Proof-of-Concept for the Analgesic Effect and Thermoregulatory Safety of Orally Administered Multi-Target Compound SZV 1287 in Mice: A Novel Drug Candidate for Neuropathic Pain
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of SZV 1287
2.2. Preparation of Enterosolvent SZV 1287 Capsules
2.3. Mouse Experiments
2.3.1. Animals
2.3.2. Ethics
2.3.3. Nociceptive Study
Experimental Design
Experimental Model
Measurement of Mechanonociceptive Withdrawal Thresholds of the Hind Paws
Measurement of Plasma and Brain Concentrations of SZV 1287 and its Main Metabolites
HPLC-UV Analyses
HPLC-FLD Analyses
2.3.4. Thermophysiological Study
Experimental Design
Determination of Proton-Induced TRPV1 Receptor Activation in Cultured Primary Sensory Neurons
Measurement of General Locomotor Activity and Abdominal Temperature
Measurement of Colonic Temperature
2.4. Data and Statistical Analysis
3. Results
3.1. Nociceptive Study
3.1.1. SZV 1287 in Enterosolvent Capsule Significantly Decreases Neuropathic Mechanical Hyperalgesia in Mice
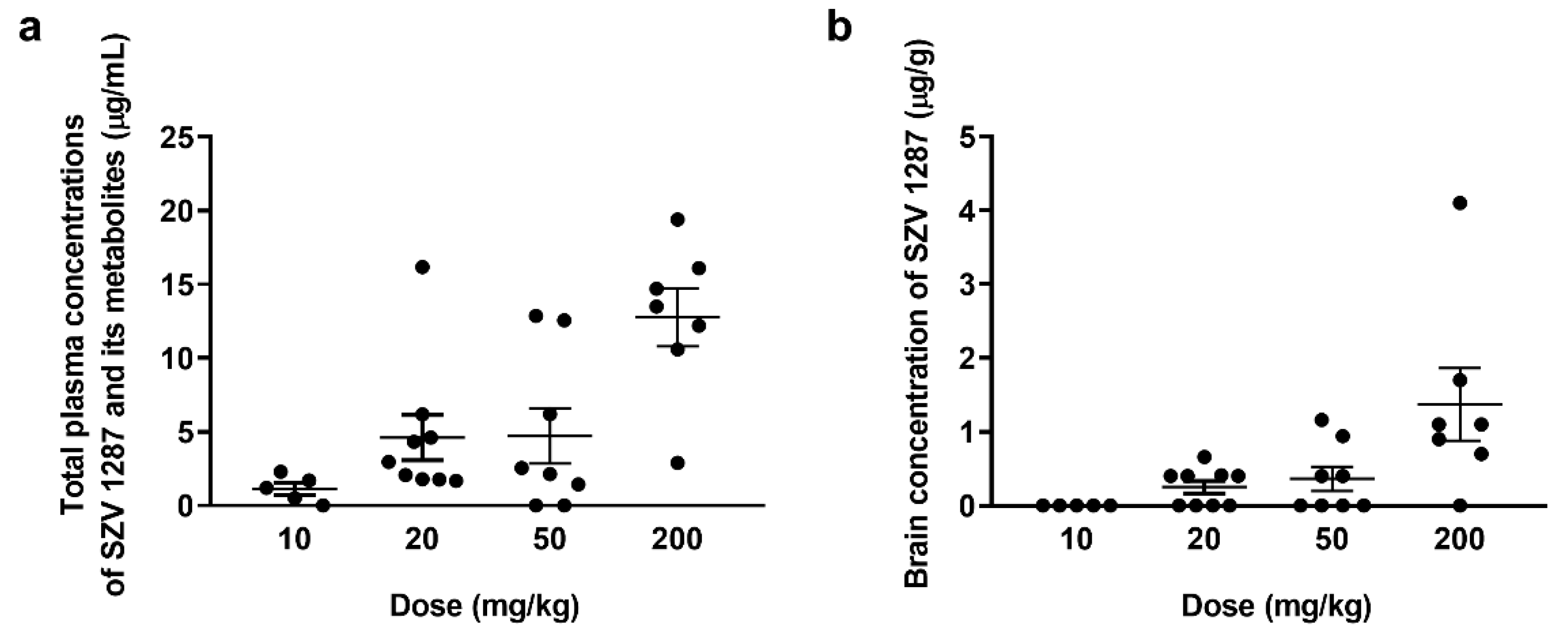
3.1.2. SZV 1287 Is Absorbed from the Enterosolvent Capsule and Penetrates into the Brain
3.1.3. SZV 1287 Reaches Its Maximal Plasma Concentration 1 H after Oral Administration of Enterosolvent Capsules
3.2. Thermophysiological Study
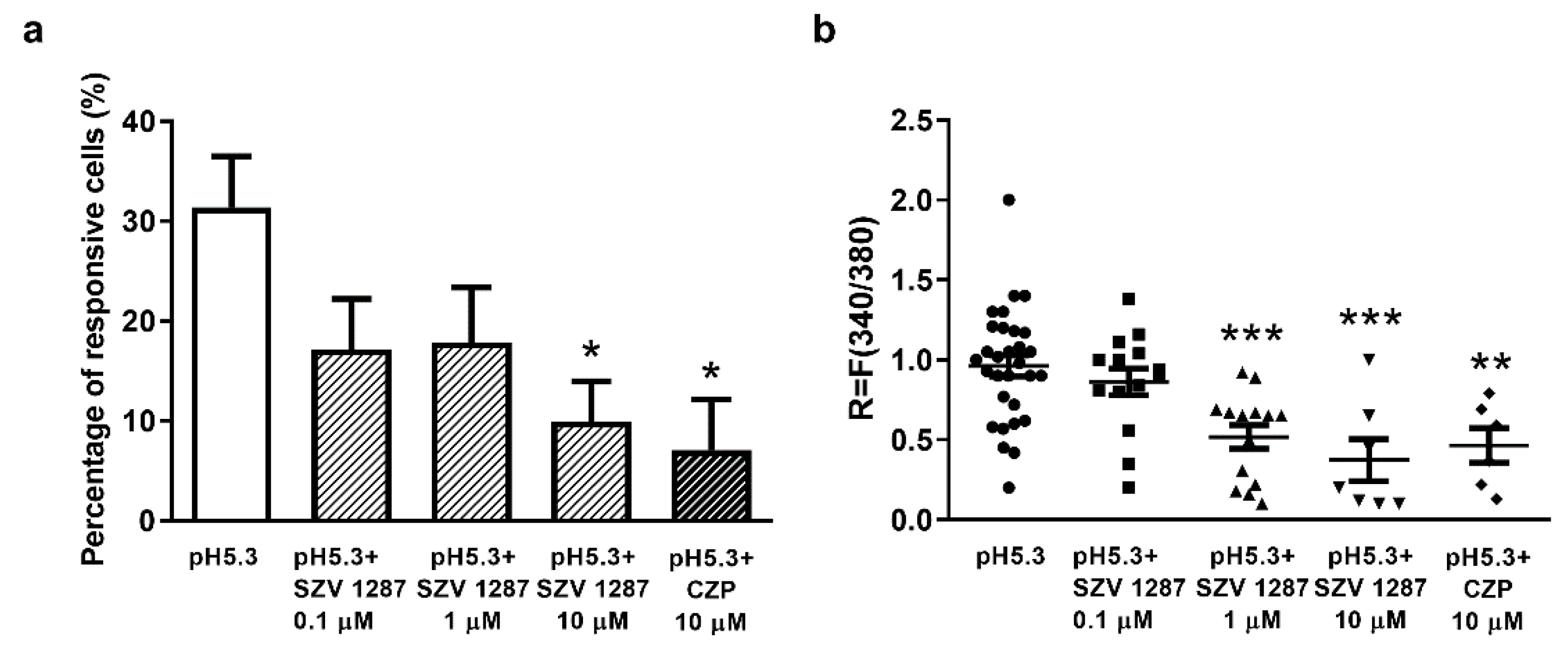
3.2.1. SZV 1287 Inhibits Proton-Induced TRPV1 Receptor Activation in Cultured Primary Sensory Neurons
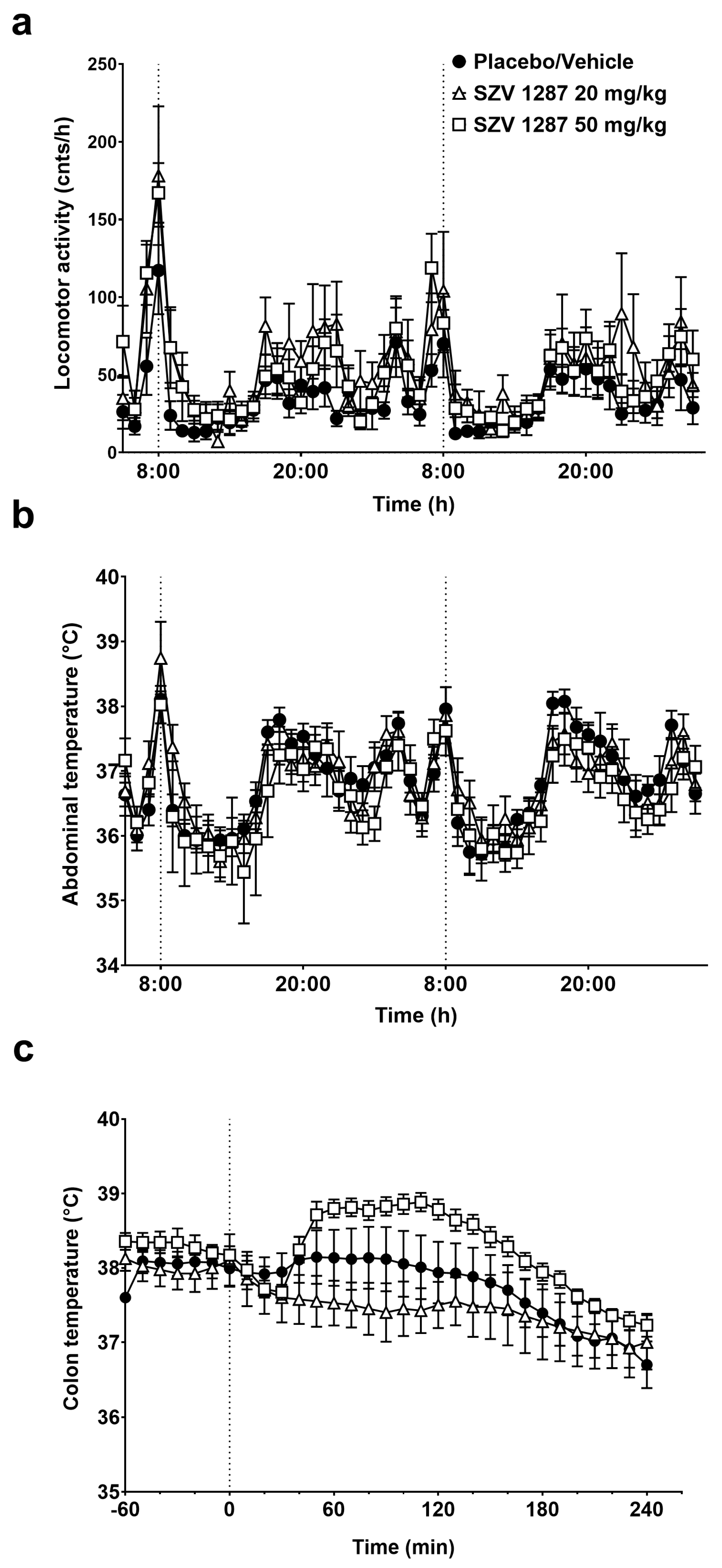
3.2.2. SZV 1287 Does Not Substantially Affect General Locomotor Activity and Deep Body Temperature Following Enterosolvent Capsule Administration in Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1. NMR Spectra Recording of SZV 1287 in Acidic Media
Appendix A.2. In Vitro Dissolution Test of Enterosolvent SZV 1287 Capsules
References
- Van Hecke, O.; Austin, S.K.; Khan, R.A.; Smith, B.; Torrance, N. Neuropathic pain in the general population: A systematic review of epidemiological studies. Pain 2014, 155, 654–662. [Google Scholar] [CrossRef]
- Attal, N.; Bouhassira, D. Pharmacotherapy of neuropathic pain. Pain 2015, 156, S104–S114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavalli, E.; Mammana, S.; Nicoletti, F.; Bramanti, P.; Mazzon, E. The neuropathic pain: An overview of the current treatment and future therapeutic approaches. Int. J. Immunopathol. Pharmacol. 2019, 33, 2058738419838383. [Google Scholar] [CrossRef] [Green Version]
- Finnerup, N.; Attal, N.; Haroutounian, S.; McNicol, E.; Baron, R.; Dworkin, R.H.; Gilron, I.; Haanpää, M.; Hansson, P.; Jensen, T.S.; et al. Pharmacotherapy for neuropathic pain in adults: A systematic review and meta-analysis. Lancet Neurol. 2015, 14, 162–173. [Google Scholar] [CrossRef] [Green Version]
- Helyes, Z.; Mátyus, P.; Tékus, V.; Scheich, B. Semicarbazide-Sensitive Amine-Oxidase Inhibitors, as Analgesics in Traumatic Neuropathy and Neurogenic Inflammation. Patent WO/2015/159/112 22 October 2015. [Google Scholar]
- Kean, W.F. Oxaprozin: Kinetic and dynamic profile in the treatment of pain. Curr. Med Res. Opin. 2004, 20, 1275–1277. [Google Scholar] [CrossRef]
- Kean, W.F.; Kean, R.; Buchanan, W.W. A critical assessment of oxaprozin clinical profile in rheumatic diseases. Inflammopharmacology 2002, 10, 241–284. [Google Scholar] [CrossRef]
- Mátyus, P.; Chai, C.L.L. Metabolism-Activated Multitargeting (MAMUT): An Innovative Multitargeting Approach to Drug Design and Development. ChemMedChem 2015, 11, 1197–1198. [Google Scholar] [CrossRef]
- Mátyus, P.; Magyar, K.; Pihlavista, M.; Gyires, K.; Wang, Y.; Woda, P.; Dunkel, P.; Tóth-Sarudy, É.; Túrós, G. Compounds for Inhibiting Semicarbazide-Sensitive Amine Oxidase (SSAO)/Vascular Adhesion Protein-1 (VAP-1) and Uses Thereof for Treatment and Prevention of Diseases. Patent WO/2010/029379 18 March 2010. [Google Scholar]
- Payrits, M.; Sághy, É.; Mátyus, P.; Czompa, A.; Ludmerczki, R.; Deme, R.; Sándor, Z.; Helyes, Z.; Szőke, É. A novel 3-(4,5-diphenyl-1,3-oxazol-2-yl)propanal oxime compound is a potent Transient Receptor Potential Ankyrin 1 and Vanilloid 1 (TRPA1 and V1) receptor antagonist. Neuroscience 2016, 324, 151–162. [Google Scholar] [CrossRef]
- Smith, D.J.; Salmi, M.; Bono, P.; Hellman, J.; Leu, T.; Jalkanen, S. Cloning of vascular adhesion protein 1 reveals a novel mul-tifunctional adhesion molecule. J. Exp. Med. 1998, 188, 17–27. [Google Scholar] [CrossRef] [Green Version]
- Andrés, N.; Lizcano, J.M.; Rodríguez, M.J.; Romera, M.; Unzeta, M.; Mahy, N. Tissue activity and cellular localization of human semicarbazide-sensitive amine oxidase. J. Histochem. Cytochem. 2001, 49, 209–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murata, M.; Noda, K.; Kawasaki, A.; Yoshida, S.; Dong, Y.; Saito, M.; Dong, Z.; Ando, R.; Mori, S.; Saito, W.; et al. Soluble Vascular Adhesion Protein-1 Mediates Spermine Oxidation as Semicarbazide-Sensitive Amine Oxidase: Possible Role in Proliferative Diabetic Retinopathy. Curr. Eye Res. 2017, 42, 1674–1683. [Google Scholar] [CrossRef]
- Salmi, M.; Jalkanen, S. Vascular Adhesion Protein-1: A Cell Surface Amine Oxidase in Translation. Antioxidants Redox Signal. 2019, 30, 314–332. [Google Scholar] [CrossRef]
- Bautista, D.M.; Pellegrino, M.; Tsunozaki, M. TRPA1: A Gatekeeper for Inflammation. Annu. Rev. Physiol. 2013, 75, 181–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Hackos, D.H. TRPA1 as a drug target—promise and challenges. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2015, 388, 451–463. [Google Scholar] [CrossRef] [Green Version]
- Pannecoeck, R.; Serruys, D.; Benmeridja, L.; Delanghe, J.R.; Van Geel, N.; Speeckaert, R.; Speeckaert, M.M. Vascular adhesion protein-1: Role in human pathology and application as a biomarker. Crit. Rev. Clin. Lab. Sci. 2015, 52, 284–300. [Google Scholar] [CrossRef]
- Vakal, S.; Jalkanen, S.; Dahlström, K.M.; Salminen, T.A. Human Copper-Containing Amine Oxidases in Drug Design and Development. Molecules 2020, 25, 1293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horváth, Á.; Menghis, A.; Botz, B.; Borbély, É.; Kemény, Á.; Tékus, V.; Csepregi, J.Z.; Mócsai, A.; Juhász, T.; Zákány, R.; et al. Analgesic and Anti-Inflammatory Effects of the Novel Semicarbazide-Sensitive Amine-Oxidase Inhibitor SzV-1287 in Chronic Arthritis Models of the Mouse. Sci. Rep. 2017, 7, 39863. [Google Scholar] [CrossRef] [PubMed]
- Horváth, Á.; Tékus, V.; Bencze, N.; Szentes, N.; Scheich, B.; Bölcskei, K.; Szőke, É.; Mócsai, A.; Tóth-Sarudy, É.; Mátyus, P.; et al. Analgesic effects of the novel semicarbazide-sensitive amine oxidase inhibitor SZV 1287 in mouse pain models with neuropathic mechanisms: Involvement of transient receptor potential vanilloid 1 and ankyrin 1 receptors. Pharmacol. Res. 2018, 131, 231–243. [Google Scholar] [CrossRef]
- Tábi, T.; Szökő, É.; Mérey, A.; Tóth, V.; Matyus, P.; Gyires, K. Study on SSAO enzyme activity and anti-inflammatory effect of SSAO inhibitors in animal model of inflammation. J. Neural Transm. 2013, 120, 963–967. [Google Scholar] [CrossRef]
- Forgács, V.; Németh, E.; Gyuricza, B.; Kis, A.; Szabó, J.P.; Mikecz, P.; Mátyus, P.; Helyes, Z.; Horváth, Á.I.; Kálai, T.; et al. Radiosynthesis and Preclinical Investigation of 11 C-Labelled 3-(4,5-Diphenyl-1,3-oxazol-2-yl)propanal Oxime ([ 11 C]SZV 1287). ChemMedChem 2020, 15, 2470–2476. [Google Scholar] [CrossRef]
- Tékus, V.; Horváth, Á.I.; Csekő, K.; Szabadfi, K.; Kovács-Valasek, A.; Dányádi, B.; Deres, L.; Halmosi, R.; Sághy, É.; Varga, Z.V.; et al. Protective effects of the novel amine-oxidase inhibitor multi-target drug SZV 1287 on streptozotocin-induced beta cell damage and diabetic complications in rats. Biomed. Pharmacother. 2021, 134, 111105. [Google Scholar] [CrossRef] [PubMed]
- Fliszár-Nyúl, E.; Faisal, Z.; Mohos, V.; Derdák, D.; Lemli, B.; Kálai, T.; Sár, C.; Zsidó, B.Z.; Hetényi, C.; Horváth, Á.I.; et al. Interaction of SZV 1287, a novel oxime analgesic drug candidate, and its metabolites with serum albumin. J. Mol. Liq. 2021, 333, 115945. [Google Scholar] [CrossRef]
- Seltzer, Z.; Dubner, R.; Shir, Y. A novel behavioral model of neuropathic pain disorders produced in rats by partial sciatic nerve injury. Pain 1990, 43, 205–218. [Google Scholar] [CrossRef]
- Malmberg, A.B.; Basbaum, A.I. Partial sciatic nerve injury in the mouse as a model of neuropathic pain: Behavioral and neu-roanatomical correlates. Pain 1998, 76, 215–222. [Google Scholar] [CrossRef]
- Sándor, K.; Elekes, K.; Szabó, Á.; Pintér, E.; Engström, M.; Wurster, S.; Szolcsányi, J.; Helyes, Z. Analgesic effects of the somatostatin sst4 receptor selective agonist J-2156 in acute and chronic pain models. Eur. J. Pharmacol. 2006, 539, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, M.; Takasu, K.; Takeuchi, Y.; Ono, H. Pain relief by gabapentin and pregabalin via supraspinal mechanisms after peripheral nerve injury. J. Neurosci. Res. 2008, 86, 3258–3264. [Google Scholar] [CrossRef]
- Fukuda, T.; Yamashita, S.; Hisano, S.; Tanaka, M. Olanzapine Attenuates Mechanical Allodynia in a Rat Model of Partial Sciatic Nerve Ligation. Korean J. Pain 2015, 28, 185–192. [Google Scholar] [CrossRef]
- Kántás, B.; Börzsei, R.; Szőke, É.; Bánhegyi, P.; Horváth, Á.; Hunyady, Á.; Borbély, É.; Hetényi, C.; Pintér, E.; Helyes, Z. Novel Drug-Like Somatostatin Receptor 4 Agonists are Potential Analgesics for Neuropathic Pain. Int. J. Mol. Sci. 2019, 20, 6245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garami, A.; Pakai, E.; McDonald, H.A.; Reilly, R.M.; Gomtsyan, A.; Corrigan, J.J.; Pinter, E.; Zhu, D.X.D.; Lehto, S.G.; Gavva, N.R.; et al. TRPV1 antagonists that cause hypothermia, instead of hyperthermia, in rodents: Compounds’ pharmacological profiles, in vivo targets, thermoeffectors recruited and implications for drug development. Acta Physiol. 2018, 223, e13038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szőke, É.; Börzsei, R.; Tóth, D.M.; Lengl, O.; Helyes, Z.; Sándor, Z.; Szolcsányi, J. Effect of lipid raft disruption on TRPV1 receptor activation of trigeminal sensory neurons and transfected cell line. Eur. J. Pharmacol. 2010, 628, 67–74. [Google Scholar] [CrossRef]
- Garami, A.; Pakai, E.; Oliveira, D.L.; Steiner, A.A.; Wanner, S.P.; Almeida, M.C.; Lesnikov, V.A.; Gavva, N.R.; Romanovsky, A.A. Thermoregulatory Phenotype of the Trpv1 Knockout Mouse: Thermoeffector Dysbalance with Hyperkinesis. J. Neurosci. 2011, 31, 1721–1733. [Google Scholar] [CrossRef] [Green Version]
- Pakai, E.; Tékus, V.; Zsiborás, C.; Rumbus, Z.; Olah, E.; Keringer, P.; Khidhir, N.; Matics, R.; Deres, L.; Ordög, K.; et al. The Neurokinin-1 Receptor Contributes to the Early Phase of Lipopolysaccharide-Induced Fever via Stimulation of Peripheral Cyclooxygenase-2 Protein Expression in Mice. Front. Immunol. 2018, 9, 166. [Google Scholar] [CrossRef] [Green Version]
- Baraldi, P.G.; Preti, D.; Materazzi, S.; Geppetti, P. Transient Receptor Potential Ankyrin 1 (TRPA1) Channel as Emerging Target for Novel Analgesics and Anti-Inflammatory Agents. J. Med. Chem. 2010, 53, 5085–5107. [Google Scholar] [CrossRef]
- DeFalco, J.; Steiger, D.; Gustafson, A.; Emerling, D.E.; Kelly, M.G.; Duncton, M.A. Oxime derivatives related to AP18: Agonists and antagonists of the TRPA1 receptor. Bioorganic Med. Chem. Lett. 2010, 20, 276–279. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Joshi, S.K.; DiDomenico, S.; Perner, R.J.; Mikusa, J.P.; Gauvin, D.M.; Segreti, J.A.; Han, P.; Zhang, X.-F.; Niforatos, W.; et al. Selective blockade of TRPA1 channel attenuates pathological pain without altering noxious cold sensation or body temperature regulation. Pain 2011, 152, 1165–1172. [Google Scholar] [CrossRef] [PubMed]
- Brito, R.; Sheth, S.; Mukherjea, D.; Rybak, L.P.; Ramkumar, V. TRPV1: A Potential Drug Target for Treating Various Diseases. Cells 2014, 3, 517–545. [Google Scholar] [CrossRef] [Green Version]
- Koivisto, A.; Jalava, N.; Bratty, R.; Pertovaara, A. TRPA1 Antagonists for Pain Relief. Pharmaceuticals 2018, 11, 117. [Google Scholar] [CrossRef] [Green Version]
- Szolcsányi, J. Effect of capsaicin on thermoregulation: An update with new aspects. Temperature 2015, 2, 277–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garami, A.; Shimansky, Y.P.; Rumbus, Z.; Vizin, R.C.L.; Farkas, N.; Hegyi, J.; Szakacs, Z.; Solymar, M.; Csenkey, A.; Chiche, D.A.; et al. Hyperthermia induced by transient receptor potential vanilloid-1 (TRPV1) antagonists in human clinical trials: In-sights from mathematical modeling and meta-analysis. Pharmacol. Ther. 2020, 208, 107474. [Google Scholar] [CrossRef] [PubMed]
- Szallasi, A.; Sheta, M. Targeting TRPV1 for pain relief: Limits, losers and laurels. Expert Opin. Investig. Drugs 2012, 21, 1351–1369. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horváth, Á.I.; Szentes, N.; Tékus, V.; Payrits, M.; Szőke, É.; Oláh, E.; Garami, A.; Fliszár-Nyúl, E.; Poór, M.; Sár, C.; et al. Proof-of-Concept for the Analgesic Effect and Thermoregulatory Safety of Orally Administered Multi-Target Compound SZV 1287 in Mice: A Novel Drug Candidate for Neuropathic Pain. Biomedicines 2021, 9, 749. https://doi.org/10.3390/biomedicines9070749
Horváth ÁI, Szentes N, Tékus V, Payrits M, Szőke É, Oláh E, Garami A, Fliszár-Nyúl E, Poór M, Sár C, et al. Proof-of-Concept for the Analgesic Effect and Thermoregulatory Safety of Orally Administered Multi-Target Compound SZV 1287 in Mice: A Novel Drug Candidate for Neuropathic Pain. Biomedicines. 2021; 9(7):749. https://doi.org/10.3390/biomedicines9070749
Chicago/Turabian StyleHorváth, Ádám István, Nikolett Szentes, Valéria Tékus, Maja Payrits, Éva Szőke, Emőke Oláh, András Garami, Eszter Fliszár-Nyúl, Miklós Poór, Cecília Sár, and et al. 2021. "Proof-of-Concept for the Analgesic Effect and Thermoregulatory Safety of Orally Administered Multi-Target Compound SZV 1287 in Mice: A Novel Drug Candidate for Neuropathic Pain" Biomedicines 9, no. 7: 749. https://doi.org/10.3390/biomedicines9070749
APA StyleHorváth, Á. I., Szentes, N., Tékus, V., Payrits, M., Szőke, É., Oláh, E., Garami, A., Fliszár-Nyúl, E., Poór, M., Sár, C., Kálai, T., Pál, S., Percze, K., Scholz, É. N., Mészáros, T., Tóth, B., Mátyus, P., & Helyes, Z. (2021). Proof-of-Concept for the Analgesic Effect and Thermoregulatory Safety of Orally Administered Multi-Target Compound SZV 1287 in Mice: A Novel Drug Candidate for Neuropathic Pain. Biomedicines, 9(7), 749. https://doi.org/10.3390/biomedicines9070749








