Alginate-Agarose Hydrogels Improve the In Vitro Differentiation of Human Dental Pulp Stem Cells in Chondrocytes. A Histological Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Hydrogel Preparation and Cell Encapsulation
2.2. Rheological and Mechanical Testing
2.3. Cell Isolation and Culture
2.4. Flow Cytometry Characterization of hDPSCs
2.5. Immunofluorescence Staining of Type I Collagen, Type II Collagen, and Aggrecan
2.6. Fluorescence Staining of F-Actin
2.7. Alginate-Agarose Scaffolds Staining with Hematoxylin-Eosin and Sirius Red
2.8. Ki67 Expression Analysis
2.9. Morphometric Analysis of Cell Aggregates
2.10. Relative Gene Expression Analysis
3. Results
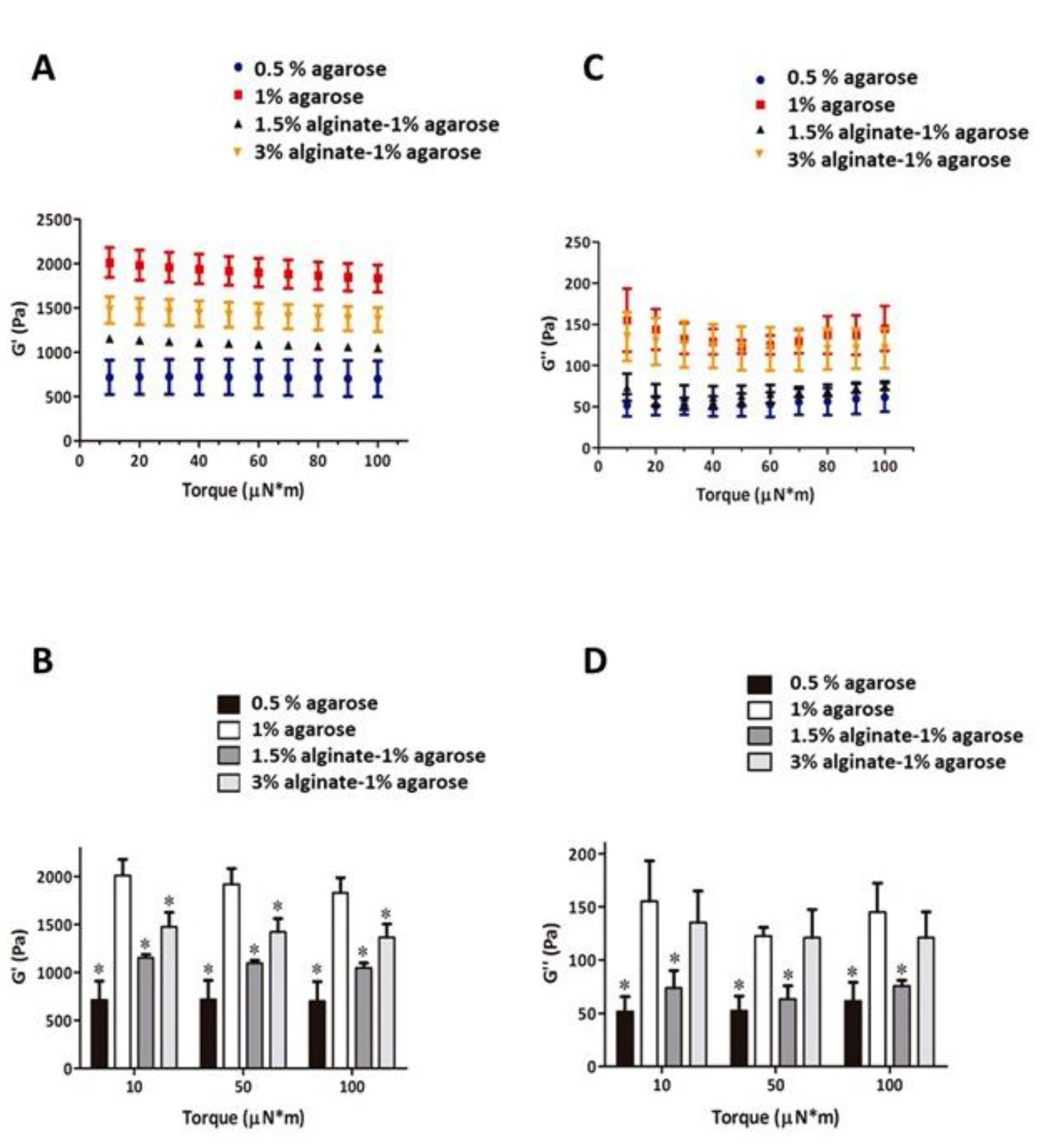
3.1. Mechanical Testing of Alginate-Agarose Hydrogels
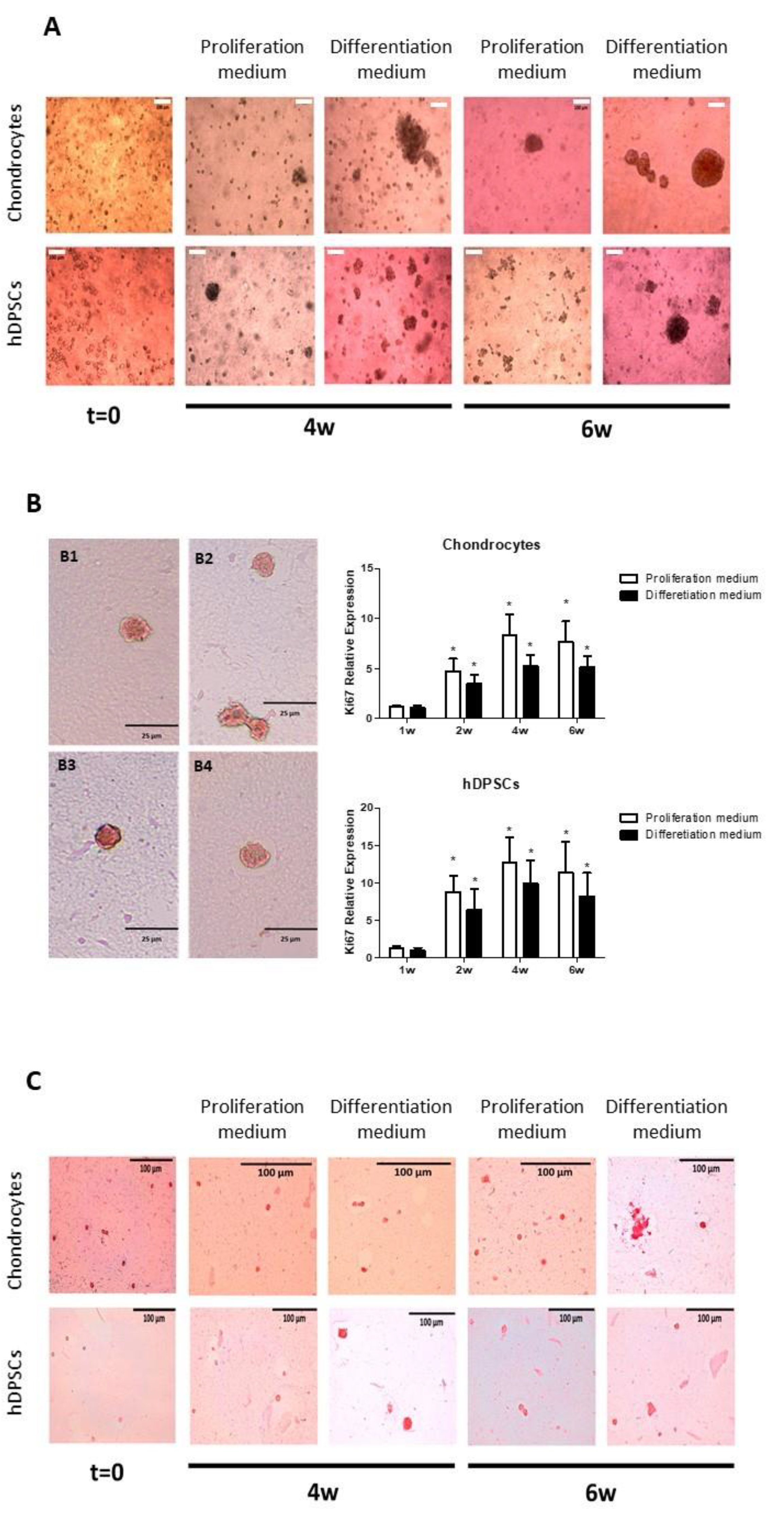
3.2. Cell Characterization and Chondrocyte Differentiation in 2D Culture
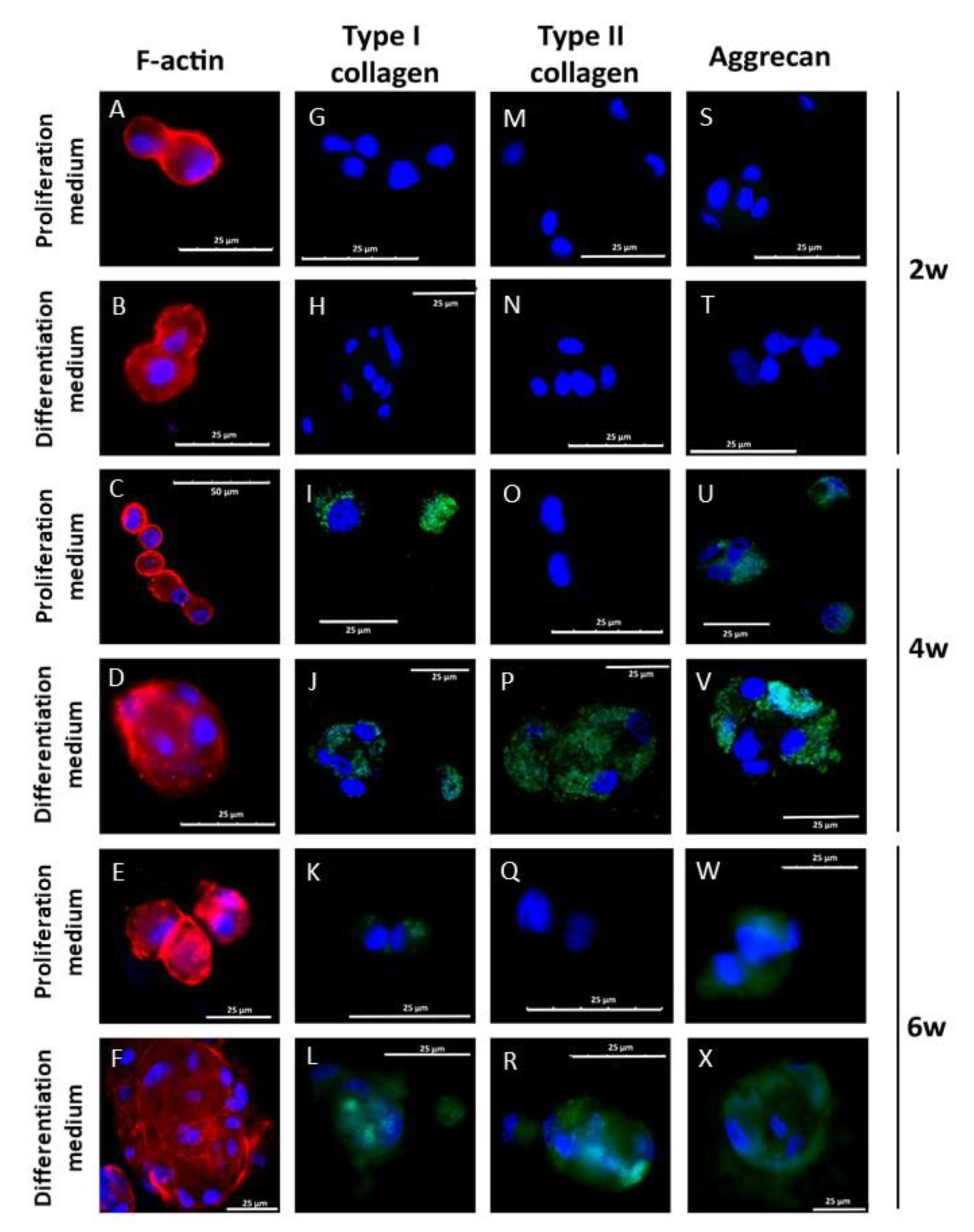
3.3. Cell Encapsulation in Alginate-Agarose Scaffolds. Cell Organization and Synthesis of Collagen
3.4. Chondral Differentiation of Chondrocytes Cultured in Alginate-Agarose Scaffolds
3.5. Chondral Differentiation of hDPSCs Cultured in Alginate-Agarose Scaffolds
3.6. Morphometric Analysis of Cell Aggregates in Alginate-Agarose Scaffolds
3.7. Analysis of the Expression of Chondrogenesis-Related Genes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ross, M.H.; Pawlina, W. Histology: A Text and Atlas: With Correlated Cell and Molecular Biology, 8th ed.; Wolters Kluwer: Philadelphia, PA, USA, 2018. [Google Scholar]
- Chapman, V.; Markides, H.; Sagar, D.R.; Xu, L.; Burston, J.J.; Mapp, P.; Kay, A.; Morris, R.H.; Kehoe, O.; El Haj, A.J. Therapeutic benefit for late, but not early, passage mesenchymal stem cells on pain behaviour in an animal model of osteoarthritis. Stem Cells Int. 2017, 2017, 1–11. [Google Scholar] [CrossRef]
- Johnstone, B.; Alini, M.; Cucchiarini, M.; Dodge, G.R.; Eglin, D.; Guilak, F.; Madry, H.; Mata, A.; Mauck, R.; Semino, C.E.; et al. Tissue engineering for articular cartilage repair—The state of the art. Eur. Cells Mater. 2013, 25, 248–267. [Google Scholar] [CrossRef]
- Clavé, A.; Potel, J.; Servien, E.; Neyret, P.; Dubrana, F.; Stindel, E. Third-generation autologous chondrocyte implantation versus mosaicplasty for knee cartilage injury: 2-year randomized trial. J. Orthop. Res. 2016, 34, 658–665. [Google Scholar] [CrossRef] [Green Version]
- López-Marcial, G.R.; Zeng, A.Y.; Osuna, C.; Dennis, J.; García, J.M.; O’Connell, G.D. Agarose-based hydrogels as suitable bioprinting materials for tissue engineering. ACS Biomater. Sci. Eng. 2018, 4, 3610–3616. [Google Scholar] [CrossRef]
- Khan, F.; Ahmad, S.R. Polysaccharides and their derivatives for versatile tissue engineering application. Macromol. Biosci. 2013, 13, 395–421. [Google Scholar] [CrossRef] [PubMed]
- Steinert, A.F.; Ghivizzani, S.C.; Rethwilm, A.; Tuan, R.S.; Evans, C.H.; Nöth, U. Major biological obstacles for persistent cell-based regeneration of articular cartilage. Arthritis Res. Ther. 2007, 9, 213–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, Y.; Yan, W. Mesenchymal stem cell sheet encapsulated cartilage debris provides great potential for cartilage defects repair in osteoarthritis. Med. Hypotheses 2012, 79, 420–421. [Google Scholar] [CrossRef] [PubMed]
- Heng, B.C.; Cao, T.; Lee, E.H. Directing stem cell differentiation into the chondrogenic lineage in vitro. Stem Cells 2004, 22, 1152–1167. [Google Scholar] [CrossRef] [PubMed]
- Sancilio, S.; Gallorini, M.; Di Nisio, C.; Marsich, E.; Di Pietro, R.; Schweikl, H.; Cataldi, A. Alginate/hydroxyapatite-based nanocomposite scaffolds for bone tissue engineering improve dental pulp biomineralization and differentiation. Stem Cells Int. 2018, 2018, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 13625–13630. [Google Scholar] [CrossRef] [Green Version]
- Gronthos, S.; Brahim, J.; Li, W.; Fisher, L.W.; Cherman, N.; Boyde, A.; DenBesten, P.; Robey, P.G.; Shi, S. Stem cell properties of human dental pulp stem cells. J. Dent. Res. 2002, 81, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Miura, M.; Gronthos, S.; Zhao, M.; Lu, B.; Fisher, L.W.; Robey, P.; Shi, S. SHED: Stem cells from human exfoliated deciduous teeth. Proc. Natl. Acad. Sci. USA 2003, 100, 5807–5812. [Google Scholar] [CrossRef] [Green Version]
- Sakai, K.; Yamamoto, A.; Matsubara, K.; Nakamura, S.; Naruse, M.; Yamagata, M.; Sakamoto, K.; Tauchi, R.; Wakao, N.; Imagama, S.; et al. Human dental pulp-derived stem cells promote locomotor recovery after complete transection of the rat spinal cord by multiple neuro-regenerative mechanisms. J. Clin. Investig. 2012, 122, 80–90. [Google Scholar] [CrossRef]
- Chen, K.; Xiong, H.; Xu, N.; Shen, Y.; Huang, Y.; Liu, C. Chondrogenic potential of stem cells from human exfoliated deciduous teeth in vitro and in vivo. Acta Odontol. Scand. 2014, 72, 664–672. [Google Scholar] [CrossRef]
- Dai, J.; Wang, J.; Lu, J.; Zou, D.; Sun, H.; Dong, Y.; Yu, H.; Zhang, L.; Yang, T.; Zhang, X.; et al. The effect of co-culturing costal chondrocytes and dental pulp stem cells combined with exogenous FGF9 protein on chondrogenesis and ossification in engineered cartilage. Biomaterials 2012, 33, 7699–7711. [Google Scholar] [CrossRef]
- Martín-De-Llano, J.J.; Mata, M.; Peydró, S.; Peydró, A.; Carda, C. Dentin tubule orientation determines odontoblastic differentiation in vitro: A morphological study. PLoS ONE 2019, 14, e0215780. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Lu, Z.; Wu, H.; Li, W.; Zheng, L.; Zhao, J. Collagen-alginate as bioink for three-dimensional (3D) cell printing based cartilage tissue engineering. Mater. Sci. Eng. C 2018, 83, 195–201. [Google Scholar] [CrossRef]
- Costantini, M.; Idaszek, J.; Szöke, K.; Jaroszewicz, J.; Dentini, M.; Barbetta, A.; Brinchmann, J.; Swieszkowski, W. 3D bioprinting of BM-MSCs-loaded ECM biomimetic hydrogels for in vitro neocartilage formation. Biofabrication 2016, 8, 035002. [Google Scholar] [CrossRef] [PubMed]
- Daly, A.; Critchley, S.E.; Rencsok, E.M.; Kelly, D.J. A comparison of different bioinks for 3D bioprinting of fibrocartilage and hyaline cartilage. Biofabrication 2016, 8, 045002. [Google Scholar] [CrossRef]
- Mata, M.; Milian, L.; Oliver, M.; Zurriaga, J.; Sancho-Tello, M.; De Llano, J.J.M.; Carda, C. In vivo articular cartilage regeneration using human dental pulp stem cells cultured in an alginate scaffold: A preliminary study. Stem Cells Int. 2017, 2017, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Rastogi, P.; Kandasubramanian, B. Review of alginate-based hydrogel bioprinting for application in tissue engineering. Biofabrication 2019, 11, 042001. [Google Scholar] [CrossRef]
- Zarrintaj, P.; Bakhshandeh, B.; Rezaeian, I.; Heshmatian, B.; Ganjali, M.R. A novel electroactive agarose-aniline pentamer platform as a potential candidate for neural tissue engineering. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, K.M.; Lee, S.Y.; Joung, Y.K.; Na, J.S.; Lee, M.C.; Park, K.D. Thermosensitive chitosan–Pluronic hydrogel as an injectable cell delivery carrier for cartilage regeneration. Acta Biomater. 2009, 5, 1956–1965. [Google Scholar] [CrossRef] [PubMed]
- Salati, M.A.; Khazai, J.; Tahmuri, A.M.; Samadi, A.; Taghizadeh, A.; Taghizadeh, M.; Zarrintaj, P.; Ramsey, J.D.; Habibzadeh, S.; Seidi, F.; et al. Agarose-based biomaterials: Opportunities and challenges in cartilage tissue engineering. Polymers 2020, 12, 1150. [Google Scholar] [CrossRef] [PubMed]
- Heo, D.N.; Kim, H.-J.; Lee, D.; Kim, H.; Lee, S.J.; Lee, H.-R.; Kwon, I.K.; Do, S.H. Comparison of polysaccharides in articular cartilage regeneration associated with chondrogenic and autophagy-related gene expression. Int. J. Biol. Macromol. 2020, 146, 922–930. [Google Scholar] [CrossRef]
- Selmi, T.A.S.; Verdonk, P.C.M.; Chambat, P.; Dubrana, F.; Potel, J.-F.; Barnouin, L.; Neyret, P. Autologous chondrocyte implantation in a novel alginate-agarose hydrogel. J. Bone Jt. Surg. Br. Vol. 2008, 90, 597–604. [Google Scholar] [CrossRef] [PubMed]
- De Ceuninck, F.; Lesur, C.; Pastoureau, P.; Caliez, A.; Sabatini, M. Culture of chondrocytes in alginate beads. In Cartilage and Osteoarthritis; Sabatini, M., Pastoureau, P., De Ceuninck, F., Eds.; Humana Press: New Jersey, NJ, USA, 2004; pp. 15–22. [Google Scholar]
- Bonhome-Espinosa, A.B.; Campos, F.; Herrera, D.D.; Sánchez-López, J.D.; Schaub, S.; Durán, J.D.; Lopez-Lopez, M.T.; Carriel, V. In vitro characterization of a novel magnetic fibrin-agarose hydrogel for cartilage tissue engineering. J. Mech. Behav. Biomed. Mater. 2020, 104, 103619. [Google Scholar] [CrossRef] [PubMed]
- Mezger, T.G. The Rheology Handbook: For Users of Rotational and Oscillatory Rheometers, 2nd ed.; Vincentz Network Publishing: Hannover, Germany, 2006. [Google Scholar]
- Prihantono, P.; Hatta, M.; Binekada, C.; Sampepajung, D.; Haryasena, H.; Nelwan, B.; Islam, A.A.; Usman, A.N. Ki-67 expression by immunohistochemistry and quantitative real-time polymerase chain reaction as predictor of clinical response to neoadjuvant chemotherapy in locally advanced breast cancer. J. Oncol. 2017, 2017, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogura, T.; Tsuchiya, A.; Minas, T.; Mizuno, S. Methods of high integrity RNA extraction from cell/agarose construct. BMC Res. Notes 2015, 8, 644. [Google Scholar] [CrossRef] [Green Version]
- Milian, L.; Mata, M.; Alcacer, J.; Oliver, M.; Sancho-Tello, M.; De Llano, J.J.M.; Camps, C.; Galbis, J.; Carretero, J.; Carda, C. Cannabinoid receptor expression in non-small cell lung cancer. Effectiveness of tetrahydrocannabinol and cannabidiol inhibiting cell proliferation and epithelial-mesenchymal transition in vitro. PLoS ONE 2020, 15, e0228909. [Google Scholar] [CrossRef] [Green Version]
- Masuda, K.; Sah, R.L.; Hejna, M.J.; Thonar, E.J.-M.A. A novel two-step method for the formation of tissue-engineered cartilage by mature bovine chondrocytes: The alginate-recovered-chondrocyte (ARC) method. J. Orthop. Res. 2003, 21, 139–148. [Google Scholar] [CrossRef]
- Re’Em, T.; Tsur-Gang, O.; Cohen, S. The effect of immobilized RGD peptide in macroporous alginate scaffolds on TGFβ1-induced chondrogenesis of human mesenchymal stem cells. Biomaterials 2010, 31, 6746–6755. [Google Scholar] [CrossRef]
- Escobar, L.; Ivirico, M.; Kuyinu, E.; Nairab, L.S.; Laurencin, C.T. Regenerative engineering for knee osteoarthritis treatment: Biomaterials and cell-based technologies. Engineering 2017, 3, 16–27. [Google Scholar] [CrossRef]
- Jakob, M.; Démarteau, O.; Schäfer, D.; Hintermann, B.; Dick, W.; Heberer, M.; Martin, I. Specific growth factors during the expansion and redifferentiation of adult human articular chondrocytes enhance chondrogenesis and cartilaginous tissue formation in vitro. J. Cell. Biochem. 2001, 81, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Von Der Mark, K.; Gauss, V.; Von Der Mark, H.; Müller, P. Relationship between cell shape and type of collagen synthesised as chondrocytes lose their cartilage phenotype in culture. Nat. Cell Biol. 1977, 267, 531–532. [Google Scholar] [CrossRef]
- Lin, P.-S.; Chang, H.-H.; Yeh, C.-Y.; Chang, M.-C.; Chan, C.-P.; Kuo, H.-Y.; Liu, H.-C.; Liao, W.-C.; Jeng, P.-Y.; Yeung, S.-Y.; et al. Transforming growth factor beta 1 increases collagen content and stimulates procollagen I and tissue inhibitor of metalloproteinase-1 production of dental pulp cells: Role of MEK/ERK and activin receptor-like kinase-5/smad signaling. J. Formos. Med. Assoc. 2017, 116, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Belflower, R.M.; Dong, Y.-F.; Schwarz, E.M.; O’Keefe, R.J.; Drissi, H. Runx1/AML1/Cbfa2 mediates onset of mesenchymal cell differentiation toward chondrogenesis. J. Bone Miner. Res. 2005, 20, 1624–1636. [Google Scholar] [CrossRef] [PubMed]
- Lian, J.B.; Balint, E.; Javed, A.; Drissi, H.; Vitti, R.; Quinlan, E.J.; Zhang, L.; Van Wijnen, A.J.; Stein, J.L.; Speck, N.; et al. Runx1/AML1 hematopoietic transcription factor contributes to skeletal development in vivo. J. Cell. Physiol. 2003, 196, 301–311. [Google Scholar] [CrossRef]
- Yano, F.; Ohba, S.; Murahashi, Y.; Tanaka, S.; Saito, T.; Chung, U.-I. Runx1 contributes to articular cartilage maintenance by enhancement of cartilage matrix production and suppression of hypertrophic differentiation. Sci. Rep. 2019, 9, 7666. [Google Scholar] [CrossRef]
- Knuth, C.A.; Kiernan, C.H.; Cabeza, V.P.; Lehmann, J.; Witte-Bouma, J.; Berge, D.T.; Brama, P.A.; Wolvius, E.B.; Strabbing, E.M.; Koudstaal, M.; et al. Isolating pediatric mesenchymal stem cells with enhanced expansion and differentiation capabilities. Tissue Eng. Part C Methods 2018, 24, 313–321. [Google Scholar] [CrossRef]
- Bachmann, B.; Spitz, S.; Schädl, B.; Teuschl, A.; Redl, H.; Nürnberger, S.; Ertl, P. Stiffness matters: Fine-tuned hydrogel elasticity alters chondrogenic redifferentiation. Front. Bioeng. Biotechnol. 2020, 8, 373. [Google Scholar] [CrossRef] [PubMed]
- Hoemann, C.; Sun, J.; Légaré, A.; McKee, M.; Buschmann, M. Tissue engineering of cartilage using an injectable and adhesive chitosan-based cell-delivery vehicle. Osteoarthr. Cartil. 2005, 13, 318–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeLise, A.; Fischer, L.; Tuan, R. Cellular interactions and signaling in cartilage development. Osteoarthr. Cartil. 2000, 8, 309–334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Limraksasin, P.; Kosaka, Y.; Zhang, M.; Horie, N.; Kondo, T.; Okawa, H.; Yamada, M.; Egusa, H. Shaking culture enhances chondrogenic differentiation of mouse induced pluripotent stem cell constructs. Sci. Rep. 2020, 10, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Solorio, L.D.; Fu, A.S.; Hernández-Irizarry, R.; Alsberg, E. Chondrogenic differentiation of human mesenchymal stem cell aggregates via controlled release of TGF-β1 from incorporated polymer microspheres. J. Biomed. Mater. Res. Part A 2009, 92, 1139–1144. [Google Scholar] [CrossRef] [Green Version]
- Furukawa, K.S.; Suenaga, H.; Toita, K.; Numata, A.; Tanaka, J.; Ushida, T.; Sakai, Y.; Tateishi, T. Rapid and large-scale formation of chondrocyte aggregates by rotational culture. Cell Transplant. 2003, 12, 475–479. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.-C.; Lin, C.-Y.; Wang, H.-S.; Lyu, S.-R. Matrix metalloproteases and tissue inhibitors of metalloproteinases in medial plica and pannus-like tissue contribute to knee osteoarthritis progression. PLoS ONE 2013, 8, e79662. [Google Scholar] [CrossRef] [Green Version]





| Gene ID | Gene Name | Assay Number | 2D or 3D Cultures |
|---|---|---|---|
| ACAN | Aggrecan | Hs00153936_m1 | 2D and 3D |
| COL10A1 | Collagen Type X Alpha 1 Chain | Hs00166657_m1 | 2D and 3D |
| COL1A1 | Collagen Type I Alpha 1 Chain | Hs00164004_m1 | 2D and 3D |
| COL2A1 | Collagen Type II Alpha 1 Chain | Hs00264051_m1 | 2D and 3D |
| GAPDH | Glyceraldehyde-3-Phosphate Dehydrogenase | 4325793 | 2D and 3D |
| HIF1A | Hypoxia Inducible Factor 1 Subunit Alpha | Hs00153153_m1 | 3D |
| MMP1 | Matrix Metallopeptidase 1 | Hs00899658_m1 | 3D |
| MMP13 | Matrix Metallopeptidase 13 | Hs00233992_m1 | 3D |
| MMP2 | Matrix Metallopeptidase 2 | Hs01548727_m1 | 3D |
| RUNX1 | RUNX Family Transcription Factor 1 | Hs01021971_m1 | 3D |
| SOX5 | SRY-Box Transcription Factor 5 | Hs01552796_m1 | 3D |
| SOX6 | SRY-Box Transcription Factor 6 | Hs00264525_m1 | 3D |
| SOX9 | SRY-Box Transcription Factor 9 | Hs00165814_m1 | 2D and 3D |
| VEGFA | Vascular Endothelial Growth Factor A | Hs00900055_m1 | 2D and 3D |
| [Alginate] (%) | [Agarose] (%) | G’ (Pa) | G’’ (Pa) | E (Pa) |
|---|---|---|---|---|
| 1.5 | 0 | NA | NA | NA |
| 3 | 0 | NA | NA | NA |
| 0 | 0.5 | 718.09 ± 199.03 | 52.41 ± 13.74 | 2160.01 |
| 0 | 1 | 1856.02 ± 212.39 | 123.31 ± 7.36 | 5580.34 |
| 1.5 | 1 | 1177.78 ± 104.23 | 72.08 ± 14.49 | 3539.95 |
| 3 | 1 | 1247.27 ± 210.81 | 101.78 ± 27.27 | 3754.26 |
| Chondrocytes | hDPSCs | |||
|---|---|---|---|---|
| PM | DM | PM | DM | |
| Number of aggregates (n) | 8 ± 0.7 | 93 ± 11.2 (p = 0.009) * | 73 ± 0.6 | 38 ± 0.2 (p = 0.0009) * |
| Number of cells per aggregate (n) | 4.9 ± 4.9 | 6.9 ± 1.4 (p = 0.219) | 4.0 ± 0.4 | 6.8 ± 3.1 (p = 0.108) |
| MaximumØ of aggregates (µm) | 42.5 ± 30.4 | 55.8 ± 8.8 (p = 0.205) | 29.2 ± 2.5 | 50.2 ± 16.9 (p = 0.044) * |
| Numberof non-aggregate-forming cells (n) | 61 ± 8.2 | 154 ±17.9 (p = 0.033) * | 247 ± 22 | 93 ± 8.2 (p = 0.0001) * |
| Total number of cells (n) | 107 ± 11 | 817 ± 76 (p = 0.007) * | 547 ± 37 | 287 ± 21 (p = 0.0003) * |
| CellØ (µm) | 33.6 ± 4.8 | 32.4 ± 3.4 (p = 0.544) | 24.4 ± 1.9 | 27.6 ± 7.2 (p = 0.152) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliver-Ferrándiz, M.; Milián, L.; Sancho-Tello, M.; Martín de Llano, J.J.; Gisbert Roca, F.; Martínez-Ramos, C.; Carda, C.; Mata, M. Alginate-Agarose Hydrogels Improve the In Vitro Differentiation of Human Dental Pulp Stem Cells in Chondrocytes. A Histological Study. Biomedicines 2021, 9, 834. https://doi.org/10.3390/biomedicines9070834
Oliver-Ferrándiz M, Milián L, Sancho-Tello M, Martín de Llano JJ, Gisbert Roca F, Martínez-Ramos C, Carda C, Mata M. Alginate-Agarose Hydrogels Improve the In Vitro Differentiation of Human Dental Pulp Stem Cells in Chondrocytes. A Histological Study. Biomedicines. 2021; 9(7):834. https://doi.org/10.3390/biomedicines9070834
Chicago/Turabian StyleOliver-Ferrándiz, María, Lara Milián, María Sancho-Tello, José Javier Martín de Llano, Fernando Gisbert Roca, Cristina Martínez-Ramos, Carmen Carda, and Manuel Mata. 2021. "Alginate-Agarose Hydrogels Improve the In Vitro Differentiation of Human Dental Pulp Stem Cells in Chondrocytes. A Histological Study" Biomedicines 9, no. 7: 834. https://doi.org/10.3390/biomedicines9070834
APA StyleOliver-Ferrándiz, M., Milián, L., Sancho-Tello, M., Martín de Llano, J. J., Gisbert Roca, F., Martínez-Ramos, C., Carda, C., & Mata, M. (2021). Alginate-Agarose Hydrogels Improve the In Vitro Differentiation of Human Dental Pulp Stem Cells in Chondrocytes. A Histological Study. Biomedicines, 9(7), 834. https://doi.org/10.3390/biomedicines9070834










