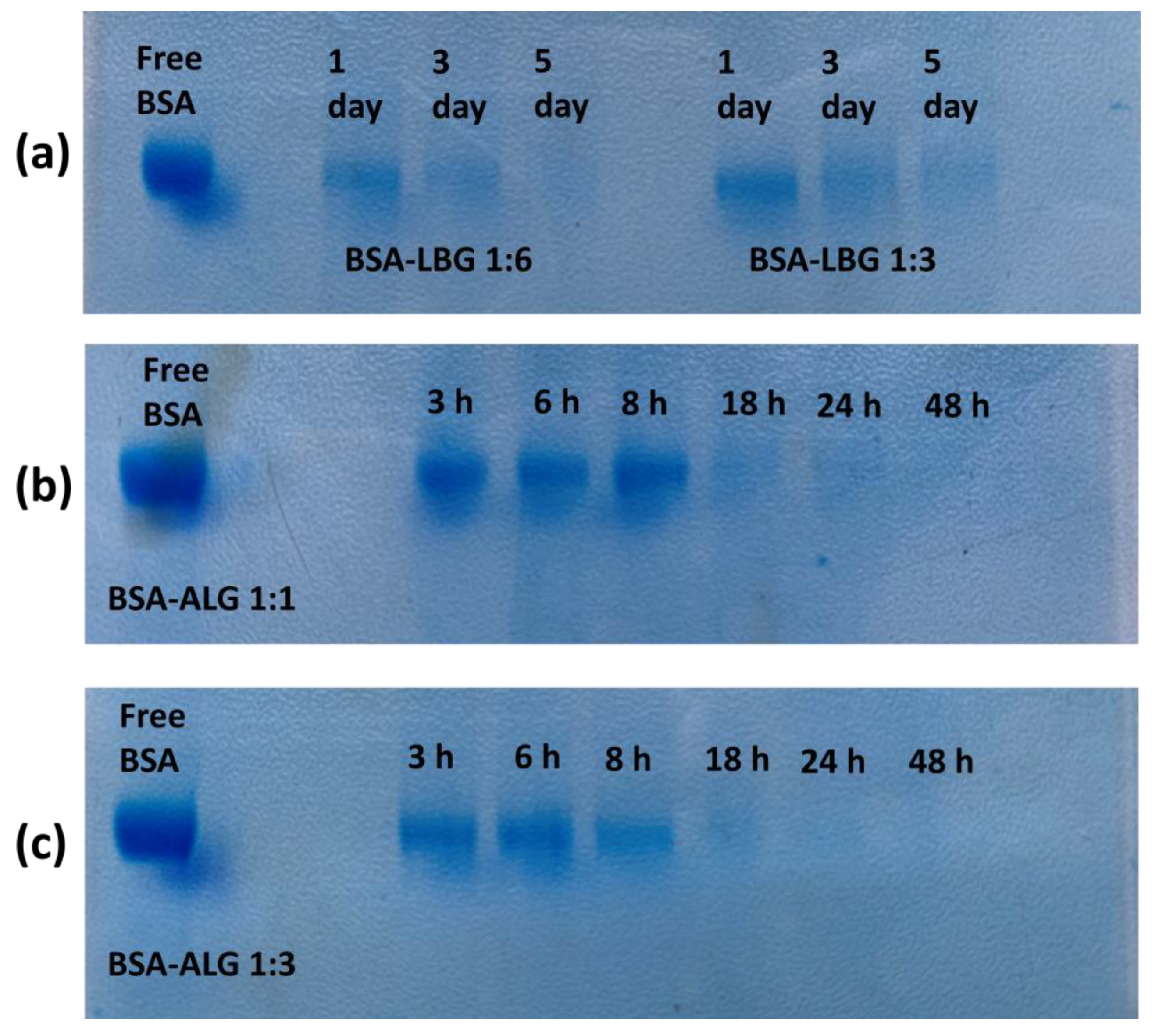
2.2.6. Electrophoretic Analysis
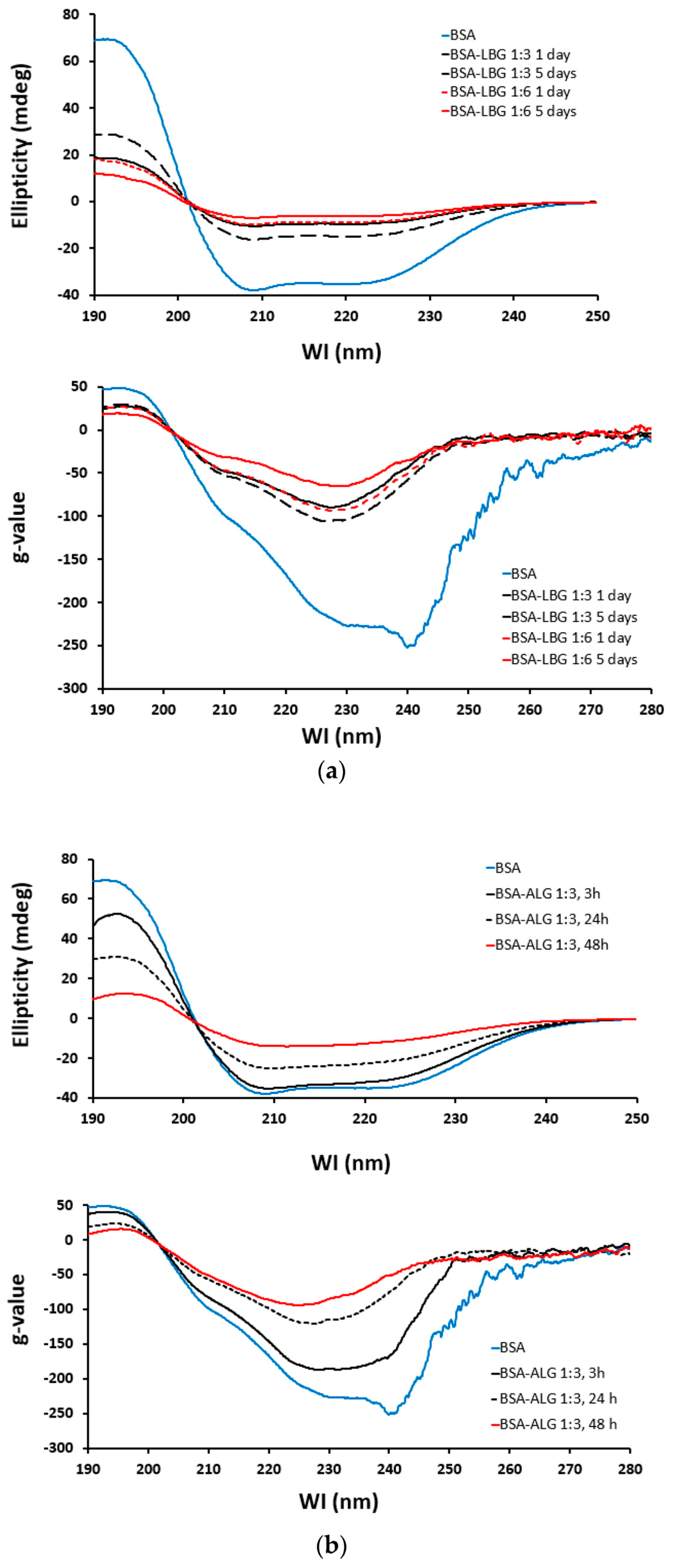
2.2.7. Circular Dichroism (CD) Spectroscopy
2.2.8. Solubility Improvement of Apigenin
3. Results
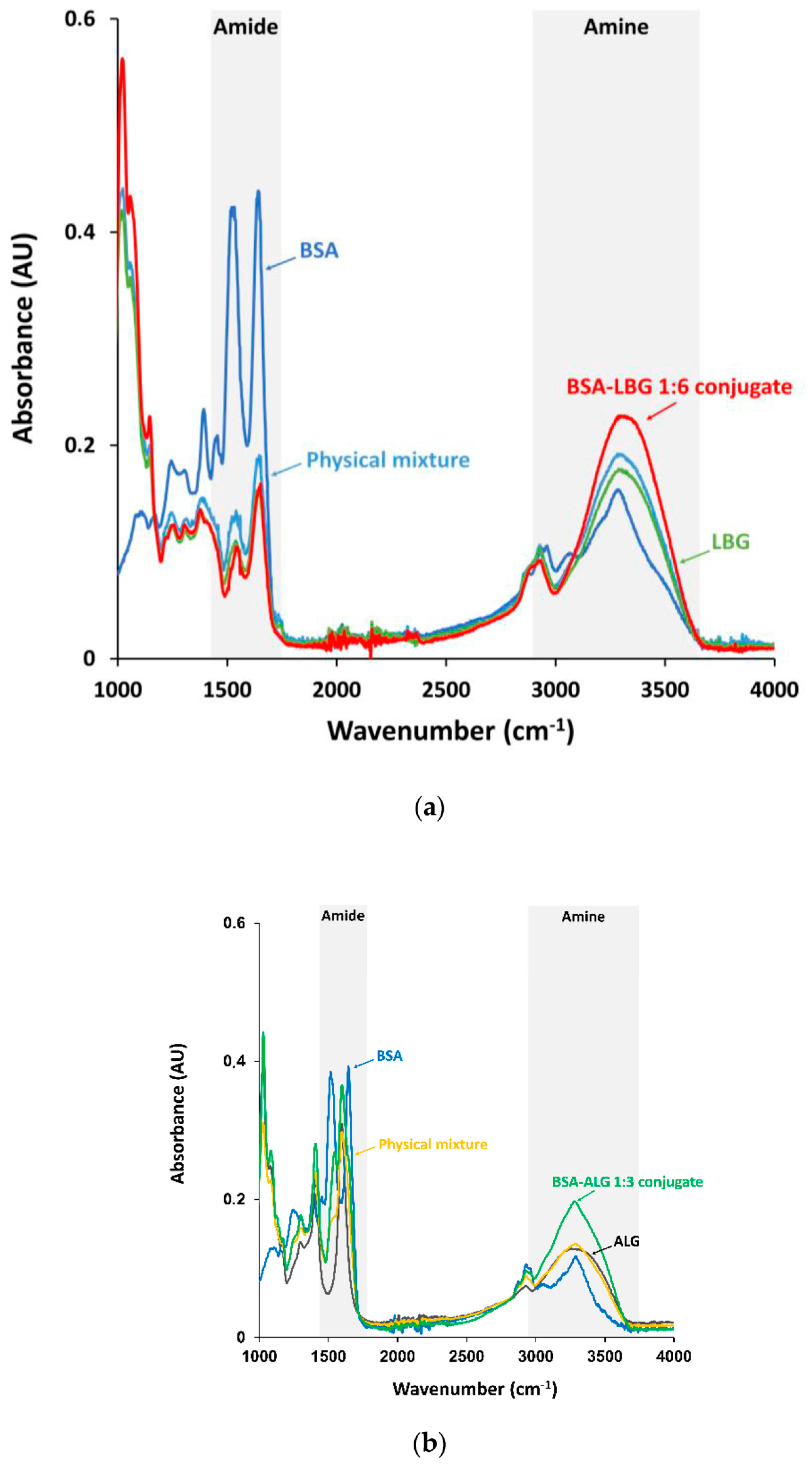
3.1. FTIR Measurements
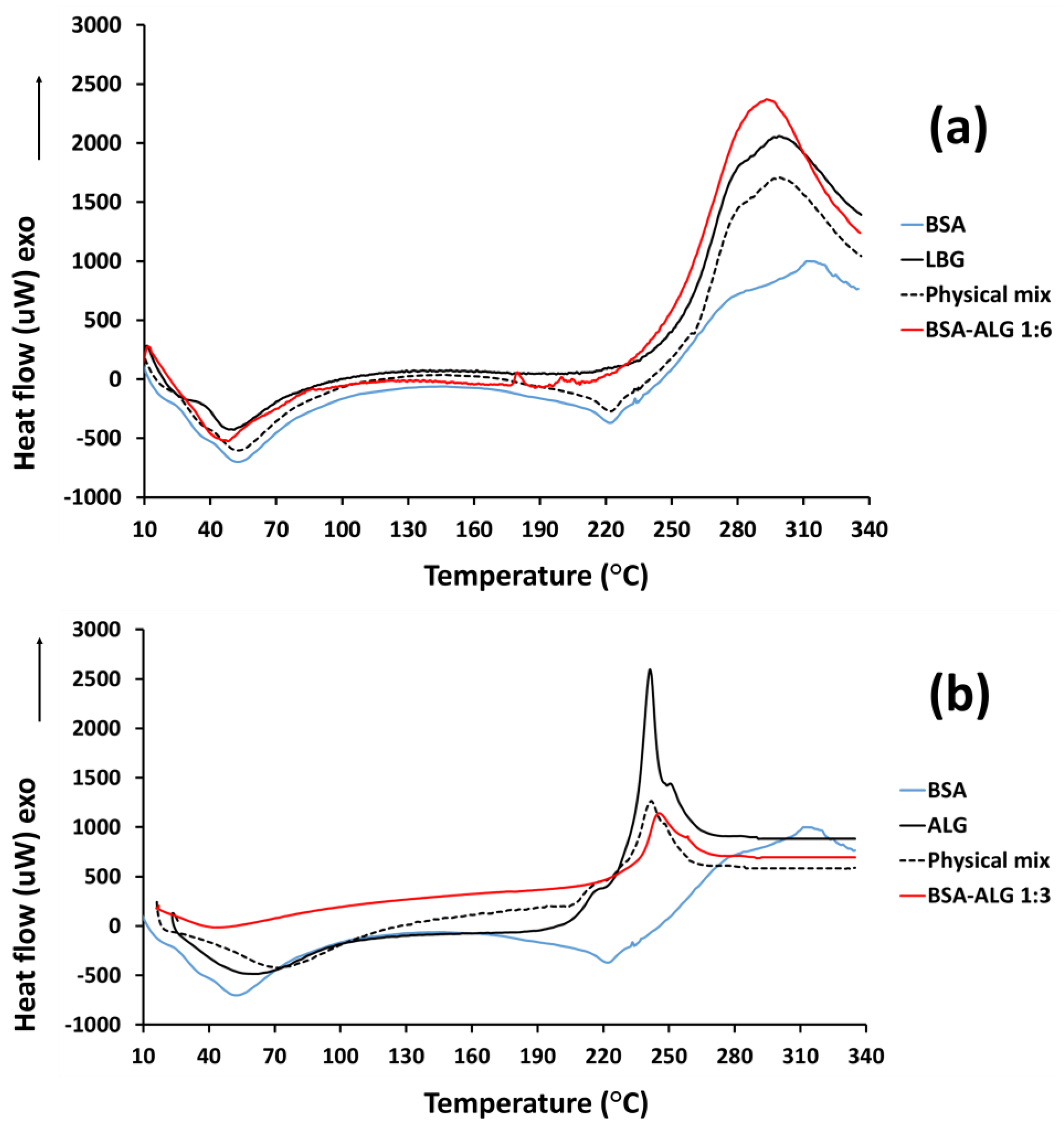
3.2. DSC Measurements
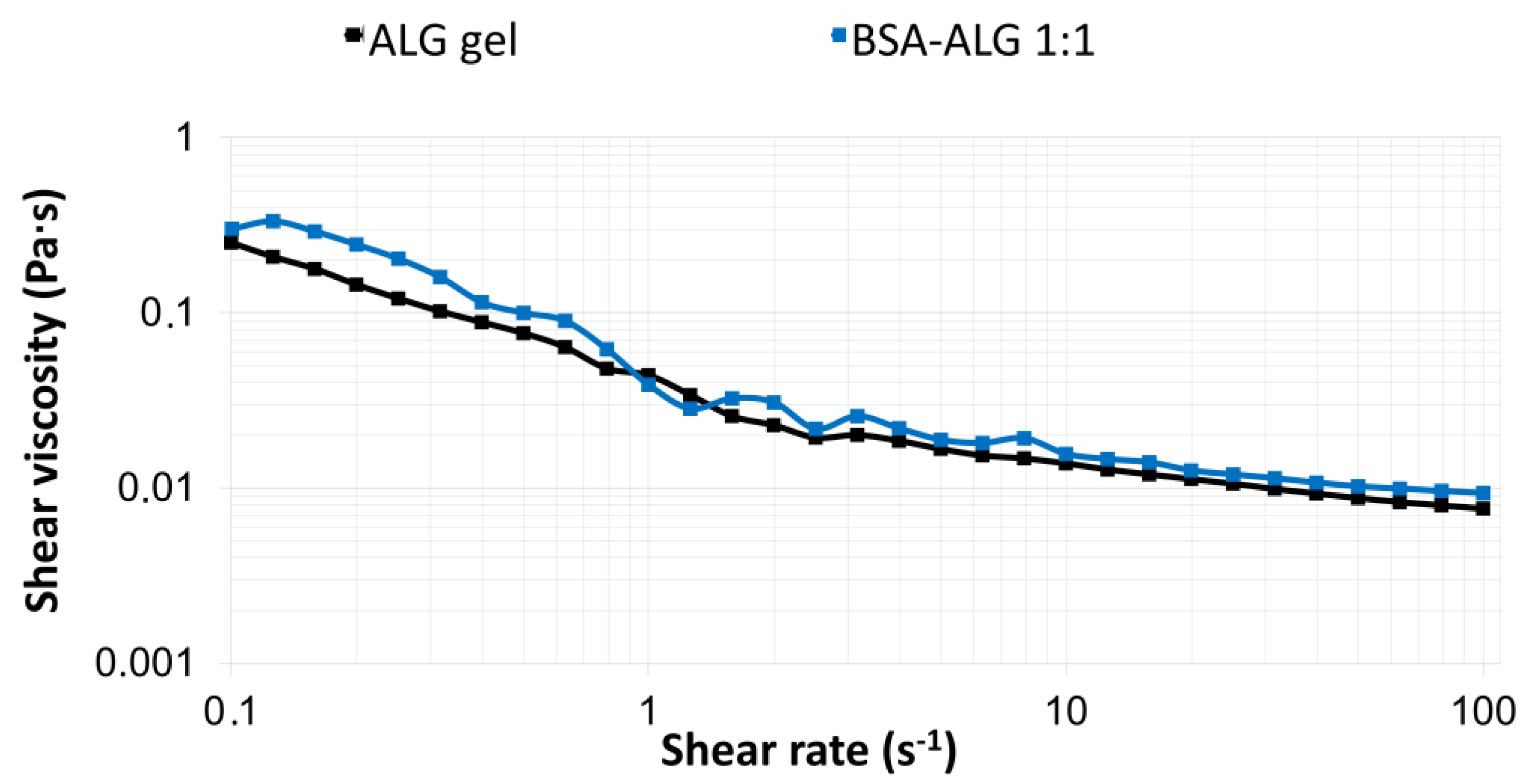
3.3. Rheological Measurements
3.4. Fluorescence Spectroscopy Measurements
3.5. Determination of Available Amino Groups
3.6. Electrophoretic Analysis
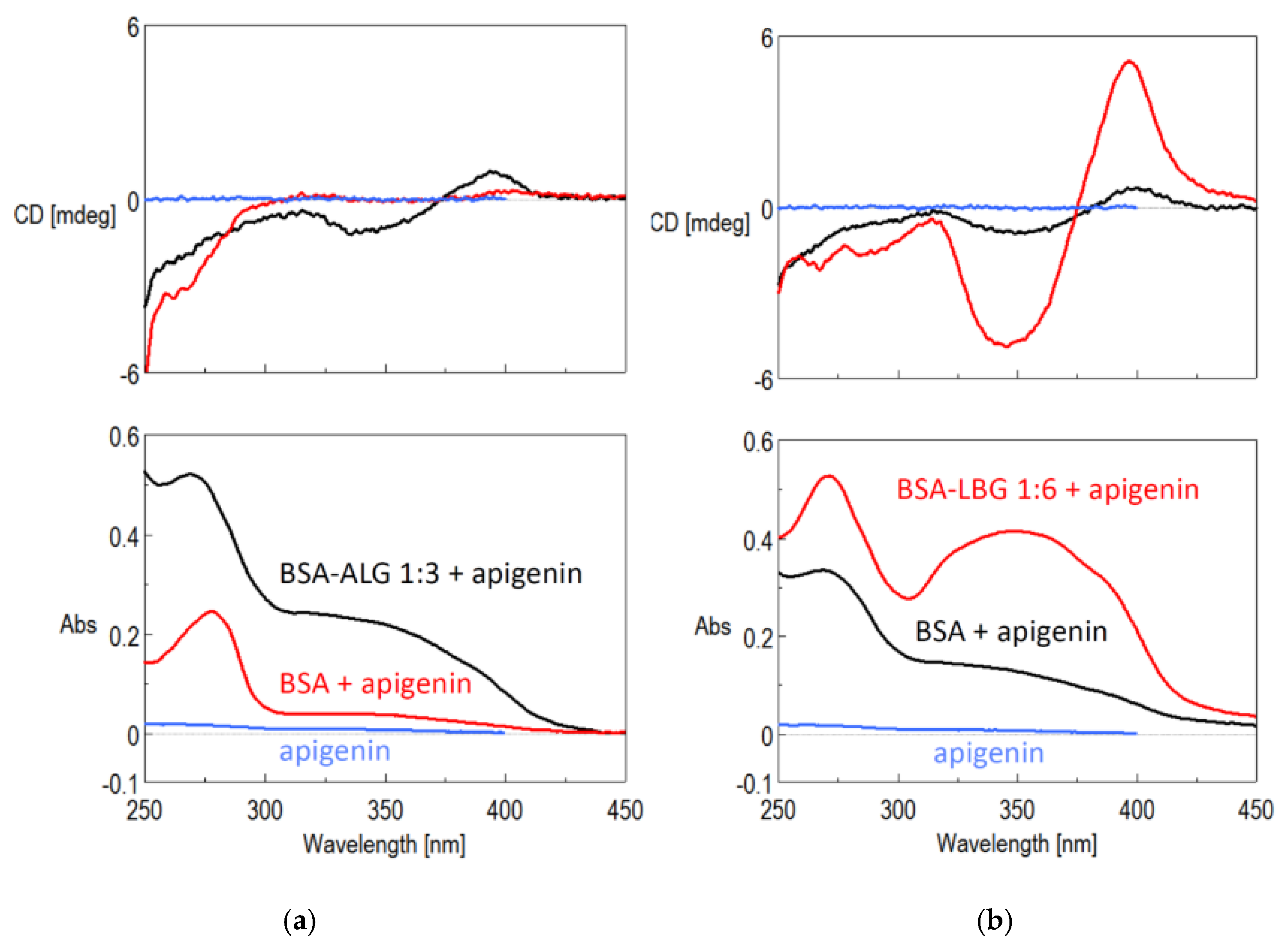
3.7. Circular Dichroism (CD) Spectroscopy
3.8. Solubility Improvement of Apigenin
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALG | Alginate |
| BSA | Bovine serum albumin |
| CD | Circular dichroism |
| ECD | Electronic circular dichroism |
| DSC | Differential scanning calorimetry |
| FTIR | Fourier transform infrared spectroscopy |
| HSA | Human serum albumin |
| ICD | Induced circular dichroism |
| IR | Infrared |
| LBG | Locust bean gum |
| MRP | Maillard reaction products |
| OPA assay | Ortho-phthaldialdehyde assay |
| PAS staining method | Periodic acid–Schiff staining method |
| SDS–PAGE | Sodium dodecyl sulfate–polyacrylamide gel electrophoresis |
| Trp | Tryptophan |
References
- Karimi, M.; Bahrami, S.; Ravari, S.B.; Zangabad, P.S.; Mirshekari, H.; Bozorgomid, M.; Shahreza, S.; Sori, M.; Hamblin, M.R. Albumin nanostructures as advanced drug delivery systems. Expert Opin. Drug Deliv. 2016, 13, 1609–1623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gelamo, E.L.; Tabak, M. Spectroscopic studies on the interaction of bovine (BSA) and human (HSA) serum albumins with ionic surfactants. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2000, 56, 2255–2271. [Google Scholar] [CrossRef]
- Solá, R.J.; Griebenow, K. Glycosylation of therapeutic proteins: An effective strategy to optimize efficacy. BioDrugs 2010, 24, 9–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, B.; Guan, X.; Li, Y.; Shang, S.; Li, J.; Tan, Z. Protein Glycoengineering: An Approach for Improving Protein Properties. Front. Chem. 2020, 8, 622. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.J.; Sun, C.; Xu, X.B.; Zhou, D.Y.; Song, L.; Zhu, B.W. Improving the functional properties of bovine serum albumin-glucose conjugates in natural deep eutectic solvents. Food Chem. 2020, 328, 127122. [Google Scholar] [CrossRef]
- Li, K.; Woo, M.W.; Patel, H.; Selomulya, C. Enhancing the stability of protein-polysaccharides emulsions via Maillard reaction for better oil encapsulation in spray-dried powders by pH adjustment. Food Hydrocoll. 2017, 69, 121–131. [Google Scholar] [CrossRef]
- Kato, A. Industrial Applications of Maillard-Type Protein-Polysaccharide Conjugates. Food Sci. Technol. Res. 2002, 8, 193–199. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.J.; Choi, S.J.; Shin, W.S.; Moon, T.W. Emulsifying properties of bovine serum albumin-galactomannan conjugates. J. Agric. Food Chem. 2003, 51, 1049–1056. [Google Scholar] [CrossRef]
- Barbosa, J.M.; Ushikubo, F.Y.; de Figueiredo Furtado, G.; Cunha, R.L. Oil in water emulsions stabilized by maillard conjugates of sodium caseinate-locust bean gum. J. Dispers. Sci. Technol. 2019, 40, 634–645. [Google Scholar] [CrossRef]
- Ledesma-Osuna, A.I.; Ramos-Clamont, G.; Guzman-Partida, A.M.; Vazquez-Moreno, L. Conjugates of bovine serum albumin with chitin oligosaccharides prepared through the maillard reaction. J. Agric. Food Chem. 2010, 58, 12000–12005. [Google Scholar] [CrossRef]
- Nasrollahzadeh, F.; Varidi, M.; Koocheki, A.; Hadizadeh, F. Effect of microwave and conventional heating on structural, functional and antioxidant properties of bovine serum albumin-maltodextrin conjugates through Maillard reaction. Food Res. Int. 2017, 100, 289–297. [Google Scholar] [CrossRef]
- Armstrong, H.J.; Hill, S.E.; Schrooyen, P.; Mitchell, J.R. A comparison of the viscoelastic properties of conventional and Maillardprotein gels. J. Texture Stud. 1994, 25, 285–298. [Google Scholar] [CrossRef]
- Koyyada, A.; Orsu, P. Natural gum polysaccharides as efficient tissue engineering and drug delivery biopolymers. J. Drug Deliv. Sci. Technol. 2021, 63, 102431. [Google Scholar] [CrossRef]
- Jacob, J.; Haponiuk, J.T.; Thomas, S.; Gopi, S. Biopolymer based nanomaterials in drug delivery systems: A review. Mater. Today Chem. 2018, 9, 43–55. [Google Scholar] [CrossRef]
- Barak, S.; Mudgil, D. Locust bean gum: Processing, properties and food applications-A review. Int. J. Biol. Macromol. 2014, 66, 74–80. [Google Scholar] [CrossRef]
- Sheskey, P.J.; Cook, W.G.; Cable, C.G. Handbook of Pharmaceutical Excipients, 8th ed.; Pharmaceutical Press: London, UK, 2017; pp. 2167–2168. [Google Scholar]
- Mandel, K.G.; Daggy, B.P.; Brodie, D.A.; Jacoby, H.I. Review article: Alginate-raft formulations in the treatment of heartburn and acid reflux. Aliment. Pharm. Ther. 2000, 14, 669–690. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Han, Q.; Shi, J.; Zhang, H.; Wang, Y. Structural, thermal and rheological characterization of bovine serum albumin binding with sodium alginate. J. Mol. Liq. 2020, 299, 112123. [Google Scholar] [CrossRef]
- Ali, F.; Rahul; Naz, F.; Jyoti, S.; Siddique, Y.H. Health functionality of apigenin: A review. Int. J. Food Prop. 2017, 20, 1197–1238. [Google Scholar] [CrossRef]
- Shakeel, F.; Alshehri, S.; Ibrahim, M.A.; Elzayat, E.M.; Altamimi, M.A.; Mohsin, K.; Alanazi, F.K.; Alsarra, I.A. Solubility and thermodynamic parameters of apigenin in different neat solvents at different temperatures. J. Mol. Liq. 2017, 234, 73–80. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, D.; Huang, Y.; Gao, Y.; Qian, S. Biopharmaceutics classification and intestinal absorption study of apigenin. Int. J. Pharm. 2012, 436, 311–317. [Google Scholar] [CrossRef]
- Wang, B.; Qin, Q.; Chang, M.; Li, S.; Shi, X.; Xu, G. Molecular interaction study of flavonoids with human serum albumin using native mass spectrometry and molecular modeling. Anal. Bioanal. Chem. 2018, 410, 827–837. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Sun, Y.; Ding, L.; Wang, Y.; Gao, Z.; Wu, Z.; Wang, S.; Li, W.; Bi, Y. Mechanism evaluation of the interactions between flavonoids and bovine serum albumin based on multi-spectroscopy, molecular docking and Q-TOF HR-MS analyses. Food Chem. 2016, 203, 150–157. [Google Scholar] [CrossRef]
- Khoder, M.; Gbormoi, H.K.; Ryan, A.; Karam, A.; Alany, R.G. Potential use of the maillard reaction for pharmaceutical applications: Gastric and intestinal controlled release alginate-albumin beads. Pharmaceutics 2019, 11, 83. [Google Scholar] [CrossRef] [Green Version]
- Nooshkam, M.; Varidi, M.; Verma, D.K. Functional and biological properties of Maillard conjugates and their potential application in medical and food: A review. Food Res. Int. 2020, 131, 109003. [Google Scholar] [CrossRef]
- Kuzan, A. Toxicity of advanced glycation end products (Review). Biomed. Rep. 2021, 14, 1–8. [Google Scholar] [CrossRef]
- Lee, Y.Y.; Tang, T.K.; Phuah, E.T.; Alitheen, N.B.M.; Tan, C.P.; Lai, O.M. New functionalities of Maillard reaction products as emulsifiers and encapsulating agents, and the processing parameters: A brief review. J. Sci. Food Agric. 2017, 97, 1379–1385. [Google Scholar] [CrossRef]
- Duan, P.; Kandemir, N.; Wang, J.; Chen, J. Rheological Characterization of Alginate Based Hydrogels for Tissue Engineering. MRS Adv. 2017, 2, 1309–1314. [Google Scholar] [CrossRef] [Green Version]
- Guo, P.; Liu, D.; Subramanyam, K.; Wang, B.; Yang, J.; Huang, J.; Auguste, D.T.; Moses, M.A. Nanoparticle elasticity directs tumor uptake. Nat. Commun. 2018, 9, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.N.; Liu, Y.; Niu, L.Y.; Zhao, C.P. Spectroscopic studies on the interaction of bovine serum albumin with surfactants and apigenin. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 94, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Jian, W.; Wang, L.; Wu, L.; Sun, Y.M. Physicochemical properties of bovine serum albumin-glucose and bovine serum albumin-mannose conjugates prepared by pulsed electric fields treatment. Molecules 2018, 23, 570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.Y.; Shin, W.S. Characterisation of bovine serum albumin-fucoidan conjugates prepared via the Maillard reaction. Food Chem. 2015, 173, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Green¢eld, N.J. Applications of circular dichroism in protein and peptide analysis. Trends 1999, 18, 236–244. [Google Scholar] [CrossRef]
- McPhie, P. Circular dichroism studies on proteins in films and in solution: Estimation of secondary structure by g-factor analysis. Anal. Biochem. 2001, 293, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Tedesco, D.; Bertucci, C. Induced circular dichroism as a tool to investigate the binding of drugs to carrier proteins: Classic approaches and new trends. J. Pharm. Biomed. Anal. 2015, 113, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Kiss, E.; Mirzahosseini, A.; Hubert, Á.; Ambrus, A.; Őrfi, L.; Horváth, P. DNA binding of sunitinib: Spectroscopic evidence via circular dichroism and nuclear magnetic resonance. J. Pharm. Biomed. Anal. 2018, 150, 355–361. [Google Scholar] [CrossRef]
- Liu, L.; Liu, Y.; Xiao, A.; Mei, S.; Xie, Y. Influence of human serum albumin glycation on the binding affinities for natural flavonoids. Open Chem. 2019, 17, 806–812. [Google Scholar] [CrossRef]
| Conjugates | Molar Ratio | Time |
|---|---|---|
| BSA-LBG | 1:3 and 1:6 | 1, 3, 5 days |
| BSA-ALG | 1:1, 1:3 | 3, 6, 8, 18, 24, 48 h |
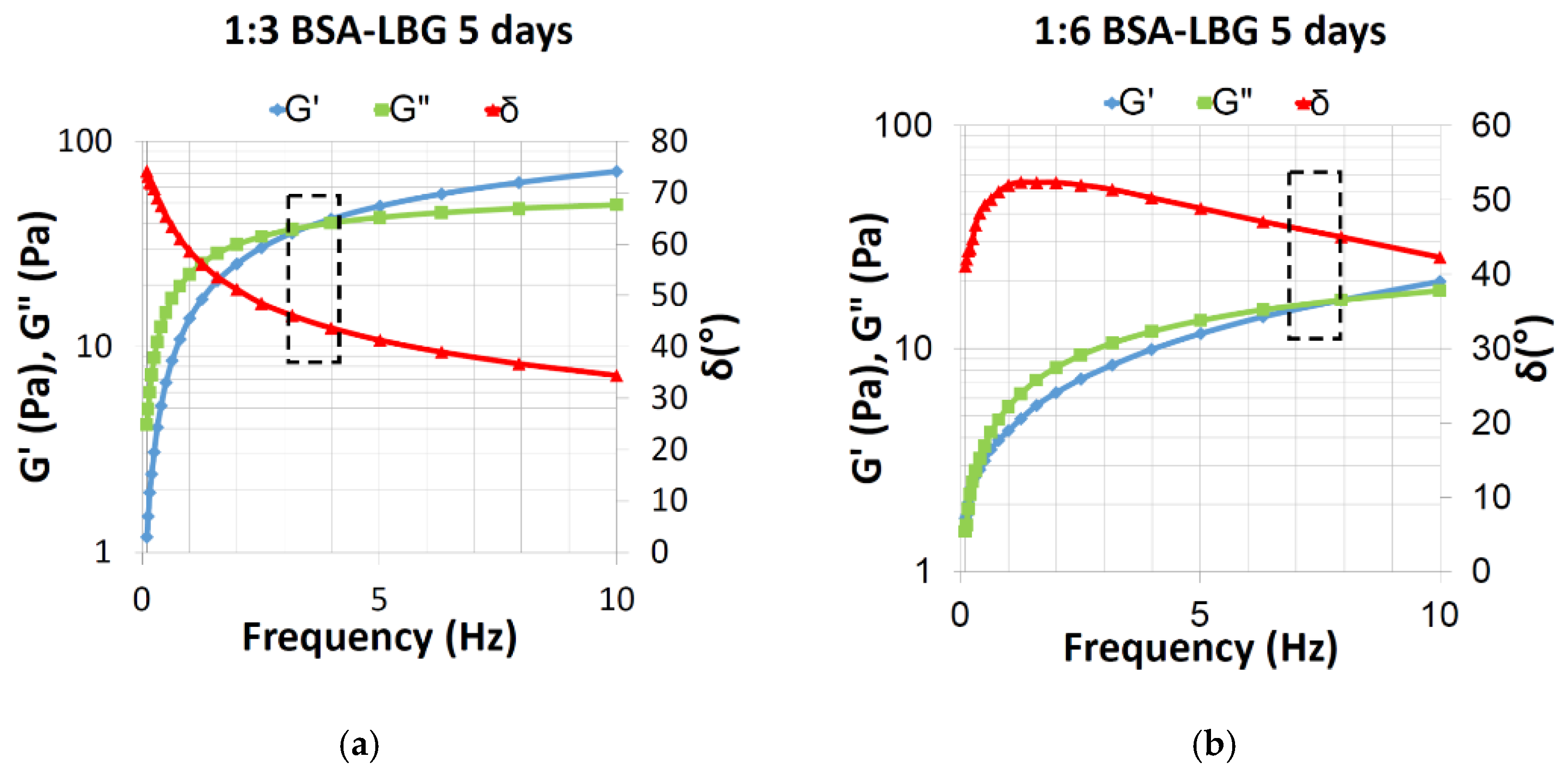
| Sample | δ (°) | G’ (Pa) | G’’ (Pa) |
|---|---|---|---|
| BSA-LBG 1:3 | 43.7 | 42.01 | 40.14 |
| 46.14 | 35.93 | 37.38 | |
| 45 | 38.77 | 38.66 | |
| BSA-LBG 1:6 | 48.2 | 2.866 | 3.206 |
| 46.63 | 2.677 | 2.833 | |
| 45 | 2.48 | 2.45 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pápay, Z.E.; Magramane, S.; Király, M.; Szalkai, P.; Ludányi, K.; Horváth, P.; Antal, I. Optimization and Development of Albumin–Biopolymer Bioconjugates with Solubility-Improving Properties. Biomedicines 2021, 9, 737. https://doi.org/10.3390/biomedicines9070737
Pápay ZE, Magramane S, Király M, Szalkai P, Ludányi K, Horváth P, Antal I. Optimization and Development of Albumin–Biopolymer Bioconjugates with Solubility-Improving Properties. Biomedicines. 2021; 9(7):737. https://doi.org/10.3390/biomedicines9070737
Chicago/Turabian StylePápay, Zsófia Edit, Sabrina Magramane, Márton Király, Petra Szalkai, Krisztina Ludányi, Péter Horváth, and István Antal. 2021. "Optimization and Development of Albumin–Biopolymer Bioconjugates with Solubility-Improving Properties" Biomedicines 9, no. 7: 737. https://doi.org/10.3390/biomedicines9070737
APA StylePápay, Z. E., Magramane, S., Király, M., Szalkai, P., Ludányi, K., Horváth, P., & Antal, I. (2021). Optimization and Development of Albumin–Biopolymer Bioconjugates with Solubility-Improving Properties. Biomedicines, 9(7), 737. https://doi.org/10.3390/biomedicines9070737







