Poly (ADP-Ribose) Polymerase Inhibitor, ABT888, Improved Cisplatin Effect in Human Oral Cell Carcinoma
Abstract
:1. Introduction
2. Material and Methods
2.1. Culture of Cell Lines Derived from Oral Carcinoma
2.2. MTT Assay
2.3. RNA Isolation, cDNA Synthesis and Real-Time Quantitative PCR Amplification
2.4. Western Blot Analysis
2.5. Immunofluorescence Staining
2.6. Xenograft Model
2.7. HE Staining
2.8. Materials
2.9. Statistical Analysis
3. Results
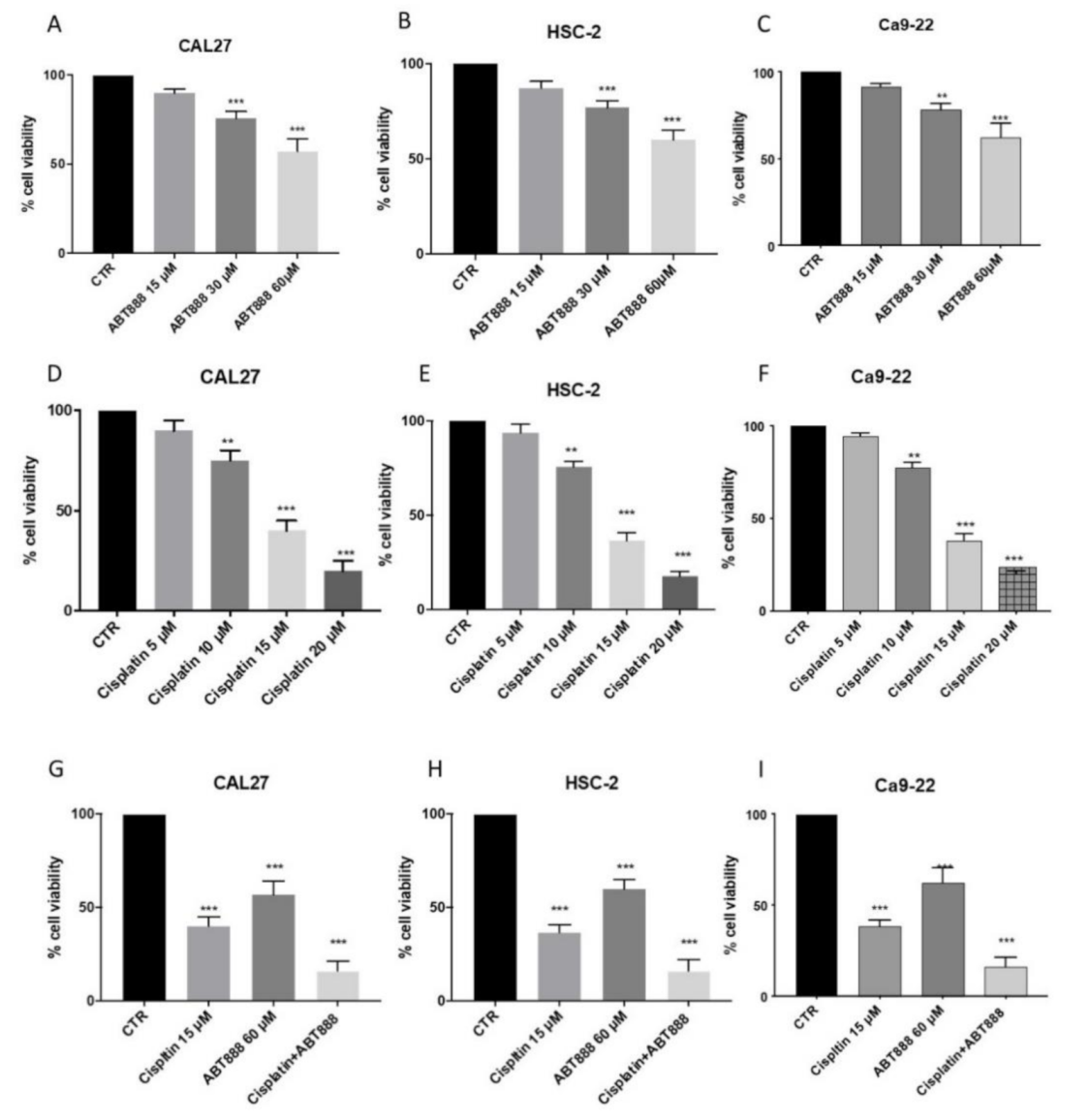
3.1. Effects of ABT888 on Cell Viability
3.2. ABT888 Significantly Enhances the Cytotoxic Effect of Cisplatin in Oral Squamous Cell Carcinoma
3.3. Effects of ABT888 and Cisplatin on Apoptosis
3.4. Effect of ABT888 and Cisplatin on Apoptosis Pathway in CAL27, HSC-2 and Ca9-22 Cells
3.5. Evaluation of Mechanism of Action of ABT888
3.6. Effect of ABT888 and Cisplatin on RAD51 Levels using an Immunofluorescence assay
3.7. Evaluation of BIPV5 and PFT-α on CAL27 Cell Viability Alone or in Association with ABT888 and Cisplatin
3.8. Effect of ABT888 and Cisplatin on Necrosis Pathway
3.9. Effect of ABT888 and Cisplatin on Tumor Growth in CAL27-Xenograft Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bézivin, C.; Tomasi, S.; Lohézic-Le Dévéhat, F.; Boustie, J. Cytotoxic activity of some lichen extracts on murine and human cancer cell lines. Phytomedicine 2003, 10, 499–503. [Google Scholar] [CrossRef]
- Jimenez, L.; Jayakar, S.K.; Ow, T.J.; Segall, J.E. Mechanisms of Invasion in Head and Neck Cancer. Arch. Pathol. Lab. Med. 2015, 139, 1334–1348. [Google Scholar] [CrossRef] [Green Version]
- Shrestha, A.D.; Vedsted, P.; Kallestrup, P.; Neupane, D. Prevalence and incidence of oral cancer in low- and middle-income countries: A scoping review. Eur. J. Cancer Care 2020, 29, e13207. [Google Scholar] [CrossRef]
- Ghantous, Y.; Yaffi, V.; Abu-Elnaaj, I. Oral cavity cancer: Epidemiology and early diagnosis. Refu’at Ha-peh Veha-Shinayim (1993) 2015, 32, 55–63. [Google Scholar]
- Ali, J.; Sabiha, B.; Jan, H.U.; Haider, S.A.; Khan, A.A.; Ali, S.S. Genetic etiology of oral cancer. Oral Oncol. 2017, 70, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Haddad, R.I.; Shin, D.M. Recent Advances in Head and Neck Cancer. N. Engl. J. Med. 2008, 359, 1143–1154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falzone, L.; Salomone, S.; Libra, M. Evolution of Cancer Pharmacological Treatments at the Turn of the Third Millennium. Front. Pharmacol. 2018, 9, 1300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vogelstein, B.; Kinzler, K.W. Cancer genes and the pathways they control. Nat. Med. 2004, 10, 789–799. [Google Scholar] [CrossRef]
- Wang, X.; Weaver, D.T. The ups and downs of DNA repair biomarkers for PARP inhibitor therapies. Am. J. Cancer Res. 2010, 1, 301–327. [Google Scholar]
- Hottiger, M.; Boothby, M.; Koch-Nolte, F.; Lüscher, B.; Martin, N.M.B.; Plummer, R.; Wang, Z.-Q.; Ziegler, M. Progress in the Function and Regulation of ADP-Ribosylation. Sci. Signal. 2011, 4. [Google Scholar] [CrossRef]
- Chaitanya, G.V.; Alexander, J.S.; Babu, P.P. PARP-1 cleavage fragments: Signatures of cell-death proteases in neurodegeneration. Cell Commun. Signal. 2010, 8, 31. [Google Scholar] [CrossRef] [Green Version]
- Michelena, J.; Lezaja, A.; Teloni, F.; Schmid, T.; Imhof, R.; Altmeyer, M. Analysis of PARP inhibitor toxicity by multidimensional fluorescence microscopy reveals mechanisms of sensitivity and resistance. Nat. Commun. 2018, 9, 1–16. [Google Scholar] [CrossRef]
- Plummer, R.; Jones, C.; Middleton, M.; Wilson, R.; Evans, J.; Olsen, A.; Curtin, N.; Boddy, A.; McHugh, P.; Newell, D.; et al. Phase I Study of the Poly(ADP-Ribose) Polymerase Inhibitor, AG014699, in Combination with Temozolomide in Patients with Advanced Solid Tumors. Clin. Cancer Res. 2008, 14, 7917–7923. [Google Scholar] [CrossRef] [Green Version]
- Del Conte, G.; Sessa, C.; Von Moos, R.; Viganò, L.; Digena, T.; Locatelli, A.; Gallerani, E.; Fasolo, A.; Tessari, A.; Cathomas, R.; et al. Phase I study of olaparib in combination with liposomal doxorubicin in patients with advanced solid tumours. Br. J. Cancer 2014, 111, 651–659. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.F.; Tolaney, S.M.; Birrer, M.; Fleming, G.F.; Buss, M.K.; Dahlberg, S.; Lee, H.; Whalen, C.; Tyburski, K.; Winer, E.; et al. A Phase 1 trial of the poly(ADP-ribose) polymerase inhibitor olaparib (AZD2281) in combination with the anti-angiogenic cediranib (AZD2171) in recurrent epithelial ovarian or triple-negative breast cancer. Eur. J. Cancer 2013, 49, 2972–2978. [Google Scholar] [CrossRef] [Green Version]
- Machado, C.B.; Da Silva, E.L.; Filho, M.O.D.M.; De Moraes, M.E.A.; Moreira-Nunes, C.A. PARP Inhibitors as Therapeutic Options for Tyrosine Kinase-dependent Leukemia: A Review. Anticancer. Res. 2020, 40, 3055–3063. [Google Scholar] [CrossRef] [PubMed]
- Joshi, P.M.; Sutor, S.L.; Huntoon, C.J.; Karnitz, L.M. Ovarian Cancer-associated Mutations Disable Catalytic Activity of CDK12, a Kinase That Promotes Homologous Recombination Repair and Resistance to Cisplatin and Poly(ADP-ribose) Polymerase Inhibitors. J. Biol. Chem. 2014, 289, 9247–9253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stopsack, K.H. Efficacy of PARP Inhibition in Metastatic Castration-resistant Prostate Cancer is Very Different with Non-BRCA DNA Repair Alterations: Reconstructing Prespecified Endpoints for Cohort B from the Phase 3 PROfound Trial of Olaparib. Eur. Urol. 2020, 79, 442–445. [Google Scholar] [CrossRef]
- Murai, J.; Zhang, Y.; Morris, J.; Ji, J.; Takeda, S.; Doroshow, J.H.; Pommier, Y. Rationale for Poly(ADP-ribose) Polymerase (PARP) Inhibitors in Combination Therapy with Camptothecins or Temozolomide Based on PARP Trapping versus Catalytic Inhibition. J. Pharmacol. Exp. Ther. 2014, 349, 408–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, P.; Altamura, S.; Boueres, J.; Ferrigno, F.; Fonsi, M.; Giomini, C.; Lamartina, S.; Monteagudo, E.; Ontoria, J.M.; Orsale, M.V.; et al. Discovery of 2-{4-[(3S)-Piperidin-3-yl]phenyl}-2H-indazole-7-carboxamide (MK-4827): A Novel Oral Poly(ADP-ribose)polymerase (PARP) Inhibitor Efficacious in BRCA-1 and -2 Mutant Tumors. J. Med. Chem. 2009, 52, 7170–7185. [Google Scholar] [CrossRef]
- Yasukawa, M.; Fujihara, H.; Fujimori, H.; Kawaguchi, K.; Yamada, H.; Nakayama, R.; Yamamoto, N.; Kishi, Y.; Hamada, Y.; Masutani, M. Synergetic Effects of PARP Inhibitor AZD2281 and Cisplatin in Oral Squamous Cell Carcinoma in Vitro and in Vivo. Int. J. Mol. Sci. 2016, 17, 272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jelinic, P.; Levine, D.A. New Insights into PARP Inhibitors’ Effect on Cell Cycle and Homology-Directed DNA Damage Repair. Mol. Cancer Ther. 2014, 13, 1645–1654. [Google Scholar] [CrossRef] [Green Version]
- Donaldson, J.G. Immunofluorescence Staining. Curr. Protoc. Cell Biol. 2015, 69, 431–437. [Google Scholar] [CrossRef]
- Peyraud, F.; Italiano, A. Combined PARP Inhibition and Immune Checkpoint Therapy in Solid Tumors. Cancers 2020, 12, 1502. [Google Scholar] [CrossRef] [PubMed]
- Cardnell, R.J.; Feng, Y.; Diao, L.; Fan, Y.-H.; Masrorpour, F.; Wang, J.; Shen, Y.; Mills, G.B.; Minna, J.D.; Heymach, J.V.; et al. Proteomic Markers of DNA Repair and PI3K Pathway Activation Predict Response to the PARP Inhibitor BMN 673 in Small Cell Lung Cancer. Clin. Cancer Res. 2013, 19, 6322–6328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ui, T.; Morishima, K.; Saito, S.; Sakuma, Y.; Fujii, H.; Hosoya, Y.; Ishikawa, S.; Aburatani, H.; Fukayama, M.; Niki, T.; et al. The HSP90 inhibitor 17-N-allylamino-17-demethoxy geldanamycin (17-AAG) synergizes with cisplatin and induces apoptosis in cisplatin-resistant esophageal squamous cell carcinoma cell lines via the Akt/XIAP pathway. Oncol. Rep. 2013, 31, 619–624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esposito, E.; Iacono, A.; Muià, C.; Crisafulli, C.; Raso, G.M.; Bramanti, P.; Meli, R.; Cuzzocrea, S. Signal transduction pathways involved in protective effects of melatonin in C6 glioma cells. J. Pineal Res. 2007, 44, 78–87. [Google Scholar] [CrossRef]
- Hsu, H.-W.; De Necochea-Campion, R.; Williams, V.; Duerksen-Hughes, P.J.; Simental, A.A.; Ferris, R.L.; Chen, C.-S.; Mirshahidi, S. Linifanib (ABT-869), enhances cytotoxicity with poly (ADP-ribose) polymerase inhibitor, veliparib (ABT-888), in head and neck carcinoma cells. Oral Oncol. 2014, 50, 662–669. [Google Scholar] [CrossRef]
- Campolo, M.; Casili, G.; Lanza, M.; Filippone, A.; Paterniti, I.; Cuzzocrea, S.; Esposito, E. Multiple mechanisms of dimethyl fumarate in amyloid β-induced neurotoxicity in human neuronal cells. J. Cell. Mol. Med. 2018, 22, 1081–1094. [Google Scholar] [CrossRef]
- Sulaiman, M.K.; Chu, Z.; Blanco, V.M.; Vallabhapurapu, S.D.; Franco, R.S.; Qi, X. SapC-DOPS nanovesicles induce Smac- and Bax-dependent apoptosis through mitochondrial activation in neuroblastomas. Mol. Cancer 2015, 14, 78. [Google Scholar] [CrossRef] [Green Version]
- Sohn, D.; Graupner, V.; Neise, D.; Essmann, F.; Schulzeosthoff, K.; Janicke, R.U. Pifithrin-α protects against DNA damage-induced apoptosis downstream of mitochondria independent of p53. Cell Death Differ. 2009, 16, 869–878. [Google Scholar] [CrossRef] [Green Version]
- Irrera, N.; D’Ascola, A.; Pallio, G.; Bitto, A.; Mannino, F.; Arcoraci, V.; Rottura, M.; Ieni, A.; Minutoli, L.; Metro, D.; et al. β-Caryophyllene Inhibits Cell Proliferation through a Direct Modulation of CB2 Receptors in Glioblastoma Cells. Cancers 2020, 12, 1038. [Google Scholar] [CrossRef] [Green Version]
- Elena, T.; Rosanna, D.P.; Emanuela, M.; Esposito, E.; Virginia, M.; Salvatore, C. Anti-Inflammatory Effects of Adrenomedullin on Acute Lung Injury Induced by Carrageenan in Mice. Mediat. Inflamm. 2012, 2012, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Siracusa, R.; Paterniti, I.; Impellizzeri, D.; Cordaro, M.; Crupi, R.; Navarra, M.; Cuzzocrea, S.; Esposito, E. The Association of Palmitoylethanolamide with Luteolin Decreases Neuroinflammation and Stimulates Autophagy in Parkinson’s Disease Model. CNS Neurol. Disord. Drug Targets 2015, 14, 1350–1366. [Google Scholar] [CrossRef] [PubMed]
- Palma, J.P.; Wang, Y.-C.; Rodriguez, L.E.; Montgomery, D.; Ellis, P.A.; Bukofzer, G.; Niquette, A.; Liu, X.; Shi, Y.; Lasko, L.; et al. ABT-888 Confers Broad In vivo Activity in Combination with Temozolomide in Diverse Tumors. Clin. Cancer Res. 2009, 15, 7277–7290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casili, G.; Campolo, M.; Lanza, M.; Filippone, A.; Scuderi, S.; Messina, S.; Ardizzone, A.; Esposito, E.; Paterniti, I. Role of ABT888, a Novel Poly(ADP-Ribose) Polymerase (PARP) Inhibitor in Countering Autophagy and Apoptotic Processes Associated to Spinal Cord Injury. Mol. Neurobiol. 2020, 57, 4394–4407. [Google Scholar] [CrossRef]
- Waszkowycz, B.; Smith, K.M.; McGonagle, A.E.; Jordan, A.M.; Acton, B.; Fairweather, E.E.; Griffiths, L.A.; Hamilton, N.M.; Hamilton, N.S.; Hitchin, J.R.; et al. Cell-Active Small Molecule Inhibitors of the DNA-Damage Repair Enzyme Poly(ADP-ribose) Glycohydrolase (PARG): Discovery and Optimization of Orally Bioavailable Quinazolinedione Sulfonamides. J. Med. Chem. 2018, 61, 10767–10792. [Google Scholar] [CrossRef]
- Morales, J.C.; Li, L.; Fattah, F.J.; Dong, Y.; Bey, E.A.; Patel, M.; Gao, J.; Boothman, D.A. Review of Poly (ADP-ribose) Polymerase (PARP) Mechanisms of Action and Rationale for Targeting in Cancer and Other Diseases. Crit. Rev. Eukaryot. Gene Expr. 2014, 24, 15–28. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Huang, S.; Zeng, L.; Li, K.; Yang, L.; Gao, S.; Guan, C.; Zhang, S.; Lao, X.; Liao, G.; et al. Necroptosis in head and neck squamous cell carcinoma: Characterization of clinicopathological relevance and in vitro cell model. Cell Death Dis. 2020, 11, 1–17. [Google Scholar] [CrossRef]
- Berghe, T.V.; Linkermann, A.; Jouan, S.; Walczak, H.; Vandenabeele, P. Regulated necrosis: The expanding network of non-apoptotic cell death pathways. Nat. Rev. Mol. Cell Biol. 2014, 15, 135–147. [Google Scholar] [CrossRef]
- Min, A.; Im, S.-A. PARP Inhibitors as Therapeutics: Beyond Modulation of PARylation. Cancers 2020, 12, 394. [Google Scholar] [CrossRef] [Green Version]
- Pascal, J.M. The comings and goings of PARP-1 in response to DNA damage. DNA Repair 2018, 71, 177–182. [Google Scholar] [CrossRef]
- Chuang, H.-C.; Kapuriya, N.; Kulp, S.K.; Chen, C.-S.; Shapiro, C.L. Differential anti-proliferative activities of poly(ADP-ribose) polymerase (PARP) inhibitors in triple-negative breast cancer cells. Breast Cancer Res. Treat. 2012, 134, 649–659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hastak, K.; Alli, E.; Ford, J.M. Synergistic Chemosensitivity of Triple-Negative Breast Cancer Cell Lines to Poly(ADP-Ribose) Polymerase Inhibition, Gemcitabine, and Cisplatin. Cancer Res. 2010, 70, 7970–7980. [Google Scholar] [CrossRef] [Green Version]
- Brenner, J.C.; Feng, F.Y.; Han, S.; Patel, S.; Goyal, S.V.; Bou-Maroun, L.M.; Liu, M.; Lonigro, R.; Prensner, J.; Tomlins, S.A.; et al. PARP-1 Inhibition as a Targeted Strategy to Treat Ewing’s Sarcoma. Cancer Res. 2012, 72, 1608–1613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daniel, R.A.; Rozanska, A.L.; Thomas, H.D.; Mulligan, E.A.; Drew, Y.; Castelbuono, D.J.; Hostomsky, Z.; Plummer, E.R.; Boddy, A.; Tweddle, D.A.; et al. Inhibition of Poly(ADP-Ribose) Polymerase-1 Enhances Temozolomide and Topotecan Activity against Childhood Neuroblastoma. Clin. Cancer Res. 2009, 15, 1241–1249. [Google Scholar] [CrossRef] [Green Version]
- Reyes-Zurita, F.J.; Pachon-Pena, G.; Lizarraga, D.; Rufino-Palomares, E.E.; Cascante, M.; Lupianez, J.A. The natural triterpene maslinic acid induces apoptosis in HT29 colon cancer cells by a JNK-p53-dependent mechanism. BMC Cancer 2011, 11, 154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, Y.; Wu, X. Peg3/Pw1 promotes p53-mediated apoptosis by inducing Bax translocation from cytosol to mitochondria. Proc. Natl. Acad. Sci. USA 2000, 97, 12050–12055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harikumar, K.B.; Kuttan, G.; Kuttan, R. Phyllanthus amarus Inhibits Cell Growth and Induces Apoptosis in Dalton’s Lymphoma Ascites Cells Through Activation of Caspase-3 and Downregulation of Bcl-2. Integr. Cancer Ther. 2009, 8, 190–194. [Google Scholar] [CrossRef]
- Shah, M.; Schwartz, G.K. Cell cycle-mediated drug resistance: An emerging concept in cancer therapy. Clin. Cancer Res. 2001, 7, 2168–2181. [Google Scholar] [PubMed]
- Santarelli, A.; Mascitti, M.; Lo Russo, L.; Sartini, D.; Troiano, G.; Emanuelli, M.; Lo Muzio, L. Survivin-Based Treatment Strategies for Squamous Cell Carcinoma. Int. J. Mol. Sci. 2018, 19, 2168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, G.; Lin, S.-Y. Exploiting the homologous recombination DNA repair network for targeted cancer therapy. World. J. Clin. Oncol. 2011, 2, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Moynahan, M.E.; Jasin, M. Mitotic homologous recombination maintains genomic stability and suppresses tumorigenesis. Nat. Rev. Mol. Cell Biol. 2010, 11, 196–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]









| Gene | Forward Primer 5’-3’ | Reverse Primer 3’-5’ |
|---|---|---|
| Bcl-2 | GAGGATTGTGGCCTTCTTTGAG | AGCCTCCGTTATCCTGGATC |
| Bax | GGACGAACTGGACAGTAACATG | GCAAAGTAGAAAGGGCGGACA |
| p53 | AGAGTCTATAGGCCCACCCC | GCTCGACGTAGGATCTGAC |
| Caspase-3 | CTGAGGCATGGTGAAGAAGGA | GTCCAGTTCTGTACCACGGCA |
| Caspase-9 | TGCGAACTAACAGGCAAGCA | GTCTGAACCTCTCTGGTTTGC |
| Β-actin | GACTTCGAGCAAGAGATGG | AGCACTGTGTGGCGTACAG |
| Antibodies | Catalogue Number | Company | Source | Dilutions |
|---|---|---|---|---|
| Bax | sc-7480 | Santa Cruz Biotechnology; Dallas, TX, USA. | Mouse | 1:500 |
| Bcl2 | sc-7382 | Santa Cruz Biotechnology; Dallas, TX, USA. | Mouse | 1:500 |
| p53 | sc-126 | Santa Cruz Biotechnology; Dallas, TX, USA. | Mouse | 1:500 |
| Caspase-3 | sc-56053 | Santa Cruz Biotechnology; Dallas, TX, USA. | Mouse | 1:500 |
| PARP-1 | sc-8007 | Santa Cruz Biotechnology; Dallas, TX, USA. | Mouse | 1:500 |
| PARG | sc-398563 | Santa Cruz Biotechnology; Dallas, TX, USA. | Mouse | 1:500 |
| Rad51 | sc-398587 | Santa Cruz Biotechnology; Dallas, TX, USA. | Mouse | 1:500 |
| VEGF | sc-7269 | Santa Cruz Biotechnology; Dallas, TX, USA. | Mouse | 1:500 |
| γH2Ax | sc-517336 | Santa Cruz Biotechnology; Dallas, TX, USA. | Mouse | 1:500 |
| β-actin | sc-47778 | Santa Cruz Biotechnology; Dallas, TX, USA. | Mouse | 1:500 |
| MLKL | ab184718 | Abcam, Cambridge, UK. | Rabbit | 1:500 |
| RIP1 | 3493 | Cell Signaling Technology, USA. | Rabbit | 1:500 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paterniti, I.; Scuderi, S.A.; Casili, G.; Lanza, M.; Mare, M.; Giuffrida, R.; Colarossi, C.; Portelli, M.; Cuzzocrea, S.; Esposito, E. Poly (ADP-Ribose) Polymerase Inhibitor, ABT888, Improved Cisplatin Effect in Human Oral Cell Carcinoma. Biomedicines 2021, 9, 771. https://doi.org/10.3390/biomedicines9070771
Paterniti I, Scuderi SA, Casili G, Lanza M, Mare M, Giuffrida R, Colarossi C, Portelli M, Cuzzocrea S, Esposito E. Poly (ADP-Ribose) Polymerase Inhibitor, ABT888, Improved Cisplatin Effect in Human Oral Cell Carcinoma. Biomedicines. 2021; 9(7):771. https://doi.org/10.3390/biomedicines9070771
Chicago/Turabian StylePaterniti, Irene, Sarah Adriana Scuderi, Giovanna Casili, Marika Lanza, Marzia Mare, Raffella Giuffrida, Cristina Colarossi, Marco Portelli, Salvatore Cuzzocrea, and Emanuela Esposito. 2021. "Poly (ADP-Ribose) Polymerase Inhibitor, ABT888, Improved Cisplatin Effect in Human Oral Cell Carcinoma" Biomedicines 9, no. 7: 771. https://doi.org/10.3390/biomedicines9070771
APA StylePaterniti, I., Scuderi, S. A., Casili, G., Lanza, M., Mare, M., Giuffrida, R., Colarossi, C., Portelli, M., Cuzzocrea, S., & Esposito, E. (2021). Poly (ADP-Ribose) Polymerase Inhibitor, ABT888, Improved Cisplatin Effect in Human Oral Cell Carcinoma. Biomedicines, 9(7), 771. https://doi.org/10.3390/biomedicines9070771











